Abstract
Objective
The objective of this study was to investigate the correlation between in vivo δ-tocotrienol (DT3) pharmacokinetics, pharmacodynamics and radiation protection, and to evaluate the effect of DT3 pre-treatment on radiation-induced alterations in apoptotic and autophagic pathways.
Methods
We evaluated pharmacokinetics (plasma, 0.5 to 12 h) and pharmacodynamics (peripheral blood indices; day 3, 7, 10 and 14) after a single subcutaneous injection of 300 mg kg−1 DT3 in unirradiated CD2F1 mice. Next, we monitored 30-day post-irradiation survival (9.25 Gy) and haematopoietic recovery of DT3-treated mice (7 Gy) exposed to cobalt-60 γ-irradiation. The effects of DT3 on irradiated bone marrow apoptosis and autophagy were determined by analyses of key caspases (3, 7, 9 and 8), beclin-1 and light chain 3 conversion.
Results
Plasma concentration of DT3 reached ∼195 µM (Cmax) 1 h after injection (Tmax), and DT3 was eliminated from plasma 12 h later. In unirradiated mice, DT3 significantly increased white blood cells (WBCs), neutrophils, lymphocytes (day 3 post DT3 injection) and platelets (day 7) by 1.5- to 2-fold, over vehicle-treated control. DT3 pre-treatment improved 30-day survival to 100% (∼15% in control) and accelerated recovery of reticulocytes, platelets, WBCs, neutrophils, lymphocytes and monocytes in peripheral blood. DT3 reduced activation of caspase-8, caspase-3 and caspase-7, inherent to apoptosis, while increasing autophagy-related beclin-1 expression in irradiated bone marrow.
Conclusion
These data indicate that DT3 stimulates multilineage haematopoiesis, protects against radiation-induced apoptosis downstream of the mitochondria and stimulates cytoprotective autophagy. Apart from a potent antioxidant activity, DT3 may elicit survival advantage following irradiation by enhancing haematopoiesis and modulating signalling pathways.
Radiation therapy is one of the most effective modalities for the treatment and cure of neoplastic diseases. However, normal tissue injury continues to be a limiting factor for radiation oncologists, who strive to improve the therapeutic index through radioprotection of the non-cancerous milieu. The risk of radiological terrorism resulting in mass casualties heightens these medical concerns [1]. In this regard, the haematopoietic system represents one of the major dose-limiting tissues owing to its high radiation sensitivity, characterised by persistent decreases in end-cells, compromised immune function, and increased susceptibility to infections and haemorrhage [2]. Several classes of compounds have been investigated for potential radiation countermeasure activity, including immunomodulators, free radical scavengers, haematopoietic cytokines, modulators of signal transduction and antiapoptotic agents [3-4]. Pharmacological interventions to counter radiation-induced pancytopenia and injury following intentional or accidental exposures are urgently required, despite advances in basic research.
A promising strategy for reducing normal tissue injury during therapeutic or accidental scenarios is the use of protectants that can alleviate the toxic effects of radiation. Recently, the antioxidant δ-tocotrienol (DT3), a vitamin E isoform, was identified as a significant radioprotectant with a dose reduction factor (DRF) of 1.27, as well as a radiomitigant (DRF=1.1) [5]. In related studies, DT3 increased cell survival, regeneration of haematopoietic microfoci and lineage–/Sca-1+/ckit+ stem/progenitor cells in irradiated mouse bone marrow, and protected human CD34+ cells from radiation-induced damage [6]. The observed protection was attributed to signal transduction pathways involving activation of extracellular signal regulated kinase 1/2 (Erk 1/2), and upregulation of mammalian target of rapamycin (mTOR) and its downstream effectors. Clearly, the radioprotective efficacy of DT3 depends not only on its antioxidant properties, but also on its stimulatory effects on the haematopoietic tissue through Erk-activated progenitor cell proliferation and haematological regeneration. Consequently, we evaluated DT3 pharmacokinetics and pharmacodynamics (haematological indices) in unirradiated mice, and the outcome of DT3 administration on radiation-induced pancytopenia in peripheral blood. Another potential mechanism of radioprotection by DT3 could be through alteration of radiation-induced signalling processes inherent to apoptotic and autophagic pathways in the haematopoietic tissue. Along with apoptosis (programmed cell death-I, PCD-I), autophagy was initially described as an alternative type of programmed cell death (PCD-II), although several in vivo studies support its role in cell survival [7]. Because the proapoptotic effects of tocotrienols, including DT3, are highly selective for malignant cells [8-10], and DT3 increased autophagy in rat pancreatic stellate cells [11], we investigated the effects of DT3 on radiation-induced apoptotic and autophagic signalling in mouse bone marrow. To this end, we evaluated specific markers of the caspase cascade (apoptosis), beclin-1 and microtubule-associated protein light chain 3 (LC3; autophagy). We report here that DT3 stimulated haematopoiesis in normal, unirradiated animals, prevented radiation lethality, and accelerated recovery of radiation-ablated haematopoietic tissue. This effect could, in part, be attributed to modulation of key apoptotic and autophagic molecules in irradiated mouse bone marrow cells.
Methods and materials
All chemicals, unless otherwise stated, were purchased from Sigma-Aldrich (St Louis, MO) and were used according to the manufacturer's instructions. All instruments were calibrated and maintained in accordance with routine quality-control procedures.
Animal care and handling
All animal procedures were reviewed and approved by the Armed Forces Radiobiology Research Institute (AFRRI) Institutional Animal Care and Use Committee using principles outlined in the National Research Council's Guide for the Care and Use of Laboratory Animals [12]. Male CD2F1 mice (Harlan Laboratories, Dublin, VA) aged 8–9 weeks were maintained at the AFRRI vivarium. These animals were evaluated using microbiological, serological and histopathological tests, and were determined to be disease- and pathogen-free during the quarantine period. Healthy animals were housed four per box in conventional sterile polycarbonate boxes with filter covers (Microisolator; Lab Products Inc., Seaford, DE) and autoclaved hardwood chip bedding. Mice had access to Harlan Teklad Rodent diet 8604 (Purina Mills, St Louis, MO) and acidified water (pH 2.5–3.0) ad libitum. Animal rooms were maintained at 21±2 °C and 50±10% relative humidity with 10–15 cycles of fresh air hourly and a 12 h light/dark cycle.
Irradiation
Unanaesthetised mice were irradiated bilaterally at AFRRI's cobalt-60 γ-irradiation facility. During irradiation, the animals were placed in well-ventilated Plexiglas chambers made specifically for mouse irradiation. The mid-line dose to the animals was delivered at a dose rate of 0.6 Gy min−1. An alanine/electron spin resonance (ESR) dosimetry system (American Society for Testing and Material Standard E 1607) was used to measure dose rates (to water) in the cores of acrylic mouse phantoms. Phantoms were 3 inches long and 1 inch in diameter, and were all located in empty compartments of the exposure rack. ESR signals were measured with a calibration curve based on standard calibration dosemeters provided by the National Institute of Standard and Technology (NIST, Gaithersburg, MD). The accuracy of the calibration curve was verified by intercomparison with the National Physical Laboratory (NPL) in the United Kingdom. The only corrections applied to the dose rates in phantoms were for decay of the cobalt-60 source and for a small difference in mass–energy absorption coefficients for water and soft tissue. The radiation field was uniform within ±2%.
Experimental design
Drug formulation and administration
DT3 was purchased from Yasoo Health Inc. (Johnson City, TN; >95% purity by nuclear magnetic resonance). The vehicle for subcutaneous (sc) administration was polyethylene glycol 400 (PEG-400)/5% Tween-80 (v/v); vehicle controls received 0.1 ml of PEG-400/5% Tween-80 and the drug-treated animals received different concentrations of DT3 in 0.1 ml of the vehicle at the nape of the neck with a 25-gauge (G) needle.
Assessment of in vivo pharmacokinetics and pharmacodynamics
To determine plasma concentration of DT3, age- and weight-matched mice were injected with a single sc injection of 300 mg kg−1 DT3 in 0.1 ml of the vehicle; this dose afforded maximum radioprotection in earlier studies [5]. For each time point, blood was collected from the caudal vena cava from 6–8 mice under isoflurane anaesthesia (0, 0.5, 1, 2, 4, 8 and 12 h after drug administration) using heparinised needles. Blood (0.6–1.0 ml) was collected in a Sarstedt microtubes coated with 1.6 mg ethylenediaminetetra-acetic acid (EDTA) per millilitre of blood (Sarstedt AG & Co., Numbrect, Germany), centrifuged at 900 g for 6 min at 4 °C and the plasma collected with sterile Pasteur pipettes. Plasma was stored at −20 °C until the time of analysis.
Preparation of plasma samples
The liquid–liquid extraction of DT3 was adapted from Nagy et al [13]. Stock standards were prepared in 1–1.5 ml of acetonitrile. The appropriate amount of DT3 working solution was added to pre-labelled tubes to create stock solutions, resulting in final concentrations of 1, 5, 50, 100, 200 and 300 µM for the standards. Aliquots of plasma samples collected from mice (220–300 µl) were transferred to 15 ml glass tubes and combined with 700 µl of 0.2% pyrogallol prior to storage at −20 °C. After thawing, 1 ml chilled ethanol was added to the samples followed by brief vortexing to precipitate proteins and extract DT3. Aliquots of 4 ml hexane containing butylated hydroxytoluene (BHT) (0.05 mg ml−1) were added to each sample, vortexed for 90 s and centrifuged at 1000 g for 3 min at 4 °C. Aliquots of 3.8 ml of the organic layer were transferred to another set of pre-labelled glass tubes (13×100 mm) and the supernatant evaporated to dryness using a vacuum centrifuge. This extraction process was repeated using fresh aliquots of hexane-BHT, with resulting organic layers being combined with corresponding dried extracts from the first extraction. The pellets were reconstituted with 150–160 µl of degassed acetonitrile, mixed for 10 s, sonicated for 30 min and mixed again for 20 s; 70 µl of the acetonitrile was transferred into a limited volume glass insert within an amber vial.
Determination of δ-tocotrienol levels by HPLC
Analysis of DT3 was performed according to the method adapted from Osakada et al [14]. An aliquot of 50 µl was injected into the high-performance liquid chromatography (HPLC) system (equipped with a 20A-T solvent pump, autosampler, and in-line 20AD UV and 10Rxl fluorescent detectors; Prominence, Shimadzu, Columbia, MD). The pump was run at a flow rate of 0.8 ml min−1 and the mobile phase was composed of acetonitrile:water (90:10). The total run time was 9 min and chromatographic separation was carried out by a Kinetex C18 reverse phase column (2.6 µm, 4.6 mm×10 cm; Phenomenex, Terrance, CA). The eluate was monitored for peaks by the UV detector set at 294 nm, the fluorescence detector set at an excitation wavelength of 296 nm and emission wavelength of 330 nm. Calibrations were linear for standard samples for the determination of DT3. The pharmacokinetic parameters for DT3 following single-dose administration were determined using non-compartmental methods.
Pharmacodynamic effects of DT3 were assessed by comparing complete blood counts (CBC) and differentials—red blood cell (RBC), white blood cell (WBC), absolute neutrophil counts, lymphocyte and platelet—with the control/vehicle-treated animals. For these studies, blood samples were collected in tubes containing EDTA at 3, 7, 10, 14 and 21 days following DT3 administration and processed as described below.
Survival studies
Mice were injected with 300 mg kg−1 DT3 sc, before exposure to radiation at an LD90/30 dose (9.25 Gy). Mice were monitored for 30 days following irradiation, and the number of moribund/dead mice recorded twice daily.
Haematological studies of peripheral blood
We used a lower radiation dose (7 Gy) in the haematological studies as this dose induced severe myelosuppression without accompanying lethality, allowing for sufficient sampling numbers at the later post-exposure times [5]. CD2F1 male mice pre-treated with 300 mg kg−1 DT3 sc 24 h prior to total body irradiation (TBI) with 7 Gy were euthanised on days 3, 7, 10, 14, 21 and 28 post-TBI. Blood from eight mice per group per time point was analysed for CBC with differential. Blood (0.6–1.0 ml) was obtained by cardiac puncture using a heparinised syringe and a 23-G needle from mice anesthetised with isoflurane (Hospira Inc., Lake Forest, IL), transferred immediately into precoded EDTA (Sigma-Aldrich, St Louis, MO) tubes and mixed gently on a rotary shaker until analysis. The coded tubes were analysed for RBCs, WBCs, ANCs, monocytes, lymphocytes, reticulocytes, haemoglobin and platelets using the ADVIA 2120 (Siemens Medical Solutions Diagnostics, Dublin, Ireland), and data were generated using Medical Solutions software, v. 5.9.
Immunoblotting
Bone marrow cells from irradiated and DT3-treated mice were collected at 4, 8 and 24 h after irradiation. Cells from femurs of two mice were pooled (n=6) and each sample set processed separately. Protein content was determined by standard techniques, and equal concentrations of protein lysates from each group were separated on Tris-Glycine gels (Invitrogen, Carlsbad, CA) and transferred to a polyvinylidene membrane using standard protocols. Membranes were blocked for 60 min at room temperature and incubated overnight at 4 °C in buffer (Tris-buffered saline solution including 0.1% Tween 20 and 5% skimmed milk powder) and 1:1000 diluted antibodies. Antibodies for cleaved caspase-3, caspase-3, cleaved caspase-7, caspase-7, caspase-8, caspase-9, cytochrome c, LC3A/B, beclin-1 and actin were purchased from Cell Signaling (Danvers, MA). Detection of primary antibodies was performed with a 1:2500 diluted horseradish peroxidase-conjugated secondary antibody (Santa-Cruz Biotechnology, Santa Cruz, CA). Blots were developed using an enhanced chemiluminescence kit (Thermo Scientific, Rockford, IL) and visualised using a Fujifilm Image Reader (LAS-3000 photoimager; Fujifilm Photo Film Co., Tokyo, Japan). Quantification of protein expression was determined by densitometry of digitised images using LAS-3000 software.
Statistical analysis
For pharmacokinetic studies, maximum plasma concentration (Cmax) and time to reach maximum plasma concentration (Tmax) were obtained directly from the data.
All survival data were compared using Fisher's exact test (one-tailed) and the generalised Savage (Mantel–Cox) procedure (BMDP Statistical Software Inc., Los Angeles, CA). Haematological parameters were graphed as mean ± standard error of mean. These data were analysed using two-way analysis of variance (ANOVA). If the overall ANOVA was significant, follow-up tests were performed for the simple main effect of the group at each time using linear contrasts and Student's t-test. A p-value of <0.05 was considered significant.
Results
Determination of δ-tocotrienol pharmacokinetics in vivo
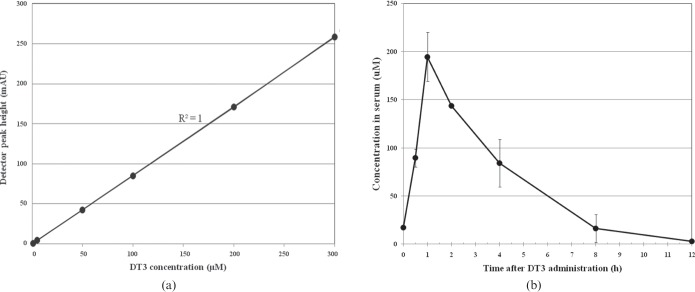
First, we standardised the HPLC with conditions for detection of pure DT3 in the linear range with fluorescence and UV detection. The peak for DT3 was expressed in the chromatogram with a retention time of 6.8±0.5 min, with a mean absolute recovery value of more than 95%. The calibration curve for DT3 was found to be linear in the range of 1–300 µM (r2=0.999) (Figure 1a).
Figure 1.
(a) Calibration curve for a range of δ-tocotrienol (DT3) standards. (b) Plasma concentration–time curve of DT3 after subcutaneous injection of 300 mg kg−1 DT3 in 0.1 ml 5% Tween 80 in polyethylene glycol 400 (PEG-400) analysed by high-performance liquid chromatography (see “Methods and materials” for details). Each data point represents mean±standard error of mean (n=6). Peak plasma concentration of DT3 was 194±25.4 µM l−1 and time required to reach plasma peak was 1 h.
The plasma concentration–time curve for DT3 following a single sc administration of 300 mg kg−1 to CD2F1 mice is depicted in Figure 1b. The peak plasma concentration (Cmax) of DT3 was 194±25.4 µM l−1, while the time required to reach the peak plasma concentration (Tmax) was 1 h. The plasma half-life (t1/2) was 1.8 h; and DT3 was cleared from plasma within 12 h after administration.
δ-tocotrienol stimulation of haematopoiesis
DT3 substantially altered various haematological constituents in non-irradiated mouse peripheral blood on day 3 following drug administration (Table 1). Although the erythrocyte counts were not significantly higher than controls on days 3, 7 and 10 after DT3 treatment, the haematocrit and haemoglobin levels were significantly lower on day 3 (p<0.05–0.001), and returned to normal values by day 10. On the other hand, the total leukocyte count increased from 3.25×103 cells µl−1 to 5.9×103 cells µl−1 on day 3, largely due to an increase in neutrophil counts (∼threefold increase; p<0.001), and increased lymphocyte counts (p<0.01). Further, DT3 induced significantly higher neutrophil counts until day 7 after injection (p<0.05). Platelet levels were similar to vehicle controls on day 3, but were elevated significantly in the DT3-treated group on day 7 after administration. All haematological indices returned to baseline values by day 10 after DT3 injection. Thus, a single injection of 300 mg kg−1 DT3 significantly elevated end-cells of different lineages, indicating a stimulatory effect on haematopoiesis.
Table 1. Pharmacodynamics of peripheral blood indices following a subcutaneous injection of 300 mg kg−1 DT3.
| Parameter studied | Days after injection of DT3 |
|||||
| Day 3 |
Day 7 |
Day 10 |
||||
| Vehicle | DT3 | Vehicle | DT3 | Vehicle | DT3 | |
| RBC (106 cells µl−1) | 9.96±0.18 | 9.65±0.05 | 9.65±0.2 | 9.8±0.18 | 8.97±0.91 | 9.17±0.15 |
| Haematocrit (%) | 42.54±0.86 | 40.11±0.39a | 41.18±0.95 | 40.61±0.70 | 37.70±0.67 | 38.34±0.69 |
| Haemoglobin (µg µl−1) | 15.31±0.22 | 14.39±0.08c | 15.05±0.20 | 14.80±0.22 | 14.84±0.41 | 14.58±0.27 |
| Reticulocytes (103 cells µl−1) | 185.40±11.06 | 166.85±7.36 | 166.86±11.09 | 158.58±11.07 | 142.9±10.98 | 145.74±9.38 |
| Platelets (103 cells µl−1) | 1448.75±24.34 | 1440.63±35.38 | 1513.88±15.48 | 1575.63±26.34a | 1474.80±61.90 | 1557.60±36.90 |
| Total leukocytes (103 cells µl−1) | 3.25±0.31 | 5.90±0.43c | 4.74±0.48 | 5.94±0.30 | 4.58±0.41 | 5.3±0.47 |
| Neutrophils (103 cells µl−1) | 0.72±0.07 | 2.43±0.43c | 0.98±0.10 | 1.36±0.09a | 1.05±0.05 | 1.27±0.15 |
| Lymphocytes (103 cells µl−1) | 2.26±0.24 | 3.10±0.30b | 3.28±0.35 | 4.06±0.23 | 3.44±0.33 | 3.55±0.36 |
| Monocytes (103 cells µl−1) | 0.10±0.02 | 0.20±0.05 | 0.19±0.03 | 0.22±0.03 | 0.18±0.04 | 0.20±0.04 |
| Eosinophils (103 cells µl−1) | 0.10±0.02 | 0.07±0.01 | 0.19±0.04 | 0.17±0.04 | 0.21±0.04 | 0.20±0.03 |
| Basophils (103 cells µl−1) | 0.10±0.02 | 0.07±0.01 | 0.19±0.04 | 0.17±0.04 | 0.26±0.15 | 0.11±0.04 |
DT3, δ-tocotrienol; RBC, red blood cell.
Values are mean±standard error; n=8 per group.
ap<0.05 compared with vehicle control by ANOVA.
bp<0.01 compared with vehicle control by ANOVA.
cp<0.001 compared with vehicle control by ANOVA.
δ-tocotrienol pre-treatment abrogates radiation-induced lethality
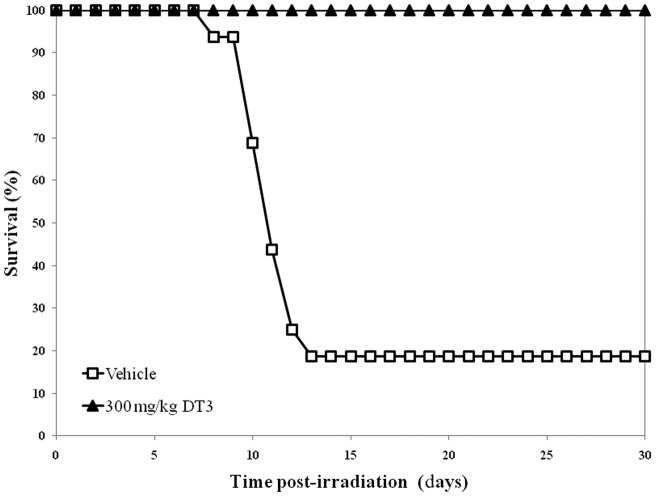
We evaluated different times of DT3 administration with respect to exposure time for optimum survival benefit in lethally irradiated (9.25 Gy) mice; detailed survival and DRF studies are reported elsewhere [5]. Mice injected with the vehicle and then irradiated exhibited signs of radiation sickness (anorexia, lethargy, diarrhoea, weight loss, delacrymation and facial oedema) followed by mortality (Figure 2). Only 16% of vehicle-treated animals survived 30 days after TBI. When DT3 was injected 1 h prior to TBI, only 30% of the animals survived [5]. However, when 300 mg kg−1 DT3 was administered 24 h prior to irradiation, survival increased to 100% (p<0.001). Therefore, in all subsequent experiments we used 300 mg kg−1 DT3, administered 24 h prior to exposure, as the optimal schedule for drug treatment in relation to irradiation time.
Figure 2.
Protection against radiation-induced lethality by administration of 300 mg kg−1 δ-tocotrienol (DT3), subcutaneous, assayed by monitoring 30-day post-irradiation survival of CD2F1 mice.
δ-tocotrienol alleviates radiation-induced pancytopenia
Normal values for each parameter were calculated by averaging counts from 24 age- and weight-matched CD2F1 mice. Analyses by two-way ANOVA are significant (p<0.001) between the irradiated and DT3-treated groups, and over time for all variables. Pair-wise comparisons between the groups are presented in Table 2.
Table 2. δ-tocotrienol-induced haematological alterations in irradiated mouse peripheral blood sampled at 3–28 days after total body irradiation.
| Parameter studied | Days after irradiation (7 Gy) |
|||||||||||
| Day 3 |
Day 7 |
Day 10 |
Day 14 |
Day 21 |
Day 28 |
|||||||
| Vehicle | DT3 | Vehicle | DT3 | Vehicle | DT3 | Vehicle | DT3 | Vehicle | DT3 | Vehicle | DT3 | |
| RBC (106 cells µl−1) | 8.95 (0.40) | 8.40a (0.52) | 7.61 (0.74) | 7.68 (0.42) | 7.20 (0.31) | 7.74b (0.34) | 7.32 (0.42) | 7.87a (0.97) | 8.63 (0.52) | 9.12 (0.42) | 8.37 (0.35) | 8.55 (0.31) |
| Haemoglobin (µg µl−1) | 13.36 (0.61) | 12.76a (0.54) | 12.19 (0.77) | 10.90 (3.63) | 11.23 (0.43) | 12.06b (0.51) | 8.02 (0.96) | 8.38 (0.80) | 13.96 (0.85) | 14.63 (0.57) | 13.33 (0.29) | 13.74 (0.46) |
| Reticulocytes (103 cells µl−1) | 2.86 (1.24) | 3.91 (2.06) | 5.60 (2.15) | 13.28c (5.12) | 26.20 (11.18) | 141.25c (72.76) | 132.44 (39.99) | 204.08 (118.52) | 436.51 (190.46) | 225.69b (38.92) | 191.54 (14.59) | 169.73 (22.80) |
| Platelets (103 cells µl−1) | 1209.50 (30.30) | 1413.50c (27.60) | 267.63 (16.39) | 237.00a (7.72) | 84.63 (10.73) | 546.50c (135.07) | 256.43 (48.51) | 598.88a (159.57) | 611.75 (54.05) | 962.25c (68.99) | 801.00 (49.25) | 1125.88c (28.21) |
| Total leukocytes (103 cells µl−1) | 0.21 (0.03) | 0.15a (0.01) | 0.28 (0.03) | 0.56c (0.07) | 0.20 (0.02) | 0.58c (0.12) | 0.65 (0.06) | 1.33 (0.38) | 1.26 (0.21) | 2.83b (0.50) | 1.40 (0.13) | 2.10b (0.19) |
| Neutrophils (103 cells µl−1) | 0.06 (0.01) | 0.05 (0.0) | 0.11 (0.01) | 0.32c (0.04) | 0.06 (0.01) | 0.29c (0.07) | 0.12 (0.02) | 0.55a (0.20) | 0.46 (0.11) | 1.6c (0.35) | 0.72 (0.09) | 0.93a (0.03) |
| Lymphocytes (103 cells µl−1) | 0.11 (0.02) | 0.06a (0.01) | 0.12 (0.02) | 0.17 (0.03) | 0.11 (0.02) | 0.19b (0.03) | 0.48 (0.03) | 0.60a (0.12) | 0.59 (0.08) | 0.91a (0.12) | 0.59 (0.07) | 1.07c (0.11) |
| Monocytes (103 cells µl−1) | 0.0 (0.0) | 0.0 (0.0) | 0.01 (0.0) | 0.03c (0.01) | 0.0 (0.0) | 0.04c (0.01) | 0.02 (0.0) | 0.08a (0.03) | 0.07 (0.01) | 0.13b (0.02) | 0.08 (0.01) | 0.12a (0.01) |
| Eosinophils (103 cells µl−1) | 0.04 (0.01) | 0.03 (0.01) | 0.03 (0.0) | 0.03 (0.0) | 0.02 (0.0) | 0.05c (0.01) | 0.02 (0.0) | 0.07a (0.02) | 0.08 (0.01) | 0.14c (0.01) | 0.07 (0.01) | 0.09 (0.07) |
| Basophils (103 cells µl−1) | 0.01 (0.0) | 0.01 (0.0) | 0.01 (0.0) | 0.01 (0.0) | 0.01 (0.01) | 0.02a (0.0) | 0.03 (0.01) | 0.04 (0.01) | 0.05 (0.0) | 0.05 (0.01) | 0.02 (0.01) | 0.07c (0.07) |
DT3, δ-tocotrienol; RBC, red blood cell.
Values are mean, standard error in parentheses; n=8 per group. Data analysed using Student’s t-test.
ap<0.05 compared with irradiated control.
bp<0.01 compared with irradiated control.
cp<0.001 compared with irradiated control.
The erythrocyte count in naïve CD2F1 mice was 9.53×106±0.47 cells µl−1. Irradiation with 7 Gy significantly reduced erythrocytes on days 7–28 in the vehicle-treated group (p<0.05–0.01; Table 2). The nadir was seen on day 10 with erythrocyte counts falling to 7.2×106 cells µl−1; however, normal levels were not attained by day 28 post TBI (8.4×106 cells µl−1). Similarly, haematocrit values (not shown) and haemoglobin levels also decreased significantly in the irradiated controls, the nadirs falling on days 10 and 14; complete recovery was not observed even on day 28 after exposure. The effect of radiation on reticulocyte counts was more immediate; the cell count fell to 2.9×103 cells µl−1 on day 3 post TBI (165×103±12.3 cells µl−1 in naïve controls). On day 21 after exposure, the irradiated controls had high reticulocytes compared with naïve counts (436×103 cells µl−1; p<0.001); however, baseline values were reached by day 28. Pre-treatment with DT3 resulted in significantly higher erythrocyte, haematocrit and haemoglobin levels on day 10 than irradiated controls (p<0.05–0.001). Although reticulocyte counts were significantly reduced in the DT3-treated group on day 3, these counts returned to normal levels by day 10, indicative of accelerated erythropoietic recovery.
Platelet counts in the irradiated group decreased significantly on day 3 and reached a nadir on day 10 (84.63×10−3 cells µl−1, compared with 1479×103±18.9 cells µl−1 in normal mice). Recovery was incomplete on day 28 after TBI, with significantly lower platelet counts (801×103 cells µl−1, p<0.001 compared with the naïve group; Table 2). DT3 pre-treatment significantly increased platelet counts at all time points studied, and blunted the platelet nadir seen on day 10 (546×103 cells µl−1; p<0.001 compared with irradiated control). The platelet levels in DT3-treated groups were significantly higher than irradiated controls on all post-irradiation sampling days, but were lower than naïve controls on day 28, implying that a longer time is required for platelet recovery.
A marked decline in the total leukocyte count was observed on day 3 in the vehicle-treated, 7 Gy-irradiated group (0.21×103 cells µl−1 compared with 4.19×103±0.47 cells µl−1 in naïve mice). A gradual increase was seen between days 3 and 14, but the day 14 counts (1.4×103 cells µl−1) were still significantly lower than the naïve leukocyte counts on day 28. The normal range for neutrophils, lymphocytes and monocytes were 0.91±0.29, 3±0.37 and 0.16±0.03×103 cells µl−1, respectively. Following exposure, the neutrophil, lymphocyte and monocyte counts in the irradiation-alone mice were at the lowest point on day 3, and did not achieve baseline values even on day 28 after TBI. Although irradiation with 7 Gy produced a nadir in neutrophil, lymphocyte and monocyte counts on day 3 post TBI, the DT3-treated group demonstrated significantly higher cell counts than the irradiated controls on days 7–28 (p<0.05–0.001), demonstrating faster recovery of the leukopoietic lineage.
Pre-treatment with δ-tocotrienol reduces radiation-induced activation of caspase-3, caspase-7 and caspase-8, but not caspase-9 or cytochrome c
We evaluated changes in apoptotic markers consequent to ionising radiation (Figure 3), and the influence of DT3 on expression of these proteins at 4, 8 and 24 h after TBI. Results demonstrating the effect of radiation on activation of caspase-8, a key modulator of the death receptor mediated (extrinsic) apoptosis pathway, are presented in Figure 4a. Radiation resulted in a marked increase in active caspase-8 expression (lower band) 4 h after TBI in bone marrow. Active caspase-8 remained significantly elevated throughout the time course of the experiments, reaching a peak at 24 h after exposure. DT3 pre-treatment significantly reduced the activation of caspase-8, as seen in the reduced intensity of the band at all time points compared with the irradiated controls.
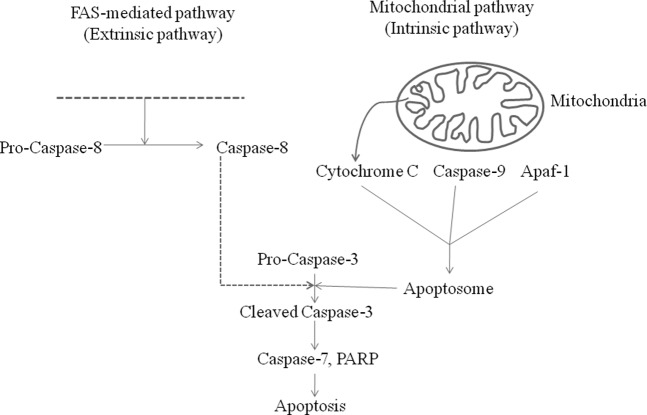
Figure 3.
Schematic representation of the extrinsic and intrinsic apoptosis pathways showing the position of various intermediates of interest in this study. Please note that the diagram does not include all components involved in these processes. Apaf-1, apoptosis-activating factor-1; PARP, poly(ADP-ribose) polymerase.
Figure 4.
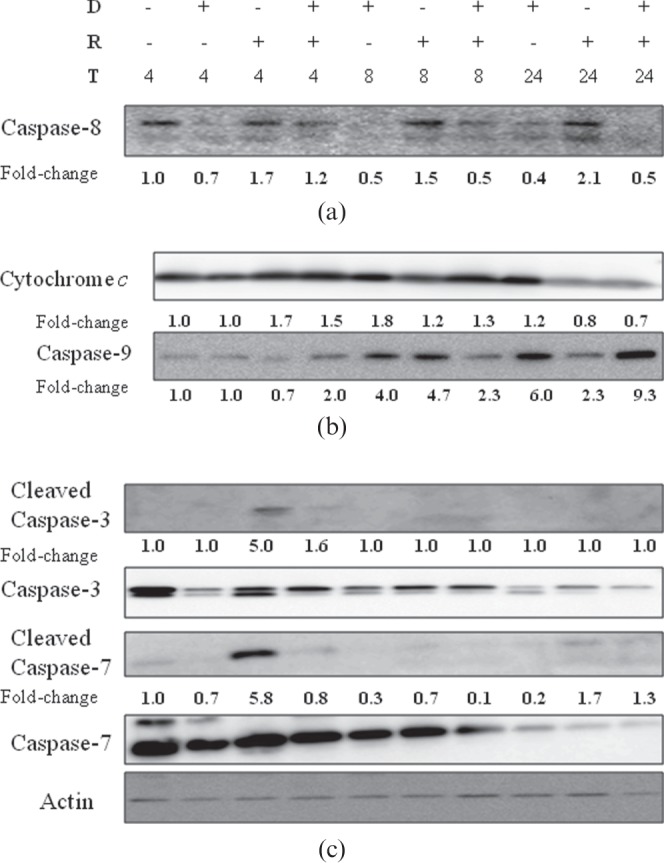
Effect of δ-tocotrienol (DT3) on expression of apoptotic marker proteins in irradiated bone marrow. (a) DT3 reduced radiation-induced changes of activated caspase-8 (lower band) at 4, 8 and 24 h after exposure. (b) DT3 did not alter translocation of cytochrome c from mitochondria into the cytosol, but increased caspase-9 expression 24 h after total body irradiation (TBI). (c) Effector caspase-3 and caspase-7 activity was inhibited by DT3 pre-treatment at all times. Whole-cell lysates were collected 4, 8 and 24 h after irradiated and subject to immunoblot analysis using appropriate antibodies. Actin was run as internal protein loading control. Representative images from 2–4 replicates. D, δ-tocotrienol; R, radiation; T, time (h, post TBI).
We also analysed cytochrome c and caspase-9, two mediators of the mitochondrial (intrinsic) apoptotic pathway. DT3 treatment alone induced release of cytochrome c at 8 and 24 h with reference to exposure time and also elevated expression of caspase-9. Upon irradiation, cytochrome c was released into the cytosol (Figure 4b) and detected at 4 h; this expression was sustained up to 8 h after exposure. DT3 did not significantly alter the expression of radiation-induced cytochrome c release from the mitochondria, indicating that DT3 may not modulate pathways upstream of the mictochondria. We validated these findings by evaluating the alteration of JC-1 expression in mitochondria of bone marrow cells 4, 8 and 24 h post TBI by flow cytometry, and found that DT3 did not significantly alter radiation-induced mitochondrial membrane permeability (not shown). Since release of cytochrome c is known to subsequently activate caspases, we also examined the expression of caspase-9. Radiation alone upregulated the caspase-9 expression at 8 h post TBI in the bone marrow (∼4.5-fold over control), and was 2.3-fold more than control levels at 24 h (Figure 4b). Significantly, DT3 pre-treatment increased accumulation of caspase-9 ∼twofold at 4 h, decreased it at 8 h and increased it to ninefold 24 h after exposure compared with vehicle-treated controls.
Caspase-3 is an effector caspase occurring downstream of both the extrinsic and intrinsic apoptotic pathways. It has been documented as a reliable index for apoptotic responses in several tissues, including the bone marrow. Phosphorylation of the inactive procaspase-3 into its active cleaved caspase-3 form was associated with conversion of caspase-7 to the active cleaved form (Figure 4c). Irradiation resulted in significantly higher levels of cleaved caspase-3 and caspase-7, 4 h after TBI; pre-treatment with DT3 significantly reduced activation of both caspase-3 and caspase-7 at 4 h (Figure 4c).
δ-tocotrienol upregulates beclin-1 expression and microtubule-associated protein light chain 3 conversion in bone marrow cells
We further investigated whether DT3 also altered the radiation-induced activation of autophagy in mouse bone marrow using beclin-1 and conversion of LC3 from form I to form II, as markers. As determined by immunoblotting (Figure 5a), DT3 pre-treatment increased the expression of beclin-1 at 8 h post-irradiation. The LC3-II form is expressed constitutively in normal bone marrow cells (Figure 5b). Compared with vehicle-treated controls, treatment with DT3 alone increased the expression of LC3-II at 4 h, but decreased LC3-II levels at 8 h. Both LC3-I and -II forms reduced to ∼0.2 compared with normal expression, at 24 h after sham-irradiation, in DT3-treated mice (Figure 5c). In the irradiated animals, LC3-I and -II expression were reduced at 8 h, while the LC3-I form increased to ∼1.7-fold at 24 h. Pre-treatment with DT3 24 h prior to TBI resulted in marked decreases in both LC3-I and -II forms at 4 h, and increases at 8 h. At 24 h, LC3-I form expression was greater than in irradiated control, and 2.2-fold higher than non-irradiated/sham-treated mice. The lack of clear conversion of LC3-I to the LC3-II form over the time points studied could be due to different rates of turnover for these proteins.
Figure 5.
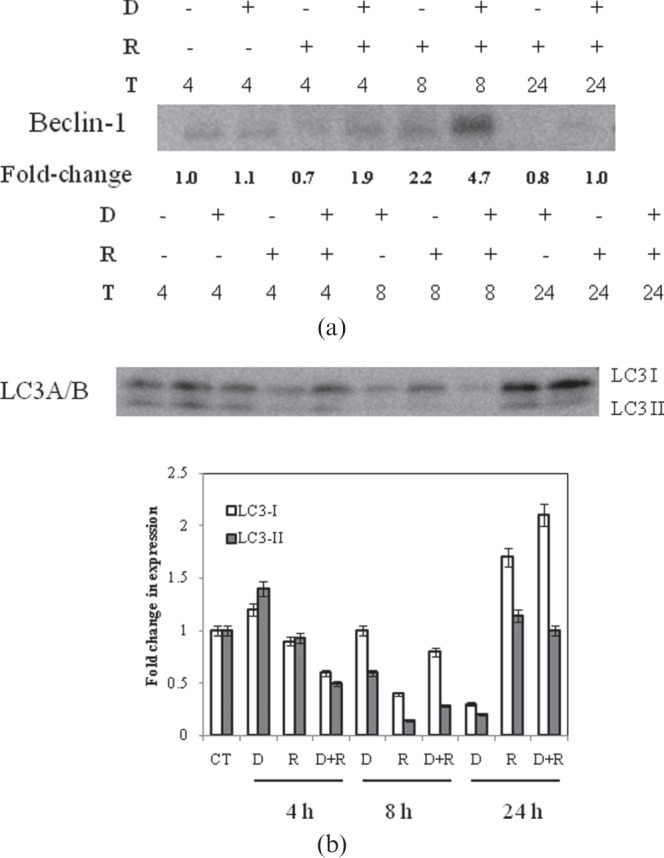
Effect of δ-tocotrienol (DT3) on naïve and irradiated bone marrow expression of autophagy-associated proteins. (a) Beclin-1 expression and (b) quantitation of microtubule-associated protein light chain 3 (LC3) conversion was determined by immunoblotting as described under “Materials and methods”. (c) Densitometry analysis of representative LC3 blot normalised to control values. Representative images from 2–4 replicates. D, δ-tocotrienol; R, radiation; T, time (h, after total body irradiation).
Discussion
Exposure to ionising radiation causes a series of physiological changes known as acute radiation syndrome (ARS), the severity of which is related to the exposure dose and dose rate [1]. The haematopoietic tissue is a rapidly proliferating, self-renewing organ, which is highly vulnerable to radiation injury, making it a major dose-limiting organ for triage following radiological accidents [15], and the status of bone marrow determines the course of treatment with radiation and/or chemotherapy [16,17]. Therefore, a major research goal in radiation biology and oncology has been the identification of protectants that can ameliorate radiation-induced ARS.
We have previously demonstrated significant protective and mitigatory effects of DT3 against radiation-induced oxidative stress and lethality [5]. The present study assessed the relationship between pharmacokinetics of DT3 and its pharmacodynamic properties, to support the development of DT3 as a radiation countermeasure. We examined pharmacokinetics using the dose and route of DT3 that showed optimum efficacy as a radiation protectant; thus 300 mg kg−1 DT3 administered sc has a half-life (t1/2) of 1.8 h, and is cleared from plasma within 12 h after a single dose. This is comparable with a mouse study wherein an oral dose of DT3 (100 mg kg−1) had a t1/2 of 3.5 h [18], and a clinical study with a t1/2 of 2.3 h for humans [19]. The slightly longer half-lives reported in these studies could be because orally administered DT3 requires a longer time interval for absorption by the intestine and entry into systemic circulation, as opposed to the sc route. The plasma clearance time for DT3 is 12 h after sc administration; this finding is similar to previous reports on the pharmacokinetics of DT3 [18,19] and confirms the rapid clearance of DT3 from plasma.
The pharmacodynamic effects of a single dose of DT3 was seen 3–7 days after drug administration in the haematopoietic tissue as increased end-cell counts of lymphoid, myeloid and megakaryocytic lineages. Previous studies have shown that treatment with vitamin E increased the number of colony forming units of erythroid precursors (CFU-E), enhanced erythropoiesis and haemoglobin levels, and corrected experimentally induced anaemia in laboratory animals [20-22]. Our data indicate that the stimulatory effect of DT3 is not limited to erythropoiesis, but extends to leukopoiesis and megakaryopoiesis. Indeed, in related studies, DT3 treatment increased progenitor cells and clonogenicity (CFU-E, CFU-GM and CFU-GEMM) in bone marrow of non-irradiated mice [6]. Several reports have shown that pharmacokinetics of DT3 vary in different tissues; in the pancreatic tissue, which contains high levels of adipocytes, DT3 level peaked at 8 h [23]. Adipocytes are the most abundant cells in bone marrow [24], and we hypothesise that uptake of DT3 by bone marrow tissue might result in a longer half-life and clearance, prolonging the time apportioned for DT3 to modulate cell survival pathways and haematopoiesis. HPLC analysis of DT3 content in bone marrow cells is necessary to test this theory.
Earlier, we reported that a single dose of DT3 protected against post-irradiation lethality in mice, with a DRF of 1.27 [5]. The survival studies presented here corroborate our earlier findings and, further, suggest that tissue-specific DT3 concentrations, rather than plasma DT3 concentration, may be responsible for the pronounced radioprotection observed following DT3 pre-treatment. The cause of death in mice exposed to TBI can be deduced from the time course of mouse survival; death from haematopoietic syndrome peaks between day 10 and 15 after exposure, and therefore 30-day survival studies following exposure are used to evaluate protection against bone marrow death [25]. In the present study, ∼80% of vehicle-treated animals died between days 10 and 14 following exposure to 9.25 Gy. This indicated that the primary cause of death was haematopoietic toxicity and that DT3 prevented lethality by protecting bone marrow. The data presented here on radiation-induced haematological deficits confirm the marked effect of DT3 on blood-forming tissue.
Significant reduction in haematological indices following irradiation reported here is consistent with earlier reports on radiation-induced ablation of bone marrow [26,27]. Irradiation severely depleted reticulocytes, WBCs, neutrophils, lymphocytes, platelets and haematocrit counts, an effect that persisted 21–28 days after exposure. This outcome may be attributed to the clastogenic effects of ionising radiation, which include destruction of mature circulating cells, loss of cells from the circulation by haemorrhage or leakage through capillary walls, and reduced reconstitution due to destruction of the stem/progenitor pool [28]. Lacking DNA, RBCs are highly radioresistant; therefore, the effects of irradiation on RBC counts are not as pronounced. Further, the long circulating lifespan of the RBCs makes anaemia less of an issue in the early period after radiation exposure [29]. However, radiation-induced neutropaenia and thrombocytopenia can result in opportunistic infections and aberrant haemorrhagic complications within 10 days of exposure, leading to lethality [30] or interruption of radiotherapy [31]. DT3 pre-treatment accelerated the reconstitution of peripheral blood cells, including WBCs, neutrophils, platelets and lymphocytes, indicative of a multilineage recovery.
Initially, we attributed this recovery to a potent antioxidant activity of DT3 [5], together with stimulation of haematopoietic progenitors and activation of pro-survival pathways [6]. Here, we posed the question: does DT3 treatment also modulate apoptotic and autophagic signal transduction pathways in mouse bone marrow? To this end, we evaluated several key proteins involved in the apoptotic and autophagic pathways.
DT3 pre-treatment without subsequent irradiation did not activate caspase-8, caspase-3 or caspase-7, although cytochrome c release and caspase-9 expression were increased. This finding supports earlier studies, where tocotrienols (including DT3) did not injure normal, non-neoplastic cells [10,11], but were pro-apoptotic in a variety of malignant cells, such as liver [8,32], breast [9,33], colon [34], prostate [10] and pancreas [17]. In the present study, we report that irradiation of the whole animal induced a concomitant initiation of the extrinsic and intrinsic apoptotic pathways in CD2F1 mouse bone marrow. Increased activation of caspase-8 and caspase-9, accompanied by cytochrome c release from mitochondria, is a signature event of apoptosis, and compatible with radiation-induced triggering of the death receptors and mitochondrial perturbation. Cytochrome c associates with procaspase-9 and Apaf-1 (apoptotic protease activating factor 1), and ATP, resulting in the cleavage of caspase-9. Cleaved caspase-8 and caspase-9 converge on the executor caspase-3, triggering its activation and committing the cell to apoptotic death by further cleaving caspase-6 and caspase-7, and poly(ADP-ribose) polymerase [35]. DT3 pre-treatment reduced activation of caspase-8, and hence the extrinsic pathway, but we did not see any reduction of radiation-induced cytochrome c release and caspase-9 levels. However, downstream apoptotic executor molecules caspase-3 and caspase-7 were effectively suppressed by DT3.
Intriguingly, we observed that in DT3-treated and irradiated bone marrow, the expression of caspase-9 increased substantially over both normal and irradiated controls. Because a recent publication suggested that inhibition of caspase-9 could prevent the autophagic flux and enhanced cell death due to blockage of cytoprotective autophagy [36], we asked what, if any, was the correlation between caspase-9 overexpression and the autophagic process?
Along with apoptosis, autophagy represents an alternative type of programmed cell death (PCD-II) [37]. Nevertheless, recent insights into autophagy indicate that this may be a response that promotes cell survival [38,39]. Some evidence even suggests that autophagy might be a protective mechanism for cells exposed to ionising radiation [40]. Recently, an autophagy-inducing drug (carbamazepine) was reported to be a radiation protectant and mitigator [41]. Our immunoblot data demonstrate upregulation of beclin-1 by DT3, and a complex effect on LC3-I and LC3-II in irradiated bone marrow, indicative of a possible increase in autophagy. These data suggest that DT3-induced autophagy could function by improving cell survival, and a causal role of caspase-9 is indicated; on the other hand, the precise role of autophagy in radiation protection is not known. Further experiments, including techniques such as electron microscopy and green fluorescent protein-LC3, are required to further examine this possibility.
In summary, the current paper reports several novel findings. First, plasma pharmacokinetic peak of DT3 was 1 h, with a t1/2 of 1.8 h; however, the increase in peripheral blood counts was observed 3–7 days after the initial drug administration in non-irradiated mice. In irradiated animals, the potent radioprotection observed can be linked to DT3-induced accelerated recovery of haematopoiesis. Finally, DT3 suppressed apoptotic death pathways and modulated autophagic markers, indicating that cytoprotection may have spared haematopoietic stem and progenitor cells from radiation clastogenicity.
Acknowledgments
The authors thank Dr Mark H. Whitnall for critical reviews, Dr Cara Olsen for statistical support and Mr Harley Clinton for haematological analysis of blood samples.
Footnotes
This study was supported by grants from the National Institute of Allergy and Infectious Diseases and Defense Threat Reduction Agency.
References
- 1.Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Internal Med 2004;140:1037–51 [DOI] [PubMed] [Google Scholar]
- 2.Coleman CN, Stone HB, Moulder JE, Pellmar TC. Medicine. Modulation of radiation injury. Science 2004;304:693–4 [DOI] [PubMed] [Google Scholar]
- 3.Moulder JE. Post-irradiation approaches to treatment of radiation injuries in the context of radiological terrorism and radiation accidents: a review. Int J Radiat Biol 2004;80:3–10 [DOI] [PubMed] [Google Scholar]
- 4.Weiss JF, Landauer MR. History and development of radiation-protective agents. Int J Radiat Biol 2009;85:539–73 [DOI] [PubMed] [Google Scholar]
- 5.Satyamitra MM, Kulkarni S, Ghosh SP, Mullaney CP, Condliffe D, Srinivasan V. Hematopoietic recovery and amelioration of radiation-induced lethality by the vitamin E isoform delta-tocotrienol. Radiat Res 2011;175:736–45 [DOI] [PubMed] [Google Scholar]
- 6.Li XH, Fu D, Latif NH, Mullaney CP, Ney PH, Mog SR, et al. Delta-tocotrienol protects mouse and human hematopoietic progenitors from gamma-irradiation through extracellular signal-regulated kinase/mammalian target of rapamycin signaling. Haematologica 2010;95:1996–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cecconi F, Levine B. The Role of Autophagy in Mammalian development: cell makeover rather than cell death. Dev Cell 2008;15:344–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Har CH, Keong CK. Effects of tocotrienols on cell viability and apoptosis in normal murine liver cells (BNL CL.2) and liver cancer cells (BNL 1ME A.7R.1), in vitro. Asia Pac J Clin Nutr 2005;14:374–80 [PubMed] [Google Scholar]
- 9.Shun MC, Yu W, Gapor A, Parsons R, Atkinson J, Sanders BG, et al. Pro-apoptotic mechanisms of action of a novel vitamin E analog (alpha-TEA) and a naturally occurring form of vitamin E (delta-tocotrienol) in MDA-MB-435 human breast cancer cells. Nutr Cancer 2004;48:95–105 [DOI] [PubMed] [Google Scholar]
- 10.Srivastava JK, Gupta S. Tocotrienol-rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem Biophys Res Commun 2006;346:447–53 [DOI] [PubMed] [Google Scholar]
- 11.Rickmann M, Vaquero EC, Malagelada JR, Molero X. Tocotrienols induce apoptosis and autophagy in rat pancreatic stellate cells through the mitochondrial death pathway. Gastroenterology 2007;132:2518–32 [DOI] [PubMed] [Google Scholar]
- 12.National Research Council Guide for the care and use of laboratory animals. 8th edition. Washington DC: The National Academies Press; 2011 [PubMed] [Google Scholar]
- 13.Nagy K, Courtet-Compondu MC, Holst B, Kussmann M. Comprehensive analysis of vitamin E constituents in human plasma by liquid chromatography-mass spectrometry. Anal Chem 2007;79:7087–96 [DOI] [PubMed] [Google Scholar]
- 14.Osakada F, Hashino A, Kume T, Katsuki H, Kaneko S, Akaike A. Alpha-tocotrienol provides the most potent neuroprotection among vitamin E analogs on cultured striatal neurons. Neuropharmacol 2004;47:904–15 [DOI] [PubMed] [Google Scholar]
- 15.Berger ME, Christensen DM, Lowry PC, Jones OW, Wiley AL. Medical management of radiation injuries: current approaches. Occ Med 2006;56:162–72 [DOI] [PubMed] [Google Scholar]
- 16.Casamassima F, Ruggiero C, Caramella D, Tinacci E, Villari N, Ruggiero M. Hematopoietic bone marrow recovery after radiation therapy: MRI evaluation. Blood 1989;73:1677–81 [PubMed] [Google Scholar]
- 17.Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, et al. Hematopoietic stem cell compartment: Acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys 1995;31:1319–39 [DOI] [PubMed] [Google Scholar]
- 18.Husain K, Francois RA, Yamauchi T, Sebti S, Malafa MP. Delta-tocotrienol is the most bioactive natural tocotrienol in the prevention of pancreatic cancer transformation. Am Assoc Cancer Res 2008;49:3826 [Google Scholar]
- 19.Yap SP, Yuen KH, Wong JW. Pharmacokinetics and bioavailability of alpha-, gamma- and delta-tocotrienols under different food status. J Pharm Pharmacol 2001;53:67–71 [DOI] [PubMed] [Google Scholar]
- 20.Gogu SR, Lertora JJ, George WJ, Hyslop NE, Agrawal KC. Protection of zidovudine-induced toxicity against murine erythroid progenitor cells by vitamin E. Exp Hematol 1991;19:649–52 [PubMed] [Google Scholar]
- 21.Bartholomew A, Latshaw D, Swayne DE. Changes in blood chemistry, hematology, and histology caused by a selenium/vitamin E deficiency and recovery in chicks. Biol Trace Elem Res 1998;62:7–16 [DOI] [PubMed] [Google Scholar]
- 22.Cherdyntseva N, Shishkina A, Butorin I, Murase H, Gervas P, Kagiya TV. Effect of tocopherol-monoglucoside (TMG), a water-soluble glycosylated derivate of vitamin E, on hematopoietic recovery in irradiated mice. J Radiat Res (Tokyo) 2005;46:37–41 [DOI] [PubMed] [Google Scholar]
- 23.Husain K, Francois RA, Hutchinson SZ, Neuger AM, Lush R, Coppola D, et al. Vitamin E delta-tocotrienol levels in tumor and pancreatic tissue of mice after oral administration. Pharmacology 2009;83:157–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trudel G, Payne M, Mädler B, Ramachandran N, Lecompte M, Wade C, et al. Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. J Appl Physiol 2009;107:540–8 [DOI] [PubMed] [Google Scholar]
- 25.Brown JM, Hall EJ, Hirst DG, Kinsella TJ, Kligerman MM, Mitchell JB, et al. Chemical modification of radiation and chemotherapy. Am J Clin Oncol 1988;11:288–303 [DOI] [PubMed] [Google Scholar]
- 26.Ghosh SP, Kulkarni S, Hieber K, Toles R, Romanyukha L, Kao TC, et al. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int J Radiat Biol 2009;85:598–606 [DOI] [PubMed] [Google Scholar]
- 27.Wambi C, Sanzari J, Wan XS, Nuth M, Davis J, Ko YH, et al. Dietary antioxidants protect hematopoietic cells and improve animal survival after total-body irradiation. Radiat Res 2008;169:384–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall EJ, Giaccia AJ. Radiobiology for the radiologist. Philadelphia, PA: Lippincott Williams & Wilkins; 2006 [Google Scholar]
- 29.Goodman JW, Smith LH. Erythrocyte life span in normal mice and in radiation bone marrow chimeras. Am J Physiol 1961;200:764–70 [DOI] [PubMed] [Google Scholar]
- 30.MacVittie TJ, Farese AM, Herodin F, Grab LB, Baum CM, McKearn JP. Combination therapy for radiation-induced bone marrow aplasia in nonhuman primates using synthokine SC-55494 and recombinant human granulocyte colony-stimulating factor. Blood 1996;87:4129–35 [PubMed] [Google Scholar]
- 31.Mac Manus M, Lamborn K, Khan W, Varghese A, Graef L, Knox S. Radiotherapy-associated neutropenia and thrombocytopenia: analysis of risk factors and development of a predictive model. Blood 1997;89:2303–10 [PubMed] [Google Scholar]
- 32.Wada S, Satomi Y, Murakoshi M, Noguchi N, Yoshikawa T, Nishino H. Tumor suppressive effects of tocotrienol in vivo and in vitro. Cancer Lett 2005;229:181–91 [DOI] [PubMed] [Google Scholar]
- 33.McIntyre BS, Briski KP, Tirmenstein MA, Fariss MW, Gapor A, Sylvester PW. Antiproliferative and apoptotic effects of tocopherols and tocotrienols on normal mouse mammary epithelial cells. Lipids 2000;35:171–80 [DOI] [PubMed] [Google Scholar]
- 34.Agarwal MK, Agarwal ML, Athar M, Gupta S. Tocotrienol-rich fraction of palm oil activates p53, modulates Bax/Bcl2 ratio and induces apoptosis independent of cell cycle association. Cell Cycle 2004;3:205–11 [DOI] [PubMed] [Google Scholar]
- 35.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006;25:4798–811 [DOI] [PubMed] [Google Scholar]
- 36.Jeong HS, Choi HY, Lee ER, Kim JH, Jeon K, Lee HJ, et al. Involvement of caspase-9 in autophagy-mediated cell survival pathway. Biochim Biophys Acta 2011;1813:80–90 [DOI] [PubMed] [Google Scholar]
- 37.Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ 2005;12Suppl. 2:1528–34 [DOI] [PubMed] [Google Scholar]
- 38.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ 2005;12Suppl. 2:1542–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ 2005;12Suppl. 2:1509–18 [DOI] [PubMed] [Google Scholar]
- 40.Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res 2001;61:439–44 [PubMed] [Google Scholar]
- 41.Kim H, Bernard ME, Flickinger J, Epperly MW, Wang H, Dixon TM, et al. The autophagy-inducing drug carbamazepine is a radiation protector and mitigator. Int J Radiat Biol 2011;87:1052–60 [DOI] [PMC free article] [PubMed] [Google Scholar]