Abstract
Objective
To study the in vitro and in vivo (abdomen) variability of apparent diffusion coefficient (ADC) measurements at 1.5 T using a free-breathing multislice diffusion-weighted (DW) MRI sequence.
Methods
DW MRI images were obtained using a multislice spin-echo echo-planar imaging sequence with b-values=0, 100, 200, 500, 750 and 1000 s mm−2. A flood-field phantom was imaged at regular intervals over 100 days, and 10 times on the same day on 2 occasions. 10 healthy volunteers were imaged on two separate occasions. Mono-exponential ADC maps were fitted excluding b=0. Paired analysis was carried out on the liver, spleen, kidney and gallbladder using multiple regions of interest (ROIs) and volumes of interest (VOIs).
Results
The in vitro coefficient of variation was 1.3% over 100 days, and 0.5% and 1.0% for both the daily experiments. In vivo, there was no statistical difference in the group mean ADC value between visits for any organ. Using ROIs, the coefficient of reproducibility was 20.0% for the kidney, 21.0% for the gallbladder, 24.7% for the liver and 28.0% for the spleen. For VOIs, values fall to 7.7%, 6.4%, 8.6% and 9.6%, respectively.
Conclusion
Good in vitro repeatability of ADC measurements provided a sound basis for in vivo measurement. In vivo variability is higher and when considering single measurements in the abdomen as a whole, only changes in ADC value greater than 23.1% would be statistically significant using a two-dimensional ROI. This value is substantially lower (7.9%) if large three-dimensional VOIs are considered.
Diffusion-weighted (DW) MRI is based on the Brownian motion of water in biological tissues [1,2]. The technique has played a preponderant role in neuro-imaging over the last two decades and it is known to detect small changes before they are apparent on anatomical imaging [3,4].
In recent years DW MRI has been increasingly used in other parts of the body, demonstrating great diagnostic potential in cancer imaging. To date, DW MRI has been successfully used for tissue characterisation and tumour staging. However, the apparent diffusion coefficient (ADC) is a potential biomarker that could be used to monitor treatment response or evaluate post-therapeutic changes. Details of the clinical use of DW MRI can be found in the 2009 consensus paper [5] or in general and organ-specific review articles [6-8].
While DW MRI is a potentially powerful tool in diagnostic oncology, the lack of uniform protocols for imaging and data analysis hinder its clinical implementation. Large differences in ADC values are reported in the literature depending on the acquisition parameters, in particular the choice of b-values (e.g. see [9] for ADC values in the kidney or Table 1 for the liver). The 2009 consensus and recommendation paper [5] highlighted the importance of quality analysis, validation and reproducibility studies. Although there are some emerging reproducibility and repeatability data in the abdomen [15,19-22], a recent review by Taouli and Koh [7] highlights the need for further work in this area. Recently, coefficients of variability of around 14% were published for both solid tumours [22] and bone marrow [23]. Other studies seem to indicate that only ADC changes of over 27% [20] or 30% [21] are significant. Substantial variations in ADC values have also been found between different scanners and vendors [24-26], further highlighting the difficulty of setting up multicentre trials.
Table 1. Apparent diffusion coefficient values measured in normal liver at 1.5 T.
| Reference | Mean ADC (10−3 mm2 s−1) | Standard deviation | Range | Number of subjects | b-values (s mm−2) | Comments |
| Taouli et al [10] | 1.60 | 0.13 | 1.44–1.88 | 10 v | 0, 500 | Conventional |
| 1.52 | 0.15 | 1.28–180 | With parallel imaging | |||
| 1.51 | 0.21 | 1.27–1.99 | Diffusion tensor/parallel imaging | |||
| Mürtz et al [11] | 0.92–0.96a | 0.09–0.14 | 0.62–1.20 | 12 v | 50, 300, 700, 1000, 1300 | Pulse triggered |
| 1.03–1.14 | 0.22–0.40 | 0.67–2.57 | Non-triggered | |||
| Kim et al [12] | 1.05/1.02b | 0.30/0.25 | 6 v/126 p | 3, 57, 192, 408, 517, 850 | ||
| 1.55/1.16 | 0.37/0.42 | 3, 57, 192, 408, 192, 408 | ||||
| 4.8/3.55 | 2.37/1.75 | 3, 57 | ||||
| Ichikawa et al [13] | 2.28 | 1.23 | 46 p | 1.6, 55 | ||
| Taouli et al [14] | 1.83 | 0.36 | 1.4–2.55 | 66 p | 0, 500 | |
| 1.51 | 0.49 | 1.12–2.71 | 0, 134, 267, 400 | |||
| Kwee et al [15] | 1.60/1.62/1.57c | 0.14/0.18/0.15 | 11 v | 0, 500 | Breath-hold | |
| 2.13/2.27/2.07 | 0.33/0.47/0.43 | Respiratory triggered | ||||
| 1.65/1.62/1.65 | 0.09/0.16/0.17 | Free breathing (7 mm slice) | ||||
| 1.64/1.66/1.57 | 0.13/0.11/0.19 | Free breathing (5 mm slice) | ||||
| Yamada et al [16] | 0.87 | 0.26 | 78 p | 30, 300, 900,1100 | ADC | |
| 0.76 | 0.27 | Diffusion coefficient (DC) | ||||
| Müller et al [17] | 1.39 | 0.16 | 10 v+9 p | 8 b-values; bmax 328–454 | ||
| Namimato et al [18] | 0.69 | 0.31 | 51 p | 30, 1200 | ||
| This study | 1.04 | 0.05 | 0.95–1.11 | 10 v | 100, 200, 500, 750, 1000 | Free breathing |
ADC, apparent diffusion coefficient; p, patients; v, volunteers.
In studies including patients, only ADC values relating to measurements performed in normal liver are quoted here.
aValue range for 3 directions.
bVolunteers/patients.
cEach sequence repeated three times.
In preparation for a study on renal cell carcinoma at our centre, we required information on the variability of a free-breathing multislice DW MRI sequence. As these tumours are relatively large and heterogeneous, we were particularly interested in the variability of both large volumes on multiple slices and smaller regions on individual images.
Methods and materials
Study population
After giving informed consent, 10 healthy volunteers (7 females, 3 males; age 32.3±4.6 years, range 26–42 years) were imaged, in accordance with local ethics regulations, on two occasions (second visit 5.8±1.9 days later, range 5–11 days).
MRI diffusion sequence and apparent diffusion coefficient calculation
Scans were performed on a 1.5 T Achieva system (Philips Medical Systems, Best, The Netherlands) in conjunction with a four-element body coil array. DW MRI images were obtained using a free-breathing multislice spin-echo echo-planar imaging (EPI) sequence: repetition time (TR) 5300–5800 ms, echo time (TE) 62 ms, EPI factor 60, three averages, field-of-view (FOV) 400–450 mm, rectangular FOV 75%, matrix 112×256, 28–35 slices to cover the abdomen from the diaphragm to the iliac crest, slice thickness 6 mm, slice gap 1 mm. Six motion probing gradients with b-values of 0, 100, 200, 500, 750 and 1000 s mm−2 were applied in three orthogonal directions and trace images were synthesised for each b-value using the mean of three orthogonal directions. ADC maps were calculated on a pixel-by-pixel basis using a mono-exponential fit, and b=0 was excluded from the calculation in order to eliminate perfusion effects. Two analyses were run, one on volumes of interest (VOIs) and one on multiple smaller regions of interest (ROIs). VOI analysis was performed for the following organs: entire spleen, entire gallbladder, kidney (renal parenchyma) and liver (part of the right hepatic lobe, approximately segments V and VI). For the ROI analysis circular ROIs (area 4 cm2, or 1–2 cm2 for the gallbladder) were placed on different slices (i.e. never two ROIs in the same organ on the same slice). The numbers of ROIs used were: liver, 5; kidney, 6 (3 in each); spleen, 3; gallbladder, 2. The ROIs were then copied to the nearest corresponding anatomical position on the second visit images. For both methods, data analysis was carried out on matched pairs for each organ to investigate ADC change between visits and calculate the coefficient of repeatability expressed as a percentage of the mean.
The intra- and interobserver variability was assessed for the VOI analysis on the dataset from the first visit by analysing the difference between matched pairs of the results obtained by one reader on two occasions and two independent readers, respectively.
Phantom imaging
A large (5 l) copper sulphate solution ([CuSo4]=3×10−3 mol l−1, [NaCl]=3.4×10−2 mol l−1) phantom was imaged 10 times on two different occasions, and also at regular intervals over a period of 3 months. To minimise the effect of temperature, the phantom was stored in the scanner room and was imaged only when the room temperature was 17±0.5 °C. For multiple image acquisitions on the same occasion, the phantom was removed from the scanner and repositioned between each acquisition.
The sequence was identical to the sequence described above for in vivo imaging but used a reduced number of slices (20). For analysis, a circular ROI covering 90% of the cross-section of the bottle was selected on each slice. The coefficient of variation (CV; %) was calculated as CV=(SD/mean)×100 (where SD is the standard deviation of the mean) for the daily repeat experiments and over the 3-month period. CV was also calculated for each slice and each sample.
Results
Phantom
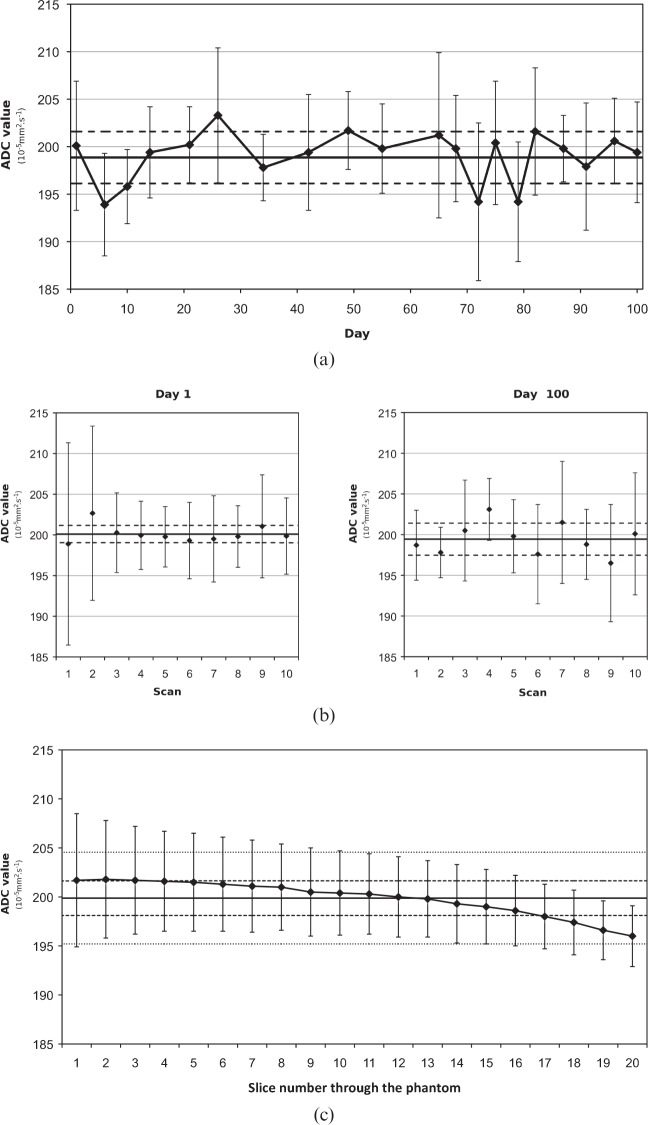
The ADC values were stable over the studied period (Figure 1) and no significant image artefact was observed. On the first day, the mean ADC of the phantom was 200.1±1.0×10−5 mm2 s−1 with a CV of 0.5% (10 measurements). On day 100, the mean ADC of the phantom was 199.4±2.0×10−5 mm2 s−1 with a CV of 1.0% (10 measurements). The mean ADC over the 3-month period was 199.0±2.6×10−5 mm2 s−1 with a CV of 1.3%. The mean intraslice CV was 3.2±1.4% (range 1.0–6.9%). The mean sample CV was 2.9±1.0% (range 1.5–6.3%). A small gradient in ADC value was always observed between one end of the phantom and the other along the z-axis of the scanner; an example measurement is displayed in Figure 1c).
Figure 1.
In vitro apparent diffusion coefficient (ADC) measurements. (a) Variability over 100 days with horizontal lines showing the mean (solid) and standard deviation (dashed) over the period. (b) Daily repeats with horizontal lines showing the mean and standard deviation of the 10 scans. (c) Example of phantom ADC measurements where points are the mean ADC values for each slice, the solid line is the mean for the whole phantom, the dashed lines show the standard deviation for the slices and dotted lines show the standard deviation of all pixels.
In vivo study
Both inter- and intraobserver variability were small. For intraobserver variability, the mean difference between pairs was −0.1±1.5% and for interobserver it was −0.2±2.5%, leading to coefficients of repeatability of 3.0% and 4.8%, respectively.
No statistical difference was found between the two visits using a paired t-test for the two types of analysis.
The ADC values obtained for the liver, spleen, kidney and gallbladder using the VOI method are displayed in Table 2. The mean volumes of the VOIs were gallbladder 18±5, liver 331±93, kidney 148±27 and spleen 168±47 cm3. The mean change in ADC value (expressed as a percentage to facilitate comparison between organs) between the two visits for all organs and the two types of analyses (VOI and multiple ROIs) was always small (between −1.5% and +1.0%; Table 3).
Table 2. Mean apparent diffusion coefficient (10−3 mm2 s−1) values, standard deviation and range of the different organs calculated using the volume of interest method.
| Organ | Visit 1 |
Visit 2 |
p-value | ||||
| ADC | SD | Range | ADC | SD | Range | ||
| Liver | 1.05 | 0.04 | 0.95–1.09 | 1.06 | 0.07 | 0.96–1.18 | 0.56 |
| Kidney | 1.76 | 0.08 | 1.65–1.91 | 1.76 | 0.10 | 1.63–1.93 | 0.85 |
| Spleen | 0.81 | 0.06 | 0.72–0.91 | 0.82 | 0.07 | 0.70–0.91 | 0.86 |
| Gallbladder | 2.93 | 0.20 | 2.65–3.36 | 2.91 | 0.21 | 2.60–3.39 | 0.67 |
ADC, apparent diffusion coefficient; SD, standard deviation.
No significant difference was found between visits for any of the organs (p-value for a two-tailed matched-pair t-test).
Table 3. Paired analysis of the difference in apparent diffusion coefficient value between the two visits for the different organs.
| Organ | Volumes of interest analysis |
Regions of interest analysis |
||||||||||
| Mean of difference (10−6 mm2 s−1) | SD (10−6 mm2 s−1) | Mean of % change | SD (%) | r (%) | p-value | Mean of difference (10−6 mm2 s−1) | SD (10−6 mm2 s−1) | Mean of % change | SD (%) | r (%) | p-value | |
| Liver | 9 | 47 | 0.85 | 4.4 | 8.6 | 0.56 | −5 | 125 | 0.82 | 12.6 | 24.7 | 0.79 |
| Kidney | −3 | 70 | −0.14 | 4.0 | 7.7 | 0.85 | 3.5 | 178 | 0.6 | 10.2 | 20.0 | 0.88 |
| Spleen | 2 | 40 | 0.28 | 4.9 | 9.6 | 0.86 | −19 | 117 | −1.3 | 14.3 | 28.0 | 0.39 |
| Gallbladder | −13 | 95 | −0.4 | 3.2 | 6.4 | 0.67 | −40 | 290 | −0.70 | 10.7 | 21.0 | 0.55 |
ADC, apparent diffusion coefficient; SD, standard deviation.
r=coefficient of reproducibility, p-value for two-tailed matched-pair t-test.
The standard deviation of the mean change and the coefficient of reproducibility (r=1.96×SD; %) are also given in Table 3.
Discussion
Phantom
The in vitro reproducibility of the ADC measurement was good, with a daily CV of less than 1% and a CV of less than 1.5% over 3 months. Our results compare favourably with those published by Delakis et al [27]. Although not stated, the CV for their more dilute CuSO4 phantom can be calculated to be 2.3% (b=0 and b=500) and 2.5% (b=0 and b=1000), whereas the CVs for their sucrose solution are 3.6% and 3.5%, respectively. Delakis et al [27] retrospectively normalised their results to a reference temperature (21 °C), which may have contributed to the higher CV. Recently, Pierpaoli et al [28] reported a CV of 2% for a polyvinylpyrrolidone phantom and Chenevert et al [25,26] reported a reproducibility coefficient of 3–5% for iced water phantoms. The latter also reported a spatial dependence along the z-axis [25].
In vivo apparent diffusion coefficient measurement
ADC values reported in the literature vary substantially but the values reported here are similar to those quoted by other authors using comparable protocols, in particular the b-values. For the liver, reported values range from 0.69 to 4.8×10−3 mm2 s−1 (Table 1). However, our value of 1.04±0.05×10−3 mm2 s−1 is very similar to volunteer studies that have used a range of b-values that exclude 0 and include high values, in particular Mürtz et al [11] (between 1.03±0.22 and 1.14±0.40×10−3 mm2 s−1) and Kim et al [12] (1.05±0.30×10−3 mm2 s−1).
Diffusion in the kidneys was extensively reviewed by Zhang et al [9], who reported a large variability in published ADC values, ranging from 1.64±0.09 to 4.07±0.74×10−3 mm2 s−1. Once more, the choice of b-values has a considerable influence on the resulting ADC values, and studies comparable with ours (encompassing the whole renal parenchyma and including high b-values >700) all measured ADC values lower than 2×10−3 mm2 s−1 (range 1.64–1.92×10−3 mm2 s−1), similar to those found here (1.76±0.08×10−3 mm2 s−1). ADC values for the gallbladder and the spleen are also comparable with those reported in the literature [11,16-18].
Apparent diffusion coefficient repeatability
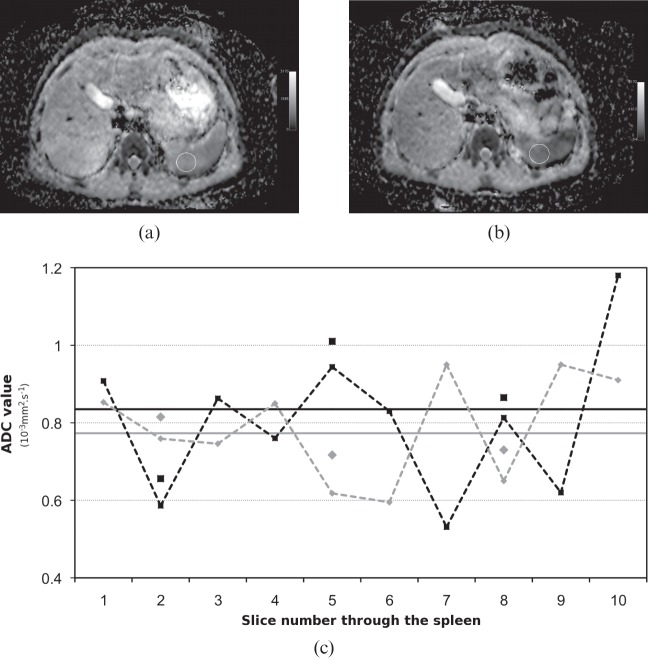
For the VOI analysis, the standard deviation of the ADC value in the spleen, liver and kidney was 4–5%, and slightly lower in the gallbladder (Table 2). Only changes greater than the coefficient of reproducibility would be statistically significant. This implies that only changes in mean ADC value over the organ greater than 6.4% for the gallbladder, 7.7% for the kidney, 8.6% for the liver and 9.6% for the spleen would be statistically significant (>1.96×SD). For multiple two-dimensional ROIs, the standard deviation of the mean of the percentage difference between visits was much higher at over 10% in all organs. This implies that, for a small ROI, only changes >20.0% for the kidney, 21.0% for the gallbladder, 24.7% for the liver and 28.0% for the spleen would be statistically significant. For multislice acquisitions, such as those used here, variations in ADC values between slices are often observed within an organ. These ADC variations are less likely to affect three-dimensional volumes as any differences between (and within) slices are likely to be averaged over the large VOI. An example for the spleen is shown in Figure 2. However, when using smaller two-dimensional ROIs, less in-slice averaging and no interslice averaging is present, which explains why the values required for statistical significance are higher for ROI analysis. Fluctuations in ADC values between slices were more noticeable in the spleen, thus explaining its higher (28%) coefficient of reproducibility. If we were to consider all organs indiscriminately, only changes greater than 7.9% would be significant when considering VOIs and greater than 23.1% when considering ROIs.
Figure 2.
Example of apparent diffusion coefficient (ADC) measurements through the spleen. (a) ADC map of slice 5 through the spleen, showing a region of interest (ROI) used for analysis. (b) Anatomically equivalent slice on the repeat visit. (c) ADC values through the spleen (black, visit 1; grey; visit 2). Solid line, mean of the entire organ (volume of interest analysis); dashed lines, mean ADC value for the spleen in this slice; individual points, mean ADC value for the ROI.
Although the coefficients of reproducibility for the ROI analysis are larger than those for the VOI analysis, they are in line with published literature. Kim et al [21] suggested that changes of less than 30% fall within measurement error for hepatic tumours, while Braithwaite et al [20] consider that treatment changes of less than 27% for abdominal ADC values at 3 T “will not be clinically detectable with confidence with one acquisition in a single individual”. In contrast, Messiou et al [23] measured a coefficient of reproducibility of 14.8% in bone marrow. This could be due to reduced patient motion (respiratory and/or peristaltic) in the regions analysed (L5 vertebral body and left iliac bone). Very good reproducibility (4.8%) was recently presented in metastatic and ovarian peritoneal tumours [29]. It was, however, somewhat mitigated by a high inter- and intra-observer variability (11.4% and 13.7%, respectively).
Koh et al [22] reported a coefficient of reproducibility of approximately 14% for solid tumours using a volume analysis. Although this is greater than our average values, it is nonetheless consistent; the volumes analysed in their study were on average smaller than the ones used here (apart from the gallbladder) and varied considerably in size (106±103 cm3, range 10.0–400.9 cm3). Both the size and the position of lesions are known to influence the reproducibility, with larger regions being more reproducible [21].
Conclusion
In summary, the in vitro repeatability of ADC measurements was good, with a coefficient of variation of <1.5%. In vivo variability was much higher and, if considering a single ROI measurement in the abdomen, only changes greater than 23.1% would be statistically significant. This value is substantially lower (7.9%) if volumes are considered.
Footnotes
MEM is partly funded (20%) by the UK National Institute for Health Research.
References
- 1.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168:497–505 [DOI] [PubMed] [Google Scholar]
- 2.Le Bihan D. Molecular diffusion nuclear magnetic resonance imaging. Magn Reson Q 1991;7:1–30 [PubMed] [Google Scholar]
- 3.Yoshikawa K, Nakata Y, Yamada K, Nakagawa M. Early pathological changes in the parkinsonian brain demonstrated by diffusion tensor MRI. J Neurol Neurosurg Psychiatry 2004;75:481–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston KC, Wagner DP, Wang XQ, Newman GC, Thijs V, Sen S, et al. Validation of an acute ischemic stroke model: does diffusion-weighted imaging lesion volume offer a clinically significant improvement in prediction of outcome? Stroke 2007;38:1820–5 [DOI] [PubMed] [Google Scholar]
- 5.Padhani AR, Liu G, Mu-Koh D, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 2009;11:102–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh D-M, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in Oncology. AJR Am J Roentgenol 2007;188:1622–35 [DOI] [PubMed] [Google Scholar]
- 7.Taouli B, Koh D-M. Diffusion-weighted MR imaging of the liver. Radiology 2010;254:47–66 [DOI] [PubMed] [Google Scholar]
- 8.Sugita R, Ito K, Fujita N, Takahashi S. Diffusion-weighted MRI in abdominal oncology: clinical applications. World J Gastroenterol 2010;21:832–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang JL, Sigmund EE, Chandarana H, Rusinek H, Chen Q, Vivier P-H , et al. Variability of renal apparent diffusion coefficients: limitations of the mono-exponential model for diffusion quantification. Radiology 2010;254:783–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taouli B, Martin AJ, Qayyum A, Merriman RB, Vigneron D, Yeh BM, Coakley FV. Parallel imaging and diffusion tensor imaging for diffusion-weighted MRI of the liver: preliminary experience in healthy volunteers. AJR Am J Roentgenol 2004;183:677–80 [DOI] [PubMed] [Google Scholar]
- 11.Mürtz P, Flacke S, Traber F, van denBrink JS, Gieseke J, Schild HH. Abdomen: diffusion weighted MR imaging with pulse-triggered single-shot sequences. Radiology 2002;224:258–64 [DOI] [PubMed] [Google Scholar]
- 12.Kim T, Murakami T, Takahashi S, Hori M, Tsuda K, Nakamura H. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am J Roentgenol 1999;173:393–8 [DOI] [PubMed] [Google Scholar]
- 13.Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with a single-shot echoplanar sequence: detection and characterization of focal hepatic lesions. AJR Am J Roentgenol 1998;170:397–402 [DOI] [PubMed] [Google Scholar]
- 14.Taouli B, Vilgrain V, Dumont E, Daire JL, Fan B, Menu Y. Evaluation of liver diffusion isotropy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences: prospective study in 66 patients. Radiology 2003;226:71–8 [DOI] [PubMed] [Google Scholar]
- 15.Kwee TC, Takahara T, Koh D-M , Nievelstein RA, Luijten PR. Comparison and reproducibility of ADC measurements in breathhold, respiratory triggered, and free-breathing diffusion-weighted MR imaging of the liver. J Magn Reson Imaging 2008;28:1141–8 [DOI] [PubMed] [Google Scholar]
- 16.Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H. Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology 1999;210:617–23 [DOI] [PubMed] [Google Scholar]
- 17.Müller MF, Prasad P, Siewert B, Nissenbaum MA, Raptopoulos V, Edelman RR. Abdominal diffusion mapping with use of a whole-body echo-planar system. Radiology 1994;190:475–8 [DOI] [PubMed] [Google Scholar]
- 18.Namimoto T, Yamashita Y, Sumi S, Tang Y, Takahashi M. Focal liver masses: characterization with diffusion-weighted echo-planar MR imaging. Radiology 1997;204:739–44 [DOI] [PubMed] [Google Scholar]
- 19.Damasio MB, Tagliafico A, Capaccio E, Cancelli C, Perrone N, Tomolillo C, et al. Diffusion-weighted MRI sequences (DW-MRI) of the kidney: normal findings, influence of hydration state and repeatability of results. Radiol Med 2008;113:214–24 [DOI] [PubMed] [Google Scholar]
- 20.Braithwaite AC, Dale BM, Boll DT, Merkle EM. Short- and midterm reproducibility of apparent diffusion coefficient measurements at 3.0-T diffusion-weighted imaging of the abdomen. Radiology 2009;250:459–65 [DOI] [PubMed] [Google Scholar]
- 21.Kim SY, Lee SS, Byun JH, Park SH, Kim JK, Park B, et al. Malignant hepatic tumors: short-term reproducibility of apparent diffusion coefficients with breath-hold and respiratory-triggered diffusion weighted MR imaging. Radiology 2010;255:815–23 [DOI] [PubMed] [Google Scholar]
- 22.Koh D-M , Blackledge M, Collins DJ, Phadani AR, Wallace T, Wilton B, et al. Reproducibility and changes in the apparent diffusion coefficients of solid tumours treated with combretastatin A4 phosphate and bevacizumab in a two-centre phase I clinical trial. Eur Radiol 2009;19:2728–38 [DOI] [PubMed] [Google Scholar]
- 23.Messiou C, Collins DJ, Morgan VA, deSouza NM. Optimising diffusion weighted MRI for imaging metastatic and myeloma bone disease and assessing reproducibility. Eur Radiol 2011;21:1713–18 [DOI] [PubMed] [Google Scholar]
- 24.Sasaki M, Yamada K, Watanabe Y, Matsui M, Ida M, Fujiwara S, et al. Variability in absolute apparent diffusion coefficient values across different platforms may be substantial: a multivendor, multi-institutional comparison study. Radiology 2008;249:624–30 [DOI] [PubMed] [Google Scholar]
- 25.Chenevert TL, Galbán CJ, Londy FJ, Meyer CR, Johnson TD, Rehemtulla A, et al. Icewater for quality control of diffusion measurements in multi-center trials. Proc Intl Soc Magn Reson Med 2011;19:844 [Google Scholar]
- 26.Chenevert TL, Galbán CJ, Ivancevic MK, Rohrer SE, Londy FJ, Kwee TC, et al. Diffusion coefficient measurement using a temperature-controlled fluid for quality control in multicenter studies. J Magn Reson Imaging 2011;34:983–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delakis I, Moore EM, Leach MO, De Wilde JP. Developing a quality control protocol for diffusion imaging on a clinical MRI system. Phys Med Biol 2004;49:1409–22 [DOI] [PubMed] [Google Scholar]
- 28.Pierpaoli C, Sarlls J, Nevo U, Basser PJ, Horkay F. Polyvinylpyrrolidone (PVP) water solutions as isotropic phantoms for diffusion MRI studies. Proc Intl Soc Magn Reson Med 2009;17:1414 [Google Scholar]
- 29.Kyriazi S, Collins DJ, Messiou C, Pennert K, Davidson RL, Giles SL, et al. Metastatic ovarian and primary peritoneal cancer: assessing chemotherapy response with diffusion-weighted MR—value of histogram analysis of apparent diffusion coefficients. Radiology 2011;261:182–9 [DOI] [PubMed] [Google Scholar]