Abstract
Background
Hyperthermia has long been used in combination with chemotherapy or radiation therapy for the treatment of superficial malignancies, in part due to its sensitizing capabilities. Patients who suffer from superficial recurrences of breast cancer have poor clinical outcomes. Skin metastases may particularly impair the quality of life due to the physical appearance, odor and bleeding.
Case presentation
A 66-year-old woman underwent mastectomy and axillary lymph node dissection for breast cancer. Nine years post-operatively, local metastases developed in the left axillary area (measuring 5 cm in diameter). Initially the tumor did not respond to radiation therapy and chemotherapy. Therefore, we added hyperthermia combined with them. Eight weeks later, the tumor became nearly flat and the patient noted improved activity in her daily life.
Conclusion
Hyperthermia may accelerate the antitumor effects of radiation therapy and chemotherapy. This treatment provides an alternative for unresectable breast cancer skin metastases.
Keywords: Breast cancer, Skin metastasis, Hyperthermia
Background
Hyperthermia has been combined with radiotherapy in an effort to improve local control, which is essential in unresectable locally advanced breast cancer (LABC). Hyperthermia’s ability to affect cells in S phase, inhibit sub-lethal damage repair, and improve oxygenation make it an attractive therapy to combine with radiation and/or chemotherapy in the hopes of synergy [1-5]. The ultimate goal of the addition of hyperthermia to treatment for LABC is improved tumor kill, which most often is assessed with the rate of clinical complete response/partial response (cCR/pPR), and if the patient undergoes surgery, pCR. In addition to the inherent biology of an individual tumor, achieving a CR with thermoradiotherapy depends on the size of the tumor, dose of radiotherapy used, and ability to adequately heat the tumor, which can be especially challenging with large burdens of unresectable disease [6].
Case presentation
A 66-year-old woman underwent mastectomy and axillary lymph node dissection for breast cancer. The histopathological findings were papillo-tubular carcinoma with metastases in two axillary lymph nodes. IHC staining showed that estrogen receptor (ER) and progestin receptor (PR) were positive, and Human Epidermal Growth Factor Receptor 2 (HER-2) oncoprotein was negative. As adjuvant therapy, doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) were administered four times and aromatase inhibitor was prescribed for five years. There was no post-mastectomy radiotherapy (PMRT). Nine years post-operatively, local metastases developed in the left axillary area. Chemotherapy (weekly paclitaxel: 80 mg/m2) and radiotherapy (total 30 Gy) were attempted, but the lesions continued to enlarge. On examination, a tumor measuring 5 cm in diameter was found in the left axillar area (Figure 1A). Therefore, we added hyperthermia combined with radiotherapy and chemotherapy. Hyperthermia was performed using a Thermotron RF-8 capacitive heating device (Yamamoto VINITA Co., Ltd., Osaka, Japan at a surface temperature of 38°C to 41°C, once a week for 50 minutes [6]. The output power ranged from 1,300 to 1,400 W. After four weeks, the surface of the tumor began to dissolve, then became gradually necrotic (Figure 1B). Along with a reduction of the tumor size, the foul odor disappeared. Eight weeks later, the tumor became nearly flat and the response was evaluated as a complete response (CR), according to the Response Evaluation Criteria in Solid Tumors Group criteria, and the patient noted improved activity in her daily life (Figure 1C).
Figure 1.
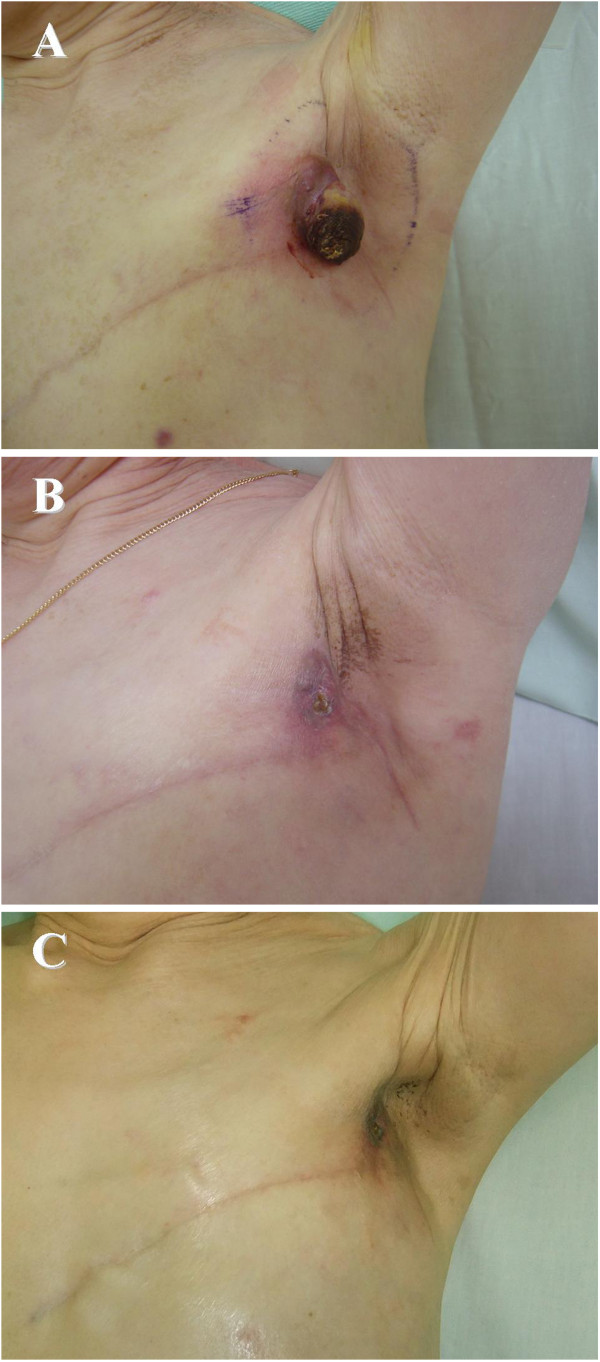
Skin metastasis from breast cancer was found in the left axillary area. (A) Before hyperthermia. The diameter of the tumor was 5 cm. Four weeks (B) and eight weeks (C) later, the tumor showed gradual shrinkage.
Discussion
Breast cancer is the most common neoplasm to metastasize to the skin. Skin metastases impair activities of daily life due to the physical appearance, odor and bleeding. In the present study, chemotherapy (weekly paclitaxel: 80 mg/m2) and radiotherapy (total 30 Gy) were attempted, but the lesions continued to enlarge. On examination, a tumor measuring 5 cm in diameter was found in the left axillar area (Figure 1A). Therefore, we added hyperthermia combined with the other therapies. In general, the first-line therapy for lymphadenopathy is radiotherapy in which the area was irradiated(total 30 Gy). Thus, we selected hyperthermia, as the radiotherapy (total 30 Gy) was already applied to the area of axillary lymphadenopathy. As a result, we succeeded in controlling these symptoms through the combined application of chemo-radiation therapy and hyperthermia, which resulted in a greatly improved quality of life.
Hyperthermia has been investigated in several randomized and non-randomized clinical trials for cancer therapy [7-11]. Hyperthermia can increase the therapeutic ratio by enhancing the permeability of tumor blood vessels to liposomes. In pre-clinical studies it has been demonstrated that the rate of liposomal extravasation is enhanced four- to eight-fold for temperatures in the target range of this trial [12]. In cats with soft tissue sarcomas, hyperthermia enhanced radio-labeled liposomal uptake by 4- to 16-fold compared to normothermia [13]. Hyperthermia also increases oxygen levels within the tumor, which is critical to the effectiveness of radiation and chemotherapy [14-17]. In a clinical setting, some recent studies [18,19] showed that neoadjuvant chemotherapy combined with hyperthermia is a feasible and well-tolerated treatment strategy in breast cancer patients. In this study [18], 19 of 44 patients were deemed inoperable at the initial assessment. Fourteen of these patients had inflammatory disease. Only five patients were candidates for breast-conserving surgery (BCS). Eight patients elected to have BCS, although 16 were eligible after reassessment following neoadjuvant treatment. At surgery, 32 patients (73%) were found to have axillary lymph node involvement. No patients progressed during neoadjuvant therapy. A complete pathological response was seen in four patients (9%). The combined pathological response was 60% (CR: 9%, and PR: 51%). Therefore, there is a possibility that the addition of HT to preoperative chemotherapy increases cCR and pCR rates more so than chemotherapy alone. In our previous studies, patients with pCR to chemotherapy had a favorable prognosis [20,21]. Thus, the goal of adding hyperthermia to radiotherapy and/or chemotherapy is to increase response rates, and hopefully local control and disease-free survival.
Conclusions
In conclusion, multidisciplinary therapy, such as hyperthermia, radiotherapy and chemotherapy, may be a useful and effective method for the treatment of progressive breast cancer.
Consent
Written consent was obtained from the patient for publication of this study and the related photos.
Abbreviations
BCS: Breast-conserving surgery; cCR: Clinical complete response; CR: Complete response.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
DY, YT, NS, and KK performed chemotherapy as a team. TI and CY treated the patient using hyperthermia. DY drafted the manuscript. MY carried the data acquisition. All authors read and approved the final manuscript.
Contributor Information
Daigo Yamamoto, Email: yamamotd@hirakata.ac.jp.
Toshio Inui, Email: yamamotd@hirakata.kmu.ac.jp.
Yu Tsubota, Email: amamotd@hirakata.kmu.ac.jp.
Noriko Sueoka, Email: yamamotd@hirakata.kmu.ac.jp.
Chizuko Yamamoto, Email: yamamotd@hirakata.kmu.ac.jp.
Kayoko Kuwana, Email: yamamotd@hirakata.kmu.ac.jp.
Mitsuo Yamamoto, Email: yamamotd@hirakata.kmu.ac.jp.
Acknowledgements
We would like to thank staff in Inui Clinic for assisting in the preparation of this manuscript.
References
- Westra A, Dewey WC. Variation in sensitivity to heat shock during the cell-cycle of Chinese hamster cells in vitro. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;19:467–477. doi: 10.1080/09553007114550601. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. Int J Radiat Biol. 2001;77:399–408. doi: 10.1080/09553000010024687. [DOI] [PubMed] [Google Scholar]
- Raaphorst GP, Ng CE, Yang DP. Thermal radiosensitization and repair inhibition in human melanoma cells: a comparison of survival and DNA double strand breaks. Int J Hyperthermia. 1999;15:17–27. doi: 10.1080/026567399285828. [DOI] [PubMed] [Google Scholar]
- Dewhirst MW. Concepts of oxygen transport at the microcirculatory level. Semin Radiat Oncol. 1998;8:143–150. doi: 10.1016/S1053-4296(98)80040-4. [DOI] [PubMed] [Google Scholar]
- Zagar TM, Oleson JR, Vujaskovic Z, Dewhirst MW, Craciunescu OI, Blackwell KL, Prosnitz LR, Jones EL. Hyperthermia for locally advanced breast cancer. Int J Hyperthermia. 2010;26:618–624. doi: 10.3109/02656736.2010.501051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uto Y, Hori H, Kubo K, Ichihashi M, Sakamoto N, Mette M, Inui T. GcMAF: our next generation immunotherapy. Nature. 2012;485:S67–S70. [Google Scholar]
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23:3079–3085. doi: 10.1200/JCO.2005.05.520. [DOI] [PubMed] [Google Scholar]
- Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, van Putten WLJ, van Rhoon GC, van Dijk JDP, Gonzalez Gonzalez D. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. Int J Radiat Oncol Biol Phys. 1996;35:731–744. doi: 10.1016/0360-3016(96)00154-X. [DOI] [PubMed] [Google Scholar]
- Lee HK, Antell AG, Perez CA, Straube WL, Ramachandran G, Myerson RJ, Emami B, Molmenti EP, Buckner A, Lockett MA. Superficial hyperthermia and irradiation for recurrent breast carcinoma of the chest wall: Prognostic factors in 196 tumors. Int J Radiat Oncol Biol Phys. 1998;40:365–375. doi: 10.1016/S0360-3016(97)00740-2. [DOI] [PubMed] [Google Scholar]
- Hehr T, Lamprecht U, Glocker S, Classen J, Paulsen F, Budach W, Bamberg M. Thermoradiotherapy for locally recurrent breast cancer with skin involvement. Int J Hyperthermia. 2001;17:291–301. doi: 10.1080/02656730110049538. [DOI] [PubMed] [Google Scholar]
- Barnes JA, Dix DJ, Collins BW, Luft C, Allen JW. Expression of inducible Hsp70 enhances the proliferation of MCF-7 breast cancer cells and protects against the cytotoxic effects of hyperthermia. Cell Stress Chaperones. 2001;6:316–325. doi: 10.1379/1466-1268(2001)006<0316:EOIHET>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery. Effect of particle size. Cancer Res. 2000;60:4440–4445. [PubMed] [Google Scholar]
- Matteucci ML, Anyarambhatla G, Rosner G, Azuma C, Fisher PE, Dewhirst MW, Needham D, Thrall DE. Hyperthermia increases accumulation of technetium-99 m-labeled liposomes in feline sarcomas. Clin Cancer Res. 2000;6:3748–3755. [PubMed] [Google Scholar]
- Griffin RJ, Okajima K, Barrios B, Song CW. Mild temperature hyperthermia combined with carbogen breathing increases tumor partial pressure of oxygen (pO2) and radio-sensitivity. Cancer Res. 1996;56:5590–5593. [PubMed] [Google Scholar]
- Song CW, Shakil A, Osborn JL, Iwata K. Tumour oxygenation is increased by hyperthermia at mild temperatures. Int J Hyperthermia. 1996;12:367–373. doi: 10.3109/02656739609022525. [DOI] [PubMed] [Google Scholar]
- Vujaskovic Z, Poulson JM, Gaskin AA, Thrall DE, Page RL, Charles HC, MacFall JR, Brizel DM, Meyer RE, Prescott DM. Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int J Radiat Oncol Biol Phys. 2000;46:179–185. doi: 10.1016/S0360-3016(99)00362-4. [DOI] [PubMed] [Google Scholar]
- Thrall DE, Larue SM, Pruitt AF, Case B, Dewhirst MW. Changes in tumour oxygenation during fractionated hyperthermia and radiation therapy in spontaneous canine sarcomas. Int J Hyperthermia. 2006;22:365–373. doi: 10.1080/02656730600836386. [DOI] [PubMed] [Google Scholar]
- Vujaskovic Z, Kim DW, Jones E, Lan L, McCall L, Dewhirst MW, Craciunescu O, Stauffer P, Liotcheva V, Betof A, Blackwell K. A phase I/II study of neoadjuvant liposomal doxorubicin, paclitaxel, and hyperthermia in locally advanced breast cancer. Int J Hyperthermia. 2010;26:514–521. doi: 10.3109/02656731003639364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craciunescu OI, Thrall DE, Vujaskovic Z, Dewhirst MW. Magnetic resonance imaging: a potential tool in assessing the addition of hyperthermia to neoadjuvant therapy in patients with locally advanced breast cancer. Int J Hyperthermia. 2010;26:625–637. doi: 10.3109/02656736.2010.499526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase S, Yamamoto D, Kuroda Y, Kawaguchi T, Kitamura K, Odagiri H, Teramoto S, Akazawa K, Nagumo Y. Phase II trial of preoperative chemotherapy for breast cancer: Japan Breast Cancer Research Network (JBCRN)-02 trial. Anticancer Res. 2011;31:1483–1487. [PubMed] [Google Scholar]
- Yamamoto D, Iwase S, Yoshida H, Kuroda Y, Yamamoto C, Kitamura K, Odagiri H, Nagumo Y. Efficacy of meloxicam in combination with preoperative chemotherapy for breast cancer - Japan Breast Cancer Research Network (JBCRN) 02–1 trial. Anticancer Res. 2011;31:3567–3571. [PubMed] [Google Scholar]


