Abstract
Objectives
The objective of this study was to determine the role of diffusion-weighted imaging (DWI) in uterine artery embolisation (UAE), and to assess the apparent diffusion coefficient (ADC) of the dominant fibroid and its relationship to contrast enhancement and fibroid volume reduction.
Methods
We carried out a retrospective study of 15 patients who underwent UAE. Calculations were performed at baseline and 6 months post-embolisation. Fibroid ADC (expressed in 10−3 mm2 s−1) was calculated using b=0 and b=1000 DWI values. Fibroid enhancement was compared with background myometrium by measuring signal-difference-to-noise ratio (SDNR). Fibroid volume was calculated using a prolate ellipse formula.
Results
There was a significant reduction (p<0.001) in fibroid ADC at 6 months (0.48; standard deviation, SD=0.26) as compared with baseline (1.01; SD=0.39). No significant change (p=0.07) was identified in 6-month myometrial ADC (1.09; SD=0.28) as compared with baseline (1.24; SD=0.20). Moderately strong and significant positive correlation was identified between baseline ADC and 6-month percentage volume reduction of the fibroid (correlation=0.66, p=0.007). No correlation was identified between SDNR and ADC at baseline or 6 months (r=0.01, p=0.97 and r=−0.13, p=0.64, respectively) or SDNR and percentage volume reduction at 6 months (correlation r=0.18, p=0.51).
Conclusion
Baseline ADC of dominant fibroids shows a moderately strong correlation with subsequent volume reduction at 6 months following UAE. No correlation was identified between ADC values and contrast enhancement on the baseline or 6-month scans. Further prospective evaluation is needed before DWI can be utilised in clinical practice.
Advances in knowledge
DWI imaging may provide additional information about UAE and possibly help to predict uterine volume reduction.
Uterine fibroids are the most common tumours of the female reproductive system [1] and can be associated with symptoms such as pain, menorrhagia and impaired fertility [2].
Uterine artery embolisation (UAE) is a minimally invasive technique for treatment of fibroids in patients wishing to avoid surgery. It also potentially preserves fertility. The procedure involves injecting an embolic agent into both uterine arteries, thereby producing ischaemic necrosis of the fibroids. The necrotic fibroid mass undergoes shrinkage over the subsequent few months [3].
As a part of treatment planning and follow-up, contrast-enhanced MRI (CEMRI) scans are now routinely performed in most centres. CEMRI scans are useful to evaluate size, location, number and vascularity of the fibroids, and also to exclude other pelvic pathology, which may be best treated by other methods (e.g. adenomyosis). CEMRI post-embolisation (at approximately 6 months) helps evaluate response to treatment by measuring the degree of fibroid infarction and volume reduction.
Diffusion-weighted imaging (DWI) is a technique for characterising the motion of water within different tissues within the body [4]. Movement of water molecules in biological tissue is restricted because their motion is limited by interaction with cell membranes and molecules. Motion of water molecules is more restricted in tissues with a high cellular density and intact cell membranes than in a less cellular environment, where there is greater mobility of water molecules.
Described by Stejskal and Tanner [5], DWI involves a modified spin-echo T2 sequence, to which a symmetrical pair of diffusion sensitising gradients is applied around the 180° refocusing pulse. Static molecules obtain phase information from the first diffusion gradient, but this information will be rephased by the second gradient without a change in the measured signal intensity. Mobile water molecules acquire different phase information from the first gradient, but, because of their motion, their signal will not be completely rephased by the second gradient, leading to a signal loss. Hence, the motion of water molecules is detected as attenuation of the measured signal intensity on DWI.
The sensitivity of the DWI sequence to the movement of water molecules can be varied by changing the gradient amplitude, the duration of the applied gradient and the time interval between the paired gradients. Changing the parameter known as the “b” value easily varies this “diffusion sensitivity”, which is proportional to these three factors [6].
To quantitatively characterise the DWI images, they are acquired with least two different b-values. The slope of a logarithmic plot between the b-values on the x-axis and the relative signal intensity on the y-axis gives us the apparent diffusion coefficient (ADC) value in units of mm2 s−1. More intuitively, a low ADC value indicates low diffusion.
Although DWI is a well-established technique in cerebral imaging [7,8], its application to body imaging is relatively new. However, it is now gaining clinical acceptance in imaging pelvic malignancies and fibroids [9].
There has been very little work investigating what role (if any) is played by DWI over and above the standard MRI sequences in UAE. Some early work has suggested that DWI can serve as an index of measuring changes after embolisation [10] and that it may help predict volume reduction [11]. The aim of this feasibility study was to assess whether using DWI offered any additional information over and above standard MRI sequences.
Methods and materials
DWI sequences were added to the standard fibroid MRI imaging protocol in 2009. A retrospective analysis of the initial 18 patients in the first year was made in 2011. 3 patients were excluded owing to suboptimal images, leaving 15 for analysis. In all three excluded cases, there was significant motion artefact preventing image subtraction and impeding accurate selection of regions of interest ROIs for calculation of ADC values.
Two experienced operators performed UAE, and a standard technique was employed using polyvinyl alcohol particles (500–700 µm; Cook Medical, Bloomington, IN), embolising both uterine arteries to complete stasis.
Imaging protocol
MRI scans were obtained at baseline (pre-UAE) and usually at around 6 months post UAE. Imaging was carried out on a 1.5 T Signa HDxt (GE Healthcare, Waukesha, WI) MRI system using the following sequences: sagittal and coronal T2 weighted fast spin-echo images, three separate coronal oblique diffusion-weighted imaging sequences with b-values of 0 and 1000 s mm−2 and sagittal three-dimensional liver acquisition with volume acquisition images pre- and post-contrast using a bolus track technique, thereby achieving maximal contrast opacification.
Image analysis
All analysis was carried out on a GE workstation (ADW 4.0; GE Healthcare). Only the dominant fibroid was evaluated in each patient. The following variables were assessed pre- and post-UAE: fibroid volume, degree of relative contrast enhancement compared with the uterus and ADC measurement. In addition, the ADC value for the myometrium was also calculated.
Analysis of diffusion-weighted images
Two radiologists and an MRI physicist undertook imaging review. Independent evaluation was undertaken in all cases, with a consensus opinion in case of disagreement. The b=0 and b=1000 diffusion images were analysed using FuncTool v. 4.4.05 software (GE Healthcare) on the GE workstation and used to generate a corresponding ADC map. To calculate the ADC value, an ROI was drawn encompassing the dominant fibroid, but taking care to avoid the myometrium (to avoid any partial volume averaging), and an ADC value was calculated. From the same data set, ADC values were also calculated for the myometrium (Figure 1). The ROI for this was used at the same location on the pre- and post-embolisation scans. The ROI varied between 10 and 18 mm, depending on myometrial thickness.
Figure 1.
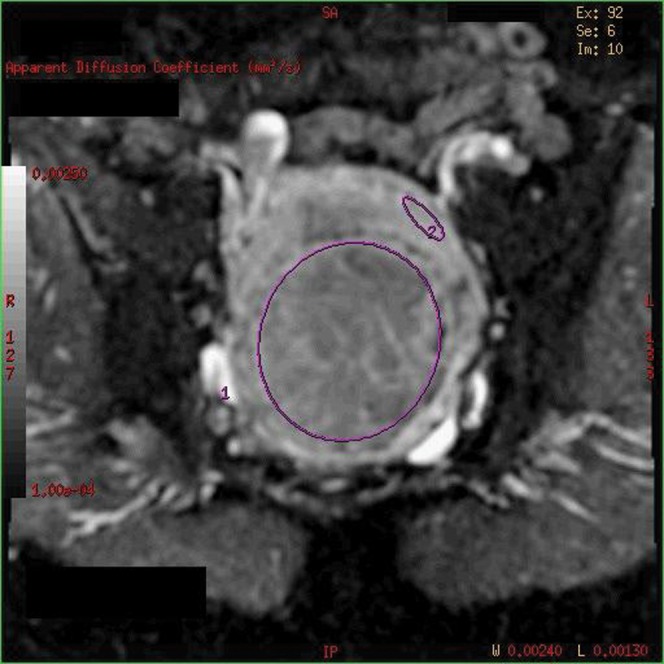
b=1000 diffusion-weighted image showing regions of interest on the fibroid and myometrium for calculation of ADC values.
Analysis of contrast enhancement
The signal-difference-to-noise ratio (SDNR) technique advocated by Pijl et al [12] was used to objectively measure contrast enhancement. The pre-contrast image was subtracted from the post-contrast image to exclude background signal from tissue. An ROI was then drawn on the subtracted image encompassing the dominant fibroid and the average signal intensity on a single slice was recorded, showing the maximum volume of the fibroid. ROIs were also drawn on the myometrium and extracorporeal air on the subtracted images. This was repeated for each of the two studies (i.e. pre and post UAE) and the SDNR values were calculated as follows:
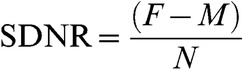 |
where F is the signal intensity of the fibroid, M is the signal intensity of the myometrium and N is the signal intensity of the extracorporeal air [7]. This value provides a measure of the degree of enhancement of the fibroid relative to the myometrium. The pre-embolisation fibroid usually demonstrates lower signal intensity than the myometrium and is expected to show a significant reduction in signal post-embolisation as compared with the myometrium. Negative values were therefore expected at the time of calculation. Figure 2 demonstrates the technique of SDNR measurement.
Figure 2.
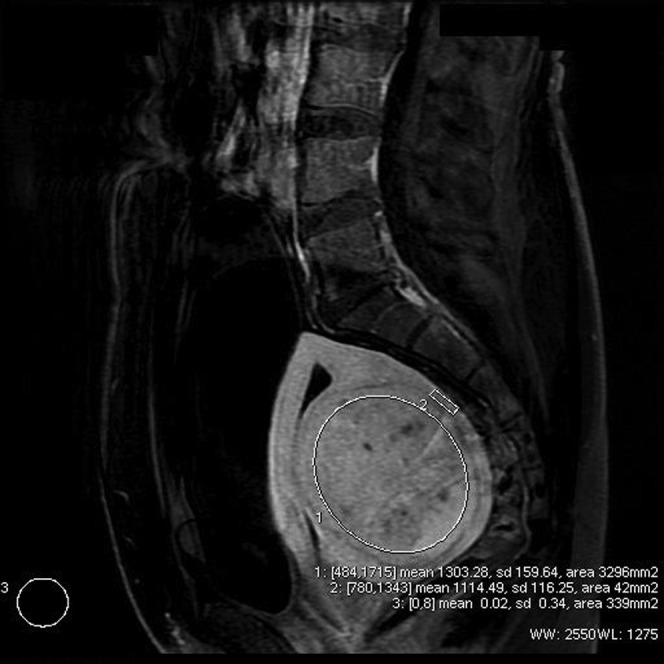
Subtracted image showing region of interest for calculation of signal-difference-to-noise ratio values.
Volume measurements
The dimensions of the fibroids were measured along the anteroposterior (AP), craniocaudal (CC) and the transverse long axis (TR). The volume of the dominant fibroid was calculated using a standard prolate ellipse formula (AP×CC×TR×0.5233) [13,14].
Statistical analysis
All patient demographic data is presented as mean and standard deviation (SD). Pearson correlation was used to determine the strength of the relationship between pairs of variables, including ADC values, SDNR and percentage volume reduction, and calculated pre and post embolisation.
A p-value <0.05 was taken to indicate a significant relationship between two variables. Paired t-tests were used to compare mean ADC levels pre and post embolisation.
Results
All 15 patients underwent a technically successful UAE procedure. Mean patient age was 40 years (range 33–52 years). The mean delay between the baseline MRI and UAE was 61 days (SD 39) and mean time to MRI follow-up was 174 days (SD 21 days). Mean baseline dominant fibroid volume was 263 cm3 (SD 170 cm3) and reduced to 130 cm3 (SD 107 cm3), a reduction of 51% on the 6-month MRI scans.
Apparent diffusion coefficient
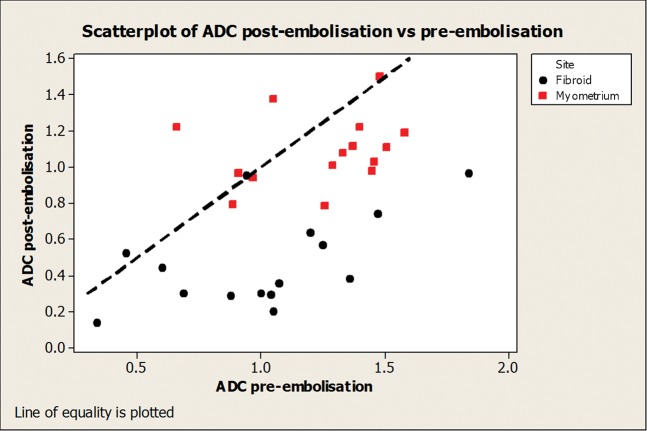
The following ADC values are quoted in ×10−3 mm2 s−1. The mean baseline ADC value of the dominant fibroid was 1.01 (SD 0.39), reducing to 0.48 (SD 0.26) post-embolisation. This was found to be a significant reduction (p<0.001). Mean baseline ADC value of the myometrium was 1.24 (SD 0.28), reducing to 1.09 (SD 0.20) at 6 months. This was not statistically significant (p=0.07). Mean reduction in ADC value was noted to be significantly greater for the dominant fibroid than the myometrium (mean difference 0.39; 95% confidence interval 0.14–0.63; p=0.005; Figure 3).
Figure 3.
Scatter plot showing distribution of apparent diffusion coefficient values pre and post embolisation for the dominant fibroid and myometrium.
Correlation coefficients were calculated between ADC values and volume reduction, baseline (r=0.66, p=0.007) and 6 months (r=0.17, p=0.54), showing a significant correlation between baseline ADC values and subsequent volume reduction.
Contrast enhancement (signal-difference-to-noise ratio)
Mean signal intensity of the fibroid on the subtracted images was 1043.2 (SD 350.1) pre embolisation and 92.6 (SD 164.5) post embolisation. Mean signal intensity of the myometrium was 1326.8 (SD 277.1) pre embolisation and 1231.1 (SD 348.1) post embolisation. Extracorporeal noise was measured at 6.6 (SD 3.1) and 8.3 (SD 4.3) pre and post embolisation, respectively.
Mean SDNR of the fibroid was −58.4 (SD 60.2) pre-embolisation and decreased to −165.6 (SD 82.5) following embolisation. Our SDNR values were negative as the fibroid signal dropped dramatically following embolisation, whereas the myometrial signal only underwent minor change. As this value is a ratio of measurement of contrast enhancement of the fibroid relative to the myometrium and the extracorporeal noise, a decrease in value post-embolisation indicates decreased signal intensity of the fibroid on the post-embolisation contrast scans.
Correlation was assessed between percentage volume reduction and baseline SDNR values (correlation r=0.18, p=0.51) and 6-month SDNR values (correlation r=0.06, p=0.84), showing no significant relationship between contrast enhancement and volume change.
No correlation was identified between ADC values and contrast enhancement (SDNR) on the baseline (r=0.01, p=0.97), nor the 6-month scans (r=−0.13 and p=0.64).
Discussion
We found a significant reduction in the mean ADC value of the dominant fibroid following embolisation. This reduction in ADC suggests that cellular integrity is disrupted, causing cellular dehydration [10,11,15]. Histologically, it is known that fibroids, which are not degenerate, are composed of densely packed smooth muscle cells with varying amounts of intervening collagen [16]. Prior to embolisation, these clusters of cells with intact cell membranes result in a restricted pattern of diffusion, which decreases following infarction.
We also noted a slight reduction in the mean ADC value of the myometrium following embolisation. This was unexpected, and may reflect a degree of myometrial damage or, given the small ROI for measurement (10–18 mm), a technical problem.
Reduction of myometrial ADC value is significantly less than the dominant fibroid (p=0.005), suggesting that myometrial reperfusion occurs. Perfusion studies performed immediately after UAE have shown maintained myometrial perfusion [17].
A moderately strong and significantly positive correlation was identified (r=0.66, p=0.007) between the baseline ADC values and subsequent dominant fibroid shrinkage. The exact reason for this is not clear; however, the degree of volume reduction may depend to some extent on the intrinsic histological nature of the fibroid. It has been suggested that this may relate to the degree of hyaline degeneration of fibroids [11], and this is a possibility, particularly since there is no way of differentiating these from non-degenerate fibroids on standard MRI sequences [18].
We did not identify any significant correlation between post-embolisation ADC values and percentage volume reduction (r=0.17, p=0.54). This is probably secondary to loss of the cellular architecture following embolisation, which reduces ADC values.
Our study used SDNR to objectively measure the degree of contrast enhancement. This technique has been advocated for quantitative measurement of focal masses on MRI [12] and has been used in the evaluation of pituitary lesions [19], and permits an objective measure of contrast enhancement, rather than a subjective score.
Using the above technique, we did not identify any correlation between the ADC value and SDNR, pre- or post-embolisation. A recently published study that utilised a slightly different measure of contrast enhancement (signal measurement on dynamic contrast imaging) has also failed to show any correlation between the two parameters [11]. However, one previous study, where a subjective percentage scale of enhancement scoring was used, did show a positive correlation between post-embolisation ADC value and degree of baseline contrast enhancement [10]. This difference is difficult to explain, but may arise because of different study methodology.
ADC value measures net movement of water molecules within tissue, and is therefore dependent on its histological nature and its cellular/fibrous content. Signal intensity measures degree of contrast enhancement, which in turn reflects extracellular contrast distribution. Both of these measure different values, and this is likely to be a plausible explanation for the lack of correlation between these two variables in our study.
We did not identify any significant correlation between percentage volume reduction and SDNR pre- or post-embolisation. It has also been demonstrated on perfusion imaging that poorly perfused and well-perfused leiomyomas show no difference in mean volume reduction [17], and lack of correlation between degree of contrast enhancement and volume reduction is not different from previous studies [17,20].
There were several limitations to our study. It was retrospective and only included 15 patients, lacking power to detect true correlations between pairs of variables. We chose to assess only the dominant fibroid rather than the total fibroid burden. Our ADC calculations were based only on b=1000 values as we felt that there may be perfusion-related discrepancy at lower b-values. The signal-to-noise ratio in the b=1000 images were adequate for ADC calculation. Given the small ROI for calculation, the ADC values of the myometrium may have been prone to a degree of sampling error.
In conclusion, we have shown a significant positive correlation between baseline ADC values and subsequent dominant fibroid volume reduction. Larger prospective studies are necessary to confirm whether diffusion-weighted MRI offers any useful additional benefit to standard CEMRI in UAE.
References
- 1.Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract Res Obstet Gynaecol 2008;22:571–88 [DOI] [PubMed] [Google Scholar]
- 2.Walker WJ, Pelage JP, Sutton C. Fibroid embolization. Clin Radiol 2002;57:325–31 [DOI] [PubMed] [Google Scholar]
- 3.Kroncke TJ. Imaging before and after uterine artery embolization. Radiologe 2008;48:639–48 [DOI] [PubMed] [Google Scholar]
- 4.Kwee TC, Takahara T, Ochiai R, Nievelstein RA, Luijten PR. Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol 2008;18:1937–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stejskal EO, Tanner JE. Spin diffusion measurements: spin-echo in the presence of a time dependent field gradient. J Chem Phys 1965;42:288–92 [Google Scholar]
- 6.Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 2007;188:1622–35 [DOI] [PubMed] [Google Scholar]
- 7.Muir KW, Buchan A, Von Kummer R, Rother J, Baron JC. Imaging of acute stroke. Lancet Neurol 2006;5:755–68 [DOI] [PubMed] [Google Scholar]
- 8.Barber PA, Darby DG, Desmond PM, Gerraty RP, Yang Q, Li T, et al. Identification of major ischemic change. Diffusion-weighted imaging versus computed tomography. Stroke 1999;30:2059–65 [DOI] [PubMed] [Google Scholar]
- 9.Whittaker CS, Coady A, Culver L, Rustin G, Padwick M, Padhani AR. Diffusion-weighted MR imaging of female pelvic tumors: a pictorial review. Radiographics 2009;29:759–74 [DOI] [PubMed] [Google Scholar]
- 10.Liapi E, Kamel IR, Bluemke DA, Jacobs MA, Kim HS. Assessment of response of uterine fibroids and myometrium to embolisation using diffusion-weighted echoplanar MR imaging. J Comput Assist Tomogr 2005;29:83–6 [DOI] [PubMed] [Google Scholar]
- 11.Hecht EM, Do RK, Kang SK, Bennett GL, Babb JS, Clark TW. Diffusion-weighted imaging for prediction of volumetric response of leiomyomas following uterine artery embolization: a preliminary study. J Magn Reson Imaging 2011;33:641–6 [DOI] [PubMed] [Google Scholar]
- 12.Pijl ME, Doornbos J, van Houwelingen HC, Tollenaar RA, Bloem JL. Quantitative analysis of focal masses at MR imaging: a plea for standardisation. Radiology 2004;231:737–44 [DOI] [PubMed] [Google Scholar]
- 13.Katsumori T, Kasahara T, Kin Y, Nozaki T. Infarction of uterine fibroids after embolization: relationship between postprocedural enhanced MRI findings and long-term clinical outcomes. Cardiovasc Intervent Radiol 2008;31:66–72 [DOI] [PubMed] [Google Scholar]
- 14.Broekmans FJ, Heitbrink MA, Hompes PG, Schoute E, Falke T, Schoemaker J. Quantitative MRI of uterine leiomyomas during triptorelin treatment: reproducibility of volume assessment and predictability of treatment response. Magn Reson Imaging 1996;14:1127–35 [DOI] [PubMed] [Google Scholar]
- 15.Jacobs MA, Herskovits EH, Kim HS. Uterine fibroids: diffusion weighted MR imaging for monitoring therapy with focused ultrasound surgery—preliminary study. Radiology 2005;236:196–203 [DOI] [PubMed] [Google Scholar]
- 16.Prayson RA, Hart WR. Pathologic considerations of uterine smooth muscle tumors. Obstet Gynecol Clin North Am 1995;22:637–57 [PubMed] [Google Scholar]
- 17.De Souza NM, Williams AD. Uterine arterial embolization for leiomyomas: perfusion and volume changes at MR imaging and relation to clinical outcome. Radiology 2002;222:367–74 [DOI] [PubMed] [Google Scholar]
- 18.Murase E, Siegelman ES, Outwater EK, Perez-Jaffe LA, Tureck RW. Uterine leiomyomas: histopathologic features, MR imaging findings, differential diagnosis, and treatment. Radiographics 1999;19:1179–97 [DOI] [PubMed] [Google Scholar]
- 19.Stobo DB, Lindsay RS, Connell JM, Dunn L, Forbes KP. Initial experience of 3 Tesla versus conventional field strength magnetic resonance imaging of small functioning pituitary tumours. Clin Endocrinol (Oxf) 2011;75:678–84 [DOI] [PubMed] [Google Scholar]
- 20.Burn PR, McCall JM, Chinn RJ, Vashisht A, Smith JR, Healy JC. Uterine fibroleiomyoma: MR imaging appearances before and after embolization of uterine arteries. Radiology 2000;214:729–34 [DOI] [PubMed] [Google Scholar]