SUMMARY
Mutations that cause Intellectual Disability (ID) and Autism Spectrum Disorder (ASD) are commonly found in genes that encode for synaptic proteins. However, it remains unclear how mutations that disrupt synapse function impact intellectual ability. In the SYNGAP1 mouse model of ID/ASD, we found that dendritic spine synapses develop prematurely during the early postnatal period. Premature spine maturation dramatically enhanced excitability in the developing hippocampus, which corresponded with the emergence of behavioral abnormalities. Inducing SYNGAP1 mutations after critical developmental windows closed had minimal impact on spine synapse function, while repairing these pathogenic mutations in adulthood did not improve behavior and cognition. These data demonstrate that SynGAP protein acts as a critical developmental repressor of neural excitability that promotes the development of life-long cognitive abilities. We propose that the pace of dendritic spine synapse maturation in early life is a critical determinant of normal intellectual development.
INTRODUCTION
Disruptions to the molecular mechanisms controlling glutamatergic synapse structure and function are believed to underlie certain neurodevelopmental disorders of cognition, such as ID and ASD, which are two disorders that are often co-diagnosed in afflicted children (Bear et al., 2004; Penzes et al., 2011; Ramocki and Zoghbi, 2008; Sudhof, 2008). Deleterious mutations in synaptic proteins are linked to these disorders and many animal models display deficits related to synapse structure and/or function (Gauthier et al., 2011; Gilman et al., 2011; Guilmatre et al., 2009; Hamdan et al., 2011a; Hamdan et al., 2011b; Hamdan et al., 2009; Sudhof, 2008). However, it remains largely unknown how synaptic dysfunction resulting from pathogenic mutations during development impacts circuit function and behavior. This is a particularly important consideration in ID and autism spectrum disorder (ASD) because these brain disorders are often first diagnosed in very young children. Disruption of excitatory/inhibitory (E/I) balance is emerging as a common neurophysiological phenotype common to many brain disorders, including ID and ASD (Rubenstein and Merzenich, 2003). Recently, it was demonstrated that increasing neural excitation is sufficient to disrupt cognition and sociability (Yizhar et al., 2011). Therefore, genetic mutations that selectively increase glutamatergic synaptic strength in pyramidal neurons would be expected to significantly impact E/I balance, information processing and behavior, particularly during early postnatal development when GABAergic interneuron systems are still maturing (Danglot et al., 2006).
Recently, autosomal dominant de novo mutations in SYNGAP1 that lead to truncation of the full-length protein were reported as a cause of sporadic ID in ~4% of screened cases (Hamdan et al., 2011a; Hamdan et al., 2009; Krepischi et al., 2010). All identified patients with SYNGAP1 haploinsufficiency have moderate to severe forms of ID, and several of these patients also have an ASD (Hamdan et al., 2011a; Pinto et al., 2010). Interestingly, these patients present with non-syndromic ID, as there are no physical abnormalities other than those observed in the cognitive/behavioral domain. Thus, de novo mutations that disrupt SYNGAP1 are highly pathogenic and selectively impact brain function. Early prevalence data indicates that these mutations are unexpectedly common (predicted to be > 1 million afflicted individuals world-wide and more prevalent than Fragile X syndrome), underscoring the impact that SYNGAP1 has on cognitive development (Hamdan et al., 2011a; Hamdan et al., 2011b; Hamdan et al., 2009).
SYNGAP1 encodes a synaptic RasGAP (SynGAP) that is largely localized to dendritic spines in neocortical pyramidal neurons (Chen et al., 1998; Kim et al., 1998; Zhang et al., 1999), where it suppresses signaling pathways linked to NMDA receptor (NMDAR) mediated synaptic plasticity and AMPA receptor (AMPAR) membrane insertion (Kim et al., 2005; Krapivinsky et al., 2004; Rumbaugh et al., 2006). This is a complicated gene with alternative transcriptional start sites and several alternatively spliced C-terminal exons that result in many possible isoforms of SynGAP (Chen et al., 1998; Kim et al., 1998). Not surprisingly, the impact of SynGAP protein expression in neurons is unclear. Both the N- and C- termini expression can influence SynGAP protein function, and depending on the variant expressed, SynGAP can either stimulate (Rumbaugh et al., 2006) or suppress dendritic spine synapse function (McMahon et al., 2012). In addition, disrupting SynGAP expression in dissociated hippocampal neurons can enhance dendritic spine function (Kim et al., 2005; Rumbaugh et al., 2006) or suppress it (Krapivinsky et al., 2004). Based on these data, it is difficult to predict how inactivating mutations of SYNGAP1 would impact the development of brain circuits and the cognitive modalities subserved by them. Regardless, considering that this protein is restricted to dendritic spines and copy number variation directly impacts cognition, mice that harbor syngap1 truncating mutations provide an excellent model to study how a genetic mutation influences synaptic maturation and cognitive development. Interestingly, adult SynGAP Heterozygous knockout mice (Hets), which model human SYNGAP1 haploinsufficiency and offer construct validity, are reported to have normal synaptic transmission and only modest defects in synaptic plasticity (Kim et al., 2003; Komiyama et al., 2002). Despite the lack of pervasive functional synaptic defects in adulthood, these animals have profound cognitive abnormalities (Guo et al., 2009; Komiyama et al., 2002; Muhia et al., 2010). These data suggest that SynGAP’s role in regulating synapse development may be particularly important to cognitive and behavioral maturation. However, the role of this critical gene in brain development remains largely unexplored. Therefore, we hypothesized that SYNGAP1 haploinsufficiency is particularly disruptive to neonatal dendritic spine synapse development, which, as a consequence, contributes to deficits in cognition and behavior.
In this study, we found that a mouse model of human SYNGAP1 haploinsufficiency had glutamatergic synapses that matured at an accelerated rate during the first few weeks of neonatal development. Loss of this essential glutamatergic synapse repressor dramatically disrupted E/I balance in neural networks that support cognition and behavior and these effects were linked to life-long intellectual disability. These studies provide a neurophysiological mechanism linking abnormal glutamatergic synapse maturation during development to enduring abnormalities in behaviors indicative of neurodevelopmental disorders.
RESULTS
SYNGAP1 haploinsufficiency accelerates the maturation of hippocampal synaptic function
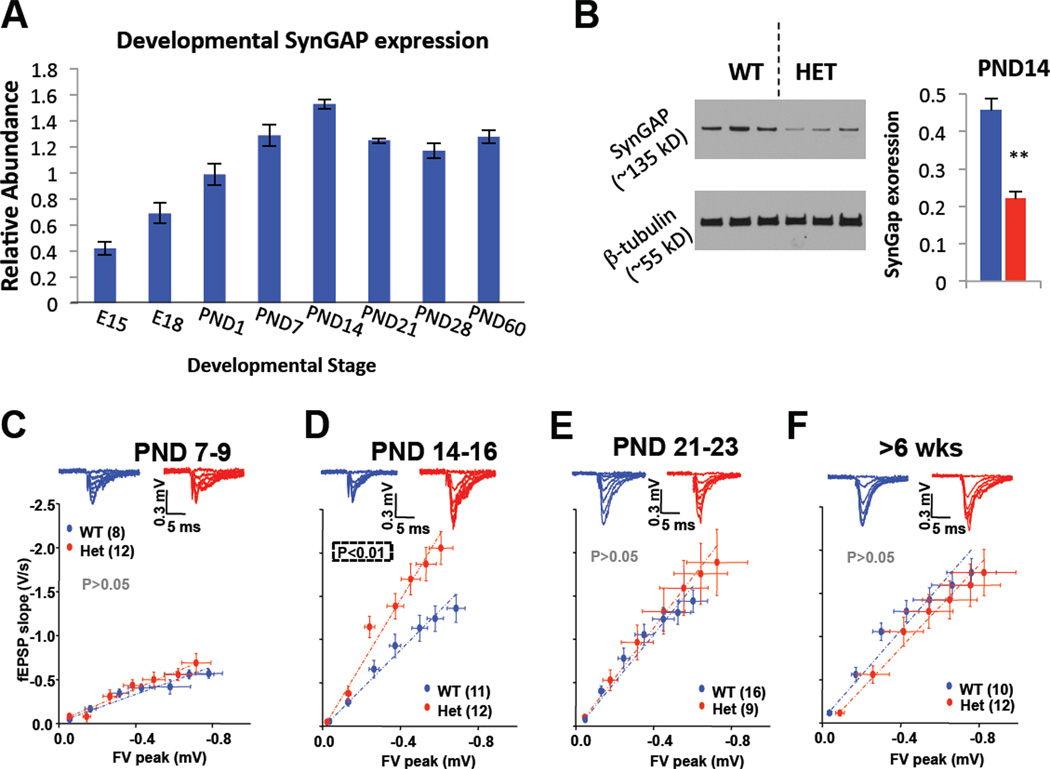
SynGAP is expressed throughout the forebrain, with particularly high levels in the hippocampus (Porter et al., 2005). The hippocampus is a central mediator of cognition and memory, as it receives and integrates information from sensory cortices that is then relayed to associational regions. Indeed, development of the hippocampus is disrupted in ID and ASD patients (Saitoh et al., 2001). SynGAP expression in the hippocampus of WT mice peaks around postnatal day (PND) 14 (Fig. 1A), suggesting that this period of brain development may be vulnerable to the reduced levels of full-length SynGAP protein expressed in Het mice (Fig. 1B). We began to probe for possible hippocampal circuit dysfunction in Het mice by measuring synaptic transmission in the medial perforant path (MPP) of the dentate gyrus (DG), the major input pathway into the hippocampus. Synaptic function was normal at very young ages (~PND9), but transmission increased dramatically in Het mice by PND14 (Fig. 1C-D). Interestingly, synaptic function was again equivalent between genotypes by PND21 and later (Fig. 1EF), indicating that SynGAP controls the trajectory of synapse maturation during a particularly critical period (PND10-20) of hippocampal development.
Figure 1. A restricted period of elevated excitatory synaptic transmission in developing SynGAP mutants.
A) SYNGAP1 transcript levels were measured in WT C57/Bl6J mice by qPCR throughout development. Relative abundance was calculated by normalizing SynGAP transcript levels to GAPDH levels, which was previously determined to not change during development. B) Hippocampi from WT (n=3) and Het (n=3) PND14 mice were extracted and probed for SynGAP and β-tubulin expression; (ANOVA, F(1,5)=28.2, p=.006). C-F) Representative traces of field EPSPs and summary graphs of input-output relationships from the MPP input into the DGNs measured during different developmental epochs. Significance was determined using an ANOVA to compare slopes after linear regression. n = slices. Error bars represent SEM.
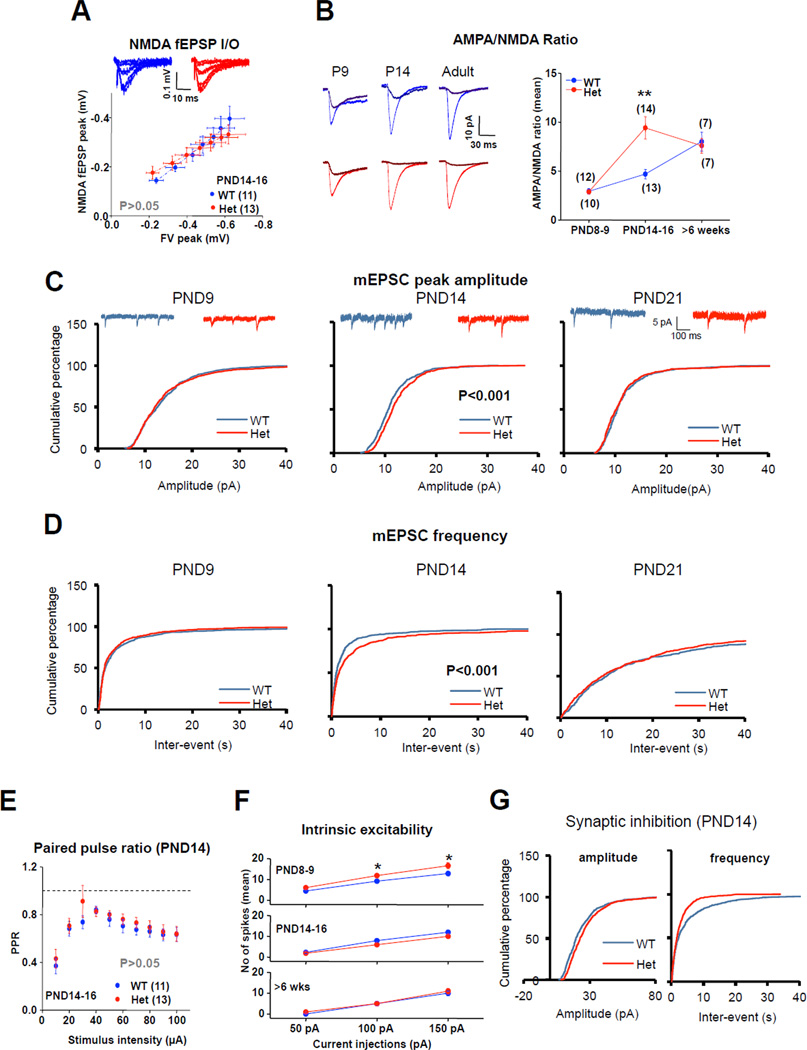
We next sought to better understand the neurophysiological mechanism responsible for enhanced synaptic function during development. NMDAR-evoked synaptic transmission was unchanged in PND14 Het mice (Fig. 2A), suggesting that the elevated synaptic transmission at PND14 was mediated by a postsynaptic increase in the sensitivity of AMPARs to evoked glutamate release. Consistent with this idea, we observed a selective increase in the ratio of AMPA/NMDA currents during whole-cell recordings of DG granule neurons (DGNs) only in PND14 Het mice (Fig. 2B). We found no differences in the rise or decay of these evoked currents at any age tested (Figure S1). These data also demonstrate that pathogenic SYNGAP1 mutations cause a premature acquisition of adult levels of functional AMPARs. We also observed a selective increase in mEPSC amplitude and frequency in SYNGAP1 Het mice at PND14, which also normalized by PND21 (Fig. 2C-D). Synaptic disruptions first seen at PND 14 were largely specific to postsynaptic function because we did not detect changes in MPP release probability (Fig. 2E) or intrinsic spiking of DGNs at this age (Fig. 2F). We observed no changes in resting membrane potential or input resistance at any age tested (Figure S1). Interestingly, we observed a significant increase in mIPSC frequency and amplitude in DGNs (Fig. 2G). Considering that SYNGAP is not expressed at inhibitory synapses (Chen et al., 1998; Kim et al., 1998), these data suggest the intriguing possibility that changes to the inhibitory system represent a compensatory response to increased excitation caused by elevated postsynaptic dendritic spine synapse AMPAR function in these neurons (Lau and Murthy, 2012).
Figure 2. Developmental disruptions to Het synaptic transmission are caused by enhanced sensitivity of AMPA receptors to released glutamate.
A) Isolated NMDAR-mediated fEPSPs at PND14-16. n = slices. B) Representative traces (left) and summary data of AMPA/NMDA ratios evoked from mild stimulation of MPP in patch-clamped DGGCs (ANOVA; PND7-9: F1,20= 0.031, p>0.05; PND14-16: F1,25=13.76, P<0.01; Adult: F1,12=0.118, P>0.05). C) Cumulative percentage of mEPSC amplitude from PND9 (n=800), PND14 (n=1300), PND21 (n=500). Note an increase in mEPSC amplitude only at PND14 (P<0.05; 2-sample K-S test). D) Summary of cumulative percentage of mEPSC inter-event interval, which is reduced at PND14 (P<0.05; 2-sample K-S test). E) Paired-Pulse ratio in WT and SynGAP Hets at PND 14-16 (RMANOVA; F1,23=0.898; P>0.05). F) Intrinsic excitability of DGGNs observed by variable current injections into patch-clamped cells at PND14-16 [RMANOVA (PND8-9; F1,24= 5.139, P<0.05; PND14-16: F1,40=2.280, P>0.05; >6weeks: F1,11= 0.353, P>0.05; asterisks denote significance after Bonferroni Post Hoc test, P<0.05). n=neurons. G) Cumulative probability of mIPSC amplitude (P<0.05, 2-sample K-S test) and frequency (P<0.05, 2-sample K-S test), respectively, from PND14; n=700. Error bars represent SEM.
SYNGAP1 haploinsufficiency disrupts dendritic spine dynamics during the 2nd postnatal week
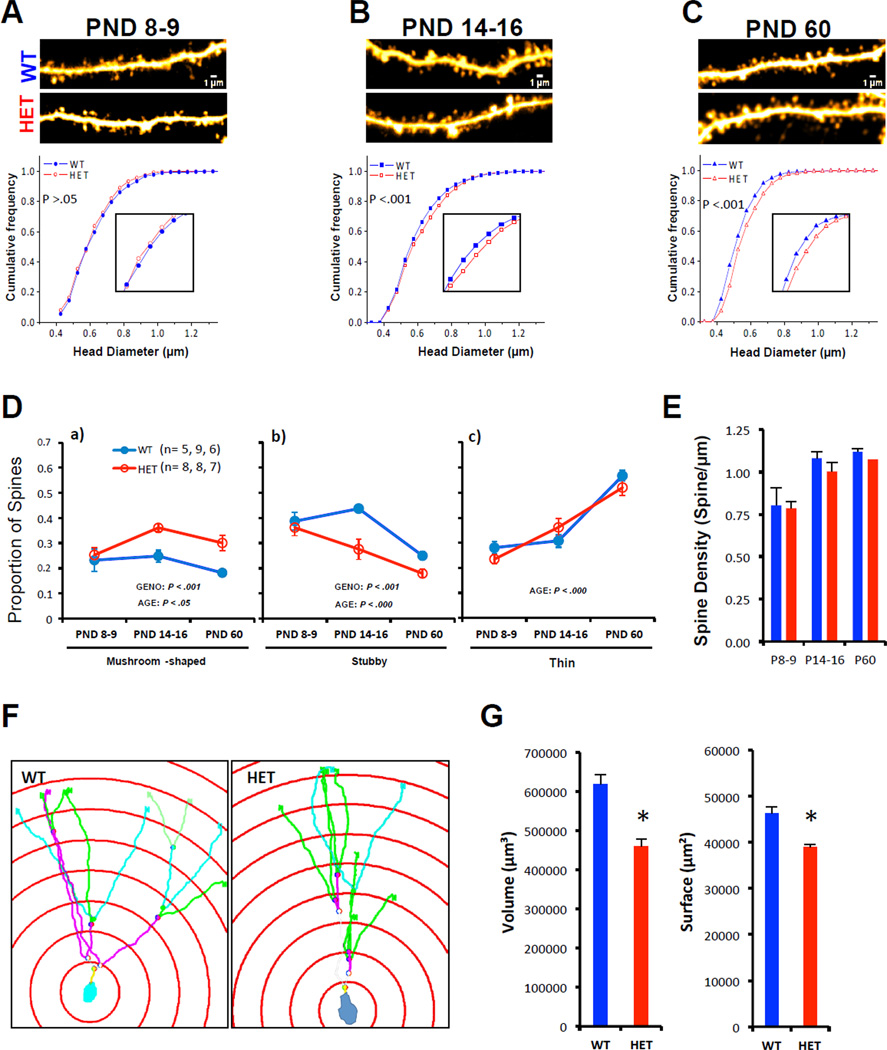
In pyramidal neurons, spine structure is tightly correlated with synapse function (Matsuzaki et al., 2001; Noguchi et al., 2011). Thus, we next sought to determine if SYNGAP1 haploinsufficiency disrupted the development of DGN dendritic spine structure over the same time-course as that observed for synaptic function. At PND9, SynGAP mutants had normal dendritic spine structure (Fig. 3A), but spines became larger relative to WT by PND14 (Fig. 3B). These abnormalities persisted into adulthood (Fig. 3C), which is consistent with the “spine dysfunction” theory of cognitive disorders (Penzes et al., 2011). The disruption to spine head size altered the distribution of spine classes in Het mice, resulting in more mushroom-type spines and fewer stubby spines beginning in the second postnatal week (Fig. 3D). We did not observe differences in spine density (Fig. 3E) at any stage of development in the DG, indicating that synapse density in the hippocampus is not affected by SYNGAP1 haploinsufficiency at the ages tested. Importantly, we confirmed that SYNGAP1 haploinsufficiency alters dendritic spine size in early development by characterizing these structures from internally perfused and fixed Thy1-GFP SYNGAP1 Hets (Figure S2). Additional structural abnormalities were observed at PND14. While dendritic arborization was unchanged in SYNGAP1 Hets (not shown), we did observe a decrease in the spatial volume occupied by individual DGN dendritic trees (Fig. 3F-G).
Figure 3. Emergence of abnormal spine size and shape in SYNGAP1 mutants during the second postnatal week.
A-C) Representative multiphoton excitation images of dendrites (scale bar = 1 µm) obtained in live acute brain slices in both WT and SYNGAP1 Hets. Cumulative frequency curves of spine head diameter in both groups across three stages of development [PND8-9, WT (n=687 spines) vs. Het (n=687); PND14-16, WT (n=1650) vs. Het (n=1650); PND60, WT (n= 963) vs. Het (n=963); K-S test was performed because a population defined by spine head diameter results in a clear non-normal distribution. Inset shows spine diameters from 0.6–0.9 um. D) Graphs depicting the proportion of Mushroom (left), Stubby (middle) and Thin (right) spines in WT and Het mice at three different developmental stages; Mushroom: Genotype [F(1, 37)=15.243, p=.00039]; Age [F(2, 37)=4.1677, p=.02332], Stubby: Genotype [F(1, 37)=13.167, p=.00086]; Age [F(2, 37)=17.451, p=.00000], Thin: Age [F(2, 37)=52.569, p=.00000]. n= slices. E) Density of WT (blue) and Het (red) spines at three different developmental time points were calculated (ANOVA; Genotype [F(1, 38)=.98887, p=.32631]; Age [F(2, 38)=14.048, p=.00003]; Genotype × Age [F(2, 38)=.13787, p=.87164]. F) Representative examples of 3D-reconstruction and Sholl ring analysis in dentate gyrus granular neuons (PND 16). G) Histograms showing volumetric and surface extension field of dentate gyrus neurons in both WT (n=40 traced neurons from 4 animals) and Het (n=40 traced neurons from 4 animals) mice; Student t test, *p<.05. Values represent means ± SEM.
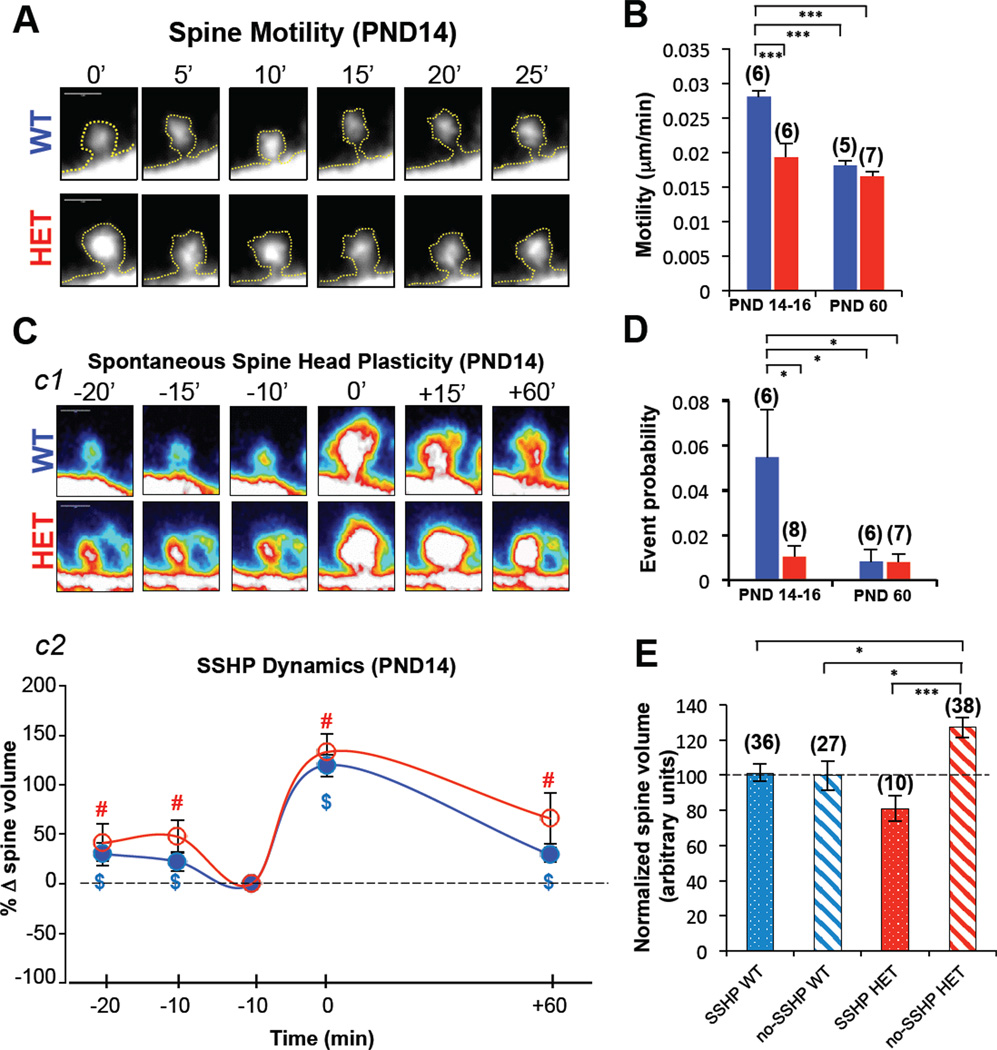
Gradual acquisition of synaptic AMPARs and subsequent functional un-silencing of glutamatergic inputs is a hallmark of early postnatal development (Kerchner and Nicoll, 2008). Therefore, premature acquisition of functional AMPARs into synapses, like that observed in SynGAP Hets, is suggestive of an aberrant acceleration of normal neurodevelopmental milestones. Therefore, we next investigated the idea that dendritic spine dynamics in SynGAP Het mice also displayed characteristics of early maturation. We observed that spines from PND14 DGNs in Het mice were significantly less motile than WT spines (Fig. 4A-B). Because spine motility rates decrease during development (Dunaevsky et al., 1999; Majewska and Sur, 2003), this measure is a mark of synapse maturation. Indeed, spines from young SynGAP Het mice appeared to have dynamics normally seen in adult animals. Spine motility rates dropped in WT animals between PND14 and adult (Fig. 4B) as expected (Dunaevsky et al., 1999; Majewska and Sur, 2003). However, motility rates did not drop in Het mice, as they already displayed adultlike motility rates by the end of the second postnatal week (Fig. 4B). Defects in spine motility and synaptic function in neonatal Het mice were linked to spine signaling abnormalities. Cofilin signaling regulates spine structure and AMPAR trafficking by controlling actin dynamics (Gu et al., 2010). Indeed, we observed that Cofilin was hyperphosphorylated in young SynGAP mutants (Figure S3). These results are consistent with signaling deficits previously reported in adult SynGAP mice (Carlisle et al., 2008; Komiyama et al., 2002).
Figure 4. SYNGAP1 haploinsufficiency disrupts developmental spine dynamics.
A) Multiphoton excitation images of an individual spine over time taken from acute slices in WT and Het mice at PND14. B) Motility of Het (red) and WT (blue) spines from slices taken at PND14-16 and PND60 [ANOVA two-way; Genotype (F(1,20)=19.439, P=.00027); Age (F(1,20)=29.338, P=.00003); Interaction (F(1,20)=9.3201, P=.00628)]; n = slices. C) c1, frames of individual spines in acute slices showing spontaneous spine head plasticity (SSHP) at PND14-16. c2, Spontaneous spine head plasticity kinetic curves of WT (n=36) and Het (n=10) spine populations demonstrating this phenomenon at PND14-16 [One-sample T-test (population mean = 100), $ = WT significance, # = Het significance, P < .05). D) Graph depicting SSHP event probability (probability that any observed spine in the brain slice would change volume >50% between 2 successive frames) in WT (blue) and Het (red) slices at PDN14-16 and PND60; [ANOVA two-way (Genotype [F(1,23)=4.6586, P=.04157]; Age [F(1,23)=5.6275, P=.02643]; Interaction [F(1,23)=4.6034, P=.04270]]; n = slices. E) Spine Head Volume measurements were made in spines that underwent plasticity (10 minutes before observation of >50% spine volume change) and spines that did not display this behavior (no-SSHP; spine chosen at random and volume measurement chosen at a randomly selected time point); thus dividing spines into two populations- plastic and not plastic; ANOVA one-way, [F(3,107)=6.720, P=.0003]. n = spines. A Bonferroni post-hoc test was applied where appropriate, *P < 0.05, ***P < 0.001. Error bars depict SEM.
During spine motility experiments, we made the surprising observation that, regardless of genotype, a subset of PND14 DGN spines displayed a novel form of structural plasticity defined by a spontaneous increase in head volume (Fig. 4C and movie S1). As a group, spines that displayed this previously unreported form of structural plasticity maintained the elevated volume for at least one hour. While the dynamics of spontaneous spine head enlargement were similar between genotypes (Fig. 4C), the frequency of spontaneous plasticity events was significantly lower in Het brain slices at PND14 (Fig. 4D). The frequency also dramatically decreased with age in WT, suggesting that this phenomenon might be related to developmental maturation of glutamatergic synapses in the DG. The frequency of plasticity events in Hets did not change between PND14 and adult time points (Fig. 4D), suggesting that spines in PND14 Het mice may have already undergone structural plasticity. In support of this idea, we observed that Het spines that did not display this plasticity were significantly larger than Het spines that did eventually enlarge (Fig. 4E). In addition, the population of PND14 Het spines that failed to enlarge was also significantly larger than both populations of WT spines (Fig. 4E). These data suggest that abnormal spine dynamics in early development may account for the persistent disruption to spine morphology observed in adult SynGAP Hets (Fig. 3A-B).
SYNGAP1 haploinsufficiency alters hippocampal information processing, E/I balance and memory
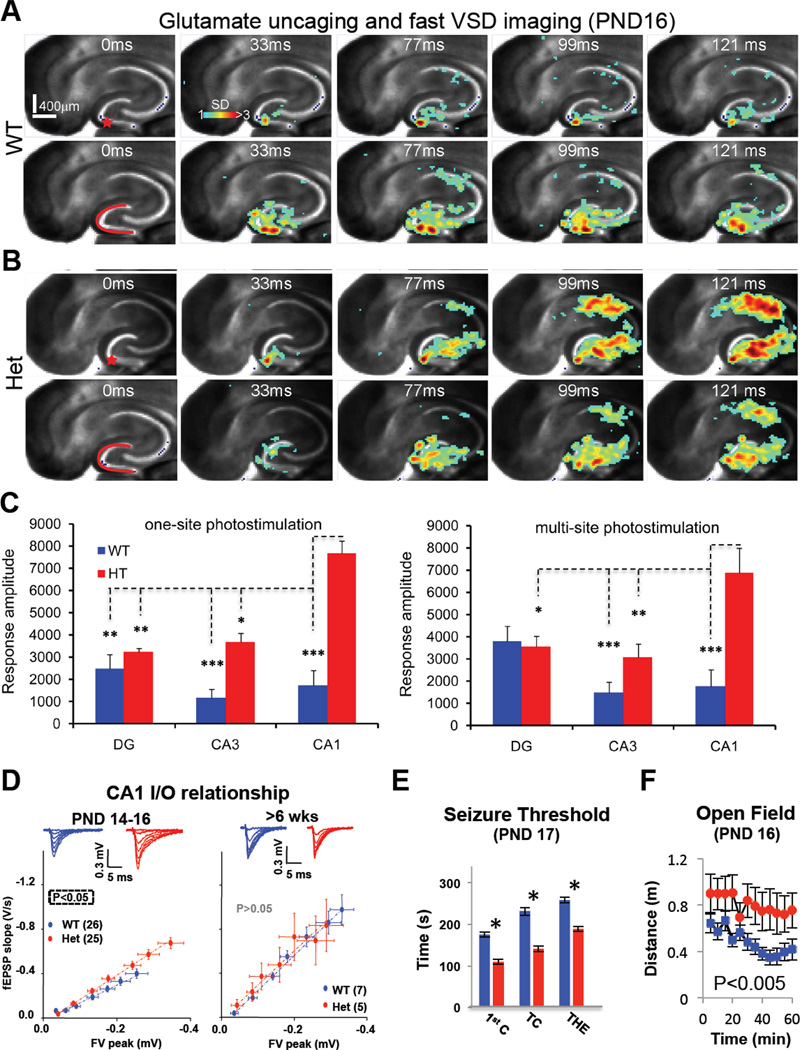
We next sought to determine if accelerated maturation of dendritic spine synapses in SynGAP Hets leads to altered excitability at the circuit level in the developing hippocampus. To directly test this idea, we performed laser photolysis of caged glutamate paired with fast voltage-sensitive dye imaging to monitor signal propagation throughout the entire hippocampus. Indeed, we found that the dynamics of signal propagation through the neonatal hippocampus were significantly different between genotypes (Fig. 5A-B). Photostimulation-evoked signals originating in the WT DG were progressively attenuated as they traveled through the tri-synaptic circuit (Fig. 5A,C and movie S2). In Het mice, however, signals originating in the DG were dramatically amplified as they spread through the hippocampus (Fig. 5B-C and movie S2), demonstrating that SYNGAP1 haploinsufficiency disrupts information processing in the hippocampus. These data also provided direct evidence that SYNGAP1 inactivating mutations shift the balance of hippocampal networks toward excitation in early development. Hyperactivity across the Het hippocampus suggested that synapses in addition to the MPP-DGN pathway would be abnormally strong. Indeed, we found that synaptic transmission in the Schaeffer Collateral pathway in Area CA1 was also abnormally strong in early development, but not in adult Hets (Fig. 5D and Figure S4), indicating that enhanced synaptic function during early neural development is a common outcome of SYNGAP1 haploinsufficiency.
Figure 5. SYNGAP1 Haploinsufficiency in young mice causes abnormal hippocampal signal processing, E/I imbalance and seizures.
A-B) Time series data of voltage sensitive dye (VSD) imaging of responses to single-site photostimulation (indicated by the red star) in DG and near-simultaneous, multi-site photostimulation across DG (indicated by the red curve), respectively, from the same PND16 WT (a) or Het (b) slice. VSD frames were acquired at 2.2 ms/frame, but are displayed at specific time points. Time progresses from left to right in the row. Color code is used to indicate VSD signal amplitudes expressed as standard deviation (SD) multiples above the mean baseline. The warmer the color, the stronger the response. C) Average response amplitude in SD units for DG, CA3 and CA1 to single-site (left) and multi-site (right) DG photostimulation from WT (n=6) and Het (n=7) slices (four animals from each genotype); ANOVA two-way: Single Site [Genotype (F(1,33)=20.8, P=0.00007); Brain Region (F(2,33)=4.27, P=.022); Interaction (F(2,33)=5.12, P=0.011)], Multi-site [Genotype (F(1,33)=13.5, P=0.00083); Brain Region (F(2,33)=4.23, P=.023); Interaction (F(2,33)=7.20, P=0.0025)]. *p<0.05, **p<0.01, *** p<0.0005 after a Bonferroni post-hoc test. D) Representative traces and pooled data of input-output relationship from the Schaffer-collateral pathway in CA1 in developing and mature SynGAP mice. Significance was determined by comparison of slopes after linear regression. E) Time taken to reach three benchmarks in fluorothyl-induced seizures in PND17 Wt (n=25) and Het (n=21) mice [ANOVA; First Clonus (1st C): [F(1,42)=41.8, P<0.001], Tonic-Clonic (TC): [F(1,42)=46.5, P<0.001], Tonic Hindlimb Extension (THE): [F(1,42)=34.0, P<0.001]]. *p<0.001 F) Activity levels of PND16 WT (n=27) and Het (n=10) mice during an exposure to a novel open field arena; RMANOVA [F(1,33)=11.2, P=0.002]. Error bars depict SEM.
Hippocampal hyper-activation indicated that SynGAP Het mice may be prone to seizures, a condition highly comorbid in patients with SYNGAP1 haploinsufficiency (Hamdan et al., 2011a; Hamdan et al., 2009). Indeed, young SynGAP Het mice had a reduced fluorothyl-induced seizure threshold (Fig. 5E) and were prone to audiogenic seizures (Table S1 and movie S3), a phenotype shared with other mouse models of neurodevelopmental disorders (Musumeci et al., 2000). We next wished to determine if behavior was altered in neonatal SynGAP mutants. Although behavioral analysis is challenging in pre-weanling mice, we did observe that activity in the open field arena was much higher in PND14 Hets compared to WT littermates (Fig. 5F). Exploratory behavior in this paradigm is guided by several factors, including spatial cognition (Dvorkin et al., 2008), and hyperactivity is associated with developmental hippocampal dysfunction (Daenen et al., 2002). Together, these data link early maturation of dendritic spine synapses and E/I imbalance to the onset of behavioral abnormalities in neonatal SynGAP Het mice.
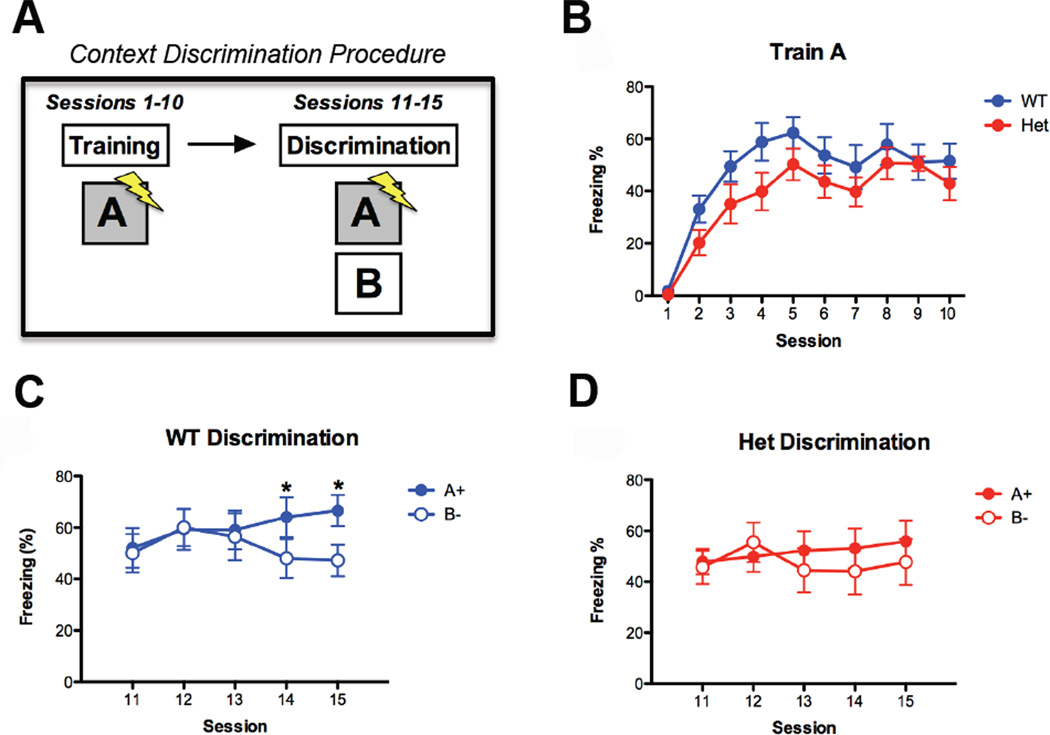
Developmental disruptions in the maturation of hippocampal circuits would be expected to cause persistent, ongoing deficits in brain function, including memory encoding (Squire, 1992). Proper DG circuit function is necessary to resolve two closely overlapping neural representations (Kesner, 2007). Experimentally, contextual discrimination is highly dependent on DG function (Sahay et al., 2011). Thus, context discrimination is an ideal paradigm to link synapse dysfunction in the DG to abnormal adult cognition. To probe for potential context discrimination deficits in SYNGAP1 haploinsufficiency, we first trained animals in a fear conditioning paradigm over several days in a unique context (A+). The animals were then exposed to the original training context (A+), followed by exposure to a slightly different contextual environment (B−) (Fig. 6A). As we reported previously (Guo et al., 2009), SynGAP Het mice exhibited normal contextual fear memory when compared to WT littermates (Fig 6B). WT mice were also able to discriminate between the similar contexts A and B, as they learned to freeze less in context B (Fig. 6C). Consistent with the idea that SynGAP Hets have functional deficits in the dentate gyrus, Het mice were unable to discriminate between the two contexts over the same period of time (Fig. 6D).
Figure 6. Adult SYNGAP1 Hets display learning deficits in a task selective for the Dentate Gyrus.
A) Schematic depicting context discrimination paradigm. B) WT (n = 10) and Het (n = 12) mice in the multi-day training paradigm. Both groups showed a significant and equivalent increase in freezing in context A across sessions (main effect of session F(9, 180) = 31.7, p < .05, no effect of genotype F(1, 20) = 2.08, p > .05, no genotype × session interaction F < 1). C) WT mice learned to discriminate the shock context (A+) from the safe environment (B−) across 5 sessions (main effect of context F(1,9) = 7.05, p < .05, context × session interaction F(4, 36) = 3.35, p < .05). D) Hets did not learn to discriminate between the shock context and safe environment across 5 sessions (no effect of context F(1, 11) = 1.72, p > .05, no context × session interaction F(4, 44) = 2.13. p > .05). * p < 0.05.
Developmental SYNGAP1 mutations lead to persistent behavioral abnormalities
The results thus far demonstrate that SYNGAP1 mutations responsible for persistent, life-long intellectual disability disrupt rodent synapse development in circuits that support cognition. However, it remains unclear if developmental synapse disruptions contribute to enduring cognitive and behavioral abnormalities. Therefore, we next performed a series of studies to link developmental synapse abnormalities to life-long cognitive disruptions.
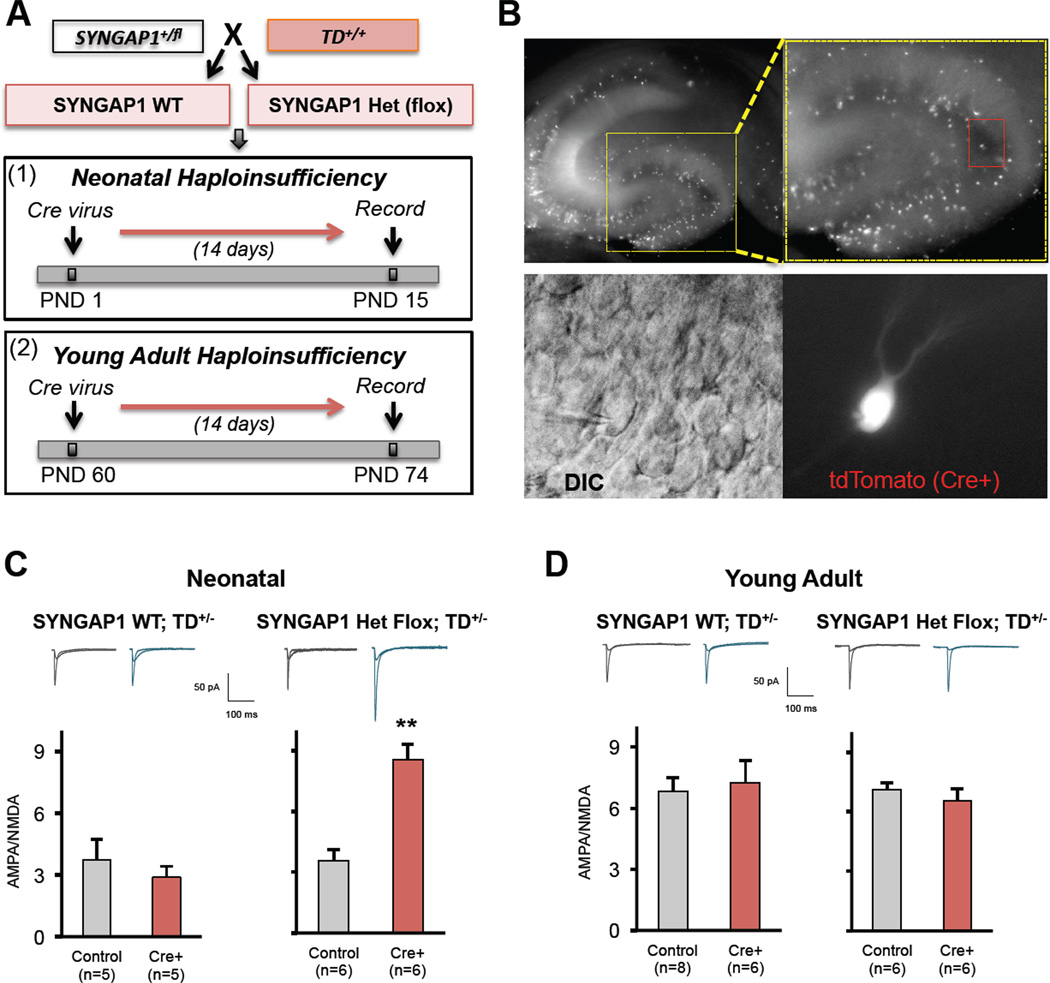
In the first set of studies, we sought to test the hypothesis that developing dendritic spine synapses are particularly sensitive to SYNGAP1 mutations. To test this idea, we engineered a conditional mouse line that allows for efficient temporal induction of SYNAGP1 haploinsufficiency. This newly engineered mouse line contained loxP sites flanking the same exons targeted in our conventional Het mouse. We confirmed that the inserted LoxP sites were correctly targeted in this mouse line and that Cre recombinase expression significantly reduced SynGAP protein levels in SYNGAP1+/fl neurons (Figure S5). We confirmed (Figure S5) a previous report (Pilpel et al., 2009) that demonstrated AAV8 psuedo-typed vector particles drive high levels of transgene expression within days of injection into the newborn mouse brain, indicating that it was technically possible to induce haploinsufficiency in the first week of life. For temporal induction of haploinsufficiency, AAV8 Cre virus particles were unilaterally injected into the hippocampus of either neonatal or adult SYNGAP1+/fl mice (Fig. 7A). To assess the effect of age-restricted haploinsufficiency, we recorded DGN AMPA/NMDA ratios from both infected (SYNGAP1 Haploinsufficiency) or uninfected (SynGAP WT) hemispheres 14 days after injections. To facilitate the identification of DGNs with haploinsufficiency, we crossed SYNGAP1 conditional KO mice with Ai9 Cre reporter mice, which express tdTomato in response to Cre activity. Indeed, two weeks after Cre virus injections, we observed a mosaic expression of tdTomato in neurons throughout the DG (Fig. 7B), confirming that the virus drives active Cre recombinase within 14 days of injection. As we had found in the conventional Het mice, PND1 virus injections into SYNGAP1+/flconditional mice resulted in a robust increase in DGN AMPA/NMDA ratios (Fig. 7C). This effect was most likely caused by altered SynGAP expression because there was no effect of Cre virus injection in SYNGAP1+/+ mice (Fig. 7C). We did not observe any other effects on synaptic or neuronal function in this neonatal haploinsufficiency experiment (not shown), indicating that a critical role of SynGAP during development is to control the maturation rate of dendritic spine synapses. Importantly, these data also demonstrate that pathogenic SYNGAP1 mutations affect synaptic maturation through cell autonomous mechanisms because we did not observe TdTomato-positive neurons outside the hippocampus. In the next experiment, virus was injected unilaterally into adult animals (Fig. 7A) to determine the effect of SYNGAP1 haploinsufficiency in the mature hippocampus. In contrast to the PND1 injections, there was no effect of Cre expression on AMPA/NMDA ratio in either the SYNGAP1+/fl or SYNGAP1+/+ mouse lines when recordings were performed two weeks after virus injection (Fig. 7D). However, we did find that adult induction of SYNGAP1 haploinsufficiency increased the intrinsic excitability of DGNs (Figure S5). This effect was not observed in either PND1 virus-injected animals (Figure S5) or in conventional adult Hets (Fig. 2F), indicating that this is a cell autonomous effect specific to adult-induced haploinsufficiency. Together, these data demonstrate that a critical period of SynGAP protein function exists in the first two weeks of hippocampal development, where this protein plays a key role in determining the rate of dendritic spine synapse development.
Figure 7. Developing, but not adult, dendritic spine synapses are cell autonomously suppressed by SynGAP protein.
A) Schematic depicting the strategy for temporal dissection of SYNAGAP1 haploinsufficiency on cell autonomous neuronal properties. Briefly, SYNGAP1 mice heterozygous for flanking LoxP (SYNGAP1+/fl) sites were crossed to homozygous Ai9 Cre reporter mice (TD+/+) and resulting offspring were injected with Cre virus at either PND1 or PND60. Whole-cell patch clamp recordings were carried out 14 days later. B) Photos were taken of an acute brain slice at PND14 with a mosaic expression of TdTomato+ neurons. Red box depicts a neuron that was successfully patched-clamped. C-D) Mean AMPA/NMDA ratios from either control (tdTomato-negative) or Cre+ (tdTomato-expressing) neurons patch-clamped in the two genotypes at the two developmental stages (neonatal = P<0.01; adults = p>0.05; Student’s t-test).
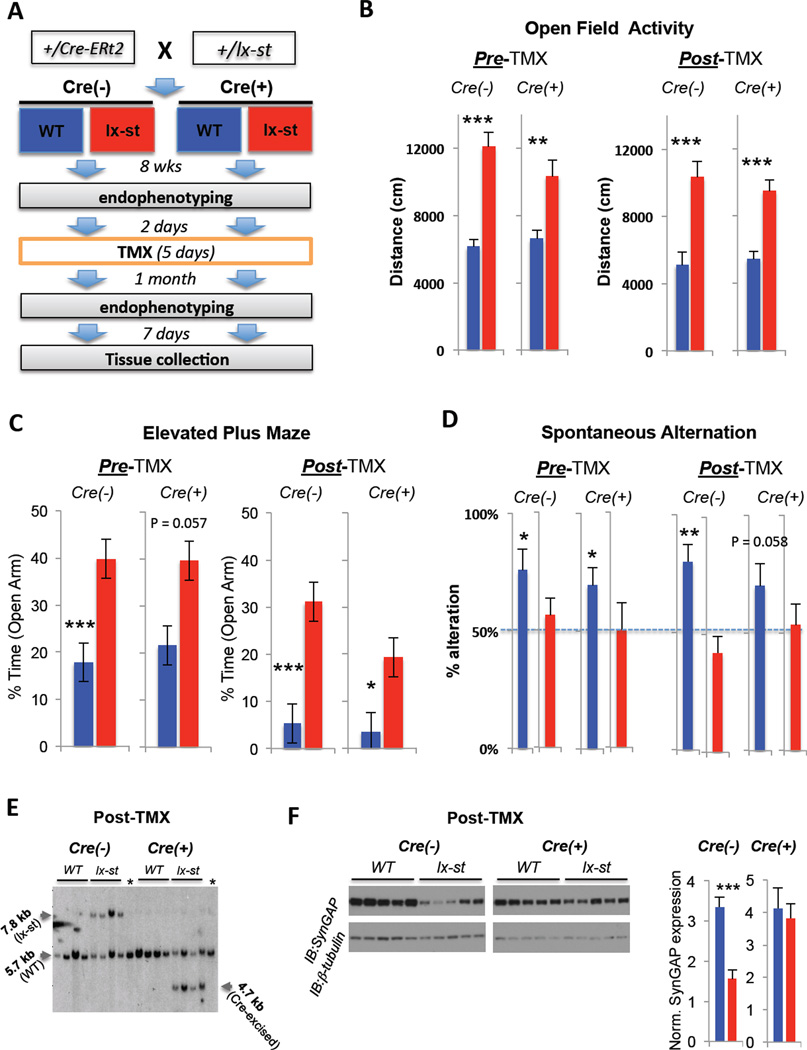
In the second series of experiments, we sought to determine if neonatal SYNGAP1 haploinsufficiency causes enduring cognitive and behavioral disability. The rationale behind this experiment was that if SYNGAP1 haploinsufficiency disrupted the organization of brain circuits during development, then rescue of SynGAP protein in adult mutants would have minimal positive impact on behavior and cognition. To test this idea, we constructed a novel mouse line that enabled conditional reversal of SYNGAP1 haploinsufficiency in adult mice. This mouse line contained a LoxP-STOP-LoxP cassette downstream of Exon 5 of the mouse SYNGAP1 gene (Figure S6). This cassette would be expected to cause a truncation of full-length protein, which is known to inactivate SynGAP (Rumbaugh et al., 2006). Indeed, all known cases of SYNGAP1 haploinsufficiency result from a truncation of the full-length protein (Hamdan et al., 2009; Hamdan et al., 2011a, Hamdan et al., 2011b) Southern blot analysis confirmed that the STOP cassette was appropriately targeted and that this targeted insertion disrupted expression of full-length SynGAP (Figure S6). Importantly, behavioral endophenotyping demonstrated that LoxP-stop Het mice had robust behavioral abnormalities similar to what we have previously published (Guo et al., 2009) in our conventional Hets (not shown). We next confirmed in cell culture experiments that the expression of Cre recombinase significantly increased SynGAP protein levels (not shown), indicating that removal of the STOP cassette could rescue SynGAP expression in a comprehensive behavioral study of adult mice. To test the effect of genetic rescue on behavioral and cognitive performance, we crossed heterozygous SYNGAP1 LoxP-Stop animals with a hemizygous inducible, and ubiquitously-expressing, Cre-ERt2 driver line (Hayashi and McMahon, 2002) previously shown to effectively rescue gene expression in adult mice (Guy et al., 2007) after tamoxifen (TMX) administration. The offspring resulting from this cross fell into four genotypes (Fig. 8A), which enabled us to test the effect of the LoxP-Stop allele while also controlling for the Cre-ERt2 background. At eight weeks of age, all animals were then endophenotyped in a mini-behavioral battery that tested for the presence of cognitive and non-cognitive behavioral abnormalities. Importantly, these behaviors were selected due to the ability to test the same animals before and after genetic rescue. The presence of the LoxP-stop cassette again resulted in behavioral abnormalities similar to that seen in our conventional SYNGAP1 Hets and the presence of CRE-ERt2 had minimal impact on the behaviors tested (Fig. 8B-D). Specifically, both Cre+ and Cre− SYNGAP1 LoxP-Stop Hets exhibited increased time in the open arm of the elevated plus maze, increased open field activity levels and the absence of spontaneous alternation in a T-maze. This latter behavior is striking, as it represents a failure of basic working memory that promotes survival (Lisman, 1999). Next, animals were injected with TMX to induce Cre-ERt2 activity, which was expected to reverse SYNGAP1 haploinsufficiency and restore SynGAP protein levels in these adult animals. One month after reversal, the same animals were again tested in the behavioral battery. Interestingly, there was no apparent effect of adult SynGAP rescue in any behaviors tested. In Open Field and Elevated Plus maze, Cre-positive LoxP-Stop Hets continued to show significant behavioral deficits relative to their WT controls (Fig. 8B-C). In addition, we compared the performance of each individual animal before and after rescue in these two tests by calculating difference scores. There was no significant difference between Cre-positive WT and LoxP-Stop Het behavior when comparing these difference scores (Figure S6), further supporting the idea that rescue did not impact behavior in Cre-positive LoxP-Stop mice. Finally, Cre-positive LoxP-Stop Hets continued to fail the spontaneous alternation tests in post-TMX trials (Fig. 8D), indicating that basic working memory in these mice did not improve after adult reversal of haploinsufficiency. Importantly, we confirmed by Southern blot that TMX injections reversed SYNGAP1 haploinsufficiency in adult Cre-positive LoxP-Stop mice (Fig. 8E) and that SynGAP protein expression was restored in these animals (Fig. 8F).
Figure 8. Rescuing SynGAP protein expression in adult mice has minimal impact on cognition and behavior.
A) Experimental scheme - Hemizygous male Cre mice were mated with female heterozygous SYNGAP1 lox stop (rescue) mice to generate four different genotypes: Cre−/WT, Cre−/lx-st, Cre+/WT, Cre+/lx-st, which were run through a behavioral battery at 8 wks, administered TMX for 5 consecutive days, retested in the behavioral battery 1 month later, and brains extracted and prepared for Southern and Western blots. B) The mice were run for 30 min sessions in a standard open field test before and after TMX administrations and analyzed for distances traveled. Students T-test: [pre-TMX, Cre− (WT (n=11) vs. Het (n=16)); Cre+ (WT (n=11) vs. Het (n=10)); post-TMX: Cre− (WT (n=10) vs. Het (n=15)); Cre+ (WT (n=10) vs. Het (n=10)); **P<0.005, ***P<0.001]. C) The mice were run in a standard 5 min elevated plus maze test before and after TMX administrations and analyzed for percent time spent in the open arms of the maze. Students T-test: [pre-TMX, Cre− (WT (n=11) vs. Het (n=16)); Cre+ (WT (n=11) vs. Het (n=10)); post-TMX: Cre− (WT (n=10) vs. Het (n=15)); Cre+ (WT (n=10) vs. Het (n=10)); *P<0.05, ***P<0.001]. D) The mice were run in a standard automated discrete-trials spontaneous alternation test three times before and after TMX administrations and analyzed for average percent alternation. Cre−/WTs and Cre+/WTs alternated significantly above chance (50%) level before and after TMX administrations, while the corresponding het groups did not. Dashed line represents chance levels of alternation (50%). Students One-Sample T-test against population mean of 50%: [pre-TMX: Cre− (WT (n=11), Het (n=16)); Cre+ (WT (n=11), Het (n=10)); post-TMX: Cre− (WT (n=10) vs. Het (n=15)); Cre+ (WT (n=10) vs. Het (n=10)); *P<0.05, **P<0.005]. E) Frontal cortical brain tissue from all four groups of mice was dissected and processed for Southern blot analysis, which confirmed that TMX excised the LoxP-Stop cassette in adult Cre-ERt2 positive animals. Four subjects from each group were randomly chosen for genetic analysis. Lanes with asterisks were loaded with C57/Bl6 positive control genomic DNA. F) Hippocampal tissues from the mice were dissected and processed for Western blot analysis of SynGAP protein levels normalized to β-tubulin after TMX administrations and behavioral testing (n=7 per group; t-test, P<0.001).
Discussion
SYNGAP1 haploinsufficiency accelerates the maturation of dendritic spine synapses during neonatal development
In this study, we report that dendritic spine synapses are profoundly impacted by SYNGAP1 haploinsufficiency during early postnatal development. This critical period of robust synaptogenesis and functional synapse maturation is marked by a steep rise in neural excitability. A major mechanism occurring during these first few weeks of rodent brain development is synapse unsilencing (Kerchner and Nicoll, 2008). While many synapse types are morphologically intact and capable of releasing glutamate, the majority of glutamaterigic post-synapses on excitatory neurons are not yet functional (Ashby and Isaac, 2011; Isaac et al., 1997; Petralia et al., 1999). Gradually, these synapses acquire functional AMPARs, driving an increase in neural circuit excitation. Our data demonstrate that an essential function of SynGAP during early brain development is to control the gain of excitatory synapses by restricting AMPA receptor accumulation apposed to presynaptic release sites, particularly in the phase of development where glutamatergic synapses gradually acquire AMPARs (e.g the first 21 days of rodent development). Mechanistically, SynGAP shapes developmental synaptic function through its synaptic GAP activity, where it suppresses many biochemical signaling cascades within dendritic spines that promote growth of synapses and insertion of AMPARs (Kim et al., 2005; Krapivinsky et al., 2004; Rumbaugh et al., 2006). This broad action of SynGAP on dendritic spine signaling arises from its central location in the NMDAR complex (Kennedy et al., 2005) and its endowment with a promiscuous GAP domain that can regulate a variety of small G-proteins (Krapivinsky et al., 2004; Pena et al., 2008). However, the role that SynGAP plays in neurons to regulate dendritic spine synapse function has been controversial because some studies report that SynGAP is a repressor of these synapses (Kim et al., 2005; Rumbaugh et al., 2006; Vazquez et al., 2004), while others report that it stimulates them (Krapivinsky et al., 2004; McMahon et al., 2012). Adding to the confusion, a recent report has demonstrated that SynGAP can enhance or inhibit dendritic spine synapse function depending on the particular isoform expressed (McMahon et al., 2012). While the roles of SynGAP may be complex at the cellular level, an important advance arising from our current study is the clear demonstration that SYNAGAP1 inactivating mutations present during early development have the net effect of de-repressing the maturation of dendritic spine synapse in the hippocampus. Thus, we propose that a core neurophysiological outcome of human SYNGAP1 haploinsufficiency is developmental hyperexcitability triggered by early dendritic spine synapse maturation.
The profound increase in glutamatergic synaptic strength caused by SYNGAP1 haploinsufficiency was only observed in an early period of hippocampal development. In addition, we observed this transient change in postsynaptic function at multiple synapses in the hippocampus, indicating that this phenomenon is widespread across neocortical dendritic spine synapses and is a primary outcome of pathogenic SYNGAP1 mutations. This effect in Het mice could arise from abnormally strong synapses that overshoot a predetermined level of function. Alternatively, because we show that SynGAP is a developmental repressor, it could also be caused by accelerated synapse development. Our findings support the latter possibility. Indeed, Het levels of postsynaptic function during the second postnatal week closely resembled that of adult WT animals, indicating that Het synapses achieve adult levels of strength earlier than their WT counterparts. This idea was further supported by the minimal impact of adult-induced SYNGAP1 haploinsufficiency on dendritic spine synapse function. Dendritic spines were less dynamic in the young Het brain, and these spines obtained adult levels of motility and plasticity earlier than WT animals. These findings are consistent with the role of SynGAP as an essential repressive factor that contributes to the developmental trajectory of glutamatergic synapse maturation (Kim et al., 2003; Rumbaugh et al., 2006; Vazquez et al., 2004). Our proposed “accelerated maturation” hypothesis also explains why others have failed to detect changes in hippocampal synaptic strength in adult SynGAP Het mice (Kim et al., 2003; Komiyama et al., 2002).
The impact of aberrant dendritic spine synapse maturation on circuit function and behavior
A major outstanding question in the field of neurodevelopmental disorders is how developmental disruptions to synapse maturation are translated into abnormal systems level alterations that impact cognition and behavior. E/I imbalance is a defining neurophysiological feature of neurodevelopmental disorders (Rubenstein and Merzenich, 2003) and elevated excitation is sufficient to disrupt cognition and sociability (Yizhar et al., 2011). We have found that the effects of SYNGAP1 haploinsufficiency on synapse maturation are translated into striking changes to E/I balance and hippocampal information processing in the early neonatal period. These data support a mechanism where premature maturation of glutamatergic synapses directly shifts the balance of networks toward aberrant excitation. These circuit-level excitability changes in neonates were accompanied by a reduced seizure threshold and elevated activity in the open field arena, suggesting that abnormal synapse maturation is directly altering behavioral performance and cognition.
Our data suggest that the large increase in neonatal hippocampal excitability occurs through selective loss of the normal repressive action of SynGAP on glutamatergic synapses in glutamatergic neurons. A selective effect on excitatory synapses during early development would be expected to produce a particularly profound hyperexcitation in the hippocampus, a brain region that is already sensitive to overstimulation due to the high level of recurrent excitation within the tri-synaptic network (Lisman, 1999). Recurrent excitation is balanced by feed forward inhibition. However, the local GABAergic networks that counteract excitation develop slowly between PND10-21 (Danglot et al., 2006), contributing to the susceptibility of the neonatal mammalian brain to seizure (Bender et al., 2004). Thus, the mammalian brain is ill-equipped to compensate for the enhanced developmental excitability caused by SYNGAP1 haploinsufficiency. Interestingly, GABAergic synaptic currents were elevated in DGNs in early development. However, because SynGAP is selectively localized to excitatory synapses (Chen et al., 1998; Kim et al., 1998), this effect is likely explained by a homoeostatic upregulation of the GABAergic system caused by elevated network excitation triggered by prematurely developing spine synapses. Thus, we propose that rapid dendritic spine synapse maturation in SYNAGAP1 mutants triggers a chain-reaction of compensatory cellular and systems-level events that may ultimately support organismal survival at the expense of intellectual development. This hypothesis presents a framework for understanding how reduced SynGAP expression in the neonate can have such a profound impact on cognitive development that persists throughout life.
In conclusion, we propose that altered E/I balance in early development, which is a major outcome of SYNAGP1 haploinsufficiency, directly contributes to cognitive and behavioral abnormalities observed in this disorder. E/I balance influences the duration and efficacy of critical period plasticity windows (Hensch, 2004), which permit refinement of connections that ultimately give rise to cognitive and behavioral modalities. Due to the pervasive disruption of synapse development and E/I balance in the neonatal SynGAP Het brain, we believe that critical windows of neural plasticity prematurely close or perhaps never open in the first place. In support of this hypothesis, the timing of synapse disruptions seen in Het mice precedes many of the known critical periods of neocortical development (Hensch, 2004). In addition, we show that dendritic spines become larger and functionally stronger earlier in development compared to WT animals. These features are characteristic of stable synapses that are less likely to be eliminated in vivo (Holtmaat et al., 2005). Most strikingly, however, SynGAP mutations cause dendritic spines to become less motile in early development. In fact, spine motility rates in young SynGAP Hets are indistinguishable from adult WT animals. The precise function of spine motility is still unknown, but there is strong evidence that it serves to promote initial wiring or rewiring of neuronal circuits during development (Konur and Yuste, 2004; Majewska and Sur, 2003; Yuste, 2011). Thus, a mutation that causes excessive developmental neural excitation, larger and stronger synaptic connections, and reduced spine motility is highly suggestive of neural networks that are initially miswired and thus resistant to later phases of experience-dependent refinement. A network with these irregular features would be expected to have altered windows of cortical development, resulting in cognitive dysfunction. In support of this idea, rescue of pathogenic SYNGAP1 mutations after critical periods close (e.g. adulthood) did not improve basic behavioral and cognitive abnormalities seen in the mouse model of the disease, suggesting that early spine synapse defects contribute to the disorganization of developing neural circuits that guide these behaviors in adulthood. Future studies will be necessary to understand precisely how developmental synapse disruptions influence the organization of neural circuits that govern intellectual and cognitive development.
EXPERIMENTAL PROCEDURES (BRIEF)
For all studies, the experimenter was blind to genotypes. The Heterozygous SynGAP KO mouse line has been described previously (Kim et al., 2003) and all studies utilized both males and females. The SYNGAP1 conditional KO line and the SYNGAP1 rescue line are described in the supplemental materials. For behavioral tests, adult animals were at least 12 weeks of age unless otherwise noted. For electrophysiological studies, acute brain slices were prepared from PND7-PND9, PND14-16, PND21-23 and 6–9 week old mice. For input-output studies, slices were prepared from one WT and one Het pair each day. Whole-cell current/voltage clamp experiments were made from visually identified DGNs in the molecular layer with glass microelectrodes with an open-tip resistance of 5–8 MΩ. Two-photon imaging of DGN dendrites was performed using a multiphoton laser-scanning microscope (Olympus FV1000MPE-TWIN, USA), equipped with a water immersion objective lens (ULTRA 25x, numerical aperture 1.05, Olympus) and FluoView software. Spines were imaged in acute slices from both Het and WT brain at PND8-9, PND14-16 and PND>60. 6–9 dendritic segments of ~20–30 µm were collected and considered for analysis. Segments were traced and each individual spine was marked and measured (spine width, length, head diameter). Spontaneous spine head plasticity was evaluated in terms of relative change in spine volume and occurrence of probability of events in both Het and WT PND 14–16 and PND>60 brain slices. We quantified relative changes in spine-head volume by measuring intensity of the spine head relative to the non-saturated parent dendrite (background signal was nominal). For voltage-sensitive dye imaging studies, optical recording of VSD signals was performed by the MiCAM02 system with a sampling rate of 2.2 ms per frame (frame resolution 88 (w)×60 (h) pixels). Stimulation was achieved by UV uncaging of MNI-glutamate. Under the 2x objective, the imaging field covered the area of 2.56×2.14 mm2 with a spatial resolution of 29.2×35.8 µm/pixel. Complete methods for each type of experiment, including all behavioral paradigms and mouse targeting strategies, are documented in the Supplemental Experimental Procedures section.
Supplementary Material
Highlights.
Pathogenic SYNGAP1 mutations promote early maturation of hippocampal spine Synapses
Mutations lead to neonatal hyperactivity of the hippocampal tri-synaptic circuit
Mutations have greatest impact during the first three weeks of development
Reversal of mutations in adults does not improve behavior and cognition
Acknowledgments
We thank Drs. Ronald Davis, Damon Page, and Fadi Hamdan, as well as members of the Rumbaugh Laboratory, for their helpful comments on this manuscript. We also thank Zachary Collier, Samuel Bacharach, Bryce Grier and Gopi Patel for assistance in context discrimination studies. This work was supported by grants to GR from the National Institute for Neurological Disorders and Stroke (R01NS064079), The Eunice Kennedy Shriver National Institute for Child Health and Human Development (R03HD060672) and the National Alliance for Research on Schizophrenia and Depression (NARSAD). The laser photo-stimulation and voltage-sensitive dye imaging part of this work was performed in XX's laboratory, supported by a grant from the National Institute for Drug Abuse (DA023700-04S1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: JPC and EDO performed electrophysiological recordings; MA performed multiphoton imaging studies and spine analysis; TKC, NJR, AGA, and BJW performed behavioral studies; YS and XX performed laser photo-stimulation and imaging studies; GR, CAM, TKC, JPC, MA, BJW, XX designed experiments; BM and TKC performed protein/mRNA studies; GR conceived the study and wrote the manuscript; GR, CAM, JPC, and TKC edited the manuscript.
References
- Ashby MC, Isaac JT. Maturation of a recurrent excitatory neocortical circuit by experience-dependent unsilencing of newly formed dendritic spines. Neuron. 2011;70:510–521. doi: 10.1016/j.neuron.2011.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends in neurosciences. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bender RA, Dube C, Baram TZ. Febrile seizures and mechanisms of epileptogenesis: insights from an animal model. Advances in experimental medicine and biology. 2004;548:213–225. doi: 10.1007/978-1-4757-6376-8_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle HJ, Manzerra P, Marcora E, Kennedy MB. SynGAP regulates steady-state and activity-dependent phosphorylation of cofilin. J Neurosci. 2008;28:13673–13683. doi: 10.1523/JNEUROSCI.4695-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Rojas-Soto M, Oguni A, Kennedy MB. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron. 1998;20:895–904. doi: 10.1016/s0896-6273(00)80471-7. [DOI] [PubMed] [Google Scholar]
- Daenen EW, Wolterink G, Gerrits MA, Van Ree JM. Amygdala or ventral hippocampal lesions at two early stages of life differentially affect open field behaviour later in life; an animal model of neurodevelopmental psychopathological disorders. Behavioural brain research. 2002;131:67–78. doi: 10.1016/s0166-4328(01)00350-3. [DOI] [PubMed] [Google Scholar]
- Danglot L, Triller A, Marty S. The development of hippocampal interneurons in rodents. Hippocampus. 2006;16:1032–1060. doi: 10.1002/hipo.20225. [DOI] [PubMed] [Google Scholar]
- Dunaevsky A, Tashiro A, Majewska A, Mason C, Yuste R. Developmental regulation of spine motility in the mammalian central nervous system. Proc Natl Acad Sci U S A. 1999;96:13438–13443. doi: 10.1073/pnas.96.23.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorkin A, Benjamini Y, Golani I. Mouse cognition-related behavior in the open-field: emergence of places of attraction. PLoS computational biology. 2008;4:e1000027. doi: 10.1371/journal.pcbi.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier J, Siddiqui TJ, Huashan P, Yokomaku D, Hamdan FF, Champagne N, Lapointe M, Spiegelman D, Noreau A, Lafreniere RG, et al. Truncating mutations in NRXN2 and NRXN1 in autism spectrum disorders and schizophrenia. Human genetics. 2011;130:563–573. doi: 10.1007/s00439-011-0975-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SR, Iossifov I, Levy D, Ronemus M, Wigler M, Vitkup D. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron. 2011;70:898–907. doi: 10.1016/j.neuron.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Lee CW, Fan Y, Komlos D, Tang X, Sun C, Yu K, Hartzell HC, Chen G, Bamburg JR, et al. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat Neurosci. 2010;13:1208–1215. doi: 10.1038/nn.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilmatre A, Dubourg C, Mosca AL, Legallic S, Goldenberg A, Drouin-Garraud V, Layet V, Rosier A, Briault S, Bonnet-Brilhault F, et al. Recurrent rearrangements in synaptic and neurodevelopmental genes and shared biologic pathways in schizophrenia, autism, and mental retardation. Archives of general psychiatry. 2009;66:947–956. doi: 10.1001/archgenpsychiatry.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Hamilton PJ, Reish NJ, Sweatt JD, Miller CA, Rumbaugh G. Reduced Expression of the NMDA Receptor-Interacting Protein SynGAP Causes Behavioral Abnormalities that Model Symptoms of Schizophrenia. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2008.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan FF, Daoud H, Piton A, Gauthier J, Dobrzeniecka S, Krebs MO, Joober R, Lacaille JC, Nadeau A, Milunsky JM, et al. De novo SYNGAP1 mutations in nonsyndromic intellectual disability and autism. Biological psychiatry. 2011a;69:898–901. doi: 10.1016/j.biopsych.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Hamdan FF, Gauthier J, Araki Y, Lin DT, Yoshizawa Y, Higashi K, Park AR, Spiegelman D, Dobrzeniecka S, Piton A, et al. Excess of de novo deleterious mutations in genes associated with glutamatergic systems in nonsyndromic intellectual disability. American journal of human genetics. 2011b;88:306–316. doi: 10.1016/j.ajhg.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan FF, Gauthier J, Spiegelman D, Noreau A, Yang Y, Pellerin S, Dobrzeniecka S, Cote M, Perreau-Linck E, Carmant L, et al. Mutations in SYNGAP1 in autosomal nonsyndromic mental retardation. The New England journal of medicine. 2009;360:599–605. doi: 10.1056/NEJMoa0805392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annual review of neuroscience. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron. 1997;18:269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- Kennedy MB, Beale HC, Carlisle HJ, Washburn LR. Integration of biochemical signalling in spines. Nat Rev Neurosci. 2005;6:423–434. doi: 10.1038/nrn1685. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nature reviews Neuroscience. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP. A behavioral analysis of dentate gyrus function. Progress in brain research. 2007;163:567–576. doi: 10.1016/S0079-6123(07)63030-1. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee HK, Takamiya K, Huganir RL. The role of synaptic GTPase-activating protein in neuronal development and synaptic plasticity. J Neurosci. 2003;23:1119–1124. doi: 10.1523/JNEUROSCI.23-04-01119.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Liao D, Lau LF, Huganir RL. SynGAP: a synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron. 1998;20:683–691. doi: 10.1016/s0896-6273(00)81008-9. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2Aand NR2B-containing NMDA receptors in and AMPA receptor Ras-ERK signaling trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Komiyama NH, Watabe AM, Carlisle HJ, Porter K, Charlesworth P, Monti J, Strathdee DJ, O'Carroll CM, Martin SJ, Morris RG, et al. SynGAP regulates ERK/MAPK signaling, synaptic plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor. J Neurosci. 2002;22:9721–9732. doi: 10.1523/JNEUROSCI.22-22-09721.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konur S, Yuste R. Developmental regulation of spine and filopodial motility in primary visual cortex: reduced effects of activity and sensory deprivation. Journal of neurobiology. 2004;59:236–246. doi: 10.1002/neu.10306. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Medina I, Krapivinsky L, Gapon S, Clapham DE. SynGAP-MUPP1-CaMKII synaptic complexes regulate p38 MAP kinase activity and NMDA receptor-dependent synaptic AMPA receptor potentiation. Neuron. 2004;43:563–574. doi: 10.1016/j.neuron.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Krepischi AC, Rosenberg C, Costa SS, Crolla JA, Huang S, Vianna-Morgante AM. A novel de novo microdeletion spanning the SYNGAP1 gene on the short arm of chromosome 6 associated with mental retardation. American journal of medical genetics Part A. 2010;152A:2376–2378. doi: 10.1002/ajmg.a.33554. [DOI] [PubMed] [Google Scholar]
- Lau CG, Murthy VN. Activity-Dependent Regulation of Inhibition via GAD67. J Neurosci. 2012;32:8521–8531. doi: 10.1523/JNEUROSCI.1245-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- Majewska A, Sur M. Motility of dendritic spines in visual cortex in vivo: changes during the critical period and effects of visual deprivation. Proc Natl Acad Sci U S A. 2003;100:16024–16029. doi: 10.1073/pnas.2636949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GCR, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nature Neuroscience. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon AC, Barnett MW, O'Leary TS, Stoney PN, Collins MO, Papadia S, Choudhary JS, Komiyama NH, Grant SG, Hardingham GE, et al. SynGAP isoforms exert opposing effects on synaptic strength. Nature communications. 2012;3:900. doi: 10.1038/ncomms1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhia M, Yee BK, Feldon J, Markopoulos F, Knuesel I. Disruption of hippocampus-regulated behavioural and cognitive processes by heterozygous constitutive deletion of SynGAP. The European journal of neuroscience. 2010;31:529–543. doi: 10.1111/j.1460-9568.2010.07079.x. [DOI] [PubMed] [Google Scholar]
- Musumeci SA, Bosco P, Calabrese G, Bakker C, De Sarro GB, Elia M, Ferri R, Oostra BA. Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia. 2000;41:19–23. doi: 10.1111/j.1528-1157.2000.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Noguchi J, Nagaoka A, Watanabe S, Ellis-Davies GC, Kitamura K, Kano M, Matsuzaki M, Kasai H. In vivo two-photon uncaging of glutamate revealing the structure-function relationships of dendritic spines in the neocortex of adult mice. The Journal of physiology. 2011;589:2447–2457. doi: 10.1113/jphysiol.2011.207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena V, Hothorn M, Eberth A, Kaschau N, Parret A, Gremer L, Bonneau F, Ahmadian MR, Scheffzek K. The C2 domain of SynGAP is essential for stimulation of the Rap GTPase reaction. EMBO reports. 2008;9:350–355. doi: 10.1038/embor.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nature Neuroscience. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- Pilpel N, Landeck N, Klugmann M, Seeburg PH, Schwarz MK. Rapid, reproducible transduction of select forebrain regions by targeted recombinant virus injection into the neonatal mouse brain. J Neurosci Methods. 2009;182:55–63. doi: 10.1016/j.jneumeth.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, Conroy J, Magalhaes TR, Correia C, Abrahams BS, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K, Komiyama NH, Vitalis T, Kind PC, Grant SG. Differential expression of two NMDA receptor interacting proteins, PSD-95 and SynGAP during mouse development. The European journal of neuroscience. 2005;21:351–362. doi: 10.1111/j.1460-9568.2005.03874.x. [DOI] [PubMed] [Google Scholar]
- Ramocki MB, Zoghbi HY. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature. 2008;455:912–918. doi: 10.1038/nature07457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain and Behavior. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh G, Adams JP, Kim JH, Huganir RL. SynGAP regulates synaptic strength and mitogen-activated protein kinases in cultured neurons. Proc Natl Acad Sci U S A. 2006;103:4344–4351. doi: 10.1073/pnas.0600084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh O, Karns CM, Courchesne E. Development of the hippocampal formation from 2 to 42 years: MRI evidence of smaller area dentata in autism. Brain: a journal of neurology. 2001;124:1317–1324. doi: 10.1093/brain/124.7.1317. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez LE, Chen HJ, Sokolova I, Knuesel I, Kennedy MB. SynGAP regulates spine formation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:8862–8872. doi: 10.1523/JNEUROSCI.3213-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R. Dendritic spines and distributed circuits. Neuron. 2011;71:772–781. doi: 10.1016/j.neuron.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Vazquez L, Apperson M, Kennedy MB. Citron binds to PSD-95 at glutamatergic synapses on inhibitory neurons in the hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:96–108. doi: 10.1523/JNEUROSCI.19-01-00096.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.