Abstract
Transcription regulation and transcript stability of a light-repressed transcript, lrtA, from the cyanobacterium Synechococcus sp. PCC 7002 were studied using ribonuclease protection assays. The transcript for lrtA was not detected in continuously illuminated cells, yet transcript levels increased when cells were placed in the dark. A lag of 20 to 30 min was seen in the accumulation of this transcript after the cells were placed in the dark. Transcript synthesis continued in the dark for 3 h and the transcript levels remained elevated for at least 7 h. The addition of 10 μm rifampicin to illuminated cells before dark adaptation inhibited the transcription of lrtA in the dark. Upon the addition of rifampicin to 3-h dark-adapted cells, lrtA transcript levels remained constant for 30 min and persisted for 3 h. A 3-h half-life was estimated in the dark, whereas a 4-min half-life was observed in the light. Extensive secondary structure was predicted for this transcript within the 5′ untranslated region, which is also present in the 5′ untranslated region of lrtA from a different cyanobacterium, Synechocystis sp. PCC 6803. Evidence suggests that lrtA transcript stability is not the result of differences in ribonuclease activity from dark to light. Small amounts of lrtA transcript were detected in illuminated cells upon the addition of 25 μg mL−1 chloramphenicol. The addition of chloramphenicol to dark-adapted cells before illumination allowed detection of the lrtA transcript for longer times in the light relative to controls without chloramphenicol. These results suggest that lrtA mRNA processing in the light is different from that in the dark and that protein synthesis is required for light repression of the lrtA transcript.
Unicellular cyanobacteria grown under autotrophic conditions show a decrease in macromolecular synthesis when cells are placed in the dark (Singer and Doolittle, 1975). This is not surprising because the main energetic process by which cyanobacteria grow, i.e. photosynthesis, ceases. Cell division and DNA replication have been reported to stop in the dark (Binder and Chisholm, 1990) and transcription and translation of most proteins are not detected (Singer and Doolittle, 1974, 1975; Tan et al., 1994). In Synechococcus sp. PCC 7002, transcripts encoding proteins that participate in the photosynthetic machinery, such as cpcBAC, apcAB, psbA, psbB, petBD, and petCA, are not detectable in dark-adapted cells, but their levels increase rapidly when the cells are exposed to light (Brand et al., 1992). Conversely, a light-repressed transcript, lrtA (Tan et al., 1994), is transcribed only in the dark. This transcript is not detected in illuminated cells and is rapidly degraded when dark-adapted cells are placed in the light. The gene encoding lrtA has been cloned and sequenced from Synechococcus sp. PCC 7002 (Tan et al., 1994) and is located on a 540-bp open reading frame within a presumed 1065-nucleotide message. The lrtA transcription start site is located 380 bp upstream from the translation start and possesses a consensus Escherichia coli promoter (Tan et al., 1994). Although the function of the lrtA gene product is unknown, it has significant sequence similarity to two different proteins. One of these proteins is a unique, chloroplast-specific small-subunit ribosomal protein, S30 (Zhou and Mache, 1989; Johnson et al., 1990; Schmidt et al., 1993), and the other is a transcription-modulator protein thought to function in the two-component bacterial regulatory system (Merrick and Coppard, 1989). The sequence similarity among these proteins suggests that lrtA may modulate either transcription and/or translation. LRTA is one of the first proteins to be synthesized upon illumination of dark-adapted cells, which further suggests that the lrtA gene product could regulate others in a timed response.
In this paper we report that the lrtA transcript is more stable in dark-adapted cells than in cells exposed to light. Also, new protein synthesis is required for light repression of the lrtA transcript. An extensive 5′ secondary structure has been determined by computer analysis, which could be responsible for the stability of the lrtA message in the cyanobacterium Synechococcus sp. PCC 7002.
MATERIALS AND METHODS
Bacterial Growth
Synechococcus sp. PCC 7002, obtained from the American Type Culture Collection (Rockville, MD), was maintained and cultured under photoautotrophic conditions using an artificial seawater medium (Widger, 1991). Water-saturated air supplemented with 3 to 5% (v/v) CO2 was bubbled into 300-mL culture tubes (42 × 3.5 cm in diameter) exposed to direct, continuous fluorescent light from two cool-white 30-W bulbs (Sylvania). Tubes were inoculated with a 5-mL aliquot from a cyanobacterial culture grown in a 50-mL flask started from a single colony grown on agar plates. Cells were grown to early log phase with an optical density of 0.8 to 1.0 at 550 nm. The cells at this concentration were used for the RPAs.
Light and Dark Adaptation
Cells grown under constant light conditions of 20 μE m−2 s−1 were considered to be light adapted, whereas cells placed in total darkness were considered to be dark adapted. Dark adaptation was for 3 h unless specified otherwise. Rifampicin was used at a final concentration of 50 μg mL−1 and CM was used at 25 μg mL−1. Both inhibitors were incubated for 10 min before the shift in the light-adaptation regimen.
Recombinant DNA Techniques
Templates for the antisense RNA probe were generated from the 895-bp BamHI fragment spanning 170 bp upstream of the lrtA transcription start to 725 bp downstream isolated from the plasmid pTX100 containing the 2.7-kb EcoRI fragment encoding the lrtA gene (Tan et al., 1994) (Fig. 1). This fragment was ligated in the BamHI site of pGEM-3Zf− (Promega), creating pHSΔlrtA9B. The fragment orientation in pHSΔlrtA9B was determined by double-stranded sequencing using universal and reverse sequencing primers. pHSΔlrtA9B was linearized with EcoRI, and the resulting DNA was used as a template for in vitro transcription of antisense RNA using the SP6 RNA polymerase. Probes for lrtA, cpcBAC, petCA, and ndhB were made using the Maxiscript II kit (Ambion, Austin, TX), according to the manufacturer's instructions, from 1 μg of DNA.
Figure 1.
The putative −35 and −10 elements, the start of transcription (determined experimentally by the method of Tan et al., 1994) (vertical arrow), and possible operator sites are shown. The probe for the RPAs is the 895-bp BamHI fragment (labeled 1) spanning the gene. Other fragments used in the RNase activity assays, labeled 1 and 2, and the entire mRNA generated from E. coli polymerase are shown. E, EcoRI; B, BamHI; and H, HindIII.
The reaction mixture was incubated at room temperature for 2 h, and 1 μL of DNase I was added and incubated for another 15 min at 37°C. Twenty microliters of gel-loading buffer (80% [v/v] formamide, 0.1% [w/v] xylene cyanol, 0.1% [w/v] bromphenol blue, and 2 mm EDTA) was added and incubated for 5 min at 90°C. The entire sample was loaded onto a 0.8-mm-thick, 8 m urea, 5% PAGE gel and electrophoresed in 1× Tris-borate-EDTA buffer at 300 V for 1 h. After electrophoresis, gels were exposed to x-ray film for 5 min and the labeled RNA was excised. The excised gel was soaked in 350 μL of 0.5 m CH3COONH4, 1 mm EDTA, and 0.1% (w/v) SDS overnight at 37°C to elute the labeled probe. An aliquot was taken for liquid-scintillation counting and the remainder was stored at −20°C. For the synthesis of the full-length sense lrtA message, Escherichia coli RNA polymerase isolated by published procedures (Burgess et al., 1975) was used, taking advantage of the near- E. coli consensus −35 and −10 promoter elements found upstream of the lrtA transcription start site. A 1.6-kb fragment was amplified by PCR using primers located at bases 480 and 2040 of the original EcoRI fragment on pTX100. The RNA polymerase reaction was done on this fragment in the presence of 150 mm KCl as a modification of the original transcription protocol described in the Maxiscript II kit. The full-length lrtA mRNA was gel purified as described above.
RPA
Synechococcus sp. PCC 7002 cells grown from 0.8 to 1.0 A550 as described were incubated in the light or in the dark. At various times 107 cells mL−1 were harvested by rapid centrifugation and solubilized in 5 m guanidine thiocyanate and 0.1 m EDTA at room temperature. Cell lysates were stored at −20°C and thawed for hybridization experiments. The cells were solubilized directly without centrifugation when timed experiments were done. Aliquots of cells were solubilized by mixing with 2 volumes of 5 m guanidine thiocyanate and 0.1 m EDTA.
Cell lysates (30 μL) were mixed with approximately 106 cpm of probe per reaction (1–3 μL depending on the specific activity of each probe used). The hybridization reactions were incubated at 45°C for 16 to 20 h. After hybridization, the samples were digested with RNase cocktail (1 mg mL−1 RNase A and 20,000 units mL−1 RNase T1) for 30 min at 37°C. The samples were further incubated with 5 μL of 20 mg mL−1 proteinase K and 20 μL of 10% (w/v) SDS for an additional 30 min. After phenol-chloroform extraction and ethanol precipitation, the recovered nucleic acids were dissolved in gel-loading buffer, incubated at 95°C for 2 min, and loaded onto a 5% PAGE gel. The samples were electrophoresed and the positions of the protected RNA fragments were determined by radiography. Results were quantitated by densitometry using an EagleEye densitometer (Stratagene). All RPA experiments were repeated a minimum of three times.
RNase Assays
Protein extracts from light- or dark-adapted cells were checked for RNase activity. Extracts were isolated as follows: 50 mL of Synechococcus sp. PCC 7002 cells (A550 = 1.0) were light or dark adapted as described and treated with lysozyme (0.5 mg/mL cells) for 20 min. The resulting spheroplasts were centrifuged and resuspended in 1 mL of lysis buffer (10% glycerol, 50 mm Tris HCl, 1 mm EDTA, 2 mm DTT, 0.5% [v/v] Triton X-100, and 1 mm PMSF, pH 7.5) and sonicated at maximum strength for three 30-s bursts, followed by cooling on ice using a probe sonicator (model 450, Branson, Danbury, CT). The cell lysates were centrifuged for 10 min at 10,000 rpm and supernatants were checked for RNase activities.
Sense or antisense RNA probes were synthesized as described above using either SP6 or T7 polymerase. Templates for the probes included the 0.89-kb BamHI fragment from lrtA (Fig. 1), the 1.6-kb BamHI fragment from petCA (Brand et al., 1992), and the 880-bp XhoI-BamHI fragment from cpcBAC (de Lorimier et al., 1984). The full-length sense lrtA probe was synthesized using E. coli RNA polymerase.
RNase activity assays were carried out using 10 μg of cell lysate from either dark- or light-adapted cells, which was mixed with 100,000 cpm of RNA probe in RNase digestion buffer (10 mm Tris-HCl, pH 7.5, 0.3 m NaCl, 5.0 mm EDTA, and 5 mm MgCl2) in a total volume of 20 μL. Each reaction was incubated at 37°C for various times before the addition of 100 μL of 0.3 m NaC2H3O2 and 1 mm EDTA containing 0.5 μg μL−1 yeast RNA. The samples were immediately extracted with phenol-chloroform and the RNA was precipitated with ethanol. The recovered nucleic acids were dissolved in gel-loading buffer and incubated at 95°C for 2 min before loading onto a 5% polyacrylamide gel. The samples were subjected to electrophoresis as described above and RNA was visualized by autoradiography.
RESULTS
lrtA Transcript Is Synthesized in the Dark but Not in the Light
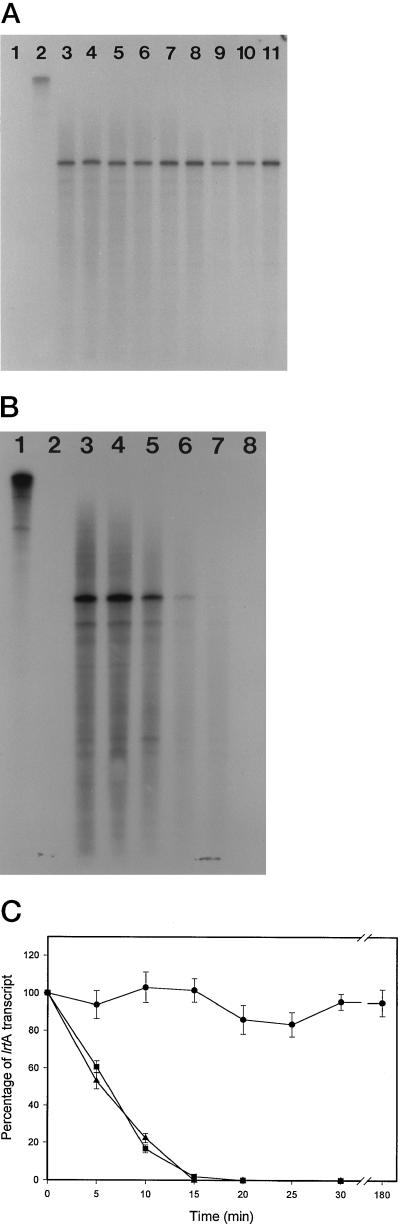
The time course for message accumulation in the dark was determined by RPA using the 895-bp BamHI lrtA fragment as a probe (Fig. 1). Aliquots were taken from preilluminated cells placed in the dark at specific time points (Fig. 2, A and B). The lrtA transcript was not detected in illuminated cells even after the radiograms were overexposed. However, the lrtA transcript was detected 20 to 30 min after the cyanobacterial cells were placed in the dark, and transcript levels continued to increased for 3 h. The message levels remained elevated for at least 7 h in the dark. Routinely, a 20- to 30-min lag from the onset of dark treatment to the detection of the transcript was seen.
Figure 2.
A, Radiogram of RPAs showing the changes in lrtA transcript levels when illuminated cyanobacteria are transferred to the dark. Lane 1, Probe plus RNase; lane 2, probe (1 μL of a 1:50 dilution from the stock used directly for the experimental samples) without RNase; lane 3, RPA from cells continuously grown in the light; lanes 4 to 12, RPAs from aliquots of illuminated cells placed in the dark and taken at 20-min intervals; lane 13, cells dark adapted for 4 h; lane 14, cells dark adapted for 6 h; and lane 15, cells dark adapted for 7 h. B, The quantitation of data from three independent experiments (including the one shown in A) plotted versus time in the dark. The level of the transcript present at each time point was quantitated using a densitometer and calculated as a percentage of a dark control (amount of transcript present in cells dark adapted for 3 h) for comparison.
The Transcript for lrtA Is More Stable in the Dark Than in the Light
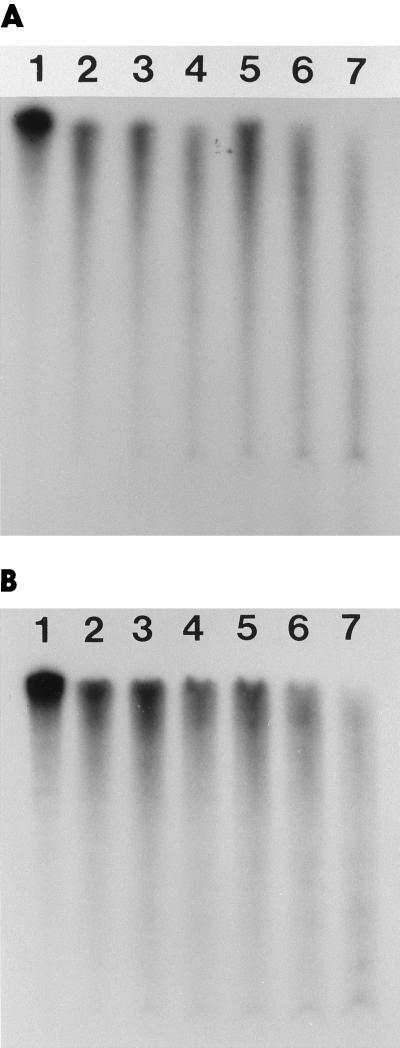
The RPA technique was used to measure the effects of rifampicin on the accumulation and stability of the lrtA transcript. Rifampicin (50 μg mL−1) was added to 3-h dark-adapted cells that were kept in the dark. Aliquots were taken from the cells at 0, 2, 5, 10, 15, 20, 25, and 30 min after the addition of rifampicin. The data showed that lrtA transcript levels in the dark remained constant for at least 30 min (Fig. 3, A and C), whereas rifampicin added at the onset of dark adaptation prevented the synthesis of the lrtA transcript (data not shown). When dark-adapted cells were exposed to light in the absence of rifampicin, a rapid decrease in transcript level was seen, with a half-life of approximately 4 min (Fig. 3, B and C). In the presence of rifampicin, a 4-min half-life again was observed for the lrtA transcript when dark-adapted cells were reexposed to light (Fig. 3C). Meanwhile, in dark-adapted cells, 100% of the message remained 3 h after the introduction of rifampicin (Fig. 3C).
Figure 3.
A, Cells were dark adapted for 3 h followed by the addition of 50 μg mL−1 rifampicin. Aliquots were taken 0, 2, 5, 10, 15, 20, 25, and 30 min after the addition of rifampicin (lanes 4–11). Lane 1, RPA from continuously illuminated cells only; lane 2, probe only (1 μL of a 1:50 dilution); and lane 3, RPA from 3-h dark-adapted cells. B, Cells were allowed to dark adapt for 3 h, and then were exposed to light in the absence of rifampicin. Aliquots were taken 0, 5, 10, 15, and 20 min (lanes 4–8) after the start of illumination. Lane 1, Probe only; lane 2, RPA from illuminated cells only; and lane 3, RPA from 3-h dark-adapted cells. C, •, Quantitation of data from three independent experiments (including the one shown in A) in which samples from dark-adapted cells, incubated with 50 μg/mL rifampicin, were assayed with RPA for lrtA transcript levels at the indicated time points in the dark; ▪, quantitation of data from B (dark-adapted cells transferred to light and assayed for transcript levels at the indicated time points); and ▴, quantitation from an experiment similar to the one shown in B, but with the addition of 50 μg/mL rifampicin to the dark-adapted cells before the onset of illumination.
RNase Activities of Cell Extracts from Dark- or Light-Adapted Cells Do Not Differ Significantly
Possible explanations for increased transcript stability in the dark include the following: (a) structured RNA components (stem loops) within the transcript are formed to protect against degradation; (b) specific protein(s) bind to the transcript, preventing degradation; and (c) in dark-adapted cells, RNase activity decreases. To explore the reasons that the lrtA transcript is more stable in the dark, differences in RNase activity between dark-adapted and illuminated cells were measured by the degradation of in vitro-labeled RNA visualized on acrylamide gels. Several RNAs were used to monitor RNase activity, including petCA (Brand et al., 1992), cpcBAC (de Lorimier et al., 1984), ndhB (M. Varughese and W.R. Widger, unpublished data), both sense and antisense lrtA fragments, and the full-length sense lrtA message. Figure 4 shows the time course for RNA digestion by cell extracts obtained from light- and dark-adapted cells. Some noticeable differences were found between the degradation rates of in vitro-generated sense lrtA RNA and those of in vivo-generated lrtA RNA. Extracts from dark-adapted cells showed increased rates of RNA degradation compared with extracts from light-adapted cells (Fig. 4A). In vivo, the lrtA message was not degraded in the dark, whereas the in vitro-synthesized lrtA message was degraded by cell extracts from both dark- and light-adapted cells. The degradation of several other mRNAs, i.e. cpcBAC (Fig. 4B), petCA, ndhB, and antisense lrtA, by cell extracts from light- and dark-adapted cells (data not shown) gave similar results. The amount of protein extract used in each set of reactions was selected to follow the degradation rates on the gels. The data from these experiments suggest that only small changes in RNase activity are seen and that these are the opposite of what would be expected if changes in RNase activity controlled lrtA transcript stability; more activity is seen in the dark than in the light. Thus, changes in general RNase activity do not appear to be the reason for the differential stability of the lrtA transcript. However, transcript stability could be caused by protection against RNase activity by protein binding to stem-loop structures.
Figure 4.
Ten-microgram aliquots of cell extracts from illuminated (lanes 2–4) or dark-adapted (lanes 5–7) cells were incubated with 100,000 cpm of full-length sense lrtA mRNA (A) or sense RNA from the 880-bp XhoI-BamHI fragment from the cpcBAC gene (B). Each reaction was allowed to take place for 30, 60, or 90 min in the light (lanes 2, 3, and 4, respectively) or in the dark (lanes 5, 6, and 7, respectively) for the experiments in both A and B. Lane 1, RNA only in both experiments.
An Extensive Secondary Structure for lrtA RNA Is Predicted
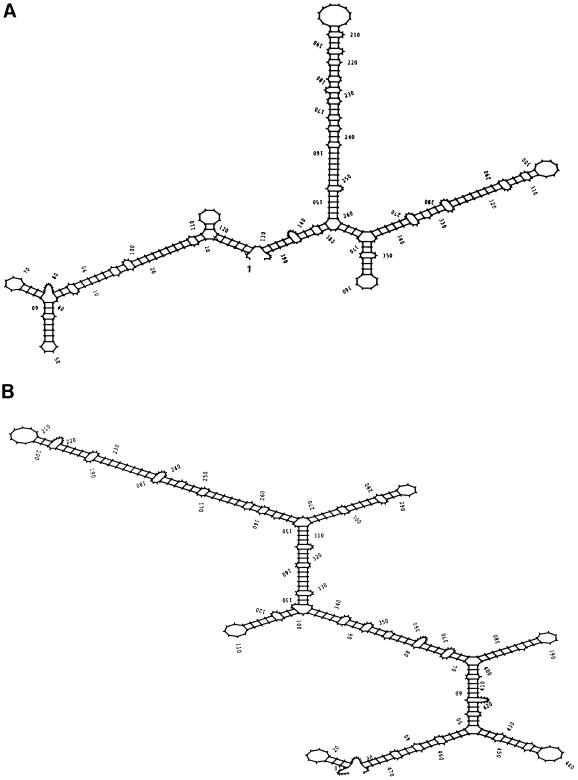
A putative stem-loop structure was predicted in the 5′ UTR of the lrtA transcript starting at nucleotide 4 from the determined 5′ start site (Fig. 5A). This structure shares features common to structures known to confer RNA stability to several prokaryotic transcripts, including ompA in E. coli (Chen et al., 1991), the ermA and ermC transcripts of Bacillus subtilis and Staphylococcus aureus (Sandler and Weisblum, 1988), and the gene 32 mRNA of phage T4 (Gorski et al., 1985). The structure predicted in the 5′ lrtA UTR is more extensive than that seen in ompA, and appears to be present in Synechocystis sp. PCC 6803 lrtA 5′ UTR (Kaneko et al., 1996) (Fig. 5B).
Figure 5.
A, Secondary structure at the 5′ UTR of lrtA from Synechococcus sp. PCC 7002. Transcription starts at position 1. B, Secondary structure at the 5′ UTR of lrtA from Synechococcus sp. PCC 6803 (Kaneko et al., 1996, and Cyanobase [http://www.kazusa.or.jp/cyano/cyano.html]). The secondary structures were predicted using the RNAfold program (Zuker and Stiegler, 1981) from the Genetics Computer Group package (Devereux, 1991).
The Synechococcus sp. PCC 7002 lrtA 5′ UTR secondary structure has a calculated ΔG of −68.4 kcal mol−1 (Zuker and Stiegler, 1981), and contains three major stem loops. The first stem loop is 124 bases long and contains an 8-base direct repeat surrounding a 6-base inverted repeat at positions 57 to 94 (Fig. 1). This stem loop, which starts at the fourth nucleotide from the transcript start site, could be involved in RNA stability, especially against degradation initiated at the 5′ end. The second stem loop is 113 bases long and is positioned in the middle of the 5′ UTR (from nucleotides +147 to +260). The third stem loop is 116 bases long, starts at nucleotide 261, and extends to nucleotide 376 (Fig. 5A). A putative ribosome-binding site sequence (AGAGA) 7 bases before the start of translation is included in this stem structure at position 368. This loop may be responsible for the observed shortened transcripts that terminate before the start of translation (Tan et al., 1994). A fourth stem loop, a typical Rho-independent transcription terminator, is located between nucleotides 1035 and 1068 downstream from the coding region (data not shown) with a ΔG of −9.6 kcal mol−1 (Zuker and Stiegler, 1981). The predicted secondary structure in the 5′ UTR of the lrtA transcript from Synechocystis sp. PCC 6803 is also extensive and has a calculated ΔG of −85.4 kcal mol−1 (Fig. 5B) (Zuker and Stiegler, 1981). There is little sequence similarity between the 5′ UTRs of Synechococcus sp. PCC 7002 and Synechocystis sp. PCC 6803; however, it is of interest that both have extensive predicted secondary structures, although they are not identical.
Protein Synthesis Is Required for lrtA Repression in the Light
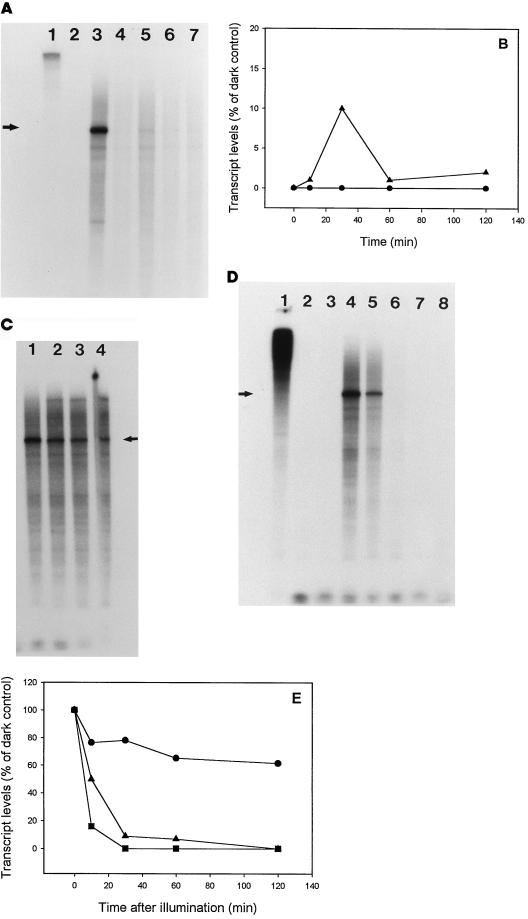
The effects of CM on the transcription of lrtA in the light and upon transition from dark to light were studied to determine if protein synthesis is required for the repression of lrtA (Fig. 6A). Aliquots were taken from continuously illuminated cells at 10, 30, 60, and 120 min after the addition of 25 μg mL−1 CM and subjected to RPA analysis. Illuminated cells in the absence of CM showed no detectable levels of the lrtA transcript (Fig. 6A, lane 2), whereas transcript levels from the same cells, only dark adapted, were easily detected (Fig. 6A, lane 3). Addition of CM to light-adapted cells followed by further incubation in the light allowed the synthesis of detectable levels of the lrtA transcript, albeit low in concentration (Fig. 6A, lanes 4–7, and B). The maximum amount of the lrtA transcript was seen 30 min after the addition of CM. Furthermore, the message was detectable for up to 2 h in the light after the addition of CM. These data were quantitated and plotted as a function of time (Fig. 6B).
Figure 6.
Effects of CM on lrtA transcription. A, Cells were placed in the light for 3 h and aliquots were analyzed for transcript levels at various times after the addition of 25 μg mL−1 CM. Lane 1, Probe only; lane 2, the RPA from 3-h-illuminated cells; lane 3, the RPA from cells dark adapted for 3 h; and lanes 4 to 7, 10, 30, 60, and 120 min after the addition of CM, respectively. B, Quantitation of the data shown in A plotted versus time as a percentage of the corresponding dark control (cells dark adapted for 3 h, lane 3 in A) (▴). For a comparison, the absence of the lrtA transcript in constant light is shown (•). C, Cells were dark adapted for 3 h, CM was added, and the cells were illuminated. Samples were taken at 10, 30, 60, and 120 min after illumination (lanes 1, 2, 3, and 4, respectively). The same probe and controls as in A were used for this experiment. D, Cells dark adapted for 3 h were incubated with 25 μg mL−1 CM and 50 μg mL−1 rifampicin for 10 min. The cells were then exposed to light. Lane 1, Probe only; lane 2, probe plus RNase; lane 3, RPA from cells grown in constant light; lane 4, RPA from 3-h dark-adapted cells; lanes 5, 6, 7, and 8, samples taken 10, 30, 60, and 120 min after the start of illumination, respectively, and subjected to RPA analysis. E, Quantitation of the data shown in C (•) and in D (▴). Some data from Figure 3B (transcript levels in cells dark adapted for 3 h and then illuminated) are shown for comparison (▪). The arrows in A, B, and C indicate the positions of the protected fragment of the lrtA message.
Addition of 25 μg mL−1 CM to dark-adapted cells, followed by illumination, allowed the detection of lrtA transcript for 2 h after illumination (Fig. 6C). This is in contrast to the 4-min half-life observed in the light without the addition of CM (Fig. 3, B and C). To determine if the CM-induced increase in transcript half-life is caused by suppression of a particular RNase or whether CM relieves the repression of transcription in the light, this experiment was repeated in the presence of both CM and rifampicin (Fig. 6D). A short half-life of 10 min was observed, suggesting that the prolonged detection of the lrtA message in the presence of CM was caused by the relief of transcription repression in the light. Quantitative data from the experiments shown in Figure 6, C and D, and Figure 3B are plotted together for a comparison (Fig. 6E).
DISCUSSION
In the present study, lrtA transcript levels were measured by RPAs in the light and in the dark. From these measurements, we have repeated and extended the initial conclusions that the lrtA transcript is not detected in the light, but is synthesized to significant levels when cells are placed in the dark (Fig. 2, A and B) (Tan et al., 1994). The lrtA transcript was shown to have an unusual stability in the dark compared with illuminated cells. Cells treated with rifampicin showed that the lrtA transcript was stable (without detectable losses for 30 min) after an initial 3-h dark incubation (Fig. 3, A and C), and nearly 100% of the message was detected 3 h after treatment (Fig. 3C). A half-life of greater than 3 h in the dark was estimated from these data. The half-life of the lrtA transcript in illuminated cells in the presence or absence of rifampicin was 4 min (Fig. 3, B and C), suggesting that the synthesis of new transcripts is not required for the rapid degradation of lrtA mRNA after illumination.
The overall RNase activities found in cell extracts from light-grown and 3-h dark-adapted cells were similar (Fig. 4, A and B). However, extracts from dark-adapted cells consistently gave slightly higher rates of RNA degradation than extracts from illuminated cells for all RNA species assayed. Sense RNA from ndhB, petCA (data not shown), lrtA, and cpcBAC (Fig. 4, A and B) showed nearly identical rates of degradation by extracts from dark-adapted cells. This suggests that in vitro-synthesized RNA is degraded differently from in vivo-generated RNA. The sense strand of lrtA was made in vitro using the E. coli polymerase (Burgess et al., 1975), and this message was degraded equally by extracts isolated from dark- or light-adapted cells. These data suggest that the increased synthesis of general RNases in the light did not cause increased lrtA transcript degradation; however, rates of in vivo RNase activity may not accurately reflect the activity measured in cellular extracts. RNase activity could be altered by isolation, causing an activation of activity in the dark or a deactivation of it in the light. Folding patterns of in vivo message compared with in vitro-synthesized message could be different, leading to digestion of the in vitro message in dark-adapted cell extracts, and ratios of RNA to RNase could also vary. These concerns are separate from the fact that measurable RNase activity of extracts does not vary substantially from light to dark.
A lag time of 20 to 30 min was seen for the appearance of the lrtA message when illuminated cells were placed in the dark (Fig. 2, A and B), suggesting that factors other than darkness are required for lrtA transcription. Repression of the transcript in the light requires protein synthesis, since the addition of CM to illuminated cells eases the transcription repression of lrtA (Fig. 6A). The data suggest that a specific protein is directly involved in lrtA light repression and that blockage of the synthesis of this repressor protein by CM allows lrtA to be transcribed.
Under autotrophic growth conditions, most other transcripts in Synechococcus sp. PCC 7002 are down-regulated in the dark, whereas lrtA behaves in the opposite manner. This behavior suggests a regulatory role for lrtA, yet no function for the gene product has been identified. Unfortunately, a specific phenotype has not been identified with the loss of the lrtA gene by mutation. Knockout mutants of lrtA grown in continuous light appear to be the same as wild type, showing no differences in growth or photosynthetic activity (Tan et al., 1994). This is not surprising because the lrtA gene product is not synthesized in the light.
When CM-treated, dark-adapted cells were placed in the light, an increase in the half-life of the transcript was observed (Fig. 6C). Significant amounts of transcript were present 1 h after illumination in the presence of CM, whereas no message was seen in 1-h-illuminated cells without CM (Fig. 3B). These data are in agreement with the results from the experiment in which CM was added to continuously illuminated cells (Fig. 6A), suggesting that CM inhibited repression of lrtA transcription. However, the apparent increased half-life in illuminated cells treated with CM could be the result of two possible mechanisms: (a) the inhibition of the synthesis of a specific RNase, or (b) the loss of transcript repression by the action of CM. To address the two possibilities, the RPA experiment was performed in the presence of both rifampicin and CM. The half-life of the transcript in the light approached 10 min, returning to times seen for untreated cells (Fig. 6D). This suggests that RNases are already present in the dark and are active, as previously intimated from the RNase activity data. No new RNase should be synthesized because most transcription and translation are not functioning. A working model suggests that the loss of a repressor protein, because of CM inhibition, leads to the production of the lrtA message. Normally, production of this light-activated (translated) repressor protein would be responsible for transcript repression in the light. The loss of this putative protein in the dark (down-regulation of transcription/translation) or in the light because of the effects of CM would allow the transcription of lrtA to proceed, which is consistent with the observed results.
Two factors govern transcript abundance: the rate of transcript formation and the rate of transcript degradation. Evidence for a repressor protein controlling transcript formation has been presented above; however, factors controlling transcript stability are unknown. The lifetimes of individual messages can vary widely within a single cell and are often regulated in response to changes in the cell's environment or growth phase. Each rate is a separate function controlled by different factors. Differences in the transcript stability of IrtA under varying conditions could be explained by (a) a specific light- induced RNase activity that selectively degrades the lrtA transcript in the light but not in the dark; (b) protein(s) binding to the lrtA transcript in the dark, leading to protection against RNase attack; or (c) changes in RNA stability (structures) by altered stem-loop conformation induced by light/dark changes in protein binding.
There appear to be no major differences in the RNase activity between cell extracts isolated from dark- and light-adapted cells that could account for the observed differential lrtA transcript stability (Fig. 4). We make this statement with the caveat that RNase activity from cell extracts may be different from that in intact cells. In addition, earlier studies in our laboratory (Brand et al., 1992) have shown that many photosynthetic transcripts, such as petBD, petCA, and cpcBAC, are transcribed only in the light and that their transcript levels are undetectable soon after the cells are placed in the dark. This observation suggests that these transcripts are not stable in the dark. However, the Synechocystis sp. PCC 6803 psbA transcript is very stable in the dark, with a half-life of 7 h, but it is unstable in the light. This behavior is controlled by photosynthetic electron transport (Mohamed and Jansson, 1991). However, the Synechocystis sp. PCC 6803 psbA transcript is transcribed only in the light and not in the dark, the opposite of the behavior of the lrtA transcript. In the same organism, the rbcLS transcript is more stable in the light than in the dark (Mohamed and Jansson, 1991). The Synechococcus sp. PCC 7942 psbA gene family is posttranscriptionally regulated by light (Golden, 1995). The psbAI and psbAIII transcripts are degraded faster under high-light conditions, and this regulation requires de novo transcription and translation after exposure to high light. In contrast to photosynthetic genes, transcripts of the rrn genes are present in both dark- and light-adapted cyanobacteria cultures (Mohamed and Jansson, 1989; Tan et al., 1994).
The lrtA 5′ UTR is 377 bases long and exhibits extensive secondary structure from base 4 to 397, with an estimated ΔG of −68.4 kcal mol−1 (Zuker and Stiegler, 1981) (Fig. 5A). The role of this putative stem-loop region is unclear. Extensive secondary structure is also predicted for the 5′ UTR of the lrtA from another cyanobacterium, Synechocystis sp. PCC 6803. The 5′ UTR of the lrtA from Synechocystis sp. PCC 6803 is 477 bp long. Comparisons of secondary structure predictions for each species show similarity even though the sequences are not conserved in the 5′ UTR of the lrtA transcript from both organisms. Although the individual stem-loop structures are different and the significance of these RNA structures is unclear, the extensive secondary structure present in the 5′ UTR of lrtA from both Synechococcus sp. PCC 7002 and Synechocystis sp. PCC 6803 suggests a similar function.
Similar RNA structures stabilize transcripts for ompA (Belasco et al., 1986; Bechhofer, 1993), papA, pufBA, and T4 phage gene 32 (Emory et al., 1992; Bechhofer, 1993). In E. coli, for example, mRNA half-lives range from a few seconds to 1 h, with an average lifetime of 2 to 4 min (Emory and Belasco, 1990). Structured elements at the 3′ and 5′ ends of phage and bacterial messages have been shown to influence mRNA stability. RNA stem-loop structures in the 5′ UTR function as 5′ stabilizers and have been identified in the ompA transcript in E. coli (Chen et al., 1991), the ermA and ermC transcripts of B. subtilis and S. aureus (Sandler and Weisblum, 1988), and the gene 32 mRNA of phage T4 (Gorski et al., 1985). 5′ cis-acting elements are important determinants of the lifetimes of Chlamydomonas reinhardtii gene transcripts (Salvador et al., 1993). Stem-loop structures at the 3′ end of a prokaryotic transcript can increase its stability by blocking the processing action of 3′ to 5′ exonucleases (for review, see Higgins et al., 1993). In plastids, 3′ inverted repeats, which can potentially form stem-loop structures, act as mRNA-processing and -stabilizing elements, but they do not terminate transcription (Stern and Gruissem, 1987).
Stabilizing an RNA structure by protein binding in the dark is an attractive hypothesis with some precedent. Chloroplast-encoded proteins, specifically the psbA message in C. reinhardtii, are expressed in the light, whereas messages are present both in the light and in the dark (Mullet, 1988; Danon and Mayfield, 1994). The expression of psbA in chloroplasts is thought to be controlled by an NADPH-dependent thioredoxin reduction of specific protein thiols, allowing this protein to bind near the 5′ untranslated end of the message. This binding mediates the onset of translation in the light (Danon and Mayfield, 1994; Mayfield et al., 1995). In Synechococcus sp. PCC 7002, lrtA is not translated in the dark to significant levels but it is translated at the onset of illumination (Tan et al., 1994). Mechanisms similar to those regulating chloroplast psbA expression may be at work, conferring transcript stability and translation regulation of the lrtA gene.
The data presented here suggest that the pathway leading to the repression of lrtA requires protein synthesis. The protein responsible for repression has not been identified, but the loss of repression by CM suggests its presence. Two important questions remain unanswered: What factors are responsible for (a) lrtA transcription in the dark while most other mRNA species are not synthesized, and (b) the increased stability of the lrtA transcript in the dark?
ACKNOWLEDGMENTS
The authors thank Dr. Costas Koumenis and Dr. Arnold Eskin for their help on the use of the RPAs and Dr. Daniel Davison for helpful discussions and review of the manuscript.
Abbreviations:
- CM
chloramphenicol
- ΔG
free energy of folding
- RPA
RNase protection assay
- UTR
untranslated region
Footnotes
This research was supported by the National Institutes of Health (grant no. GM46297), the Robert A. Welch Foundation (grant no. E-1381), and the National Science Foundation (equipment grant no. BIR 9109294).
LITERATURE CITED
- Bechhofer D (1993) 5′ mRNA stabilizers. In JG Belasco, G Brawerman, eds, Control of Messenger RNA Stability. Academic Press, New York, pp 31–52
- Belasco JG, Nilsson G, Von Gabain A, Cohen SN. The stability of E. coli gene transcripts is dependent on determinants localized to specific mRNA segments. Cell. 1986;46:245–251. doi: 10.1016/0092-8674(86)90741-5. [DOI] [PubMed] [Google Scholar]
- Binder BJ, Chisholm SW. Relationship between DNA cycle and growth rate in Synechococcus sp. strain PCC 6301. J Bacteriol. 1990;172:2313–2319. doi: 10.1128/jb.172.5.2313-2319.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S, Tan X, Widger WR. Cloning and sequencing of the petBD operon from the cyanobacterium Synechococcus sp. PCC 7002. Plant Mol Biol. 1992;20:481–491. doi: 10.1007/BF00040607. [DOI] [PubMed] [Google Scholar]
- Burgess RR, Jendridak JJ. A procedure for the rapid, large scale purification of Escherichia coli DNA-dependent RNA polymerase involving polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975;14:4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Chen L, Emory SA, Bricker AL, Bouvet P, Belasco JG. Structure and function of a bacterial mRNA stabilizer: analysis of the 5′ untranslated region of ompA mRNA. J Bacteriol. 1991;173:4578–4586. doi: 10.1128/jb.173.15.4578-4586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A, Mayfield SP. Light-regulated translation of chloroplast messenger RNAs through redox potential. Science. 1994;266:1717–1719. doi: 10.1126/science.7992056. [DOI] [PubMed] [Google Scholar]
- de Lorimier R, Bryant DA, Porter RD, Liu WY, Jay E, Stevens SE. Genes for the a and b subunits of phycocyanin. Proc Natl Acad Sci USA. 1984;81:7946–7950. doi: 10.1073/pnas.81.24.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J. The GCG Sequence Analysis Software Package, Version 7. Madison, WI: Genetics Computer Group; 1991. [Google Scholar]
- Emory SA, Belasco JG. The ompA 5′ untranslated RNA segment functions in Escherichia coli as a growth-rate-regulated mRNA stabilizer whose activity is unrelated to translational efficiency. J Bacteriol. 1990;172:4472–4481. doi: 10.1128/jb.172.8.4472-4481.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emory SA, Bouvet P, Belasco JG. A 5′-terminal stem-loop structure can stabilize mRNA in E. coli. Genes Dev. 1992;6:135–148. doi: 10.1101/gad.6.1.135. [DOI] [PubMed] [Google Scholar]
- Golden SS. Light-responsive gene expression in cyanobacteria. J Bacteriol. 1995;177:1651–1654. doi: 10.1128/jb.177.7.1651-1654.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski K, Roch GM, Prentki P, Krish HM. The stability of the bacteriophage T4 gene 32 mRNA: a 5′ leader sequence that can stabilize mRNA transcripts. Cell. 1985;43:461–469. doi: 10.1016/0092-8674(85)90176-x. [DOI] [PubMed] [Google Scholar]
- Higgins CF, Causton HC, Dance GSC, Mudd EA (1993) The role of the 3′ end in mRNA stability and decay. In JG Belasco, G Brawerman, eds, Control of Messenger RNA Stability. Academic Press, New York, pp 13–30
- Johnson CH, Kruft V, Subramanian AR. Identification of a plasmid-specific ribosomal protein in the 30S subunit of chloroplast ribosomes and isolation of the cDNA clone encoding its cytoplasmic precursor. J Biol Chem. 1990;265:12790–12795. [PubMed] [Google Scholar]
- Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S and others. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- Mayfield SP, Yohn CB, Amybeth C, Danon A. Regulation of chloroplast gene expression. Annu Rev Plant Mol Biol. 1995;46:147–166. [Google Scholar]
- Merrick MJ, Coppard JR. Mutations in genes downstream from the rpoN gene (encoding ς54) of Klebsiella pneumoniae affect expression from ς54-dependent promoters. Mol Microbiol. 1989;3:1765–1775. doi: 10.1111/j.1365-2958.1989.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Mohamed A, Jansson C. Influence of light on accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis 6803. Plant Mol Biol. 1989;13:693–700. doi: 10.1007/BF00016024. [DOI] [PubMed] [Google Scholar]
- Mohamed A, Jansson C. Photosynthetic electron transport controls degradation but not production of psbA transcripts in the cyanobacterium Synechocystis 6803. Plant Mol Biol. 1991;16:891–897. doi: 10.1007/BF00015080. [DOI] [PubMed] [Google Scholar]
- Mullet JE. Chloroplast development and gene expression. Annu Rev Plant Physiol. 1988;39:475–502. [Google Scholar]
- Salvador ML, Klein U, Bogorad L. 5′ Sequences are important positive and negative determinants of the longevity of Chlamydomonas chloroplast gene transcripts. Proc Natl Acad Sci USA. 1993;90:1556–1560. doi: 10.1073/pnas.90.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler P, Weisblum B. Erythromycin-induced stabilization of ermA messenger RNA in Staphylococcus aureus and Bacillus subtilis. J Mol Biol. 1988;203:905–915. doi: 10.1016/0022-2836(88)90116-7. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Srinivasa B, Weglohner W, Subramanian AR. A small chloroplast ribosomal protein (S31) that has no apparent counterpart in the E. coli ribosome. Biochem Mol Biol Int. 1993;29:25–31. [PubMed] [Google Scholar]
- Singer RA, Doolittle WF. Novel ribonucleic acid species accumulated in the dark in the blue green alga Anacystis nidulans. J Bacteriol. 1974;118:351–357. doi: 10.1128/jb.118.2.351-357.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer RA, Doolittle WF. Control of gene expression in blue-green algae. Nature. 1975;253:650–651. doi: 10.1038/253650a0. [DOI] [PubMed] [Google Scholar]
- Stern DB, Gruissem W. Control of plastid gene expression: 3′ inverted repeats act as mRNA processing and stabilizing elements, but do not terminate transcription. Cell. 1987;51:1145–1157. doi: 10.1016/0092-8674(87)90600-3. [DOI] [PubMed] [Google Scholar]
- Tan X, Varughese M, Widger WR. A light-repressed transcript found in Synechococcus PCC 7002 is similar to a chloroplast-specific small subunit ribosomal protein and to a transcription modulator protein associated with sigma 54. J Biol Chem. 1994;269:20905–20912. [PubMed] [Google Scholar]
- Widger WR. Photosynth Res. 1991;30:71–84. doi: 10.1007/BF00042005. [DOI] [PubMed] [Google Scholar]
- Zhou D-X, Mache R. Presence in the stroma of chloroplasts of a large pool of a ribosomal protein not structurally related to any Escherichia coli ribosomal protein. Mol Gen Genet. 1989;219:204–208. doi: 10.1007/BF00261178. [DOI] [PubMed] [Google Scholar]
- Zuker M, Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981;9:133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]