Abstract
Objective
The aim of this study was to retrospectively evaluate the technical success rates and clinical effectiveness of fluoroscopically guided nose tube drainage of mediastinal abscesses and a nasojejunum feeding tube in post-operative gastro-oesophageal anastomotic leakage (GEAL).
Methods
From January 2006 to June 2011, 18 cases of post-operative GEAL with mediastinal abscesses after oesophagectomy with intrathoracic oesophagogastric anastomotic procedures for oesophageal and cardiac carcinoma were treated by insertion of a nose drainage tube and nasojejunum feeding tube under fluoroscopic guidance. We evaluated the feasibility of two-tube insertion to facilitate leakage site closure and complete resolution of the abscess, and the patients’ nutritional benefit was also evaluated by checking the serum albumin level between pre- and post-enteral feeding via the feeding tube.
Results
The two tubes were placed successfully under fluoroscopic guidance in 18 patients (100%). The procedure time for two-tube insertion ranged from 20 to 40 min (mean 30 min). 17 patients (94%) achieved leakage site closure after two-tube insertion and had a good tolerance of two tubes in the nasal cavity. The serum albumin level was significant, increased from pre-enteral feeding (2.49±0.42 g dl−1) to the post-enteral feeding (3.58±0.47 g dl−1) via the feeding tube (p<0.001). The duration of follow-up ranged from 1 to 49 months (mean 19 months).
Conclusion
The insertion of nose tube drainage and a nasojejunum feeding tube under fluoroscopic guidance is safe, and it provides effective relief from mediastinal abscesses in GEAL after oesophagectomy. Moreover, our findings indicate that two-tube insertion may be used as a selective procedure to treat mediastinal abscesses in post-operative GEAL.
Advances in knowledge
Directive drainage of mediastinal abscesses in post-operative GEAL may be an effective treatment.
Mediastinal leakage occurs in about 7% of distal oesophageal resections and is associated with a mortality rate of up to 60% [1,2]. Although the amounts of post-operative anastomotic leakage can be rather slight, the leakage is a major source of mortality and morbidity [3]. The leading causes of death in patients with mediastinal abscesses are infection and nutritional deficiency [4,5]. Mediastinal abscesses form a potentially devastating condition in gastro-oesophageal anastomotic leakage (GEAL). Historically, intravenous antibiotics combined with surgical debridement have been the mainstay of therapy [6]. Rethoracotomy extended procedure, however, carries considerable risks for a critically ill patient [5]. Han et al [7] treated patients with mediastinal abscess with stent placement and nasal–oesophagal drainage, while percutaneous drainage was applied under the guidance of CT [8,9] or endoscopy [10,11]. Drainage may be a promising treatment for mediastinal abscess in GEAL. In this study, we determined the feasibility and effectiveness of using two-tube insertion under fluoroscopic guidance in the treatment of mediastinal abscess in GEAL. The effect of treatment was evaluated by time interval of the leaks’ closure and the effect on nutritional status was evaluated by serum albumin level.
Methods and materials
18 patients with mediastinal abscesses in post-operative GEAL underwent two-tube insertion under fluoroscopic guidance from January 2006 to June 2011. The 18 patients underwent oesophagectomy with intrathoracic oesophagogastric anastomotic procedures for oesophageal and cardiac carcinoma. 12 patients were male and 6 were female, and their ages ranged from 38 to 70 years (mean 59 years). 3 of the patients were TNM (tumour, node, metastasis) stage I oesophageal carcinoma patients, 10 were TNM stage IIa, 2 were TNM stage IIb and 3 were TNM stage III [12]. Frozen-section analysis of resected margins by a skilled pathologist was available, and resection margins were cancer-free in all cases. The retrospective study was approved by the Institutional Review Board of our hospital and the Ethics Committee of the Institute for Medical Science. The procedure was explained to all of the patients in detail, and written consent was obtained from all the patients prior to the procedure. The mean maximum diameter of leakages was 5.4 cm, which was measured by CT examination. Signs and symptoms were present in all patients (100%), and included high fever in 17 patients, shortness of breath in 11 patients, chest pain in 10 patients and arrhythmia in 3 patients. The leakages in 13 patients (72%) were confirmed by first radiographic contrast examination. Five (28%) of these patients had initially negative contrast examination results, but they were all eventually proven to have anastomotic leakage by repeated radiographic contrast examinations 4–7 days later. The leakages in all patients were confirmed by first CT examination (Figure 1). The two tubes were removed after the leak’s closure testified by CT findings. Time interval means the length of the period of treatment with the tubes.
Figure 1.
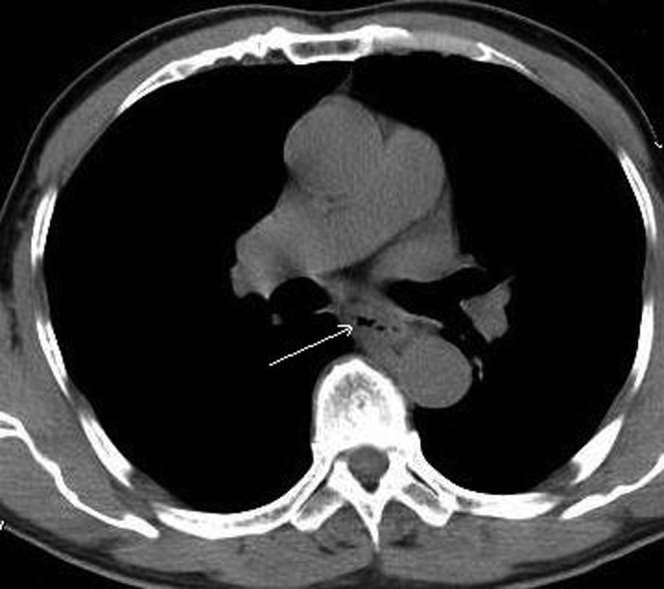
The intrathoracic anastomotic leak was confirmed by CT at 1 week post-operation. The fistula located in the posterior mediastinum (white arrow).
Interventional procedure
Nasogastric placement of drainage tube through the abscess
All the procedures were performed by two skilled interventional radiologists. The patients lay in the supine position, which allowed an easy performance of interventional manoeuvres, and optimal fluoroscopic visualisation of the leak. A 0.035-inch guidewire (Terumo, Tokyo, Japan) and a 4F H1 catheter (Terumo) were advanced into the oesophagus through one nasal ostium under fluoroscopic guidance (DSA, Angiostar; Siemens, Germany). When the H1 catheter got near to the leakage, Omnipaque (GE Healthcare, Waukesha, WI) was observed to see where the leak was. The catheter was then twisted for getting into the abscess. The fluid collection aspirated through the catheter was sent for bacterial culture. The prosthesis delivery system was then advanced over the guidewire until a 10F Flocare polyurethane unweighted feeding tube (Nutricia Export BV, Amsterdam, Netherlands) was sent into the abscess (Figure 2). We suggest that the backward distance of the tube to the leak should be <5 cm. The Flocare tube was connected to a low-pressure continuous vacuum pump, for which the negative pressure was about 8–10 mmHg. The fluid collection was observed every day for its colour and total quantity.
Figure 2.
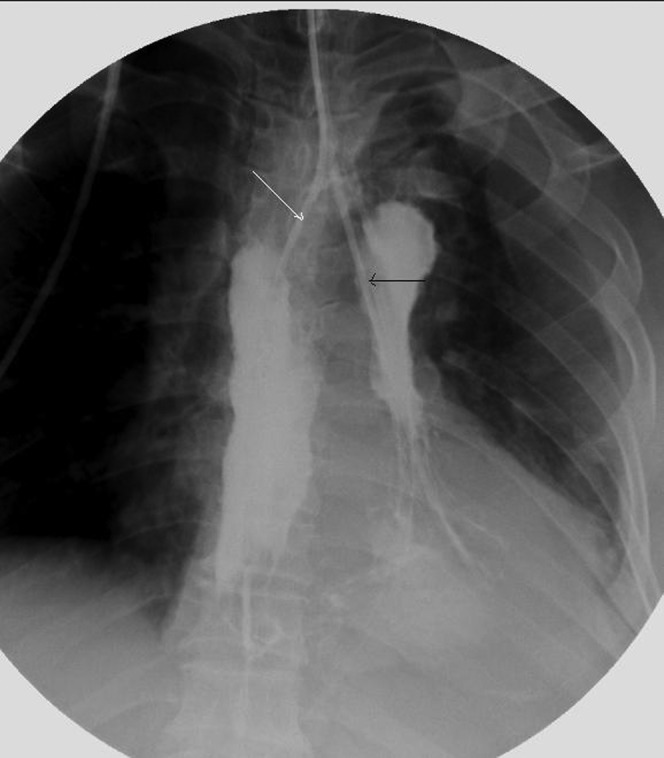
A nose drainage tube (white arrow) was inserted through the fistula with fluoroscopic guidance and the distal tip of the drainage tube was positioned at the bottom of the abscess cavity. A feeding nasojejunum tube (black arrow) was placed during interventional operation for post-operative enteral nutrition supply.
Nasogastric placement of feeding tube of jejunum
A 4F H1 catheter (Terumo) was advanced into the oesophagus through the ipsilateral nasal ostium. When the H1 catheter had got into the stomach, an Amplatz superstiff exchange guidewire (Medi-Tech/Boston Scientific, Natick, MA) was then inserted via the catheter into the distal portion of the jejunum. A 10F Flocare polyurethane unweighted feeding tube (Nutricia, Fribourg, Switzerland) was inserted into the jejunum using the guidewire. Contrast medium (Omnipaque; GE Healthcare) was injected through the Flocare feeding tube to verify the success of the manipulation. Essential nutrients were poured into the jejunum through the Flocare feeding tube.
When daily volume of the fluid drainage was reduced to 5–10 ml, the drainage tube could be gradually pulled back (Figure 3) because the adhesion between the lung and the mediastinal wall had formed already at the end of the tube. The two tubes were removed when the leakage’s closure was confirmed by CT scan. Under the fluoroscopical guiding the drainage tube was pulled back from the distal end to the proximal end of the vomica.
Figure 3.
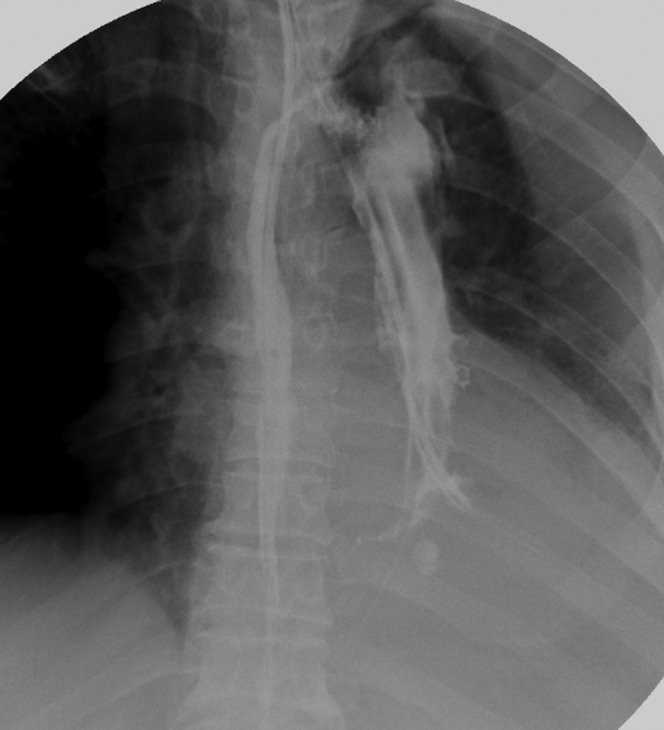
11 days post-operation, the fistula had become smaller; we changed the position of the drainage tube to keep optimum drainage.
Immediate technical success was defined as successful insertion of the two tubes without any problems. Clinical success was defined as closure of the leakage site (Figure 4), which was confirmed by follow-up CT examination (Figure 5). The patients’ nutritional benefit was evaluated by the serum albumin level change between the pre- and post-enteral feeding via the feeding tube. The differences in the means were evaluated with the use of t-test.
Figure 4.
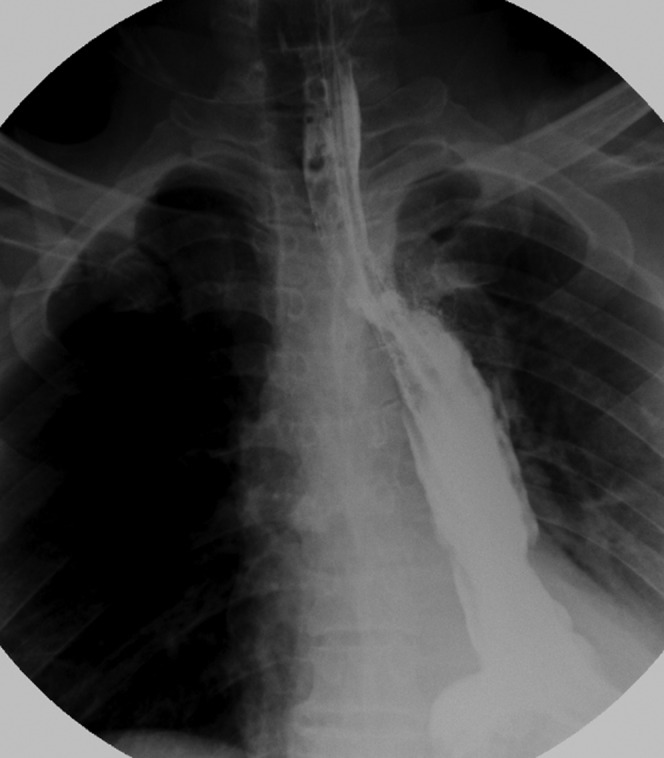
The fistula closed on 14 days post-operation. The fistula’s closure was testified by CT and gastrointestinal series.
Figure 5.
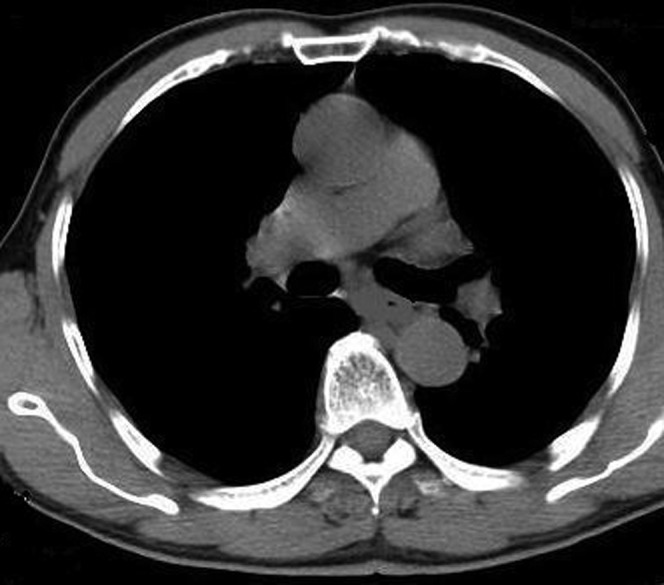
The clinical success of the operation was confirmed by follow-up CT examination.
Results
Immediate technical success was achieved in all patients (18/18 patients; Table 1). All patients had a good tolerance of two-tube in nasal cavity. Shortness of breath and nasal mucosa diabrosis were not seen in any patients.
The procedure times for two-tube insertion ranged from 20–40 min (mean 30 min). The procedure time was dependent on the detection of the leakage site. 17 patients (17/18) experienced symptomatic relief. One patient with diabetes mellitus achieved successful two-tube insertion; the leakage did not close and the patient required surgical exploration for repair of the leak. However, the patient died of sudden uncontrollable hematemesis on the fifth day after the surgical debridement. The mean time interval of the leakage treatment was 13.1±3.5 days (standard error of the mean with 95% confidence intervals). The median follow-up time was 19.3 months (range 1–49 months). No patient was diagnosed with leak recurrences.
The patient’s nutritional benefit was shown by the increasing serum albumin level after enteral feeding via the nasojejunum feeding tube. The mean serum albumin level was 2.49±0.42 g dl−1 (standard error of the mean with 95% confidence intervals; range 1.77–3.59 g dl−1) in 7 days pre-enteral feeding tube period and 3.58±0.47 g dl−1 (standard error of the mean with 95% confidence intervals; range 2.53–4.78 g dl−1) in 7 days post-enteral feeding via the feeding tube. The differences in the mean were significantly increased between pre- and post-enteral feeding via the feeding tube (p=0.00087).
Discussion
Mediastinal abscesses are a rare and potentially fatal condition. Mortality, often from overwhelming sepsis, can occur in as many as 40% of cases when the diagnosis is not readily suspected and treatment is delayed [13,14]. Mediastinal abscesses arise from a variety of causes, including head and neck infections, trauma or surgery, and so on [15-17]. Abscess formation is a life-threatening post-operative complication of oesophagogastric surgery. Mediastinal abscesses in GEAL are a special kind of pyothorax. In our opinion the key in management is adequate and effective drainage. Previous studies have revealed that most of the mediastinal abscesses were in contained leaks [18-20]. It is difficult to deal with this type of abscess by the conventional chest tube. Nasogastric placement of drainage tube through the abscess in our study may be a helpful treatment. We successfully applied the drainage tube through the leak for the treatment of mediastinal abscesses in 18 patients. 17 patients (94%) experienced symptomatic relief. The mean time interval of tube drainage was 13 days, which is similar to the 14 days found in previous studies [9,11]. More important is the leaks’ closure.
In our study one patient with diabetes mellitus received surgical exploration for repair of the leak after the failure of two-tube treatment, but he died of sudden uncontrollable hematemesis on the fifth day after the surgical debridement. Repeat operation rates for post-operative GEAL are as high as 24%, and involve resection and revisions of the original anastomosis [21,22]. While Turkyilmaz et al [23] found that the mortality rate of the second operation was high in patients who suffered from GEAL, in our opinion the patients with mediastinal abscesses in GEAL were always emaciated and could not tolerate the second surgery operation.
Some studies [24-26] concluded that stent placement has been shown to be an excellent treatment in patients with mediastinal abscess in GEAL, while others [27] revealed that certain problems might be associated with stent placement and removal: insufficient closure of the leaks, stent migration and development of strictures after stent removal, severe complications such as bleeding and food blockage, and hard removal because of the growth of granulation tissue. Han et al [7] treated the patients of mediastinal abscess with stent placement and nasal-oesophagus drainage. In our opinion, drainage is essential, while stent placement is not very suitable in our patients because the leakages in our study can be deemed as optimal.
In 1984, Gobien et al [8] described a series of 12 patients with mediastinal abscess, in 6 of whom successful percutaneous drainage was achieved. Since then, drainage has been applied in treatment of mediastinal abscesses under the guidance of CT or endoscope. But little has been written regarding the use of tube drainage under fluoroscopic guidance in the management of mediastinal abscesses [28]. The results of the present study show that two-tube drainage was technically successfully in a single setting in 17 abscess drainage attempts (94%). Percutaneous drainage under the guidance of CT determined with whether the patient can be positioned appropriately for percutaneous access, whether the abscess is sufficiently away from vital mediastinal structures to minimise the complication risks [9]. Our method can avoid the risks. Fluoroscopic placement requires less additional sedation, whereas endoscopic placement allows direct visualisation of GEAL, and it can be performed without additional X-ray support. However, the catheter used in our study has a considerably smaller diameter than the endoscopy instrument, and is thus better able to get into the narrow fistula.
The enteric feeding might contribute to the general nutritional correction from the post-surgical status, and this might contribute to recovery of mediastinal abscess and closure of anastomotic leakage. Routine jejunostomy to nutritional support is a traumatic method. But the nasogastric placement of a nutritional tube under fluoroscopic guidance can avoid injury to the jejunum. Also, total parenteral nutrition is expensive, and it has a high rate of complications. The enteral feeding allows for a high serum albumin level compared with total parenteral nutrition [29]. In our study, the patient nutrition benefit showed a significantly increased serum albumin level after enteral feeding (p<0.001). In our view, food intake can irritate the mucosa at the anastomotic site and aggravate the mediastinal abscess, because the digestive slice can continuously enter the abscess from the fistula. Ott et al [30] had reported a success rate for fluoroscopic placement of 90%, with a tube placed into the jejunum in 53% of patients and placed into the duodenum in 47% of the patients. In the present study, the tubes were placed fluoroscopically in the jejunum in all patients, and the mean procedure time was 30 min. In some studies the nasogastric decompression tube was placed routinely [28]. But in our study the nasogastric decompression tube was not placed due to the effect of the gastric acid inhibitor.
There are some limitations in our study. One of these was the retrospective and non-randomised study design. This question warrants further investigation. Another limitation was that some patients feel discomfort; however, in our study all patients had a good tolerance of the two tubes in the nasal cavity, and shortness of breath and mucosa diabrosis were not observed in any patients.
In summary, fluoroscopically guided nasal tube drainage of mediastinal abscesses and nasojejunal feeding tube use appear to be associated with high technical success and clinical effectiveness rates in our study. We suggest that two-tube insertion may be considered as an alternative therapeutic option for patients with mediastinal abscesses in GEAL.
Table 1. Data in fluoroscopically guided two-tube insertion for relief of mediastinal abscesses in GEAL.
| Number | Age (years)/sex | Stage | Operation procedure | Location | Duration (days) | Further treatment | Follow-up, months |
| 1 | 44/M | III | Left Ivor–Lewis | Posterior | 7 | No | 15 (died owing to recurrence) |
| 2 | 56/M | IIa | Left Ivor–Lewis | Posterior | 12 | No | 24 |
| 3 | 61/M | I | Left Ivor–Lewis | Anterior | 8 | No | 49 |
| 4 | 63/F | IIa | Left Ivor–Lewis | Posterior | 10 | No | 33 (died owing to recurrence) |
| 5 | 70/F | IIa | Left Ivor–Lewis | Posterior | 18 | No | 23 |
| 6 | 48/F | IIb | Left Ivor–Lewis | Posterior | 15 | No | 31 (died owing to recurrence) |
| 7 | 55/M | IIa | Left Ivor–Lewis | Posterior | 13 | No | 14 |
| 8 | 57/M | III | Left Ivor–Lewis | Posterior | 12 | No | 10 (died owing to recurrence) |
| 9 | 67/F | IIIa | Left Ivor–Lewis | Anterior | 14 | No | 21 |
| 10 | 51/F | I | Left Ivor–Lewis | Posterior | 13 | No | 33 |
| 11 | 63/M | IIa | Left Ivor–Lewis | Posterior | 17 | No | 21 (died owing to recurrence) |
| 12 | 66/M | IIa | Left Ivor–Lewis | Posterior | 9 | No | 15 |
| 13 | 70/F | III | Left Ivor–Lewis | Anterior | 11 | No | 13 (died owing to recurrence) |
| 14 | 60/M | IIa | Left Ivor–Lewis | Posterior | 12 | No | 21 |
| 15 | 57/M | I | Left Ivor–Lewis | Posterior | 15 | No | 19 |
| 16 | 59/M | IIa | Left Ivor–Lewis | Posterior | 16 | No | 18 |
| 17 | 62/M | IIa | Left Ivor–Lewis | Anterior | 21 | Rethoracotomy | 1 (died of haematemesis) |
| 18 | 38/M | IIb | Left Ivor–Lewis | Posterior | 13 | No | 25 (died owing to recurrence) |
GEAL, gastro-oesophageal anastomotic leakage; F, female; M, male.
References
- 1.Patil PK, Patel SG, Mistry RC, Deshpande RK, Desai PB. Cancer of the esophagus: esophagogastric anastomotic leak-a retrospective study of predisposing factors. J Surg Oncol 1992;49:163–7 [DOI] [PubMed] [Google Scholar]
- 2.Dewar L, Gelfand G, Finley RJ, Evans K, Inculet R, Nelems B. Factors affecting cervical anastomotic leak and stricture formation following esophagogastrectomy and gastric tube interposition. Am J Surg 1992;163:484–9 [DOI] [PubMed] [Google Scholar]
- 3.Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg 1999;230:392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urschel JD. Esophagogastrectomy anastomotic leaks complicating esophagectomy: a review. Am J Surg 1995;169:634–40 [DOI] [PubMed] [Google Scholar]
- 5.Sauvanet A, Baltar J, Le Mee J, Belghiti J. Diagnosis and conservative management of intrathoracic leakage after oesophagectomy. Br J Surg 1998;85:1446–9 [DOI] [PubMed] [Google Scholar]
- 6.Chen KC, Chen JS, Kuo SW, Huang PM, Hsu HH, Lee JM, et al. Descending necrotizing mediastinitis: a 10-year surgical experience in a single institution. J Thorac Cardiovasc Surg 2008;136:191–8 [DOI] [PubMed] [Google Scholar]
- 7.Han XW, Wu G, Li YD, Ma N, Wang YL, Gao XM. Treating mediastinoesophageal fistula with covered stent through nasal esophagus drainage tube. J Intervent Radiol 2005;14:167–9 [Google Scholar]
- 8.Gobien R, Stanley J, Gobien B, Vujic I, Pass H. Percutaneous catheter aspiration and drainage of suspected mediastinal abscesses. Radiology 1984;151:69–71 [DOI] [PubMed] [Google Scholar]
- 9.Arellano RS, Gervais DA, Mueller PR. Computed tomography-guided drainage of mediastinal abscesses: clinical experience with 23 patients. J Vasc Interv Radiol 2011;22:673–7 [DOI] [PubMed] [Google Scholar]
- 10.Segalin A, Bonavina L, Lazzerini M, De Ruberto F, Faranda C, Peracchia A. Endoscopic management of inveterate esophageal perforation and leaks. Surg Endosc 1996;10:928–32 [DOI] [PubMed] [Google Scholar]
- 11.Pross M, Manger T, Reinheckel T, Mirow L, Kunz D, Lippert H. Endoscopic treatment of clinically symptomatic leaks of thoracic esophageal anastomoses. Gastrointest Endosc 2000;51:73–6 [DOI] [PubMed] [Google Scholar]
- 12.American Joint Committeeon Cancer AJCC Cancer Staging Manual. New York, NY: Springer-Verlag; 2002 [Google Scholar]
- 13.Gorlitzer M, Grabenwoeger M, Meinhart J, Swoboda H, Oczenski W, Fiegl N, et al. Descending necrotizing mediastinitis treated with rapid sternotomy followed by vacuum assisted therapy. Ann Thorac Surg 2007;83:393–6 [DOI] [PubMed] [Google Scholar]
- 14.Misthos P, Katsaragakis S, Kakaris S, Theodorou D, Skottis I. Descending necrotizing anterior mediastinitis: analysis of survival and surgical treatment modalities. J Oral Maxillofac Surg 2007;65:635–9 [DOI] [PubMed] [Google Scholar]
- 15.Chang C, Huang J, Lai PC, Yang C. Posterior mediastinal abscess in a hemodialysis patient—a rare but life-threatening complication of Staphylococcus bacteremia. Clin Nephrol 2009;71:92–5 [DOI] [PubMed] [Google Scholar]
- 16.Church A, Sivasothy P, Parmer J, Foweraker J. Mediastinal abscess after lung transplantation secondary to Burkholderia gladioli infection. J Heart Lung Transplant 2009;28:511–14 [DOI] [PubMed] [Google Scholar]
- 17.Patel NN, Murphy G, Hamilton M. Traumatic sternal abscess with mediastinal involvement. Ann Thorac Surg 2008;86:1997. [DOI] [PubMed] [Google Scholar]
- 18.Crestanello JA, Deschamps C, Cassivi SD, Nichols FC, Allen MS, Schleck C, et al. Selective management of intrathoracic anastomotic leak after esophagectomy. J Thorac Cardiovasc Surg 2005;129:254. [DOI] [PubMed] [Google Scholar]
- 19.Whooley BP, Law S, Alexandrou A, Murthy SC, Wong J. Critical appraisal of the significance of intrathoracic anastomotic leakage after esophagectomy for cancer. Am J Surg 2001;181:198. [DOI] [PubMed] [Google Scholar]
- 20.Jiang F, Yu MF, Ren BH, Yin GW, Zhang Q, Xu L. Nasogastric placement of sump tube through the leak for the treatment of esophagogastric anastomotic leak after esophagectomy for esophageal carcinoma. J Surg Res 2011;171:448–51 [DOI] [PubMed] [Google Scholar]
- 21.Wright C, Kucharczuk J, O’Brien SM, Grab J, Allen M. Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk adjustment model. J Thorac Cardiovasc Surg 2009;137:587–96 [DOI] [PubMed] [Google Scholar]
- 22.Schubert D, Scheidbach H, Kuhn R, Wex C, Weiss G, Eder F, et al. Endoscopic treatment of thoracic esophageal anastomotic leaks by using silicone-covered, self expanding polyester stents. Gastrointest Endosc 2005;61:891–6 [DOI] [PubMed] [Google Scholar]
- 23.Turkyilmaz A, Eroglu A, Aydin Y, Tekinbas C, Muharrem Erol M, Karaoglanoglu N. The management of esophagogastric anastomotic leak after esophagectomy for esophageal carcinoma. Dis Esophagus 2009;22:119. [DOI] [PubMed] [Google Scholar]
- 24.Leers JM, Vivaldi C, Schäfer H, Bludau M, Brabender J, Lurje G, et al. Endoscopic therapy for esophageal perforation or anastomotic leak with a self-expandable metallic stent. Surg Endosc 2009;23:2258. [DOI] [PubMed] [Google Scholar]
- 25.Karbowski M, Schembre D, Kozarek R, Ayub K, Low D. Polyflex self-expanding, removable plastic stents: assessment of treatment efficacy and safety in a variety of benign and malignant conditions of the esophagus. Surg Endosc 2008;22:1326–333 [DOI] [PubMed] [Google Scholar]
- 26.Dai YY, Gretschel S, Dudeck O, Rau B, Schlag PM, Hunerbein M. Treatment of oesophageal anastomotic leaks by temporary stenting with self-expanding plastic stents. Br J Surg 2009;96:887–91 [DOI] [PubMed] [Google Scholar]
- 27.Kanatas AN, Aldouri A, Hayden JD. Anastomotic leak after oesophagectomy and stent implantation:a systematic review. Oncol Rev 2010;4:159–65 [Google Scholar]
- 28.Hu Z, Yin R, Fan X, Zhang Q, Feng C, Yuan F, et al. Treatment of intrathoracic anastomotic leak by nose fistula tube drainage after esophagectomy for cancer. Dis Esophagus 2011;24:100–7 [DOI] [PubMed] [Google Scholar]
- 29.Han YM, Kim CY, Yang DH, Kwak HS, Jin GY. Fluoroscopically guided feeding tube insertion for relief of postoperative gastrointestinal anastomotic obstruction and leakage. Cardiovasc Intervent Radiol 2006;29:395–400 [DOI] [PubMed] [Google Scholar]
- 30.Ott DJ, Mattox HE, Gelfand DW, Chen MY, Wu WC. Enteral feeding tubes: placement by using fluoroscopy and endoscopy. AJR Am J Roentgenol 1991;157:769–71 [DOI] [PubMed] [Google Scholar]


