Abstract
Objectives
To establish local diagnostic reference levels (LDRLs) at the Royal Children's Hospital (RCH) Melbourne, Parkville, Australia, for typical paediatric CT examinations and compare these with international diagnostic reference levels (DRLs) to benchmark local practice. In addition, the aim was to develop a method of analysing local scan parameters to enable identification of areas for optimisation.
Methods
A retrospective audit of patient records for paediatric CT brain, chest and abdomen/pelvis examinations was undertaken. Demographic information, examination parameters and dose indicators—volumetric CT dose index (CTDIvol) and dose–length product (DLP)—were collected for 220 patients. LDRLs were derived from mean survey values and the effective dose was estimated from DLP values. The normalised CTDIvol values, mAs values and scan length were analysed to better identify parameters that could be optimised.
Results
The LDRLs across all age categories were 18–45 mGy (CTDIvol) and 250–700 mGy cm (DLP) for brain examinations; 3–23 mGy (CTDIvol) and 100–800 mGy cm (DLP) for chest examinations; and 4–15 mGy (CTDIvol) and 150–750 mGy cm (DLP) for abdomen/pelvis examinations. Effective dose estimates were 1.0–1.6 mSv, 1.8–13.0 mSv and 2.5–10.0 mSv for brain, chest and abdomen/pelvis examinations, respectively.
Conclusion
The RCH mean CTDIvol and DLP values are similar to or lower than international DRLs. Use of low-kilovoltage protocols for body imaging in younger patients reduced the dose considerably. There exists potential for optimisation in reducing body scan lengths and justifying the selection of reference mAs values. The assessment method used here proved useful for identifying specific parameters for optimisation.
Advances in knowledge
Assessment of individual CT parameters in addition to comparison with DRLs enables identification of specific areas for CT optimisation.
In the internationally adopted system of radiation protection, a medical procedure involving the exposure of a patient to ionising radiation must be both justified and optimised [1]. A dose limit or constraint is not applicable in these situations, as the exposure will depend on the medical question being investigated, and a higher radiation risk may be warranted in cases in which there is potential for greater clinical gain. A procedure involving exposure to radiation will result in net patient benefit if undertaken according to appropriate clinical guidance that incorporates the radiation protection principles of justification and optimisation.
CT currently accounts for the largest contribution to population dose from medical procedures [2], and several studies have shown that the use of CT is increasing, including imaging of children [3-6]. Furthermore, a number of CT dose surveys [7-11] have observed substantial differences between practices for the same type of examination, suggesting that some exposures may not be suitably optimised. The extent of the variation indicates that these differences are not solely attributable to patient factors, such as size and shape, but must also result from the exposure parameters and protocols used. Therefore, optimisation of CT protocols is essential, particularly for children, who are more radiosensitive than adults [12].
The International Commission on Radiological Protection (ICRP) introduced diagnostic reference levels (DRLs) as an optimisation tool for managing dose from medical imaging procedures [1,13-15]. Regulatory authorities typically establish modality- and examination-specific DRLs at the third quartile value of the distribution of mean doses resulting from a national survey [15,16]. Reference values can also be established at a practice level, and these are generally referred to as local DRLs (LDRLs). These values should be reviewed more frequently than national DRLs, allowing greater local control and therefore increased opportunity for management and optimisation of doses. Because of the smaller sample sizes in a local survey, LDRLs are usually calculated from the mean of the local dose distribution rather than from the third quartile [16]. For CT examinations, DRLs and LDRLs are established in terms of the CT dose indicators volumetric CT dose index (CTDIvol) and/or dose–length product (DLP).
For paediatric CT examinations there are several studies that have established or proposed national DRLs [17-20]. However, there are few studies presenting LDRLs for paediatric CT [21]. Furthermore, there is limited assessment of the contribution individual protocol parameters make to reference values. The continuous advances in CT technology can make it difficult to realise the dose implications from parameter selection. Therefore, undertaking a dose survey and detailed analysis across different age groups for paediatric patients provides an opportunity to better understand the impact of local protocol parameters and patient size on dose. Assessing local practice is essential for proactive optimisation and increasing dose awareness among staff.
The aim of this study was to assess local doses for paediatric CT examinations for the purpose of optimisation. This was achieved by sampling dose indicators and scan parameters across several age groups for typical paediatric CT examinations performed at the Royal Children's Hospital (RCH) in Parkville, Melbourne, Australia. LDRLs were derived and the mean values of the dose indicators (CTDIvol and DLP) were compared with international DRLs to facilitate benchmarking. To enable optimisation, further assessment of the scan parameters and dose indicators was undertaken to isolate and investigate the influence of each factor on dose across the different age groups. Additionally, effective dose has been estimated for each age group and type of CT examination. Martin [22] recommends that effective dose is useful as a generic indicator of the radiation risk and to broadly classify the level of health detriment to a reference patient. Furthermore, several authors quote typical effective dose values for paediatric CT examinations as a method of allowing comparison between protocols and different practices [23-25].
Methods and materials
Dose survey
A retrospective audit of patient records at the RCH for CT brain, chest and abdomen/pelvis examinations was undertaken. Approval was gained from the institutional Human Research Ethics Committee to access these data. In clinical practice at the RCH, the protocol to be used for an individual patient is typically selected based on the age of the patient for head examinations and the weight of the patient for body examinations. However, body weight was not a recorded parameter, and therefore the age of the patient was used to group the records in this study. It is recommended that, when assessing doses from a patient survey at a practice level, the sample should consist of at least 10 patients [16]. In this study, samples of 20 patients were selected for each age group in each of the three study protocols. A total of 220 patient records were included in the study: 100 for CT brain examinations and 60 for each of the CT chest and abdomen/pelvis examinations, reflecting the number of age groups for each type of scan.
All scans were performed on a Somatom Sensation® 16 multidetector CT (MDCT) scanner with a Straton® X-ray tube (Siemens, Erlangen, Germany) utilising automatic tube current modulation (CARE Dose4DTM; Siemens, Erlangen, Germany). The gender, age, parameters used (tube potential, time per rotation, detector configuration, beam collimation, pitch, effective mAs) and dose indicators (CTDIvol and DLP) were recorded for each patient. All protocols are programmed into the CT scanner in terms of the patient's age and are given in Table 1. The CT protocols were not changed during the survey period. Only those patient examinations in which there were no obvious indicators that the examination was atypical were sampled. For example, patients with metal implants were excluded. For CT examinations of the brain, only those performed without contrast were included in the study. CT examinations of the chest and abdomen/pelvis were included whether they were performed with or without intravenous contrast, as all post-contrast examinations were single phase only and the same protocol was used.
Table 1. Standard CT protocol parameters by age group for brain, chest and abdomen/pelvis examinations.
| Examination | Age group | Set parameters |
|||||
| Voltage (kV) | Qrefa (mAs) | Rotation time (s) | Pitch | Detector configuration | Beam collimation (mm) | ||
| CT brain | 0–6 months | 120 | 150 | 0.75 | Axial | 12×1.5 mm | 18 |
| 6 months to 3 years | 120 | 150 | 0.75 | Axial | 12×1.5 mm | 18 | |
| 3–6 years | 120 | 200 | 0.75 | Axial | 12×1.5 mm | 18 | |
| 6–10 years | 120 | 240 | 0.75 | Axial | 12×1.5 mm | 18 | |
| >10 years | 120 | 270 | 0.75 | Axial | 12×1.5 mm | 18 | |
| CT chest | <5 years | 80 | 65 | 0.50 | 1.00 | 16×1.5 mm | 24 |
| 5–10 years | 100 | 80 | 0.50 | 1.00 | 16×1.5 mm | 24 | |
| >10 years | 120 | 80 | 0.50 | 1.00 | 16×1.5 mm | 24 | |
| CT abdomen/pelvis | <5 years | 80 | 80 | 0.50 | 1.25 | 16×1.5 mm | 24 |
| 5–10 years | 100 | 80 | 0.50 | 1.25 | 16×1.5 mm | 24 | |
| >10 years | 120 | 60 | 0.50 | 1.25 | 16×1.5 mm | 24 | |
aQref is the imaging quality reference mAs, which is a setting specific to Siemens (Erlangen, Germany) that is used for automatic tube current modulation (CARE Dose4DTM; Siemens), which is defined by the user for each protocol. This value is adjusted based on image quality requirements and the amount of noise acceptable in the image. It is defined in terms of the effective mAs (actual mAs divided by pitch).
CT dosimetry
On most modern CT scanners, the CTDIvol and DLP values are displayed as projected values following the CT localiser radiograph (also called the scout/surview/topogram/scanogram), and the itemised values resulting from each individual part of the examination and total values are displayed at the conclusion of an examination based on the scan parameters used. At RCH this final dose screen is captured for each patient so that these values could readily be collected for use in the survey. The CTDIvol values represent standardised dose measurements made in two different polymethylmethacrylate, homogeneous, cylindrical phantoms. The measurements are made at the periphery and centre of the cylinder, and weighted accordingly to take into account the varying dose distributions with depth in the phantom resulting from the beam-shaping filters in the CT scanner. Typically, a 16-cm-diameter phantom is used to represent the head of an adult or child (or child's body) and a 32-cm-diameter phantom is used to represent an adult's body. Some jurisdictions now require the phantom size for the CTDIvol and DLP calculations to be displayed on the final dose screen, although this was not a feature of the RCH scanner.
In an earlier study [26] conducted on the RCH CT scanner, the authors found that the displayed dose indicators for paediatric body examinations were based on the 32-cm-diameter phantom. This displayed dose considerably underestimated the dose measured with thermoluminescence dosemeters (TLDs) and supports the recommendation by Shrimpton and Wall [27] that all CT dose indicators should be expressed in terms of the 16-cm phantom for children regardless of age or scan location. Displayed dose indicators for head examinations were expressed in terms of the 16-cm-diameter phantom on the RCH CT scanner, which more closely reflected TLD measurements. Therefore, in this study, doses expressed in terms of the 32-cm phantom were converted to doses relative to the 16-cm phantom by multiplying by a factor of 2.08, following the scanner-specific methodology of Huda et al [28], taking into account the X-ray beam filtration used for the scan. Chapple et al [29] have similarly shown that paediatric body DLP values that have been calculated on the basis of the adult 32-cm phantom should be multiplied by a factor of 2 to be expressed relative to the 16-cm phantom. To distinguish between the values, CTDIvol,16 and DLP16 will be used for doses relative to the 16-cm phantom, and CTDIvol,32 and DLP32 for doses relative to the 32-cm phantom.
The DLP is calculated by multiplying the CTDIvol by the scan length. For helical scans, this represents the imaged length, rather than the exposed length, as it does not take into account the additional over-ranging length required for data interpolation for many CT scanners [30]. The imaged scan length was calculated from the recorded CTDIvol and DLP values for each patient in the survey.
Local diagnostic reference levels and international benchmarking
Recommendations were made regarding establishing LDRLs in terms of CTDIvol and DLP for the RCH based on mean dose values from the survey in all age groups for each type of CT examination. These values were rounded up to the nearest whole number (CTDIvol) or nearest 50 mGy·cm (DLP) to provide a user-friendly reference level that staff may become more readily familiar with. The mean values of CTDIvol and DLP were then compared with international paediatric CT DRLs. Since national DRLs are derived from the third quartile, if the mean value LDRL exceeded a corresponding 75th percentile national DRL, this indicated that further investigation and either justification of the higher value or optimisation was warranted locally.
Assessment of scan parameters
The dose indicators CTDIvol and DLP are useful quantities for providing an overview of the average X-ray beam intensity and the total dose delivered. However, they combine several factors that affect the dose to the patient and, when assessing practice at a local level, it is beneficial to isolate parameters to allow greater control over protocol optimisation. This includes separating parameters that are dependent on patient size (such as the scan length and the mAs values when automatic modulation of the tube current is employed) from parameters that are specific to the protocol (such as tube potential, beam collimation and pitch).
The use of automatic modulation of the tube current to achieve consistent image quality by compensating for patient size and varying attenuation within the body is a dose reduction tool available on modern scanners [31]. It was used for all scans sampled in this dose survey. Therefore, to examine the effects of patient size the average mAs value for an examination and the scan length were evaluated. This also allowed assessment of the user-specified reference parameter that drives the automatic adjustment of the tube current. In addition, the scan length was assessed to demonstrate any changes with age for the different types of CT examinations. Furthermore, the CTDIvol values were normalised with respect to mAs values to quantify the X-ray beam output in terms of the tube potential, beam collimation, pitch and filter regardless of the patient size.
Effective dose
The surveyed mean DLP values were utilised with commonly used published [9] age- and region-specific conversion coefficients (Table 2) to estimate the effective dose according to the 1990 recommendations of the ICRP [13]. These conversion coefficients were matched to the age groups used in this study to estimate effective dose. When some age groups included a range of ages incorporating two different age-specific conversion coefficients, these values were averaged. In the eldest age category (>10 years) for examinations of the chest and abdomen/pelvis both DLP16 and DLP32 may be applicable for the range of patient sizes in this age group, and hence effective doses were estimated from both DLP values. Adult conversion coefficients were used when converting DLP32 values to the effective dose.
Table 2. Dose–length product (DLP) to effective dose (E) conversion coefficients.
| Examination | Age groupa |
E/DLP conversion coefficient (mSv mGy−1 cm−1)b |
|||
| ICRP 60 [9] | ICRP 103 [26]c | ICRP 103 [33]d | ICRP 103 [34]d | ||
| CT brain | 0–6 months [0 years] | 0.0110 | 0.0076 | 0.0085 | 0.0130 |
| 6 months to 3 years [1 year] | 0.0067 | 0.0046 | 0.0053 | 0.0080 | |
| 3–6 years [5 years] | 0.0040 | 0.0028 | 0.0035 | 0.0050 | |
| 6–10 years [10 years] | 0.0032 | 0.0022 | 0.0027 | 0.0040 | |
| >10 years [adult] | 0.0021 | 0.0014 | 0.0019 | 0.0020 | |
| CT chest | <5 years [1 year] | 0.0260 | 0.0320 | 0.0260 | 0.0380 |
| 5–10 years [5, 10 years] | 0.0160 | 0.0190 | 0.0140 | 0.0230 | |
| >10 years [10 years] | 0.0130 (0.0140)b | 0.0160 (0.0170)b | 0.0120 (0.0150)b | 0.0190 (0.0200)b | |
| CT abdomen/pelvis | <5 years [1, 5 years] | 0.0250 | 0.0230 | 0.0220 | 0.0260 |
| 5–10 years [5, 10 years] | 0.0180 | 0.0160 | 0.0140 | 0.0180 | |
| >10 years [10 years] | 0.0150 (0.0150)b | 0.0140 (0.0140)b | 0.0120 (0.0140)b | 0.0150 (0.0150)b | |
ICRP, International Commission on Radiological Protection.
aThe age groups used in the survey were based on the categorisations used for CT protocols at Royal Children's Hospital Melbourne, Parkville, Australia. Since conversion coefficients are generally defined for a specific age, these were matched to the age groups used in the survey. The age specific to the conversion coefficients is shown in square brackets in this column. For example, for the 0- to 6-month age group for CT brain examinations in this survey, the age-specific conversion coefficient for 0-year-olds has been used. In some cases, an average of conversion coefficients was used based on the distribution of ages in the survey and, for these, two ages have been listed in the square brackets.
bAll values of the conversion coefficients are relative to the 16-cm dosimetry phantom, except those shown in round brackets, which are relative to the 32-cm dosimetry phantom and are for adults. Some of the patients in the >10 years age group will be closer in size to an adult than to a child.
cThese conversion coefficients were derived from the previous study by Brady et al [26] as described in the Methods and materials section. These values have been used to calculate the ICRP 103 effective doses given in Table 4.
dThe conversion coefficients provided by Deak et al [33] are age specific and tube potential specific. The tube potential for each protocol is given in Table 1. All body conversion coefficients in Deak et al [33] have been provided relative to the 32-cm dosimetry phantom and therefore have been divided by 2 to be expressed relative to the 16-cm dosimetry phantom, except for the values in rounded brackets. Deak et al [33] provided separate conversion coefficients for the abdomen and the pelvis. For the CT abdomen/pelvis examinations the conversion coefficients have been averaged. The conversion coefficients given for the CT abdomen/pelvis examinations for Alessio and Phillips [34] relate to the abdomen only.
There is not yet a widely adopted set of age-specific conversion coefficients for estimating effective dose according to the 2007 recommendations of the ICRP [32]. In the previous study [26] conducted by the authors on the RCH CT scanner, organ doses were measured using TLDs placed in a physical anthropomorphic phantom representing a 10-year-old child to estimate effective dose for different CT examinations according to the formalism described in ICRP 103 [32]. These estimates of effective dose were used with the displayed DLP values to derive effective dose conversion coefficients for a reference 10-year-old child. In the present study, these values were then scaled for all other age groups according to the relative ratios of the published ICRP 60 conversion coefficients [9]. This method is approximate, but suitable for the purpose of providing broad estimates of effective dose in this study using the two different ICRP recommendations [13,32]. These conversion coefficients are compared with other published ICRP 103 paediatric conversion coefficients in Table 2. Deak et al [33] provided conversion coefficients specific to a 64-MDCT scanner, and Alessio and Phillips [34] derived conversion coefficients based on adult effective doses, adjusted for paediatric sizes. It is evident that a wide range of values currently exist in the literature. The conversion coefficients based on earlier TLD measurements [26] were used for effective dose estimates in this study because they are specific to this scanner and calculated directly from a paediatric anthropomorphic phantom, rather than an adult phantom.
Results
Recommended LDRLs for CT practice at the RCH in terms of both CTDIvol and DLP based on rounded mean values are given in Table 3. These have been provided relative to both phantom sizes for the body examinations. The LDRLs in terms of CTDIvol are much higher for the brain examinations than for the body examinations, reflecting the higher X-ray beam intensity used for the brain examinations.
Table 3. Recommended local diagnostic reference levels (LDRLs) by age group for typical paediatric CT examinations.
| Examination | Age group | LDRL |
|||
| CTDIvol,32 (mGy) | DLP32 (mGy cm) | CTDIvol,16 (mGy) | DLP16 (mGy cm) | ||
| CT brain | 0–6 months | – | – | 18 | 250 |
| 6 months to 3 years | – | – | 20 | 300 | |
| 3–6 years | – | – | 30 | 450 | |
| 6–10 years | – | – | 40 | 650 | |
| >10 years | – | – | 45 | 700 | |
| CT chest | <5 years | 2 | 50 | 3 | 100 |
| 5–10 years | 5 | 150 | 11 | 300 | |
| >10 years | 12 | 400 | 23 | 800 | |
| CT abdomen/pelvis | <5 years | 2 | 100 | 4 | 150 |
| 5–10 years | 5 | 200 | 10 | 400 | |
| >10 years | 8 | 350 | 15 | 750 | |
CTDIvol,16, volumetric CT dose index relative to the 16-cm dosimetry phantom; CTDIvol,32, volumetric CT dose index relative to the 32-cm dosimetry phantom; DLP16, dose–length product relative to the 16-cm phantom; DLP32, dose–length product relative to the 32-cm phantom.
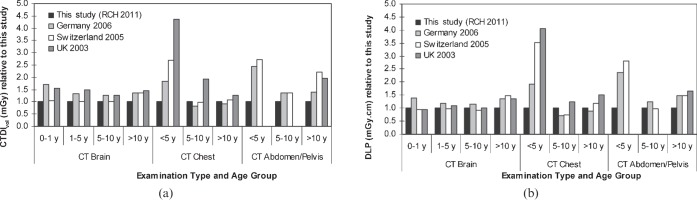
Figure 1 compares the mean values of CTDIvol and DLP with established or proposed national DRLs for Germany [17], Switzerland [18] and the UK [19]. The national values established in these countries were based on surveys that included a range of single-detector CT scanners and MDCT scanners in clinical practice at the time. All of the other surveys included the 16-MDCT scanner assessed in this study. For CT imaging of the head, the RCH CTDIvol values are all lower than the international DRLs. However, when comparing the values of DLP for CT head examinations, the Swiss DRLs are slightly lower (<10%) than the RCH values. For CT chest imaging the CTDIvol and DLP values for children under 5 years old are significantly lower at the RCH. Similarly, the RCH values in the youngest age group for CT abdomen/pelvis examinations are more than two times lower than the international values. This dose reduction is not evident in the older age groups for chest imaging.
Figure 1.
Comparison of (a) mean volumetric CT dose index (CTDIvol) values and (b) mean dose–length product (DLP) values for different age groups for common CT examinations from the paediatric dose survey undertaken in this study, at Royal Children's Hospital Melbourne, Parkville, Australia (RCH), 2011, with international diagnostic reference levels (Germany, 2006 [17]; Switzerland, 2005 [18]; the UK, 2003 [19]). The year indicates the last year of data collection in each of the studies. All values are relative to the mean values from this study. All values are relative to the 16-cm dosimetry phantom (CTDIvol,16, DLP16) except for the chest and abdomen/pelvis examinations in the >10 years age category, which are relative to the 32-cm dosimetry phantom (CTDIvol,32, DLP32).
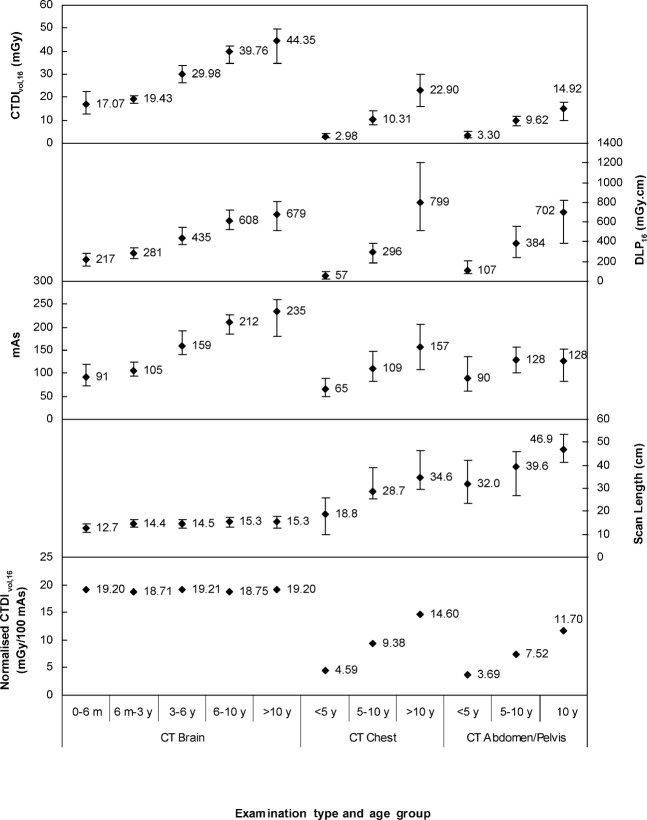
To enable identification of parameters that can be further optimised, a more detailed breakdown of the scan parameters is given in Figure 2. The range and mean values for CTDIvol,16, DLP16, actual mAs and scan length are shown for each age group and examination. The CTDIvol increased with age for all types of examination, regardless of whether the examination was of the body or head region. The DLP increased with age for each type of examination, although the increase was less for brain examinations than for body examinations. For head and chest imaging the mean mAs values were more than two times higher in the eldest age group than in the youngest age group. However, for abdomen/pelvis imaging the mean mAs value increased by <50% between the youngest age group and the middle age group, and did not change between the middle age group and the eldest age group. Scan length did not substantially change with age for brain examinations, whereas for body examinations it increased with age, particularly for the youngest age groups. Furthermore, the range of scan lengths is larger for body examinations than for head examinations.
Figure 2.
Range and mean values of volumetric CT dose index relative to the 16-cm dosimetry phantom (CTDIvol,16), dose–length product relative to the 16-cm dosimetry phantom (DLP16), mAs (actual mAs, not effective mAs) and scan length for different age groups for CT brain, chest and abdomen/pelvis examinations from the paediatric dose survey. The bottom panel shows normalised median CTDIvol,16 values. Age groups are defined in terms of months (m) or years (y).
The bottom panel of Figure 2 shows CTDIvol,16 values normalised to 100 mAs for each age group and examination. Since this quantity is independent of both the examination mAs values and the scan length, it reflects the influence on dose of the protocol parameters initially selected by the operator. Normalised CTDIvol,16 remained constant for brain examinations, but increased with age for body examinations.
Values of effective dose using ICRP 60 and ICRP 103 definitions and tissue-weighting factors were estimated using the mean DLP values (Figure 2) and age- and region-specific conversion factors (Table 2), and are given in Table 4. The ICRP 103 values of effective dose were lower than the values calculated using the ICRP 60 definition for CT brain examinations (by 31%) and CT abdomen/pelvis examinations (by 7%), but higher for CT chest examinations (by 23%). Overall, the effective dose values for CT brain examinations were lower than those for any of the body imaging, except for the effective dose for a CT chest examination in the youngest age group, which was similar to the effective dose for a CT brain examination. The effective dose values increased with age for the body examinations using DLP16. Using the DLP32 value and adult conversion coefficients resulted in approximately halving the estimate of effective dose.
Table 4. Estimated effective doses for typical paediatric CT examinations according to the International Commission on Radiological Protection (ICRP) 60 [13] and ICRP 103 [32] definitions of effective dose.
| Examination | Age group | Effective dose (mSv) ICRP 60 | Effective dose (mSv) ICRP 103 |
| CT brain | 0–6 months | 2.4 | 1.6 |
| 6 months to 3 years | 1.9 | 1.3 | |
| 3–6 years | 1.7 | 1.2 | |
| 6–10 years | 1.9 | 1.3 | |
| >10 years | 1.4 | 1.0 | |
| CT chest | <5 years | 1.5 | 1.8 |
| 5–10 years | 4.6 | 5.6 | |
| >10 years | 10 (5.4)a | 13 (6.6)a | |
| CT abdomen/pelvis | <5 years | 2.7 | 2.5 |
| 5–10 years | 6.7 | 6.3 | |
| >10 years | 11 (5.1)a | 10 (4.7)a |
aAll values of effective dose have been estimated from the mean DLP16 (dose–length product relative to the 16-cm phantom) values, except those shown in brackets, which have been estimated from the mean DLP32 (dose–length product relative to the 32-cm phantom) values. These have been included for comparison as some of the patients in the >10 years age group will be closer in size to an adult than to a child.
Discussion
Repeat surveys conducted in the UK have shown that DRLs assist in reducing radiation doses over time [19]. DRLs for paediatric CT examinations were 10–40% lower when comparing surveys several years apart [19,27]. With an established and widely adopted DRL programme, it is envisaged that at some stage doses may become relatively constant and DRLs will then be important to protect against unnecessary dose increases. In Australia, where national DRLs have yet to be established, there will be a significant initial opportunity for optimisation.
At the local level, dose surveys can be undertaken to establish LDRLs that can be compared with national DRLs. It is useful to conduct surveys based on real patients, which can be repeated at regular intervals to assess CT practice at an institution. Using the dose indicators on the CT display (CTDIvol and DLP) ensures that results will be comparable between different scanners, sites and countries, as long as the dosimetry phantom is specified. Furthermore, Heggie [35] argues that dose surveys are also an essential tool for understanding local practice when commissioning a new CT scanner to ensure that optimisation is undertaken. Therefore, LDRLs are useful to gauge changes in local practice and techniques, and the impact of new imaging technology.
Establishing typical dose levels for children is more challenging than deriving these values for adults. Parameters (and hence doses) vary considerably with size and age for children. Multiple reference values for a particular examination may be appropriate to account for the variation in size across age ranges. It has been suggested that age may not be an appropriate indicator of size in children and a method for deriving a representative “age” from a size measurement of the patient has been developed [16,36]. The thickness of the patient directly correlates with the distance that the X-ray beam travels in the patient and hence is a more direct determinant of dose [16,36-39]. However, in this study, dose records were grouped for children based on age and not size. This allowed investigation of the range of doses that may be encountered in a particular age bracket. This has been standard practice at the RCH as part of the quality assurance programme for a number of years.
Local diagnostic reference levels and international benchmarking
In all age groups for brain examinations at the RCH, the mean CTDIvol values are equal to or lower than international DRL values (Figure 1a). This implies that the protocol settings for brain scans at the RCH are well optimised and reflect current international practice. However, the dose saving evident in the RCH CTDIvol values has reduced when comparing the DLPs (Figure 1b), suggesting that the scan lengths at RCH may on average be slightly longer (<10%), offsetting the dose reduction achieved in the parameter selection for the protocols. However, there are other contributing factors that may result in a numerical difference in the dose indicators, but not the actual dose to the patient. For axial brain examinations there may be a small component of the scan that extends beyond the top of the skull and scans only air to ensure that the complete region of clinical interest is included in the scan range. For the protocol settings used at the RCH, this will be up to a maximum of a single beam collimation, or 18 mm, of extended scan length that does not directly expose the patient but increases the DLP and, hence, this may account for the observed differences in DLP values. Additionally, the definition of scan length for examinations performed with a tilted gantry will also directly affect the DLP. For example, on the scanner used in this study the scan length is measured parallel to the patient's long axis, whereas measurement of the scan length parallel to the axis of rotation would reduce the length by approximately 10%. Therefore, the actual dose to the patient has not changed, but the DLP has, depending on the scan length used.
For body imaging, the RCH mean values are considerably lower than international DRLs in terms of both CTDIvol and DLP in the under 5 years age group (Figure 1). This is most likely to be the result of the low tube potential (80 kV) employed at the RCH, which leads to a considerable dose reduction. For example, the UK survey [19] found that almost 80% of sites conducted chest imaging in this age group at 120 kV, with only 5% using 80 kV. This may also explain why the UK DRL is higher than the other values in the 5–10 years age group. Again, 120 kV is typically used in the UK, whereas at the RCH 100 kV is used in this age group. The German survey [17] found that in younger patients it was more likely that a lower tube potential would be used for chest imaging than for abdomen/pelvis scans, although in the 2–5 years age group low-tube-potential chest imaging accounted for just over 30% of the examinations.
The German DLP DRLs are lower than the RCH mean values in the two older age groups for chest imaging (Figure 1b). Furthermore, the German study included the over-ranging length in the helical scans, which was not included in the present study. Therefore, it was expected that the German DLP DRLs would be higher than the RCH mean values, which do not include this additional length. The third quartile value for the scan length, including over-ranging, in the age group 6–10 years in the German study was 22.7 cm. The mean scan length in the RCH survey for 5–10 year olds was 28.7 cm. Clearly, the RCH scan length is extended. Similarly, the Swiss DLP [18] for chest imaging is lower than the current study in the 5–10 years age group. The average scan length for 5–10 year olds in the Swiss study was 23 cm, again shorter than the current study.
All RCH mean values for abdomen/pelvis examinations across all age groups are equal to or lower than the international DRLs. In the two older age groups, the dose saving achieved through the selection of parameters, evident in the CTDIvol values at the RCH, again appears to be offset to some extent by a longer scan length when assessing the DLP values. In the 5–10 years age group the mean RCH scan length was 39.6 cm, whereas the German survey third quartile value was 31.6 cm. Similarly, in the over 10 years old category the RCH mean length was 46.9 cm and the German third quartile value was 40 cm. Again, there appears to be an opportunity for dose reduction by reviewing the scan length for the RCH examinations. However, overall, the RCH average dose values for abdomen/pelvis imaging are lower in comparison.
A comprehensive single-site Australian survey was undertaken by Watson and Coakley [21] based on paediatric protocols on a 64-MDCT scanner. A comparison with the current study shows that the majority of CTDIvol and DLP values in the Watson and Coakley study are significantly lower. This is likely to be the result of changes in technology between the 16-MDCT scanner in this study and the newer 64-MDCT scanner in their survey. It may also be the result of optimisation of protocols as some of the dose saving appears to be due to lower mAs values for the axial CT brain examinations and lower CTDIvol values for the body imaging, although it is not clear which parameters may cause this. For example, none of the protocols reported in their study are performed at the lowest available tube potential (80 kV) and the pitch is lower than the current study for all body imaging. The only doses from the present study which are comparatively lower are those for CT chest and CT abdomen/pelvis imaging in the youngest age group, performed at 80 kV. One of the significant benefits of the Watson and Coakley study is that they had access to the patients' weights, and have reported dose values in terms of both age and weight.
Assessment of scan parameters
It is useful to make comparisons with other studies and international DRLs using the dose indicators. For the current study, this has led to identification of chest imaging in older patients as one area that can be potentially optimised. However, with only the dose indicators it is difficult to ascertain the factors that require optimisation. For example, for the chest examinations, the mean DLP in the eldest age group is 14 times higher than the mean DLP for the youngest age group, whereas for the abdomen/pelvis examinations it is only a factor of 7 times higher. It is not clear which factors contribute to this significant difference and therefore a more detailed breakdown of the local scan parameters was necessary. This was achieved by assessing the normalised CTDIvol, average mAs values and scan length for each type of examination.
Normalised CT dose index
The CT brain examinations had the highest value of normalised CTDIvol (Figure 2). This is partly attributable to the narrower beam collimation than that used for body examinations, which incurs a greater dose penalty owing to overbeaming in which the unused penumbral region of the X-ray beam is proportionally higher for narrower collimations. Furthermore, less filtration is used for head scanning, which leads to a greater dose than a more filtered beam with higher beam quality for body examinations [28]. Since the tube potential, beam collimation and pitch did not change for the different age groups for brain examinations, the normalised CTDIvol remained constant. The slight variation in normalised values evident in Figure 2, despite the use of the same protocol parameters, is due to the limitations of using average mAs values for this calculation. For a fixed tube current these values would be identical.
The beam collimation and pitch remained constant between the age groups for the body examinations, although the tube potential increased over the three age groups. This is observed in the increasing normalised CTDIvol values in Figure 2. The normalised CTDIvol values for the chest and abdomen/pelvis imaging both increased by a factor of 3.2 between the youngest and oldest age groups. The chest and abdomen/pelvis normalised values for the same age groups differ only by the pitch. It is interesting that the values are similar as the two body regions have quite different attenuation and contrast characteristics. It may be expected that the CTDIvol values, which reflect the patient dose by including the mAs values, may be lower for the chest imaging than for the abdomen/pelvis imaging owing to the reduced attenuation from the air in the lungs and the increased inherent contrast in this area allowing for lower dose settings. However, this was not always the case, particularly for the oldest age group (Figure 2). Therefore, either justification or optimisation of the higher CTDIvol and normalised CTDIvol values for chest imaging relative to abdomen/pelvis imaging should be undertaken.
Tube current modulation
In all examinations assessed, the tube current modulation dose reduction tool was used (CARE Dose4D). This requires a user-defined reference mAs value (called Qref), which is set at a level to achieve the desired image quality and is expressed in terms of the effective mAs value. The tube current is modulated based on the size of the patient being scanned relative to the standard-sized patient (defined as weighing 20 kg or as aged 5 years for this scanner). Therefore, for very young/small patients it is expected that the range of mAs values for the examination will be less than the reference value, and for older/larger patients the mAs will exceed the reference mAs value. For example, for a 4-day-old baby undergoing a CT chest examination the average value for the examination was 49 mAs compared with a reference value of 65 mAs. For a 14-year-old adolescent undergoing a CT chest examination the average was 124 mAs and the reference was 80 mAs.
For the brain examinations that were undertaken with a tilted gantry, the average tube current was lower than the reference value for all age groups. Some sections of the scan had a very low tube current when the transmission length through the brain was quite small (e.g. top of the head). Overall, the mAs for the brain examinations increased significantly with age (Figure 2), by a factor of 2.6, reflecting the higher reference mAs (Table 1) required to achieve the same image quality in older patients with a more radiodense skull. The mean mAs values for the chest examinations also increased by a factor of 2.4 between the youngest and oldest age groups. Again, the increased density of the skeleton in older children and their larger size are expected to lead to the mAs increase. However, the increase in mAs was only by a factor of 1.4 for the abdomen/pelvis examinations.
For the chest examinations the increase in mAs between the two younger groups was because of the increasing reference value and/or the increasing size of the patient, whereas the increase in the oldest group was only because of larger patient size since the reference value remained constant. For abdomen/pelvis examinations the patients in the oldest group were larger, which would increase the examination mAs, but this was offset by a lower reference mAs, and hence the mean examination mAs was the same for the two oldest age groups. Therefore, the reference mAs values for body imaging should be reviewed and the relative differences between chest and abdomen/pelvis values in the same age group should be justified; otherwise, these reference values should be optimised for diagnostic image quality.
Scan length
The average scan length for brain examinations increased between the two youngest age groups (up to 3 years old), but was then fairly consistent (Figure 2). This correlates well with the rapid growth of the head in the first 2 years [39]. The change of body size as children develop is clearly evident in the longer scan lengths for the body examinations in older children (Figure 2). The greatest change in mean scan length for chest examinations occurred in the first 5 years, whereas the increase in mean scan length for abdomen/pelvis examinations was more consistent across the age groups.
The length of the scan for abdomen/pelvis examinations was 11–13 cm longer on average than for examinations of the chest for all age groups. For children under 5 years, the average chest scan length was 40% shorter than the abdomen/pelvis scan length, whereas in the older age groups it was only 25% shorter. Regardless of age, the anatomical borders of the scan seldom change. For chest examinations, the entire thorax anatomy and half of the liver are routinely included. For abdomen/pelvis examinations the typical scan range is from just above the diaphragm to the symphysis pubis. Hence, it is most likely that anatomical changes as children develop lead to differences in the comparative sizes of the chest and abdomen/pelvis in different age groups, which is reflected in the relative scan lengths from this survey.
For all scans in helical mode, the operator's planned length defines the mid-position of the first and last image (slice) to be reconstructed, and the length of the table movement for a single rotation (which varies with pitch) is automatically added to this planned length [30]. This additional half-rotation width at each end is part of the imaged length and included in the DLP calculation. An additional scan length owing to over-ranging for helical data interpolation is not included in the DLP, and van der Molen and Geleijns [30] have shown that, for the same scanner as used in this study for a CT scan with a pitch of 1.00–1.25, the over-ranging length is 5–6 cm. Therefore, the scan lengths calculated here for the body examinations represent the imaged length and underestimate the total length exposed, and consequently the effective dose.
Effective dose
The dose indicators CTDIvol and DLP are representative of the dose to cylindrical phantoms and do not take into account the relative radiosensitivity of the organs and tissues exposed or the amount of the body directly irradiated. Effective dose is designed to provide a measure of overall radiation detriment owing to stochastic effects and is to be used for prospective dose assessment to facilitate planning and optimisation [32]. While not intended for retrospective use for estimation of doses to individuals, it is utilised here as an optimisation tool that will allow comparison with similar procedures undertaken at different hospitals.
The estimates of effective dose made in this study (Table 4) differ between the ICRP 103 and ICRP 60 definitions because of the change in weighting factors for organs and tissues in the head and chest. When directly irradiated, the brain has a comparatively lower tissue weighting factor in ICRP 103, causing the brain dose estimate to decrease. In the chest region, the breast weighting factor has increased, leading to a higher effective dose for the chest examination according to ICRP 103. There is a slight decrease in effective dose estimates for the abdomen/pelvis examinations. Although the tissue weighting factor for the gonads has decreased, an overall increase in the weighting factor for the remainder of the tissues and organs tends to partially offset this. Furthermore, the conversion coefficients used in this study (Table 2) to determine the effective dose using the ICRP 103 definition were based on an abdomen/pelvis examination that did not include direct irradiation of the testes. Therefore, the gonad absorbed dose, which was averaged from the directly irradiated ovary absorbed dose and the testes absorbed dose, was small compared with the contribution from other organs and tissues [26].
Other studies have had similar findings to this study for CT examinations of the body when comparing the effect of the changes in the tissue-weighting factors on the effective dose estimates for adults [40,41] and for children [33]. However, some of these studies [33,40] show an increase in the effective dose estimate for CT brain examinations based on the ICRP 103 definition, when compared with the ICRP 60 definition, whereas this study, based on the previous work by the authors [26], shows a decrease. According to ICRP 60, in which a single remainder organ is directly irradiated and receives an equivalent dose in excess of the highest dose for the primary organs, the remainder of the weighting factor is split evenly between that organ and the rest of the remainder. This splitting rule applies to the brain dose when the brain is directly irradiated and, therefore, for calculation of the effective dose according to ICRP 60 the brain should be allocated a tissue-weighting factor of 0.025, not 0.005. The ICRP 60 effective dose estimates made by Deak et al [33] for CT brain examinations appear to apply a tissue-weighting factor of 0.005 for the brain dose and consequently underestimate the ICRP 60 effective dose. Therefore, Deak et al [33] conclude that the ICRP 103 effective dose estimate for brain examinations increases relative to the ICRP 60 estimate, which does not agree with the findings of the present study.
The study by Huda et al [40] similarly reports an increase in effective dose estimates for CT head examinations for adults according to ICRP 103, when compared with ICRP 60 effective dose estimates. They have made use of the ImPACT CT Patient Dosimetry Calculator (v. 1.0) [42] for effective dose estimates according to either ICRP 60 or ICRP 103. ImPACT does employ the ICRP splitting rule for ICRP 60 effective dose calculations. However, the effective dose estimates in ImPACT are based on Monte Carlo simulations, which use a mathematical anthropomorphic phantom. Not all of the organs and tissues specified in the ICRP 103 effective dose estimate were included in the Monte Carlo calculations and a method of substituting known organ absorbed doses has been used by ImPACT. For example, the salivary glands and oral mucosa in ImPACT have been allocated the brain absorbed dose. Since the brain absorbed dose is relatively high when directly irradiated in a CT brain examination, the salivary glands and oral mucosa are allocated this same absorbed dose, even though it is most likely that they would not be directly irradiated in this type of scan. Therefore, the ICRP 103 effective dose estimates in ImPACT are higher than the ICRP 60 effective dose estimates for a brain examination because of the inappropriately high absorbed doses allocated to these tissues, which are included only in the ICRP 103 effective dose estimate and not in the ICRP 60 effective dose estimate.
A recent study by Thomas and Wang [25] reported effective doses based on a similar paediatric patient dose survey conducted for scans performed on an eight-MDCT scanner without tube current modulation. The effective doses in their study were calculated using ICRP 60 conversion coefficients. The ICRP 60 effective dose estimates reported in the current study are predominantly lower, except for imaging of the chest in the older age groups. This appears to be the result of two factors. First, in the other study, a higher pitch (1.35) is used, which will reduce dose. Second, the mAs is lower than in the present study. Furthermore, the scan protocols reported in the Thomas and Wang [25] study demonstrate that the mAs values are consistently higher for abdomen/pelvis examinations than for chest examinations in each age group, which is not the case in the present study for body examinations in the oldest age group. Again, this is suggesting that the protocol parameters at the RCH for chest examinations should be compared with the parameters for abdomen/pelvis examinations and, when the values for chest examinations are higher than for the abdomen/pelvis examinations, these need to be clinically justified or otherwise optimised.
Conclusion
As the contribution of medical imaging to collective population dose continues to grow [2], there is greater awareness and utilisation of tools such as DRLs to reduce and manage dose. Furthermore, conducting a CT dose survey within a facility is essential for understanding and analysing local practice. The mean values from these surveys are also useful for comparison with national or even international DRLs to facilitate benchmarking and ultimately optimisation of dose.
A comparison of the average dose values at the RCH with international DRLs for common paediatric CT examinations has shown that the RCH values are typically similar or lower. In particular, the use of 80 kV for both chest and abdomen/pelvis imaging in children under 5 years old leads to a significant dose saving. There is potential for dose optimisation at the RCH by reducing scan lengths for body examinations for children over 5 years old. In the short term, a review of these protocols should be undertaken to determine whether there is appropriate justification for the extended scan lengths. Analysing the local scan parameters provided a better indication of areas that may need further optimisation. In addition to scan length, it was identified that the reference mAs values for chest imaging should be justified, particularly relative to the abdomen/pelvis examination values for the same age groups. Because of the inherent contrast in the chest and lack of attenuating tissue, it is expected that these values might be lower for chest examinations than for abdomen/pelvis examinations, but in fact in the older age group they were found to be higher.
In the future, it may be worthwhile deriving LDRLs at the RCH for common protocols based on clinical indication and/or weight. This may result in narrower ranges for the scan lengths and allow more directed LDRLs. There are many factors that need to be considered when comparing local doses with DRLs, and the dose indicators need to be properly defined in terms of the CT dosimetry phantom used and the type of examination. This study demonstrates that using several quantities isolates the influence of each factor on dose and enables the identification of protocol parameters and areas of operator technique that can be optimised.
Acknowledgment
We would like to thank Luke Wilkinson at St Vincent's Hospital, Melbourne, VIC, Australia, for useful discussions on this study.
References
- 1.International Commission on Radiological Protection Radiological protection in medicine. ICRP Publication 105. Ann ICRP 2007;37:1–64 [Google Scholar]
- 2.United Nations Scientific Committee on the Effects of Atomic Radiation Sources and effects of ionizing radiation. Vol. I. UNSCEAR 2008 report to the General Assembly. Annex A: medical radiation exposures. New York, NY: United Nations; 2010 [Google Scholar]
- 3.Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 2001;176:289–96 [DOI] [PubMed] [Google Scholar]
- 4.Wiest PW, Locken JA, Heintz PH, Mettler FA. CT scanning: a major source of radiation exposure. Semin Ultrasound CT MRI 2002;23:402–10 [DOI] [PubMed] [Google Scholar]
- 5.Blackwell CD, Gorelick M, Holmes JF, Bandyopadhyay S, Kuppermann N. Pediatric head trauma: changes in use of computed tomography in emergency departments in the United States over time. Ann Emerg Med 2007;49:320–4 [DOI] [PubMed] [Google Scholar]
- 6.Brady Z, Cain TM, Johnston PN. Paediatric CT imaging trends in Australia. J Med Imaging Radiat Oncol 2011;55:132–42 [DOI] [PubMed] [Google Scholar]
- 7.Pages J, Buls N, Osteaux M. CT doses in children: a multicentre study. Br J Radiol 2003;76:803–11 [DOI] [PubMed] [Google Scholar]
- 8.Moss M, McLean D. Paediatric and adult computed tomography practice and patient dose in Australia. Australas Radiol 2006;50:33–40 [DOI] [PubMed] [Google Scholar]
- 9.Shrimpton PC, Hillier MC, Lewis MA, Dunn M. National survey of doses from CT in the UK: 2003. Br J Radiol 2006;79:968–80 [DOI] [PubMed] [Google Scholar]
- 10.Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009;169:2078–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace AB, Goergen SK, Schick D, Soblusky T, Jolley D. Multidetector CT dose: clinical practice improvement strategies from a successful optimization program. J Am Coll Radiol 2010;7:614–24 [DOI] [PubMed] [Google Scholar]
- 12.National Research Council of the National Academies Board on Radiation Effects Research. Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2. Washington, DC: The National Academies Press; 2006 [Google Scholar]
- 13.International Commission on Radiological Protection 1990 Recommendations of the International Commission on Radiological Protection. ICRP Publication 60. Ann ICRP 1991;21:1–201 [PubMed] [Google Scholar]
- 14.International Commission on Radiological Protection Radiological protection and safety in medicine. ICRP Publication 73. Ann ICRP 1996;26:1–47 [PubMed] [Google Scholar]
- 15.International Commission on Radiological Protection Diagnostic reference levels in medical imaging: review and additional advice. ICRP Supporting Guidance 2. Ann ICRP 2001;31:33–52 [PubMed] [Google Scholar]
- 16.Institute of Physics and Engineering in Medicine Guidance on the establishment and use of diagnostic reference levels for medical X-ray examinations. IPEM Report No. 88. York, UK: IPEM; 2004 [Google Scholar]
- 17.Galanski M, Nagel HD, Stamm G. Paediatric CT exposure practice in the Federal Republic of Germany: results of a nationwide survey in 2005–2006. Hannover, Germany: Medizinische Hochschule Hannover; 2007 [Google Scholar]
- 18.Verdun FR, Gutierrez D, Vader JP, Aroua A, Alamo-Maestre LT, Bochud F, et al. CT radiation dose in children: a survey to establish age-based diagnostic reference levels in Switzerland. Eur Radiol 2008;18:1980–6 [DOI] [PubMed] [Google Scholar]
- 19.Shrimpton PC, Hillier MC, Lewis MA, Dunn M. Doses from computed tomography (CT) examinations in the UK: 2003 review. Report NRPB-W67 Chilton, UK: National Radiological Protection Board; 2005 [Google Scholar]
- 20.Yakoumakis E, Karlatira M, Gialousis G, Dimitriadis A, Makri T, Georgiou E. Effective dose variation in pediatric computed tomography: dose reference levels in Greece. Health Phys 2009;97:595–603 [DOI] [PubMed] [Google Scholar]
- 21.Watson DJ, Coakley KS. Paediatric CT reference doses based on weight and CT dosimetry phantom size: local experience using a 64-slice CT scanner. Pediatr Radiol 2010;40:693–703 [DOI] [PubMed] [Google Scholar]
- 22.Martin CJ. Effective dose: how should it be applied to medical exposures? Br J Radiol 2007;80:639–47 [DOI] [PubMed] [Google Scholar]
- 23.McLean D, Malitz N, Lewis S. Survey of effective dose levels from typical paediatric CT protocols. Australas Radiol 2003;47:135–42 [DOI] [PubMed] [Google Scholar]
- 24.Theocharopoulos N, Damilakis J, Perisinakis K, Tzedakis A, Karantanas A, Gourtsoyiannis N. Estimation of effective doses to adult and pediatric patients from multislice computed tomography: a method based on energy imparted. Med Phys 2006;33:3846–56 [DOI] [PubMed] [Google Scholar]
- 25.Thomas K, Wang B. Age-specific effective doses for pediatric MSCT examinations at a large children's hospital using DLP conversion coefficients: a simple estimation method. Pediatr Radiol 2008;38:645–56 [DOI] [PubMed] [Google Scholar]
- 26.Brady Z, Cain TM, Johnston PN. Differences in using the International Commission on Radiological Protection's publications 60 and 103 for determining effective dose in paediatric CT examinations. Radiat Meas 2011;46:2031–4 [Google Scholar]
- 27.Shrimpton PC, Wall BF. Reference doses for paediatric computed tomography. Radiat Prot Dosimetry 2000;90:249–52 [Google Scholar]
- 28.Huda W, Sterzik A, Tipnis S. X-ray beam filtration, dosimetry phantom size and CT patient dose conversion factors. Phys Med Biol 2010;55:551–61 [DOI] [PubMed] [Google Scholar]
- 29.Chapple CL, Willis S, Frame J. Effective dose in paediatric computed tomography. Phys Med Biol 2002;47:107–15 [DOI] [PubMed] [Google Scholar]
- 30.van der Molen AJ, Geleijns J. Overranging in multisection CT: quantification and relative contribution to dose-comparison of four 16-section CT scanners. Radiology 2007;242:208–16 [DOI] [PubMed] [Google Scholar]
- 31.Lewis MA, Edyvean S. Patient dose reduction in CT. Br J Radiol 2005;78:880–3 [DOI] [PubMed] [Google Scholar]
- 32.International Commission on Radiological Protection The 2007 recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann ICRP 2007;37:1–332 [DOI] [PubMed] [Google Scholar]
- 33.Deak PD, Smal Y, Kalender WA. Multisection CT protocols: sex- and age-specific conversion factors used to determine effective dose from dose-length product. Radiology 2010;257:158–66 [DOI] [PubMed] [Google Scholar]
- 34.Alessio AM, Phillips GS. A pediatric CT dose and risk estimator. Pediatr Radiol 2010;40:1816–21 [DOI] [PubMed] [Google Scholar]
- 35.Heggie JC. Patient doses in multi-slice CT and the importance of optimisation. Australas Phys Eng Sci Med 2005;28:86–96 [DOI] [PubMed] [Google Scholar]
- 36.Hart D, Wall BF, Shrimpton PC, Bungay D, Dance DR. Reference doses and patient size in paediatric radiology. Report NRPB-R318 Chilton, UK: National Radiological Protection Board; 2000 [Google Scholar]
- 37.Haaga JR. Radiation dose management: weighing risk versus benefit. AJR Am J Roentgenol 2001;177:289–91 [DOI] [PubMed] [Google Scholar]
- 38.Boone JM, Geraghty EM, Seibert JA, Wootton-Gorges SL. Dose reduction in pediatric CT: a rational approach. Radiology 2003;228:352–60 [DOI] [PubMed] [Google Scholar]
- 39.Kleinman PL, Strauss KJ, Zurakowski D, Buckley KS, Taylor GA. Patient size measured on CT images as a function of age at a tertiary care children's hospital. AJR Am J Roentgenol 2010;194:1611–19 [DOI] [PubMed] [Google Scholar]
- 40.Huda W, Magill D, He W. CT effective dose per dose length product using ICRP 103 weighting factors. Med Phys 2011;38:1261–5 [DOI] [PubMed] [Google Scholar]
- 41.Christner JA, Kofler JM, McCollough CH. Estimating effective dose for CT using dose-length product compared with using organ doses: consequences of adopting International Commission on Radiological Protection publication 103 or dual-energy scanning. AJR Am J Roentgenol 2010;194:881–9 [DOI] [PubMed] [Google Scholar]
- 42. Impactscan.org [homepage on the internet]. London, UK: ImPACT; © ImPACT 1998–2008 [cited 18 November 2011]. Available from: http://www.impactscan.org/ctdosimetry.htm. [Google Scholar]