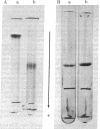
Abstract
Inherited deficiency of phosphoglycerate kinase (PGK; ATP:3-phosphoglycerate 1-phosphotransferase, EC 2.7.2.3) is associated with chronic nonspherocytic hemolytic anemia and mental disorders in man. One such variant, PGK-Uppsala, was purified to homogeneity. PGK-Uppsala had a lower-than-normal specific activity (30% of normal in the backward reaction and about 20% of normal in the forward reaction) and higher-than-normal Michaelis constants for ATP, ADP, 3-phosphoglycerate and 1,3-diphosphoglycerate. Peptide mapping analysis revealed that the structural abnormality of PGK-Uppsala is a single amino acid substitution from arginine to proline at the 206th position. Based on the known complete amino acid sequence of the normal human PGK and the three-dimensional model deduced from horse PGK, correlations between the structural and functional abnormalities of PGK-Uppsala are discussed. Structural abnormalities of PGK-II, which is an electrophoretic variant not associated with enzyme deficiency, and PGK-München, which is associated with enzyme deficiency and heat instability but not associated with hemolytic anemia, are also discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks R. D., Blake C. C., Evans P. R., Haser R., Rice D. W., Hardy G. W., Merrett M., Phillips A. W. Sequence, structure and activity of phosphoglycerate kinase: a possible hinge-bending enzyme. Nature. 1979 Jun 28;279(5716):773–777. doi: 10.1038/279773a0. [DOI] [PubMed] [Google Scholar]
- Chen S. H., Malcolm L. A., Yoshida A., Giblett E. R. Phosphoglycerate kinase: an X-linked polymorphism in man. Am J Hum Genet. 1971 Jan;23(1):87–91. [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fujii H., Krietsch W. K., Yoshida A. A single amino acid substitution (Asp leads to Asn) in a phosphoglycerate kinase variant (PGK München) associated with enzyme deficiency. J Biol Chem. 1980 Jul 10;255(13):6421–6423. [PubMed] [Google Scholar]
- Huang I. Y., Rubinfien E., Yoshida A. Complete amino acid sequence of human phosphoglycerate kinase. Isolation and amino acid sequence of tryptic peptides. J Biol Chem. 1980 Jul 10;255(13):6408–6411. [PubMed] [Google Scholar]
- Huang I. Y., Welch C. D., Yoshida A. Complete amino acid sequence of human phosphoglycerate kinase. Cyanogen bromide peptides and complete amino acid sequence. J Biol Chem. 1980 Jul 10;255(13):6412–6420. [PubMed] [Google Scholar]
- Krietsch W. K., Eber S. W., Haas B., Ruppelt W., Kuntz G. W. Characterization of a phosphoglycerate kinase deficiency variants not associated with hemolytic anemia. Am J Hum Genet. 1980 May;32(3):364–373. [PMC free article] [PubMed] [Google Scholar]
- Kuntz G. W., Eber S., Kessler W., Krietsch H., Krietsch W. K. Isolation of phosphoglycerate kinases by affinity chromatography. Eur J Biochem. 1978 Apr 17;85(2):493–501. doi: 10.1111/j.1432-1033.1978.tb12265.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Yoshida A. Micro-scale peptide mapping method for identification of variant proteins. Biochem Genet. 1971 Dec;5(6):541–547. doi: 10.1007/BF00485672. [DOI] [PubMed] [Google Scholar]
- Yoshida A. Human phosphoglycerate kinase. Methods Enzymol. 1975;42:144–148. doi: 10.1016/0076-6879(75)42108-5. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Watanabe S., Chen S. H., Giblet E. R., Malcolm L. A. Human phosphoglycerate kinase. II. Structure of a variant enzyme. J Biol Chem. 1972 Jan 25;247(2):446–449. [PubMed] [Google Scholar]
- Yoshida A., Watanabe S. Human phosphoglycerate kinase. I. Crystallization and characterization of normal enzyme. J Biol Chem. 1972 Jan 25;247(2):440–445. [PubMed] [Google Scholar]