Abstract
Objectives
The aim of this study was to determine the correlation between increasing pulmonary embolism thrombus load and right ventricular (RV) dilatation as demonstrated by CT pulmonary angiography (CTPA) and to assess the thrombus load threshold which indicates impending RV decompensation.
Methods
2425 consecutive CTPAs were retrospectively analysed. Thrombus load using a modified Miller score (MMS), RV to left ventricular (RV:LV) ratio, presence of septal shift, and pulmonary artery and aorta size were analysed in 504 positive CTPA scans and a representative cohort of 100 negative scans. Results were correlated using non-parametric analysis (two-tailed t-test or χ2 test) and Pearson’s rank correlation.
Results
Increasing thrombus load correlated with a higher RV:LV ratio, with a statistically significant difference in RV:LV ratios between the negative and positive pulmonary embolism (PE) cohorts. Larger thrombus loads (MMS ≥12 vs MMS <12) were strongly correlated with RV strain (mean RV:LV ratio, 1.323 vs 0.930; p<0.0001). Smaller thrombus loads had no significant influence on RV strain. Septal shift was also more likely with an MMS of ≥12, as was an increase in pulmonary artery diameter (r=0.221, p<0.001).
Conclusion
With increasing thrombus load in PE, there is CTPA evidence of RV decompensation with an MMS threshold of 12. This suggests a “tipping point” beyond which RV decompensation is more likely to occur. This is the first study to describe this tipping point between a thrombus load of MMS >12 and an increase in RV:LV ratio. This finding may help to improve risk stratification in patients with acute PE diagnosed by CTPA.
Acute pulmonary embolism (PE) remains a diagnostic challenge for physicians and accounts for significant morbidity and mortality in hospitalised patients. In the United Kingdom, the incidence of proven PE is 60–70 per 100 000 in the population and mortality rates range from 6% to 15%. Clinical manifestations vary widely, from asymptomatic patients with small peripheral emboli to patients who present with circulatory collapse and large thromboembolic loads who may warrant thrombolysis. Between these extremes, there is a significant group presenting with PE who have apparent clinical haemodynamic stability but demonstrate radiological findings (e.g. via echocardiography or CT pulmonary angiography) or biomarkers [such as B-type natriuretic peptide (BNP) or troponin] of right heart strain, in whom the prognosis may be poorer and for whom the role of thrombolysis has not been established [1-4]. Studies to date have demonstrated that right heart strain is associated with higher mortality than no right heart strain [5,6], and CT assessment of right heart strain correlates with echocardiographic findings [7].
CT pulmonary angiography (CTPA) has been established as the imaging modality of choice for the initial diagnosis of pulmonary thromboembolism [8,9], and is also used for assessing right ventricular (RV) afterload [10,11]. In addition, it enables quantification of thrombus load, for which a variety of scoring systems are available. These include the modified Miller score (MMS), a catheter pulmonary angiography score [12] adapted for CTPA by Bankier et al [13], and more complex systems such as the Qanadli and Mastora scores [14,15]. The aim of this study was to determine if there is a correlation between increasing thrombus load using MMS and RV dilatation as a predictor of RV failure according to CTPA findings.
Methods
The reports of 2425 consecutive CTPA scans undertaken over a 40-month period from 2001 to 2004 at the Royal Infirmary of Edinburgh, Scotland, UK, were retrospectively reviewed. All CTPA scans reported positive for the presence of PE were included in the study, and the MMS and RV and left ventricular (LV) dimensions were calculated.
To provide a comparison group, we included scans in 100 consecutive patients in whom PE was suspected but subsequently refuted by CTPA imaging. 100 matched controls were calculated to be sufficiently powered, based on the mean RV:LV ratio of 1.087 in this PE cohort [standard deviation (SD) 0.412], to achieve a true reduction in mean RV:LV ratio of 0.117, using a power of 80% and a two-sided 0.05 significance level.
Imaging protocol
The CTPA scans were performed using a multislice CT scanner without cardiac gating on a GE HiSpeed Advantage Scanner (GE Healthcare, Milwaukee, WI), using a slice thickness of 3 mm and a pitch of 1.7:1 with 1.5-mm reconstruction from the thoracic inlet to the inferior extent of the diaphragm. Approximately 100 ml of intravenous contrast material (iodine 200 mg ml−1) was injected at a rate of 3 ml s−1. CTPA scans were initially reported by the hospital’s diagnostic service. All study scans were independently reviewed by a senior radiology specialist registrar with 5 years of CTPA experience and every scan in which there was discordance in the diagnosis or extent of thrombus was also reviewed by an experienced pulmonary radiologist with 16 years of experience in CTPA reporting.
Calculation of the modified Miller score and ventricular dimensions
The MMS is a score of thrombus load proposed by Miller et al [12] for conventional angiography and adapted for CTPA scan by Bankier et al [13]. Each segmental pulmonary artery (nine on the right, seven on the left) that is fully or partly occluded by thrombus is given a score of 1. Any further proximal involved vessels score the number of segmental branches distal to that vessel, thereby giving an MMS of 0 (no thrombus) to 16 (thrombus in all segmental arteries or saddle embolism). In this study, the MMS was evaluated in consensus between observers.
The RV:LV ratio was calculated using the minor axes of the RV and LV chambers in the axial plane at the widest points between the inner surface of the free wall and the surface of the interventricular septum. The maximum dimensions of the right and left ventricles may be found at different axial scan levels. This is illustrated in Figure 1.
Figure 1.
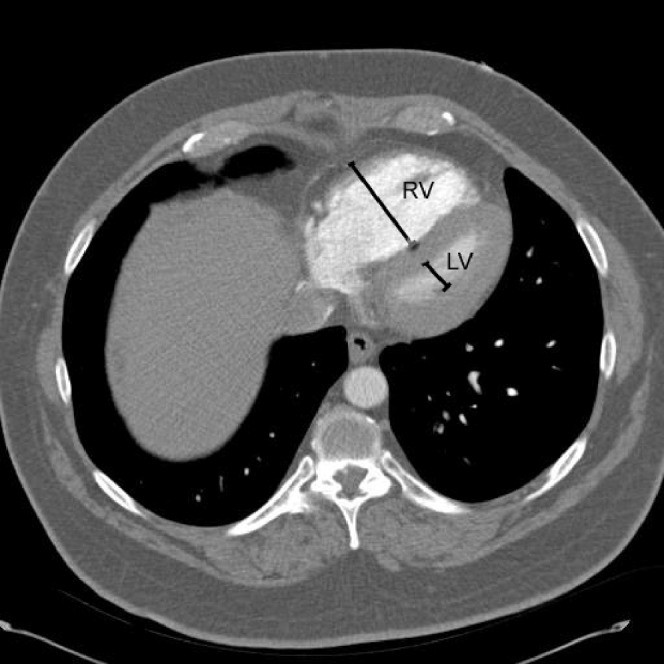
Illustration of measurement of maximum minor axis dimensions in a patient with right ventricular (RV) strain and septal shift. RV indicates the RV measurement; LV indicates the left ventricular measurement.
The presence of septal shift and the maximum pulmonary artery and ascending aortic diameter were also recorded. Finally, the presence or absence of consolidation, atelectasis or effusion was recorded.
Statistical methods
All data were analysed using SPSS® v. 13 for Windows (SPSS Inc., Chicago, IL). Data are presented as mean (standard deviation) unless otherwise stated. Non-parametric analysis was undertaken using the independent samples two-tailed t-test or χ2 test. Correlations were calculated using Pearson’s rank correlation. A significance value of p≤0.05 was applied.
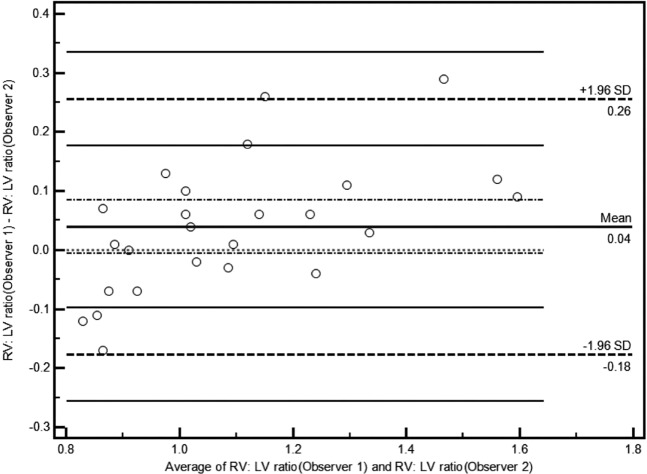
Interobserver reproducibility between the 2 observers for the RV:LV ratio was assessed on 25 scans using Bland–Altman analysis [16].
Results
During the 40-month period, 559 CTPA scans were reported as positive for PE; of these, 55 were excluded for reasons including a change in diagnosis on review or being technically suboptimal for the accurate calculation of MMS. A final total of 504 scans were included in the study. For the negative PE group, the results of 100 consecutive scans within an equivalent cohort during the study period were included.
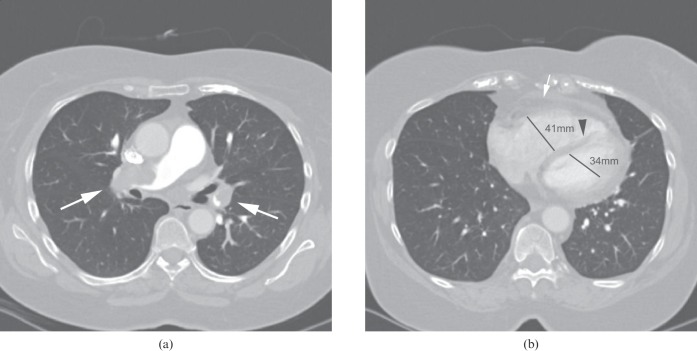
For the patients with PE, the MMS ranged from 1 to 16, with a mean MMS of 7.86. The mean RV:LV ratio was 1.087 (SD, 0.412) and the median was 0.9794 (range, 0.52–3.29). This, along with the presence of consolidation, atelectasis and pleural effusion, is demonstrated in Table 1. In the entire cohort, increasing MMS was shown to correlate with a higher RV:LV ratio (r=0.390, p<0.001) and with pulmonary artery diameter (r=0.221, p<0.001). A positive CTPA scan with increased RV:LV ratio is illustrated in Figure 2a,b.
Table 1. Comparison of patient age, RV:LV ratio and presence of consolidation, atelectasis and effusion in the PE vs non-PE cohorts.
| Parameters | PE cohort (n=504) | Non-PE cohort (n=100) | p-value |
| Mean age (years) | 63.4 (SD, 16.37) | 63.4 (SD, 16.78) | p=0.993 |
| RV:LV ratio | 1.087 (SD, 0.412) | 0.930 (SD, 0.133) | p<0.0001 |
| Atelectasis (%) | 20.2 | 22.0 | p=0.69 |
| Consolidation (%) | 28.8 | 27 | p=0.72 |
| Effusion (%) | 30.6 | 16 | p=0.003 |
PE, pulmonary embolism; RV:LV ratio, right ventricular to left ventricular ratio; SD, standard deviation.
Figure 2.
(a) Large central embolus (arrows) with MMS 16. (b) RV dilatation (white arrow indicates an RV wall measurement of >5 mm), septal straightening (arrowhead) and an increase in RV:LV ratio (maximum RV and LV diameters given) in the same patient, indicating RV strain. LV, left ventricular; MMS, modified Miller score; RV, right ventricular.
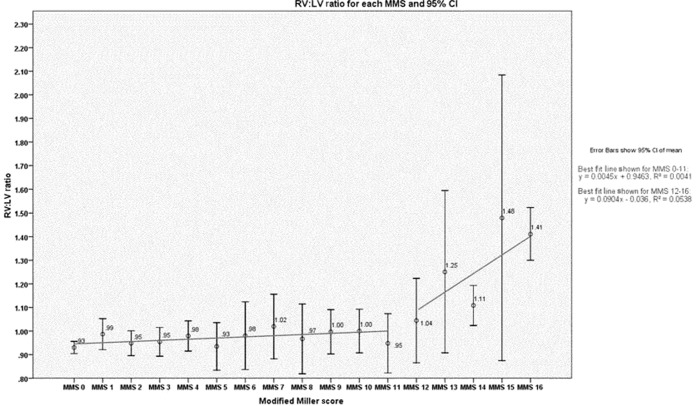
The mean RV:LV ratio remained unchanged until a thrombus load of MMS ≥12 was reached. There was no significant difference in RV:LV ratio between the patients with lower thrombus loads (MMS ≤12; n=338) and the non-PE cohort [mean RV:LV ratio, 0.971 (SD, 0.242) vs 0.930 (SD, 0.133), respectively; p=0.108], but there was a significant difference in mean ratio between patients with higher thrombus loads (MMS ≥12; n=166) and the non-PE cohort [mean RV:LV ratio, 1.323 (SD, 0.561) vs 0.930 (SD, 0.133), respectively; p<0.0001]. Multiple analyses were carried out comparing each MMS from 1 to 12; these demonstrated that there was no statistical significance between any two mean MMS scores in this cohort (independent samples t-test, all p>0.05 for all comparisons). The correlation coefficients were calculated for MMS 1–12, which demonstrated no significant correlation (r=0.0526, p=0.315). For larger thrombus loads of MMS ≥12, however, there was a significant correlation between MMS and RV:LV ratio (r=0.232, p=0.003). This association did not hold true for either pulmonary artery diameter or aortic diameter (non-significant for the MMS <12 and MMS ≥12 groups). This is demonstrated in Table 2 and in graphical form in Figure 3.
Table 2. Correlation between MMS, mean RV:LV ratio, mean pulmonary artery diameter and mean aortic diameter in the PE cohort.
| MMS score | Patients (n) | Mean RV:LV ratio | Mean pulmonary artery diameter | Mean aortic diameter |
| MMS 1 | 57 | 0.987 | 27.0 | 31.3 |
| MMS 2 | 66 | 0.948 | 27.1 | 31.7 |
| MMS 3 | 49 | 0.954 | 27.9 | 30.4 |
| MMS 4 | 39 | 0.979 | 27.7 | 31.0a |
| MMS 5 | 22 | 0.935 | 26.1 | 29.6 |
| MMS 6 | 20 | 0.980 | 26.9 | 33.1 |
| MMS 7 | 24 | 1.019 | 27.5 | 30.2 |
| MMS 8 | 12 | 0.966 | 30.5 | 33.8 |
| MMS 9 | 24 | 0.997 | 28.8 | 32.6 |
| MMS 10 | 10 | 1.000 | 29.2 | 32.8 |
| MMS 11 | 15 | 0.948 | 26.4 | 30.0b |
| MMS 12 | 18 | 1.044 | 27.7 | 31.6 |
| MMS 13 | 13 | 1.251 | 30.0 | 32.9 |
| MMS 14 | 21 | 1.108 | 28.6 | 32.7c |
| MMS 15 | 7 | 1.479 | 29.6 | 31.7 |
| MMS 16 | 107 | 1.411 | 29.8 | 32.0 |
MMS, modified Miller score; PE, pulmonary embolism; RV:LV ratio, right ventricular to left ventricular ratio.
aData available for 38 patients.
bData available for 14 patients.
cData available for 20 patients.
Figure 3.
Graph of data showing the relationship of mean RV:LV ratio with MMS—with the RV:LV ratio remaining stable until MMS ≥12 is reached—with a statistically significant higher mean ratio for patients with higher thrombus loads (MMS ≥12) than the non-PE cohort (p<0.0001). CI, confidence interval; LV, left ventricular; MMS, modified Miller score; RV, right ventricular.
Septal changes (defined as septal straightening or septal bulge into the left ventricle) were also more likely in patients with MMS ≥12 than in those with MMS ≤12 (63.8% vs 18.6%, respectively; p<0.001).
In terms of interobserver agreement, on Bland–Altman analysis of the RV:LV ratio measurements, the means and SDs between the two observers were 0.04 and 0.22, respectively. The Bland–Altman plot is shown in Figure 4.
Figure 4.
Bland–Altman plot of the RV:LV ratio as measured by the 2 study observers in 25 patients. LV, left ventricular; RV, right ventricular; SD, standard deviation.
Discussion
We believe this to be the first study to describe the relationship between thrombus load and RV:LV ratio on CTPA examination. The RV:LV ratio remains relatively unchanged until the MMS score reaches ≥12. Above this thrombus load, the right ventricle begins to dilate in keeping with decompensation, indicating a “tipping point”. This is a concept used in physics and sociology and is defined as the critical point in an evolving situation that leads to a new and potentially irreversible development [17].
Our results show that not all patients with a high thrombus load have evidence of RV decompensation. This implies that some patients with high thrombus loads are able to compensate while others are not, resulting in RV strain and dilatation visible on CTPA. This is evidenced by the large SDs seen in Figure 3 for MMS 12, 13 and 15. The mechanism for this disparity remains unclear and could be owing to a number of factors. One suggestion is that those patients without RV dilatation are decompressing their RV afterload through temporary right-to-left cardiac shunts [18]. Autopsy studies have shown prevalence of patent foramen ovale (PFO) in the general population of up to 27% [19], while echocardiography in transthoracic and transoesophageal studies has shown an incidence of PFO in the healthy population of 10–24.3% [20-22]. An unsuspected PFO may open up and allow temporary right-to-left shunting in the presence of raised right heart pressure, which may reduce the acute RV dilatation on CT. This, however, is unlikely to equate to a survival benefit: it has been shown previously that those who have a PFO in the setting of PE have an overall mortality of 33% vs 14% in the absence of a PFO, likely to be due to paradoxical embolus and secondary myocardial ischaemia [23]. Another possibility is that patients capable of RV compensation may have a better protected RV myocardium because they do not have pre-existing subclinical coronary disease. It is recognised that patients may develop RV ischaemia or infarction as a result of a significant increase in RV afterload when PE occurs [24]. Animal studies have also shown that RV muscle can hypertrophy fairly rapidly over the course of a couple of weeks in response to increased RV pressure [25]. This could provide a further explanation for why some patients may increase their RV muscle bulk in response to repeated silent earlier pulmonary emboli, enabling them to avoid RV collapse in the presence of larger thrombus loads.
There are also a small number of outliers in whom there is a raised RV:LV ratio but who have relatively low thrombus loads, reflecting the heterogeneous population in whom PE is diagnosed.
We acknowledge that criticism may arise from the choice of study protocol used. We chose to use MMS and non-cardiac gating as well as axial RV:LV ratios because these were more representative of daily radiology practice than more complicated thrombus load scores such as the Walsh [26], Qanadli or Mastora scores and volumetric RV:LV measurements. While we acknowledge that volumetric analysis of RV:LV volume has been shown to be the most reproducible measurement [27] and is slightly superior in identifying high-risk patients with adverse clinical outcomes to unidimensional measurements, it is time consuming and requires dedicated software tools which would be disadvantageous in the emergency setting [28]. With non-cardiac-gated scans, while there was a degree of cardiac motion artefact, this did not adversely affect the ventricular wall detection and measurement in diastole. As reported previously by investigators from our institution [10], the patients in the major PE group also exhibited reduced cardiac motion artefact compared with the non-PE group, which is likely to reflect ventricular dyskinesis associated with major PE [29] and allows reproducible measurements, as demonstrated by the high interobserver agreement between the two observers in our study (Bland–Altman analysis; mean, 0.04, SD, 0.22). We acknowledge the limitations of scanner protocol; however, we would not expect any inaccuracies in axial measurements to alter the correlation we have shown between clot burden and degree of RV dilatation.
This study was designed as a purely radiological study; consequently, we have not included the clinical outcomes of these patients. Studies have consistently demonstrated a poorer prognosis associated with right heart strain [5,6], and data from a number of studies have now confirmed an increased risk of mortality in patients with right heart strain evidenced through either biomarkers of cardiac injury (troponin or BNP) [30-33] or radiological and echocardiogram evidence [5,6,31]. In the cohort of patients without circulatory collapse, it is still not known whether treatment with thrombolysis is warranted; a recent study [34], currently in press, noted that while pulmonary artery obstruction scores can differentiate between patients with and without RV dysfunction, these were not correlated to adverse clinical outcome. Using only RV:LV ratio or thrombus load measurements in clinical decision making may be misleading because a significant number of patients with either no PE or lower thrombus loads may also have an increased RV:LV ratio. Further work is in progress to correlate the tipping point between thrombus load and right heart strain with eventual clinical outcome in order to help improve the existing treatment algorithms in pulmonary thromboembolism.
Conclusion
Using CT pulmonary angiography, we have demonstrated that in acute pulmonary thromboembolism, the RV:LV ratio remains relatively unchanged with increasing thrombus load until the tipping point of MMS 12 is reached; above this, RV dilatation is more likely to occur. Although further larger studies are required to correlate with clinical outcomes, these findings may contribute to the process of risk stratification for this patient group and affect treatment, such as the decision to use fibrinolytic therapy.
References
- 1.Kasper W, Konstantinides S, Giebel A, Tiede N, Krause T, Just H. Prognostic significance of right ventricular afterload stress detected by echocardiography in patients with clinically suspected pulmonary embolism. Heart 1997;77:346–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldhaber SZ, Come PC, Lee RT, Braunwald E, Parker JA, Haire WD, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet 1993;341:507–11. [DOI] [PubMed] [Google Scholar]
- 3.Kreit JW. The impact of right ventricular dysfunction in patients with pulmonary embolism. Chest 2004;125:1539–45. [DOI] [PubMed] [Google Scholar]
- 4.Task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology. Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2008;29:2276–315. [DOI] [PubMed] [Google Scholar]
- 5.Ribeiro A, Lindmaker P, Juhlin-Dannfelt A, Johnsson H, Jorfeldt L. Echocardiography Doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate. Am Heart J 1997;134:479–87. [DOI] [PubMed] [Google Scholar]
- 6.Grifoni S, Olivotto I, Cecchini P, Pieralli F, Camaiti A, Santoro G, et al. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation 2000;101:2817–22. [DOI] [PubMed] [Google Scholar]
- 7.Dogăn H, Kroft LJM, Bax JJ, Schuijf JD, van derGeest RJ, Doornbos J, et al. MDCT assessment of right ventricular systolic function. AJR Am J Roentgenol 2006;186:S366–70. [DOI] [PubMed] [Google Scholar]
- 8.British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax 2003;58:470–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology. Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2008;29:2276–315. [DOI] [PubMed] [Google Scholar]
- 10.Reid JH, Murchison JT. Acute right ventricular dilatation: a new helical CT sign of massive pulmonary thromboembolism. Clin Radiol 1998;53:694–8. [DOI] [PubMed] [Google Scholar]
- 11.Ferretti GR, Collomb D, Ravey JN, Vanzetto G, Coulomb M, Bricault I. Severity assessment of acute pulmonary embolism: role of CT angiography. Semin Roentgenol 2005;40:25–32. [DOI] [PubMed] [Google Scholar]
- 12.Miller GA, Sutton GC, Kerr IH, Bigson RV, Honey M. Comparison of streptokinase and heparin in treatment of isolated acute massive pulmonary embolism. Br Med J 1971;2:681–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bankier AA, Janata K, Fleischmann D, Kreuzer S, Mallek R, Frossard M, et al. Severity assessment of acute pulmonary embolism with spiral CT: evaluation of two modified angiographic scores and comparison with clinical data. J Thorac Imaging 1997;12:150–8. [DOI] [PubMed] [Google Scholar]
- 14.Qanadli SD, El Hajjam M, Vieillard-Baron A, Joseph T, Mesurolle B, Oliva VL, et al. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol 2001;176:1415–20. [DOI] [PubMed] [Google Scholar]
- 15.Mastora I, Remy-Jardin M, Masson P, Galland E, Delannoy V, Bauchart J, et al. Severity of acute pulmonary embolism: evaluation of a new spiral CT angiographic score in correlation with echocardiographic data. Eur Radiol 2003;13:29–35. [DOI] [PubMed] [Google Scholar]
- 16.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10. [PubMed] [Google Scholar]
- 17.whatis.com [homepage on the internet]. Needham, MA. The leading IT encyclopaedia and learning center. c2008 [updated 29 January 2006; cited 16 February 2011]. Available from: http://whatis.techtarget.com. [Google Scholar]
- 18.Miller RL, Das S, Anandarangam T, Leibowitz DW, Thomashow B, Alderson PO, et al. Relationship between patent foramen ovale and perfusion abnormalities in acute pulmonary embolism. Am J Cardiol 1997;80:377–8. [DOI] [PubMed] [Google Scholar]
- 19.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 1984;59:17–20. [DOI] [PubMed] [Google Scholar]
- 20.Lechat P, Mas JL, Lascault G. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988;318:1148–52. [DOI] [PubMed] [Google Scholar]
- 21.Hausmann D, Mügge A, Becht I, Daniel WG. Diagnosis of patent foramen ovale by transesophageal echocardiography and association with cerebral and peripheral embolic events. Am J Cardiol 1992;70:668–72. [DOI] [PubMed] [Google Scholar]
- 22.Meissner I, Khandheria BK, Heit JA, Petty GW, Sheps SG, Schwartz GL, et al. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol 2006;47:440–5. [DOI] [PubMed] [Google Scholar]
- 23.Konstantinides S, Geibel S, Kasper W, Olschewski M, Blumel L, Just H. Patent foramen ovale is an important predictor of adverse outcome in patients with major pulmonary embolism. Circulation 1998;97:1946–51. [DOI] [PubMed] [Google Scholar]
- 24.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999;353:1386–9. [DOI] [PubMed] [Google Scholar]
- 25.Pokreisz P, Marsboom G, Janssens S. Pressure overload-induced right ventricular dysfunction and remodelling in experimental pulmonary hypertension: the right heart revisited. Eur Heart J Suppl 2009;9:H75–84. [Google Scholar]
- 26.Walsh PN, Greenspan RH, Simon M, Simon AL, Myers TM, Woosley PC, et al. An angiographic severity index for pulmonary embolism. Circulation 1973;47Suppl. 2:101–8.4686587 [Google Scholar]
- 27.Kang DK, Ramos-Duran L, Schoepf UJ, Armstrong AM, Abro JA, Ravenel JG, et al. Reproducibility of CT Signs of right ventricular dysfunction in acute pulmonary embolism. AJR Am J Roentgenol 2010;194:1500–6. [DOI] [PubMed] [Google Scholar]
- 28.Henzler T, Krissak R, Reichert M, Sueselbeck T, Schoenberg SO, Fink C. Volumetric analysis of pulmonary CTA for the assessment of right ventricular dysfunction in patients with acute pulmonary embolism. Acad Radiol 2010;17:309–15. [DOI] [PubMed] [Google Scholar]
- 29.Belenkie I, Dani R, Smith ER, Tyberg JV. The importance of pericardial constraint in experimental pulmonary embolism and volume loading. Am Heart J 1992;123:733–7. [DOI] [PubMed] [Google Scholar]
- 30.Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation 2007;116:427–33. [DOI] [PubMed] [Google Scholar]
- 31.Konstantinides SV. Acute pulmonary embolism revisited. Postgrad Med J 2008;84:651–8. [DOI] [PubMed] [Google Scholar]
- 32.Kostrubiec M, Pruszczyk P, Bochowicz A, Pacho R, Szulc M, Kaczynska A, et al. Biomarker-based risk assessment model in acute pulmonary embolism. Eur Heart J 2005;26:2166–72. [DOI] [PubMed] [Google Scholar]
- 33.Binder L, Pieske B, Olschewski M, Geibel A, Klostermann B, Reiner C, et al. N-terminal pro-brain natriuretic peptide or troponin testing followed by echocardiography for risk stratification of acute pulmonary embolism. Circulation 2005;112:1573–9. [DOI] [PubMed] [Google Scholar]
- 34.Apfaltrer P, Henzler T, Meyer M, Roeger S, Haghi D, Gruettner J, et al. Correlation of CT angiographic pulmonary artery obstruction scores with right ventricular dysfunction and clinical outcome in patients with acute pulmonary embolism. Eur J Radiol [serial on the internet]. September 2011 [cited February 28 2012]. Available from: 10.1016/j.ejrad.2011.08.014. [DOI] [PubMed] [Google Scholar]