Abstract
Background
Decision support interventions have been developed to help men clarify their values and make informed decisions about prostate cancer testing, but they seldom target high-risk black and immigrant men.
Purpose
This study evaluated the efficacy of a decision support intervention focused on prostate cancer testing in a sample of predominantly immigrant black men.
Methods
Black men (N = 490) were randomized to tailored telephone education about prostate cancer testing or a control condition.
Results
Post-intervention, the intervention group had significantly greater knowledge, lower decision conflict, and greater likelihood of talking with their physician about prostate cancer testing than the control group. There were no significant intervention effects on prostate specific antigen testing, congruence between testing intention and behavior, or anxiety.
Conclusions
A tailored telephone decision support intervention can promote informed decision making about prostate cancer testing in black and predominantly immigrant men without increasing testing or anxiety.
Clinical trial
Registered in clinicaltrials.gov (NCT01415375)
Keywords: Prostate cancer screening, Patient education, Screening, African Americans, Immigrant, Informed decision making, Behavioral intervention, Randomized controlled trial
Introduction
Prostate cancer accounts for 29% of new cancers and 9% of cancer deaths among American men (1). Globally, African American and African-Caribbean men have the greatest prostate cancer incidence and mortality rate (2, 3). Early detection would appear to be a prudent preventive strategy for men of African descent, but there is insufficient evidence that the benefits of prostate cancer testing outweigh the harms (4). For example, while preliminary evidence on whether prostate cancer screening reduces mortality is mixed (5, 6), it is clear that screening can increase the odds that prostate cancer will be detected and that some men will receive invasive treatments for a cancer that would never become clinically apparent (6, 7).
Medical experts and professional organizations send mixed messages regarding prostate cancer testing, creating some confusion among patients and their physicians. The United States Preventive Services Task Force recommends against prostate-specific antigen- (PSA-) based screening in men, regardless of age (4). Other organizations recommend that men of a certain age should be informed about the risks and benefits of prostate cancer testing and share in decisions about testing based on their personal preferences (8–11). Effective and acceptable methods of promoting informed and preference-sensitive (12) testing decisions in high-risk black populations are just beginning to be explored (13, 14), and the present study adds to this literature.
In addition to racial disparities in prostate cancer-related morbidity and mortality, there are racial disparities in prostate cancer testing, awareness, and knowledge. In one study, fewer black (40%) than white (61%) men had heard of the PSA test (15). A study using Medicare claims data found the annual rate of PSA testing was 38% among white men versus 31% among black men (16). Another study found evidence that black men underestimate their prostate cancer risk (17). Thus, it is imperative to develop practical, effective methods for educating black men about prostate cancer risks and the evidence on potential benefits and harms of testing.
Numerous interventions have been developed to support men in their prostate cancer testing decisions. A systematic review (18) concluded that such interventions improve knowledge, decision conflict, and patient involvement in decision making related to prostate cancer testing. The interventions also appear to reduce prostate cancer testing interest and behavior among patients seeking routine care, but not among those specifically seeking testing. Most of the reviewed trials used clinic-based and predominantly white samples. Results from the few studies that have tested decision support interventions with black men converge with some of the findings in the review (13, 14). To our knowledge, there have been no decision support trials that include a substantial sample of high-risk, immigrant black men.
The apparently robust beneficial effects of decision support interventions on knowledge and decision conflict suggest that progress has been made in this area. However, research to date has failed to show whether interventions increase the odds that men will act according to their preferences to engage in testing or not. Within the medical community, there has been growing recognition that procedures such as prostate cancer testing, which involve options and significant tradeoffs in terms of positive and negative outcomes associated with those options, should not be implemented without taking patients’ preferences into account (12). Yet there are no published trials that use objective prostate cancer testing outcomes (e.g., medical claims of PSA testing) to examine the congruence between men’s reported testing preferences and actual behaviors. The current study addresses this significant gap in the literature.
Conceptual Framework for the Study
We developed and tested a decision support intervention based on the Ottawa Decision Support Framework, which provides an approach to supporting individuals in making high-quality decisions that are informed and consistent with their values (19). In the context of prostate cancer testing, we would add that a high-quality decision is one that is consistent with men’s preferences (20). The Ottawa framework identifies determinants of sub-optimal healthcare decisions that may be modified by decision support interventions, including: problems with perceptions of the decision (e.g., inadequate knowledge, unclear values, decisional conflict), perceptions of others (e.g., limited knowledge of others’ opinions and practices, inadequate support), and personal and external resources to make the decision (e.g., ability to talk with a physician) (21). The present intervention addressed these problematic determinants of prostate cancer testing decisions.
The intervention included print education material, discussions with a health educator, and a values clarification exercise. The different intervention components addressed men’s knowledge, values and decision conflict, and increased their ability and motivation to talk with a physician about testing. The education materials, along with the attention and encouragement by the interventionist, also informed men about the perception of prostate cancer testing among other men and medical experts and provided a source of support during their decision-making process. The project was conducted in collaboration with the participants’ labor union, so there was an inherent trust built into the communications.
Because prostate cancer testing is not universally recommended, intervention materials and communications were designed to provide accurate, balanced information that would not bias men for or against testing. The aim was to provide the information, exercises (e.g., values clarification) and encouragement that men would need to make an informed decision about testing that was consistent with their values. The intervention also aimed for efficiency and greatest impact by providing information that was tailored to men’s needs. Importantly, the intervention encouraged men to talk with their physician about prostate cancer testing and their decision to test or not, since there may be other medical considerations men should take into account in relation to this decision.
Hypotheses
The primary study outcomes were knowledge about prostate cancer and prostate cancer testing, decision conflict, talking with a physician about testing, and congruence between testing preferences and behaviors. We hypothesized that relative to men randomized to an attention control condition, men randomized to a prostate cancer decision support intervention condition would have: greater gains in knowledge about prostate cancer and prostate cancer testing; lower decision conflict; greater likelihood of talking with their doctor about prostate cancer testing; and greater likelihood of acting on their intentions to test.
Secondary study outcomes included state anxiety, rates of PSA testing, and perceived importance of risks versus benefits of prostate cancer testing. State anxiety was measured as a potential adverse effect of educating high-risk men about their risk for prostate cancer. Based on prior research (22), we hypothesized that there would be no significant differences in anxiety between conditions. PSA testing and the perceived importance of risks versus benefits of prostate cancer testing were measured to evaluate if the intervention biased men with respect to their attitudes or behaviors related to prostate cancer testing. The aim of the intervention was to provide men with an accurate and balanced perspective on the potential benefits and harms of prostate cancer testing, rather than promoting or discouraging testing. Thus, we hypothesized that there would be no significant difference between conditions in rate of PSA testing or perceived importance of risks versus benefits of prostate cancer testing.
Finally, the study included exploratory analyses of potential covariates and intervention moderators, including past history of PSA testing, education level, immigrant status, marital status, and age. Prior research (23, 24) has shown a positive association between prostate cancer testing and education, age, being married, and prior PSA testing. In the United States, immigrant men appear to be less likely to get tested for prostate cancer than non-immigrant men (25).
Method
Design
A two-group randomized controlled trial design with pretest and posttest measures was used. Participants were randomized to a decision support intervention consisting of tailored telephone education about prostate cancer testing (experimental) or fruit and vegetable consumption (attention control). Participants also received an educational pamphlet. Trained interviewers used a structured telephone interview to collect self-report data before and eight months after randomization. Medical claims for physician visits and PSA testing were tracked for 2-years post-enrollment. Participants received $20 cash or equivalent gift card. An Institutional Review Board approved the study and participants provided informed consent.
Setting and Participants
The sampling frame was constructed from a list of health insurance beneficiaries of a large healthcare workers’ union in the New York City area. Inclusion criteria included: accessible by telephone, have primary care physician, between 45- to 70-years old, and of black African descent. New York has the highest concentration of black persons in the United States (26), and nearly one-third migrated from the Caribbean (27). Exclusion criteria included a prostate cancer test in the 12 months before enrollment and history of prostate cancer.
Enrollment and Sample Size Calculations
Potential participants were randomly drawn from the sampling frame and recruited using advance letters and reply cards. Between June 2005 and August 2006, eligible participants were consented and then completed the pretest survey by phone. Following each pretest survey, the data collection interviewer emailed the Principal Investigator (SL) the contact information of the enrolled participant. The Principal Investigator used a computer-generated randomization schedule to randomize the participant and emailed the randomization assignment to the interventionist. Data collectors were blind to condition but the interventionists were not.
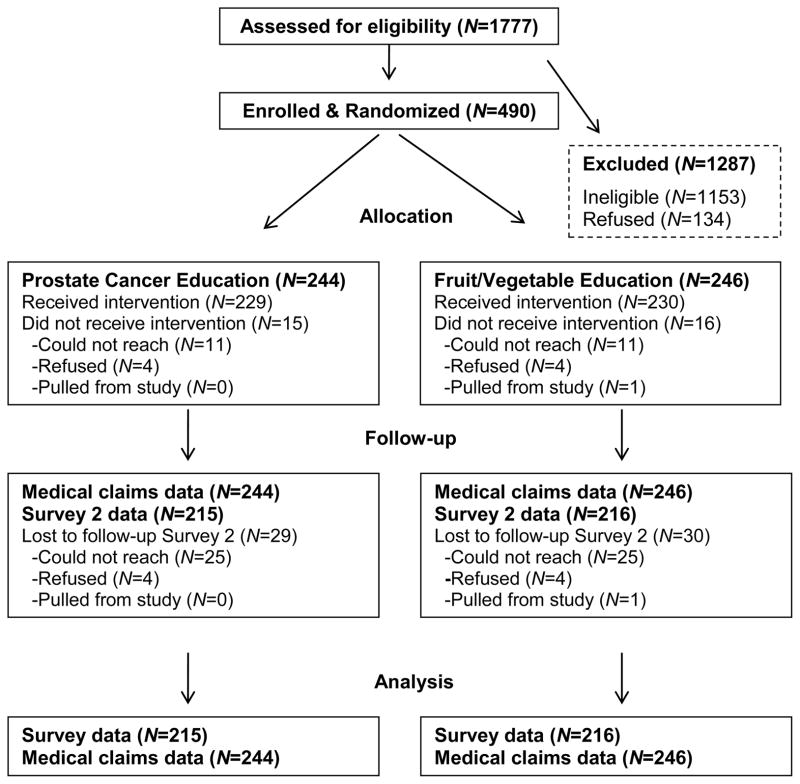
To determine sample size, we assumed an intervention effect size of .30 on primary outcomes based on prior studies (22, 28). This effect size, with 80% power and alpha set at .05 (two-tailed), required a sample of 350 (29). A sample of 490 allowed for attrition and detection of smaller effects. Randomization was conducted within three age strata (45–49, 50–54, and 55–70 years old) using the PLAN procedure of SAS (Cary, NC). The participant flow is shown in Figure 1. The response rate among eligible men was 78.5% (490/624). Attrition was low (12%) and did not vary by condition. Most (93.6%) participants received their allocated intervention, but a few could not be reached by telephone. Medical claims data on prostate cancer testing and physician visits were 100% complete.
Figure 1.
Participant flow.
Interventions
Overview
In addition to a condition-specific educational pamphlet, participants received a maximum of two tailored telephone education calls within one-month (median = 1 week) by trained graduate-level health educators. The initial call lasted an average of 20 minutes (median = 20 minutes; SD = 2.3 minutes) with a brief follow-up call (mean and median = 5 minutes; SD = 6.3 minutes) a week later (a few men could not be reached for the follow-up call: 11% in control group; 14% in experimental group). Treatment fidelity checks were conducted on 44% of calls. Trained raters listened to audiotaped calls and checked whether key points were covered and the interventionist spoke at an appropriate pace, addressed questions and probed appropriately. Mean treatment fidelity ratings were high (>99% adherence) in both conditions.
Experimental intervention
Immediately after randomization, men in the intervention group were mailed a pamphlet, Prostate Cancer: Your Life-You Decide (30), which depicts black and white physicians and laypersons describing the advantages and disadvantages of prostate cancer testing. It includes information about prostate cancer risk factors and prostate cancer tests, including potential risks and benefits of testing, and was designed for men with low literacy.
The telephone intervention began a week after randomization. The interventionists were graduate students with training in public health and health education. The Principal Investigator (SL) trained them on the intervention protocols, which were manualized. Training covered important interviewing techniques (31), such as acting professional, being polite (e.g., not interrupting), using probes (e.g., repeating a question), and avoiding leading questions. Training techniques included lectures, role-playing and feedback on recorded intervention sessions.
The telephone component of the intervention aimed to establish a rapport, provide tailored and balanced information about prostate cancer risk and tests, help men to clarify testing preferences, and prepare men to talk with their physician about prostate cancer. The emphasis on education about risk, education about the potential benefits and harms of prostate cancer screening, values clarification, and shared decision making, is consistent with practice guideline and decision support theory. Ultimately, men must decide what to do based on knowledge of the facts and their personal preferences and values. Men who are unaware of their prostate cancer risk and the potential benefits and risks of screening options are not in a position to make a quality decision about their healthcare or to exercise self-determination with respect to managing their risk. Further, because the screening decision is so complex and may be influenced by other health considerations, it is important for men to communicate with their physician about their preferences and decision. We believe that men will benefit most from decision support counseling that is administered before they talk with their physician, because most physicians do not have the time to offer the kind of detailed information that men may need. The key elements of the intervention are briefly described below.
Rapport building
Techniques to build rapport included informing men about what to expect during the study, reminding men that their opinions are valued, listening and reflecting back on what men say, and allowing men to contribute to the direction and flow of discussion.
Tailoring on knowledge and beliefs
To facilitate tailoring, the interventionist briefly assessed knowledge and beliefs related to prostate cancer and testing. The interventionist asked questions that aimed to uncover misconceptions about prostate cancer, testing and treatment options, and risk factors. For example, men were asked, “Do most men with prostate cancer die from it, or do they mostly die from other causes?” “Is it possible to have prostate cancer and not know it?” “How can a doctor know if you have prostate cancer?” When men gave an incorrect or partial response to a question, the interventionist provided tailored information to correct men’s misconceptions and probed to make sure men understood the information.
Values clarification
The interventionist described five potential risks and five potential benefits of prostate cancer testing. A sample risk item is: “Some men and doctors are concerned that medical science has not proved that prostate cancer tests save lives. So, there is a risk that testing may lead you to get treatment for a disease that would not kill you.” After each potential risk, the participant rated whether it made him less interested in getting tested (0 = no, 1 = yes, a little bit less interested, 2 = yes, a lot less interested). A sample benefit item is: “If prostate cancer is found at an early stage through testing, you may have more treatment options.” After each potential benefit, the participant rated whether it made him more interested in getting tested (0 = no, 1 = yes, a little bit more interested, 2 = yes, a lot more interested). After rating all the risks and benefits, the participant was asked: “In thinking of your choice to get tested or not, would you say that the benefits outweigh the risks of testing, the risks outweigh the benefits of testing, or they are equally important to you?”
Importance of talking with a physician
The interventionist explained that the decision to test for prostate cancer is an individual one, but one that is best done in consultation with a physician. The interventionist also explained that there might be reasons why the physician would or would not recommend prostate cancer testing after hearing the participant’s views on testing. Then the interventionist attempted to elicit a commitment from the participant to contact his physician. Probes were provided to gauge level of interest and confidence in making an appointment with the physician. If interest and confidence were low, the interventionist probed further and tried to discuss ways in which the participant could focus on motivators and overcome potential barriers.
Follow-up
A follow-up telephone call was scheduled. The interventionist indicated that the call would provide the participant an opportunity to address any questions that might arise after thinking about their discussion or any of the information in the brochure.
Attention control intervention
The attention control intervention used tailored telephone education to increase men’s knowledge of and adherence to national guidelines related to fruit and vegetable intake. Details are available elsewhere (32).
Measures
Demographics and medical history (pretest)
Self-reported demographic variables included age, race, education, immigrant and marital status. Self-reported medical history variables included whether participants had talked with a doctor about prostate cancer, whether a doctor had recommended any prostate cancer tests, and whether they had received prostate cancer tests. We also collected medical claims data on any history of PSA testing prior to enrollment.
Knowledge (pretest and posttest)
Knowledge was assessed with a 14-item index. All items were covered in the pamphlet, Prostate Cancer: Your Life-You Decide (30). Six items focused on testing (e.g., “If you have an abnormal test result on a prostate cancer test, does that mean you definitely have cancer?”), five on risk factors and epidemiology (e.g., “Are black or African American men more likely to get prostate cancer than other men?”), and three on treatment effectiveness and side effects (e.g., “Do some treatments for prostate cancer cause urinary problems?”). All items were subject to pilot testing using cognitive interviewing techniques to ensure comprehension. An expert panel of prostate cancer researchers and clinicians judged the items to be valid indicators of knowledge about prostate cancer and prostate cancer testing. Percent correct was used as the outcome measure.
Decisional conflict (posttest)
Decision conflict related to prostate cancer testing was measured using a modified version of the validated Decisional Conflict Scale (33). The 16-item version of the scale was used with the 3-level response category suggested for low-literacy populations (0 = yes; 2 = unsure or don’t know; and 4 = no). Decision conflict was assessed only at posttest because pilot testing revealed the scale was reactive (i.e., prompted men to ask questions about testing). Many men (N = 81) were still undecided about testing at posttest, so they could not answer items 10–16 which presume a completed decision. These 7 items were dropped along with items 6 and 8 in order to bring reliability up to an acceptable level (Cronbach’s alpha = .62). The remaining 7 items were averaged and multiplied by 25 to create a single score (range 0–100), with higher scores indicating a higher level of decision conflict.
Verified physician visit to discuss testing (posttest)
At pretest, participants reported whether they had ever talked with a physician about prostate cancer testing. At posttest, they reported whether they had visited their primary care physician and discussed testing since the pretest interview. A self-reported visit to discuss prostate cancer between pretest and posttest was counted if it could be verified using medical claims. Based on responses to these face-valid questions, a variable was created to indicate if men visited a physician to discuss prostate cancer testing for the first time during the intervention period (0 = no, 1 = yes).
Testing intention, benefits-to-risk ratio of testing, and verified PSA testing (posttest)
At posttest, men were asked whether they had “decided to get tested in the future for prostate cancer” (no/yes). This measure of testing intention indicates men’s preference for testing or not testing. They also were asked: “In thinking of your choice to get tested or not, would you say that the benefits of testing outweigh the risks, the risks of testing outweigh the benefits, or are they equally important to you?” To assess PSA testing objectively, medical claims were scanned for PSA procedure codes using an expert system. The report generator was blind to participants’ study condition. Claims data were retrieved for up to 2 years after pretest, allowing us to calculate cumulative 1- and 2-year follow-up PSA testing rates. Digital rectal examination (DRE) could not be verified because the test is not specific to prostate cancer testing.
Congruence (posttest)
Congruence between men’s stated intention to get tested and their actual testing behavior was measured with a categorical variable. Self-reports of men’s intention to get a PSA test at posttest were compared with the medical claims of PSA testing at 1- and 2-year follow-up. Intention-behavior agreement was coded as congruent (“1”), whereas disagreement was coded as incongruent (“0”).
Anxiety (pretest and posttest)
State anxiety was measured with a 7-item subscale of the Hospital Anxiety and Depression Scale (34). Participants rated how often they had specific anxious feelings over the prior week. Response options varied by item but were scored from 0 to 3. Total scores could range from 0 (no anxiety) to 21 (maximum anxiety). Reliability was acceptable (Cronbach’s alpha = .66 pretest, .70 posttest).
Analytic Strategy
Group differences on demographic and medical variables at pretest were tested using t-tests and Chi-Square techniques. To identify potential control variables, preliminary analyses consisting of t-tests, ANOVAs, and Chi-Square tests were used to assess the relation between participant characteristics at pretest and major outcomes at posttest. Study outcomes included a mix of continuous and categorical outcomes. In addition, some outcomes were measured at pretest and posttest, whereas others were measured only at posttest. All inferential analyses included the covariates identified in the preliminary analyses. Inferential analyses with repeated measures included baseline scores on the outcome measure as an additional covariate. Continuous outcomes were analyzed using ANCOVA techniques. For repeated measures outcomes, we also tested the overall condition-by-time interaction. Categorical outcomes were analyzed using logistic regression. Finally, exploratory analyses of potential moderators replicated the ANCOVA and logistic regression models, but included interaction terms.
A partial intention-to-treat (ITT) approach was used for inferential analyses. Participants were included in analyses even if they did not receive the allocated intervention. Strict ITT analysis requires complete data. There were no missing data at pretest. Missing data at posttest were limited to self-reports of men lost to follow-up (N = 59/490 participants; 12%). There were no statistically significant differences between those who were and were not lost to follow-up on variables shown in Table 1 or on outcome variables assessed at pretest. Given that there were no differences between individuals with complete or incomplete data and that the difference in statistical efficiency had to be less than 6.3% between the list-wise deletion approach we used and imputation methods, it was not necessary to use imputation methods. We had complete data on PSA testing and physician visits via medical claims.
Table 1.
Descriptive data (percentages) on demographic, social and medical variables at pretest in the total sample and by experimental condition (N = 490)1.
| Characteristics | Total (N = 490) | Control Group (N = 246) | Intervention Group (N = 244) |
|---|---|---|---|
| Age group, years | |||
| 45–49 | 23.9 | 24.8 | 23.0 |
| 50–54 | 27.1 | 28.0 | 26.2 |
| 55–70 | 49.0 | 47.2 | 50.8 |
| Immigrant | 83.1 | 82.0 | 84.1 |
| Education, highest level | |||
| <High school degree | 31.3 | 31.7 | 30.7 |
| High school degree | 31.8 | 30.5 | 33.2 |
| College education or degree | 36.9 | 37.8 | 36.1 |
| Married | 83.7 | 82.5 | 84.8 |
| Report ever talked with doctor about prostate cancer | 56.3 | 57.3 | 55.3 |
| Doctor ever recommend prostate cancer test | 36.9 | 34.1 | 39.8 |
| Report DRE 12+ months ago | 24.4 | 29.1 | 26.7 |
| Report PSA test 12+ months ago | 27.8 | 24.8 | 30.7 |
| PSA medical claim within year before pretest | 24.9 | 23.6 | 26.2 |
| PSA medical claim any time before pretest | 45.9 | 45.9 | 45.9 |
There were not statistically significant between-group differences on any of the variables at baseline.
Notes. DRE = digital rectal examination; PSA = prostate specific antigen.
Results
Sample Characteristics and Major Variables
Table 1 presents descriptive data on demographics and variables related to prostate cancer testing at pretest. There were no statistically significant (p<.05) associations between condition and the pretest variables shown in Table 1. The mean age was 55.04 years (SD = 6.29 years) and most men were married and immigrants, predominantly Caribbean (77.4% of immigrants).
Table 1 also shows that more than half of the men reported that a physician had talked with them about prostate cancer in the past, but less than 40% recalled a doctor recommending a prostate cancer test. Approximately a quarter of the men reported that they had had a DRE or PSA test more than 12 months before enrollment. Consistent with the eligibility criteria, no enrolled men reported a prostate cancer test within the 12 months before enrollment. However, due to an unanticipated long lag in medical claims reports, we discovered at the end of enrollment that nearly a quarter of the enrolled men had a medical claim for a PSA test in the 12 months before enrollment. The contradiction between men’s self reports and the medical claims suggest some failures of memory as well as potential involuntary testing. PSA testing is not generally recommended more than once a year among asymptomatic men without a prior elevation in PSA. Therefore, we collected follow-up PSA claims data for 2 years after pretest.
Process Variables
At posttest, more than 96% of men in both conditions reported that the interventionist was trustworthy, respectful, a good listener, and helped them to learn something about their health. There were no significant differences between groups on these variables.
Preliminary Analyses to Identify Control Variables
Two of the variables in Table 1 were related to outcomes: education level was positively related to knowledge (p<.001) and inversely related to decision conflict (p<.03) at posttest, and having had a PSA medical claim at any time prior to the pretest was associated with greater congruence between testing intentions and behaviors at 1- year and 2-year follow-up periods (p’s<.01). All inferential analyses included these variables as covariates.
Knowledge, Decision Making, Communication with Physician, and Anxiety
Continuous outcomes were analyzed first (Table 2). There was a significant effect of condition on level of decision conflict at posttest, controlling for covariates. The experimental group reported a lower level of decision conflict than the control group. There was a significant condition-by-time interaction effect on knowledge scores at posttest, controlling for covariates, F(1,427)=17.781, p<.001, partial eta squared = .04. As shown in Table 2, there was a significant effect of condition on percent correct on the knowledge index at posttest, but not at pretest, after controlling for covariates. Relative to the control group, the experimental group answered more of the knowledge questions correctly at posttest. The two items that the men in the intervention group were most likely to get correct at posttest were: (1) “Can some treatments for prostate cancer cause sexual problems?” (84.7% correctly responded, “yes”), and (2) “Are black or African American men more likely to get prostate cancer than other men?” (81.9% correctly responded, “yes”). The two items that the men in the intervention group were least likely to get correct at posttest were: (1) “Can tests for prostate cancer tell doctors if a man has a fast growing type of cancer?” (10.2% correctly responded, “no”), and (2) “Do all medical doctors agree that it is good for men to get tested for prostate cancer?” (11.6% correctly responded, “no”). There was no significant condition-by-time interaction on anxiety, controlling for covariates. As shown in Table 2, anxiety was low at pretest and posttest in both groups.
Table 2.
Adjusted means for continuous variables as a function of condition and time, with statistical results of analyses on adjusted means1.
| Outcome | Total Sample | Control | Intervention | Statistical test | Partial Eta Sq |
|---|---|---|---|---|---|
|
| |||||
| Mean (SE) | Mean (SE) | Mean (SE) | |||
| Decision conflict | |||||
| Posttest (adjusted)2 | 37.00 (1.158) | 39.85 (1.636) | 34.15 (1.639) | F(1,427) = 6.05* | .014 |
| Knowledge % correct | |||||
| Pretest (adjusted)3 | 50.6 (.008) | 49.6 (.012) | 51.7 (.012) | F(1,486)=1.64 | .003 |
| Posttest (adjusted)4 | 58.1 (.007) | 54.7 (.009) | 61.6 (.009) | F(1,426)=27.48*** | .06 |
| State anxiety | |||||
| Pretest (adjusted)3 | 2.00 (.117) | 1.95 (.165) | 2.05 (.165) | F(1,486)=0.66 | .000 |
| Posttest (adjusted)5 | 2.09 (.103) | 2.16 (.146) | 2.02 (.147) | F(1,426)=0.42 | .001 |
Notes.
For all self-report data, the pre-test sample size was 490 (246 control group; 244 experimental group), whereas the post-test sample size was 431 (216 control group; 215 experimental group). For all analyses, removing the covariates from analyses did not change the results of any of the significance tests.
Decision conflict only measured at posttest; adjusted for two covariates in model: education and any PSA claim prior to pretest.
Adjusted for two covariates in model: education and any PSA claim prior to pretest.
Adjusted for three covariates in model: education, any PSA claim prior to pretest, and percent correct on knowledge index at pretest.
Adjusted for three covariates in model: education, any PSA claim prior to pretest, and state anxiety level at pretest.
p<.05
p<.001
Categorical outcomes were analyzed next (Table 3). There was a significant effect of experimental condition on odds of speaking with a doctor about prostate cancer for the first time, controlling for covariates. At pretest, condition was unrelated to the odds of speaking with a doctor about prostate cancer, but by posttest, the odds of speaking with a doctor about prostate cancer was more than 2 times greater in the experimental group than in the control group.
Table 3.
| Outcome | Total Sample % | Control % | Intervention % | Exp(B) | 95% C.I. |
|---|---|---|---|---|---|
| Verified visit to discuss prostate cancer with physician | 12.1 | 8.3 | 15.8 | 2.127*** | 1.152 – 3.925 |
| Plan to test for prostate cancer pretest | 60.2 | 59.3 | 61.1 | 1.076 | .748 – 1.548 |
| Plan to test for prostate cancer posttest | 81.0 | 81.0 | 80.9 | .994 | .614 – 1.610 |
| Testing benefits outweigh risks posttest | 30.9 | 28.2 | 33.5 | 1.283 | .848 – 1.942 |
| Verified PSA 1-year follow-up | 45.5 | 45.9 | 45.1 | .965 | .671 – 1.386 |
| Verified PSA 2-year follow-up | 64.7 | 66.7 | 62.7 | .829 | .564 – 1.218 |
| Congruence between intention to test and verified PSA test 1-year follow-up | 56.7 | 58.1 | 55.3 | .891 | .621 – 1.279 |
| Congruence between intention to test and verified PSA test 2-year follow-up | 59.2 | 59.3 | 59.0 | .986 | .686 – 1.417 |
Notes.
For all self-report data, the pre-test sample size was 490 (246 control group; 244 experimental group), whereas the post-test sample size was 431 (216 control group; 215 experimental group). However, the sample size was 490 (246 control group; 244 experimental group) for data using medical claims records.
All analyses adjusted for the covariates education level and claims-verified PSA test prior to pretest.
p<.05
p<.01
p<.001
As shown in Table 3, there was no effect of experimental condition on the odds of planning to test for prostate cancer at pretest or posttest, controlling for covariates. At both time periods, the majority of men in both groups reported an interest in testing. At posttest, experimental condition did not affect the odds of reporting that the benefits of testing outweigh the risks. As shown in Table 3, a minority of men believed that the benefits of testing outweighed the risks. Indeed, the majority of men in both groups believed that it was equally important to consider the risks and benefits when making a testing decision (intervention: 63.3%; control: 69.0%).
PSA Testing and Congruence between Testing Intentions and PSA Testing at Follow-up
As shown in Table 1, approximately one-fourth of the sample had a PSA medical claim one year before pretest and approximately 46% had a claim at any time prior to the pretest. Yet, according to men’s self-reports, none had a PSA test in the prior year and only 28% had a PSA at more than one year before the study. As shown in Table 3, nearly 65% of men had a claims-verified PSA test at the 2-year follow-up. Table 3 also shows that experimental condition had no effect on the odds of men having a claims-verified PSA test at the 1- or 2-year follow-up, controlling for covariates. Nor was there an intervention effect on congruence between testing intention and a claims-verified PSA test at the 1- or 2-year follow-up, controlling for covariates.
Moderator Analyses
None of the demographic, social, or prior testing variables moderated the relation between experimental condition and the primary outcomes of knowledge, decision conflict, talking with doctor about prostate cancer testing, and congruence between testing intention and behavior at 1- and 2-year follow-up.
Discussion
This trial is among the few to test the efficacy of an intervention to promote informed decision making about prostate cancer testing among black men. It is the first to focus on a predominantly immigrant black sample, to track PSA testing through medical claims for 2 years, and to examine the congruence between men’s prostate cancer testing intentions and verified testing behavior. As hypothesized, relative to the control intervention, the experimental intervention resulted in significantly greater knowledge about prostate cancer and testing, a lower level of prostate cancer testing decision conflict, and a greater proportion of men talking with a physician about prostate cancer testing for the first time. Also consistent with hypotheses, the experimental intervention did not increase state anxiety, plans to get a prostate cancer test, or likelihood of PSA testing. Contrary to hypotheses, the experimental intervention did not improve the congruence between men’s prostate cancer testing intention and behavior.
Our findings generally replicate those from other studies. The systematic review by Volk and colleagues (18) concluded that most decision support interventions in this area--which generally have used samples of white men--improved knowledge, resulted in lower decision conflict, and increased patients’ involvement in decision making with their physician. Similar findings have been observed in studies with samples of black men (13, 14). With respect to knowledge, the men in the present study scored better than the men in some studies (35) but worse than others (13). Men in the intervention group were most knowledgeable at posttest on items related to the potential adverse side effects of prostate cancer treatments and race as a risk factor for prostate cancer. They were least knowledgeable about the inability of prostate cancer tests to detect fast growing cancers and the disagreement among doctors about the value of prostate cancer tests.
Overall, the experimental intervention group showed about 10 percentage points improvement in knowledge. While this was more than three times greater than the change in knowledge observed in the control condition, it is a relatively modest effect. One explanation for this modest effect is that it is difficult for men to grasp some of the more complex, nuanced arguments related to the controversy around prostate cancer testing (36). Indeed, despite presenting contrasting physicians’ views on the potential benefits and harms of testing in the brochure and explaining the testing controversy in the telephone education session, more than 88% of the men in the intervention group believed at posttest that all medical doctors agreed that it is good for men to get prostate cancer tests. Simpler and more personally relevant information, such as the unreliability of tests and race-related risk, was more likely to be learned and retained over the relatively long follow-up period. Arguably, the personally relevant information is what is most likely to activate patients to talk with their physician and consider whether testing is right for them, and the positive intervention effects on talking with a physician are consistent with this argument.
Importantly, the intervention activated men in the experimental intervention group to initiate discussions about prostate cancer with their doctor. This is an important outcome and consistent with current guidelines, which emphasize shared and informed decision making about testing (11). These discussions did not appear to lead men down the path to testing or to increase anxiety, as there were no differences in PSA testing rates or anxiety across conditions.
Men in both groups generally agreed that the risks and benefits were equally important to consider when deciding to test. This suggests that the intervention did not unduly bias men about testing. The testing intention and PSA testing data also support this conclusion. Prior studies have shown mixed results in terms of PSA testing outcomes, including increases, decreases, or null effects (18). The present findings converge with those of two studies with black samples that showed no intervention effects on testing (13, 37). However, the medical claims data showed a spike in PSA testing over time in both groups, possibly reflecting trends in which physicians’ routinely order PSA tests for men in this age group, particularly if they are black (38).
Congruence between testing intention and behavior was a major outcome of interest, because prostate cancer testing is a preference sensitive medical procedure. One potential contributor to the null effects of the intervention on congruence is the apparent practice of involuntary PSA testing. Prior research has shown that it is common for men to be unaware when a physician has ordered a PSA test (39, 40). In the present study, many men who were classified as having received a PSA based on medical claims were unaware that they had had a test. When screening is performed without patients’ knowledge or consent, it undermines preference-based decision making. In the context of an intervention, involuntary testing makes it difficult to increase congruence between men’s intentions and testing outcomes because men’s intentions are not taken into consideration at the time of testing. In order to maximize congruence between men’s intentions and testing behaviors, it may be necessary to intervene at the level of both the patient and the primary care physician. Of course, it will be critical to overcome barriers to implementing educational aids or informed decision making interventions in primary care, such as time barriers and lack of physician input into developing educational materials (41). At a minimum, physicians could give patients a handout that states a PSA test was (or will be) ordered, thereby opening the door for a conversation about the test. With the recently revised United States Preventive Services Task Force recommendations against prostate cancer screening, clinical practice guidelines and physicians’ behaviors may change. Specifically, involuntary, opportunistic screening might decline and physicians might be more attentive to patients’ preferences.
This study had a number of strengths, including a randomized controlled design, verified PSA testing and physician visit outcomes, long-term follow-up, a large community sample of black men with diverse educational and cultural backgrounds, low attrition, and assessment of congruence between reported testing intentions and claims-verified testing behavior. One innovative feature was the collaboration between an academic research team and a workers’ union health plan. This collaboration allowed us to build on the trusting relationship that men have with their union. This unique partnership between academic researchers and a healthcare union/insurer suggests a model for developing, evaluating and disseminating a variety of evidence-based cancer education and control interventions within healthcare systems. Our partner currently offers telehealth interventions for smoking cessation and chronic disease management for its beneficiaries. It would not be difficult to extend these services to include decision support interventions related to cancer screening.
The study also had limitations. First, the sample was predominantly immigrant black men living in an urban area. In some respects the mostly immigrant sample is a strength of the study given the lack of attention to immigrant black men who are at high risk for prostate cancer. Nonetheless, it is not clear whether the results from the intervention would generalize to a more diverse population of black men. Second, the sample consisted of men who all had a primary care physician and access to health insurance that covered prostate cancer tests. It is likely that PSA testing rates would be significantly lower in a sample with less access to care.
Overall, the study findings suggest that the intervention was acceptable and effective at improving prostate cancer testing knowledge, decision outcomes and doctor-patient communication among black men. The intervention was easy to implement, did not appear to bias men for or against testing, and did not arouse anxiety. The intervention failed to facilitate preference-based PSA testing, potentially because many men were being tested without their knowledge. Future interventions may benefit from a multilevel approach that provides decision aids to patients in clinics that also use informed consent procedures with prostate cancer testing.
Acknowledgments
This research was supported by grant R01 CA104223 from the National Cancer Institute of the National Institutes of Health. We acknowledge all members of the trial research team, the Data Safety and Monitoring Board members, and the research participants. We thank Adam Davey, Alfred Ashford, and Ronald Myers for their methodological and statistical advice. Preliminary data from this project were presented at the Annual Meeting of the Society of Behavioral Medicine in March 2008.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
References
- 1.American Cancer Society. Cancer Facts and Figures 2012. Atlanta, GA: American Cancer Society; 2012. [Google Scholar]
- 2.Odedina FT, Akinremi TO, Chinegwundoh F, et al. Prostate cancer disparities in Black men of African descent: a comparative literature review of prostate cancer burden among Black men in the United States, Caribbean, United Kingdom, and West Africa. Infect Agent Cancer. 2009;4 (Suppl 1):S2. doi: 10.1186/1750-9378-4-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2006. from http://seer.cancer.gov/csr/1975_2006/
- 4.Moyer VA. Screening for Prostate Cancer: U.S. Preventive Services Task Force Recommendation Statement. Annals of Internal Medicine. 2012 doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 5.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 6.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Prostate Cancer Screening in the Randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: Mortality Results after 13 Years of Follow-up. Journal of the National Cancer Institute. 2012;104:125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barry MJ, Mulley AJ., Jr Why are a high overdiagnosis probability and a long lead time for prostate cancer screening so important? Journal of the National Cancer Institute. 2009;101:362–363. doi: 10.1093/jnci/djp028. [DOI] [PubMed] [Google Scholar]
- 8.Lim LS, Sherin K. Screening for prostate cancer in U.S. men: ACPM position statement on preventive practice. Am J Prev Med. 2008;34:164–170. doi: 10.1016/j.amepre.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: prostate cancer. Journal of the National Comprehensive Cancer Network. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 10.Greene K, Albertsen PC, Babaian R. Prostate-specific antigen best practice statement: 2009 update. J Urol. 2009;182:2232–2241. doi: 10.1016/j.juro.2009.07.093. [DOI] [PubMed] [Google Scholar]
- 11.American Cancer Society. American Cancer Society guidelines for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60:70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 12.Howard K, Salkeld GP, Mann GJ, et al. The COMPASs Study: Community Preferences for Prostate cAncer Screening. Protocol for a quantitative preference study. BMJ open. 2012;2:e000587. doi: 10.1136/bmjopen-2011-000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor KL, Davis JL, 3rd, Turner RO, et al. Educating African American men about the prostate cancer screening dilemma: a randomized intervention. Cancer Epidemiology, Biomarkers & Prevention. 2006;15:2179–2188. doi: 10.1158/1055-9965.EPI-05-0417. [DOI] [PubMed] [Google Scholar]
- 14.Ellison GL, Weinrich SP, Lou M, et al. A randomized trial comparing web-based decision aids on prostate cancer knowledge for African-American men. J Natl Med Assoc. 2008;100:1139–1145. doi: 10.1016/s0027-9684(15)31481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steele CB, Miller DS, Maylahn C, Uhler RJ, Baker CT. Knowledge, attitudes, and screening practices among older men regarding prostate cancer. Am J Public Health. 2000;90:1595–1600. doi: 10.2105/ajph.90.10.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etzioni R, Berry KM, Legler JM, Shaw P. Prostate-specific antigen testing in black and white men: an analysis of medicare claims from 1991–1998. Urology. 2002;59:251–255. doi: 10.1016/s0090-4295(01)01516-3. [DOI] [PubMed] [Google Scholar]
- 17.Shavers VL, Underwood W, Moser RP. Race/ethnicity and the perception of the risk of developing prostate cancer. Am J Prev Med. 2009;37:64–67. doi: 10.1016/j.amepre.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volk RJ, Hawley ST, Kneuper S, et al. Trials of decision aids for prostate cancer screening: a systematic review. Am J Prev Med. 2007;33:428–434. doi: 10.1016/j.amepre.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 19.Doull M, O’Connor A, Jacobsen MJ, et al. Investigating the decision-making needs of HIV-positive women in Africa using the Ottawa Decision-Support Framework: Knowledge gaps and opportunities for intervention. Patient Education and Counseling. 2006;63:279–291. doi: 10.1016/j.pec.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Williams RM, Zincke NL, Turner RO, et al. Prostate cancer screening and shared decision-making preferences among African-American members of the Prince Hall Masons. Psychooncology. 2008;17:1006–1013. doi: 10.1002/pon.1318. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor AM, Fiset V, DeGrasse C, et al. Decision aids for patients considering options affecting cancer outcomes: evidence of efficacy and policy implications. J Natl Cancer Inst Monogr. 1999:67–80. doi: 10.1093/oxfordjournals.jncimonographs.a024212. [DOI] [PubMed] [Google Scholar]
- 22.Davison BJ, Kirk P, Degner LF, Hassard TH. Information and patient participation in screening for prostate cancer. Patient Education and Counseling. 1999;37:255–263. doi: 10.1016/s0738-3991(98)00123-2. [DOI] [PubMed] [Google Scholar]
- 23.Myers RE, Chodak GW, Wolf TA, et al. Adherence by African American men to prostate cancer education and early detection. Cancer. 1999;86:88–104. doi: 10.1002/(sici)1097-0142(19990701)86:1<88::aid-cncr14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 24.Ashford AR, Albert SM, Hoke G, et al. Prostate carcinoma knowledge, attitudes, and screening behavior among African-American men in Central Harlem, New York City. Cancer. 2001;91:164–172. doi: 10.1002/1097-0142(20010101)91:1<164::aid-cncr21>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 25.Consedine NS, Morgenstern AH, Kudadjie-Gyamfi E, Magai C, Neugut AI. Prostate cancer screening behavior in men from seven ethnic groups: the fear factor. Cancer Epidemiology, Biomarkers & Prevention. 2006;15:228–237. doi: 10.1158/1055-9965.EPI-05-0019. [DOI] [PubMed] [Google Scholar]
- 26.US Census Bureau. American Community Survey, Selected Population Profiles, S0201. 2004 Retrieved December 17, 2009. from http://www.census.gov/prod/2007pubs/acs-04.pdf.
- 27.Mederios KM. Immigration and America’s black popluation. Populatin Bulletin. 2007;62:1–18. [Google Scholar]
- 28.Frosch DL, Kaplan RM, Felitti V. The evaluation of two methods to facilitate shared decision making for men considering the prostate-specific antigen test. J Gen Intern Med. 2001;16:391–398. doi: 10.1046/j.1525-1497.2001.016006391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 30.Weinrich SP, Seger RE, Rao GS, et al. A decision aid for teaching limitations of prostate cancer screening. J Natl Black Nurses Assoc. 2008;19:1–11. [PubMed] [Google Scholar]
- 31.Gorden RL. Interviewing: Strategy, Techniques, and Tactics. Homewood, IL: Dorsey Press; 1980. [Google Scholar]
- 32.Wolf RL, Lepore SJ, Vandergrift JL, Basch CE, Yaroch AL. Tailored telephone education to promote awareness and adoption of fruit and vegetable recommendations among urban and mostly immigrant black men: a randomized controlled trial. Prev Med. 2009;48:32–38. doi: 10.1016/j.ypmed.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Connor A. Decisional conflict scale. (4) Retrieved November 1, 2003. from http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf.
- 34.Spielberger CD, Gorsuch RL, Lushene R, Vagge PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 35.Gattellari M, Ward JE. A community-based randomised controlled trial of three different educational resources for men about prostate cancer screening. Patient Education & Counseling. 2005;57:168–182. doi: 10.1016/j.pec.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 36.Partin MR, Nelson D, Radosevich D, et al. Randomized trial examining the effect of two prostate cancer screening educational interventions on patient knowledge, preferences, and behaviors. J Gen Intern Med. 2004;19:835–842. doi: 10.1111/j.1525-1497.2004.30047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myers RE, Daskalakis C, Cocroft J, et al. Preparing African-American men in community primary care practices to decide whether or not to have prostate cancer screening. J Natl Med Assoc. 2005;97:1143–1154. [PMC free article] [PubMed] [Google Scholar]
- 38.Farwell WR, Linder JA, Jha AK. Trends in prostate-specific antigen testing from 1995 through 2004. Arch Intern Med. 2007;167:2497–2502. doi: 10.1001/archinte.167.22.2497. [DOI] [PubMed] [Google Scholar]
- 39.Diefenbach PN, Ganz PA, Pawlow AJ, Guthrie D. Screening by the prostate-specific antigen test: what do the patients know? J Cancer Educ. 1996;11(3):9–44. doi: 10.1080/08858199609528390. [DOI] [PubMed] [Google Scholar]
- 40.Federman DG, Goyal S, Kamina A, Peduzzi P, Concato J. Informed consent for PSA screening: does it happen? Eff Clin Pract. 1999;2:152–157. [PubMed] [Google Scholar]
- 41.Steginga SK, Pinnock C, Jackson C, Gianduzzo T. Shared decision-making and informed choice for the early detection of prostate cancer in primary care. BJU International. 2005;96:1209–1210. doi: 10.1111/j.1464-410X.2005.05782.x. [DOI] [PubMed] [Google Scholar]