Abstract
Objectives
The objective of this study was to pool the lymph node metastasis rate (LNMR) in patients with thoracic oesophageal cancer (TOC) and to determine which node level should be included when undergoing radiation therapy.
Methods
Qualified studies were identified on Medline, Embase, CBM and the Cochrane Library through to the end of April 2011. Pooled estimates of LNMR were obtained through a random-effect model. Possible effect modifiers which might lead to the statistical heterogeneity were identified through meta-regression, and further subgroup analyses of factors influencing LNMR were performed.
Results
45 observational studies with a total of 18 415 patients were included in the meta-analysis. The pooled estimates of LNMR in upper, middle and lower TOC were 30.7%, 16.8% and 11.0% cervical, 42.0%, 21.1% and 10.5% upper mediastinal, 12.9%, 28.1% and 19.6% middle mediastinal, 2.6%, 7.8% and 23.0% lower mediastinal, and 9%, 21.4% and 39.9% abdominal, respectively. Lymph node metastasis most frequently happened to paratracheal, paraoesophageal, perigastric 106recR and station 7. The most obvious difference (≥15%) of LNMR between two-field and three-field lymphatic dissection occurred in cervical, paratracheal, 106recR and 108.
Conclusions
Through the meta-analysis, more useful information was obtained about clinical target volume (CTV) delineation of TOC patients treated with radiotherapy. However, our study is predominantly a description of squamous carcinoma and the results may not be valid for adenocarcinoma.
Globally, oesophageal carcinoma is the eighth most common malignancy and sixth most fatal, with approximately 460 000 new diagnoses and >380 000 deaths annually [1]. In Asia (especially China) it usually occurs as squamous cell carcinoma in the middle or upper third of the oesophagus. But in Europe and the United States, most oesophageal tumours are adenocarcinomas and most commonly arise in the distal end of the oesophagus and at the gastro-oesophageal junction [2]. Among the various treatment modalities, surgery is still the mainstay of treatment for potentially resectable oesophageal carcinoma, but consensus has not been reached on surgical approach and extent of lymph node dissection [3], that is, two-field dissection (2FLD, including mediastinal and abdominal stations) vs three-field dissection (3FLD, including cervical, mediastinal and abdominal stations).
However, surgery is inappropriate in 40–60% of patients, because most oesophageal carcinomas are in an advanced stage at diagnosis [4], and there is no clear evidence for the superiority of surgery over primary (chemo-)radiotherapy for these patients. Despite some controversy about whether post-operative radiotherapy improves survival in all cases [5,6], there seems to be a survival benefit in cases involving lymph node metastasis [7]. In patients with thoracic oesophageal carcinoma (TOC), the locoregional recurrence is still the main reason for failure [8], and the dismal prognosis primarily attributes to lymph node metastasis. As reported by many studies [9-11], the lymph node metastasis rate (LNMR) was affected by invasion depth, lymphatic vessel invasion, length, histological type and differentiation of the tumour. The sample sizes and LNMRs reported in different studies varied, so it is difficult for clinical oncologists to reach an agreement on the pattern of lymph node metastasis of TOC and to determine the optimal radiotherapy target volumes. To date, the common radiotherapy target volume for TOC has been studied often, including:
large T-shaped, bilateral supraclavicular areas, the whole mediastinum and left gastric lymph nodes included [12]
bilateral supraclavicular areas and mediastinum [13]
tumour bed only [14]
5–8 cm outside the tumour bed vertically and 2 cm horizontally without prophylactic irradiation of bilateral supraclavicular areas [15]
a small T-shaped field including bilateral lower cervical, supraclavicular areas, and the upper portion of the mediastinum [16].
Exploratory meta-analysis of observational studies may provide useful information to understand and quantify sources of variability in results across studies and become a method for assessing efficacy and effectiveness [17] of a treatment. In this work, we conducted a meta-analysis by pooling the reported LNMR data to determine the clinical parameters that may be used in the current clinical practice for the target delineation of post-operative or radical radiotherapy for patients with TOC.
Methods and materials
This meta-analysis was performed in accordance with the guidelines proposed by the Meta-analysis Of Observational Studies in Epidemiology group (MOOSE) [18].
Literature search strategy
Medline (1950–2011), EMBASE (1974–2011), CBM (1978–2011) and the Cochrane Library were searched to identify relevant published articles. The search included the following terms: ((“lymph nodes” [mesh])) AND (“esophageal neoplasm/pathology” [mesh] OR “esophageal neoplasm/radiotherapy” [mesh] OR “esophageal neoplasm/surgery” [mesh]). The computer search was supplemented with manual searches for reference lists of full text articles.
Selection criteria
Lists of articles identified through the above search strategy were further assessed. An article was included in the subsequent analysis if the following criteria were satisfied:
it included thoracic oesophageal carcinoma
it included patients undergoing surgical treatment, two-field dissection or three-field dissection
it described the lymph node metastasis of different sites in detail
it had been published
it included 50 or more patients.
To decide whether a study qualified for the analysis, two reviewers applied the inclusion criteria, to ensure the judgment is reproducible.
Data extraction
Relevant data from the qualified studies were extracted by two investigators (XD and JZ) independently. To resolve disagreement between reviewers, a third reviewer assessed all discrepant items and the majority opinion was used for analysis. The LNMRs of total, upper, middle, and lower TOC and of every detailed site were extracted from each study. Lymph nodes were named according to the guideline of the Japanese Society for Esophageal Diseases (JSED) [19], among which paraoesophageal nodes include 105, 108 and 110, paratracheal nodes include cervical and thoracic paratracheal nodes, and perigastric nodes include stations 1, 2, 3 and 4 (Table 1).
Table 1. Terminology of regional lymph node in oesophageal carcinoma by the Japanese Society for Esophageal Diseases.
| Numbering | Cervical and mediastinal lymph nodes | Numbering | Abdominal lymph nodes |
| 100 | Superficial cervical | 1 | Right cardiac |
| 101 | Paraoesophageal | 2 | Left cardiac |
| 102 | Deep cervical | 3 | Lesser curvature |
| 103 | Peripharygeal | 4 | Greater curvature |
| 104 | Supraclavicular | 7 | Left gastric artery |
| 105 | Upper thoracic paraoesophageal | 8 | Common hepatic artery |
| 106 | Thoracic paratracheal | 9 | |
| 106rec | Recurrent nerve | 10 | Coeliac artery |
| 106pre | Pretracheal | 11 | Splenic hilar |
| 106tb | Tracheobronchial | Splenic artery | |
| 107 | Subcarinal | ||
| 108 | Middle thoracic paraoesophageal | ||
| 109 | Main bronchus | ||
| 110 | Lower thoracic paraoesophageal | ||
| 111 | Supradiaphragmatic | ||
| 112 | Posterior mediastinal |
Data and statistical analysis
To obtain the pooled estimates of LNMR, the data were combined using a fixed-effects model if the results appeared homogeneous. If significant heterogeneity existed (if the χ2 test for homogeneity had p<0.10) after careful verification of the data, a random-effect meta-analysis was performed and reasons for the heterogeneity were explored through a subgroup analysis or meta-regression analysis. In addition, publication bias was assessed using tests of funnel plot asymmetry [20]. Missing data on the characteristics of the studies were handled with Rubin's multiple imputation [21].
The data were analysed in SPSS v. 19.0 (IBM Inc, Armonk, NY), and meta-analysis and meta-regression were performed in Comprehensive Meta Analysis Version 2 (Englewood, NJ).
Results
Selected articles and description of the studies
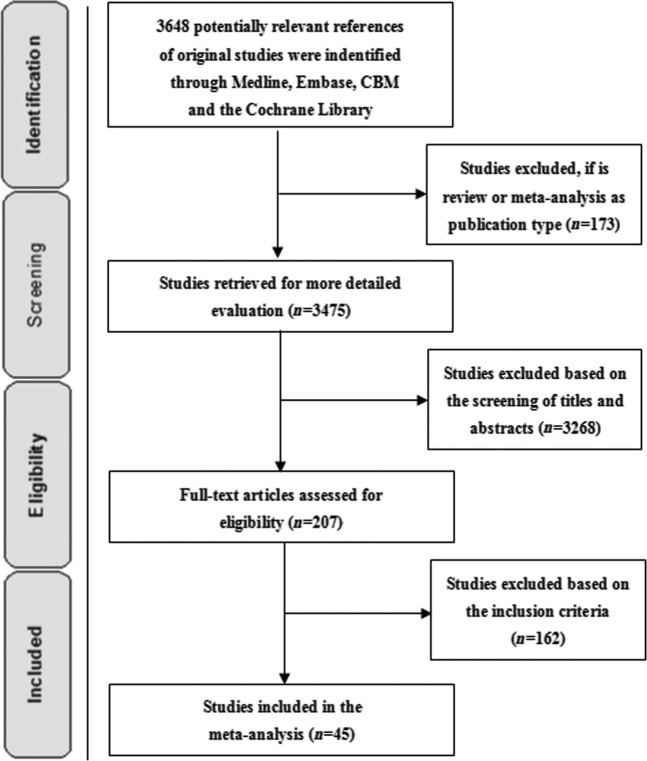
The search is illustrated in a flow diagram (Figure 1). Potentially relevant reports included 3648 articles, of which 45 [3,9-11,22-62] (n=18 415) satisfied the selection criteria. In so far as different articles and authors reported on patients in the same study at different time [25,63], only the article with detailed data was permitted, to prevent duplication [20], and the data of the same population in different studies were integrated artificially [31,32]. If the same author reported different patient populations in the same article, it was included as two studies [3]. Study characteristics for the included studies are summarised in Table 2. General characteristics for each study are listed in Table 3. No randomised controlled trials (RCTs) were found. Of the 45 studies, 29 were done in China, 11 in Japan, 4 in Europe and 1 in Russia.
Figure 1.
Flow diagram of the search results in this meta-analysis.
Table 2. Patient and tumour characteristics of 45 studies.
| Characteristic | n | % |
| No. of patients | 18 415 | |
| Sex | ||
| Male | 12 950 | 70.3 |
| Female | 5465 | 29.7 |
| Mean age | 57.6 | |
| Histology | ||
| Squamous | 92.5 | |
| Good differentiation | 22.8 | |
| Moderate differentiation | 57.3 | |
| Poor differentiation | 19.9 | |
| Non-squamous | 7.5 | |
| Tumour location | ||
| Upper | 2350 | 12.8 |
| Middle | 11 366 | 61.7 |
| Lower | 4699 | 25.5 |
| Surgical approach | ||
| Two-field | 50.3 | |
| Three-field | 49.7 | |
| Mean nodes per patient | 18.2 | |
| T stage | ||
| Tis–T1 | 11.0 | |
| T2 | 24.0 | |
| T3 | 50.3 | |
| T4 | 14.7 |
Table 3. Study and population characteristics for studies of thoracic oesophageal carcinoma.
| Study | Year | Country | Sample size | Male (%) | Mean age (years) | Mean lymph nodes | Squamous cell carcinoma (%) | Three-field dissection (%) | Upper (%) | Middle (%) | Lower (%) |
| Kawaguchi et al [22] | 1973 | Japan | 65 | 82.2 | 60.0 | 20.0 | 100.0 | 100.0 | 0 | 69.2 | 30.8 |
| Matsubara [23] | 1992 | Japan | 110 | UR (62.0) | UR (54.9) | UR (24.0) | UR (75.9) | 100.0 | 16.4 | 62.7 | 20.9 |
| Kakegawa et al [24] | 1991 | Japan | 715 | UR (78.6) | UR (54.4) | UR (14.2) | UR (96.9) | 100.0 | 11.0 | 63.1 | 25.9 |
| Fujita et al [25] | 1994 | Japan | 70 | 88.6 | 58.5 | 81.8 | 100.0 | 100.0 | 14.3 | 52.9 | 32.8 |
| Sayama et al [26] | 1994 | Japan | 226 | 85.0 | 61.4 | UR (55.5) | 95.1 | UR (100.0) | 7.5 | 56.2 | 36.3 |
| Shao et al [27] | 1994 | China | 216 | 74.9 | UR (62.8) | 7.5 | 94.0 | UR (7.4) | 18.1 | 81.9 | 0 |
| Su et al [28] | 1994 | China | 175 | UR (55.3) | UR (57.7) | 16.3 | UR (63.7) | 21.7 | 21.7 | 64.6 | 13.7 |
| Fu and Lian [29] | 1996 | China | 1063 | 77.8 | 54.0 | UR (26.7) | 100.0 | UR (99.1) | 4.1 | 72.9 | 23.0 |
| Bhansali et al [30] | 1997 | Japan | 90 | 90.0 | 58.0 | UR (59.6) | 100.0 | 100.0 | 12.2 | 54.5 | 33.3 |
| Nishimaki et al [31,32] | 1997 | Japan | 154 | 92.9 | 60.5 | 77.2 | 94.8 | 100.0 | 6.5 | 51.3 | 42.2 |
| Wang et al [33] | 1997 | China | 130 | 86.2 | 57.5 | UR (40.2) | 90.8 | UR (57.8) | 47.7 | 52.3 | 0 |
| She et al [34] | 1998 | China | 230 | 76.1 | 56.4 | 25.0 | 100.0 | 100.0 | 23.5 | 63.5 | 13.0 |
| Guo et al [35] | 1999 | China | 616 | 63.0 | UR (52.6) | UR (6.5) | 100.0 | 100.0 | 10.3 | 72.4 | 17.3 |
| Zheng et al [36] | 1999 | China | 988 | 59.2 | 56.3 | UR (3.4) | 95.1 | 0 | 12.1 | 66.1 | 21.8 |
| Wang et al [37] | 2000 | China | 243 | 72.8 | UR (61.0) | 15.7 | 100.0 | 5.3 | 17.3 | 57.2 | 25.5 |
| Xiang et al [38] | 2001 | China | 100 | 79.0 | 56.1 | 30.1 | 91.0 | 100.0 | 14.3 | 75.5 | 10.2 |
| Dresner et al [39] | 2001 | UK | 104 | 86.5 | 62.9 | 22.0 | 0.0 | 0 | 0 | 0 | 100.0 |
| Sato et al [40] | 2002 | Japan | 155 | 82.0 | 63.9 | 41.7 | 100.0 | 51.6 | 12.3 | 64.5 | 23.2 |
| An et al [41] | 2003 | China | 217 | 66.8 | 56.0 | 18.4 | 100.0 | 100.0 | 18.9 | 54.4 | 26.7 |
| He et al [42] | 2003 | China | 150 | 78.7 | UR (66.0) | UR (27.5) | 100.0 | 4.7 | 14.0 | 58.7 | 27.3 |
| Nakagawa et al [43] | 2003 | Japan | 199 | 91.5 | 61.0 | UR (52.0) | 96.5 | 100.0 | 8.0 | 59.8 | 32.2 |
| Stilidi et al [44] | 2003 | Russia | 147 | 76.9 | 57.0 | 43.0 | 94.6 | 0 | 8.8 | 58.5 | 32.7 |
| Li et al [45] | 2004 | China | 104 | 63.5 | 56.8 | UR (34.2) | 100.0 | 100.0 | 100.0 | 0 | 0 |
| Lerut et al [46] | 2004 | Belgium | 174 | 85.6 | 59.3 | 59.2 | 44.8 | 100.0 | 6.5 | 30.4 | 63.1 |
| Liu et al [47] | 2005 | China | 472 | 77.8 | 57.0 | 21.7 | 94.3 | 100.0 | 11.2 | 71.4 | 17.4 |
| Feith et al [43] | 2006 | Germany | 621 | 63.0 | 61.0 | UR (7.3) | 0 | 0 | 0 | 0 | 100.0 |
| Xu et al [49] | 2007 | China | 308 | 63.0 | 57.5 | UR (14.8) | 100.0 | 100.0 | 10.4 | 73.4 | 16.2 |
| Li et al [9] | 2007 | China | 230 | 79.6 | 55.6 | 25.3 | 100.0 | 100.0 | 15.7 | 65.2 | 19.1 |
| Sun et al [50] | 2007 | China | 152 | 58.6 | 56.1 | 21.1 | 100.0 | 100.0 | 23.7 | 51.3 | 25.0 |
| Fang et al [51] | 2007 | China | 87 | 78.2 | 59.0 | 12.4 | 100.0 | 59.8 | 27.6 | 60.9 | 11.5 |
| Xue et al [52] | 2007 | China | 1412 | 51.0 | 58.0 | UR (14.5) | 98.2 | 0 | 14.3 | 68.8 | 16.9 |
| Xiao et al [53] | 2008 | China | 549 | UR (71.0) | UR (58.8) | 17.0 | 100.0 | 0 | 13.1 | 66.1 | 20.8 |
| Liu et al [54] | 2008 | China | 886 | 72.1 | 58.2 | 6.7 | 94.7 | UR (38.6) | 12.1 | 72.7 | 15.2 |
| Wang et al [55] | 2008 | China | 161 | 59.0 | 56.0 | UR (6.6) | 90.1 | 0 | 19.9 | 55.3 | 24.8 |
| Meier et al [56] | 2008 | Germany | 111 | 82.5 | 63.0 | 37.0 | 0 | 0 | 0 | 0 | 100.0 |
| Chen et al [11] | 2009 | China | 1850 | 73.0 | 55.0 | 26.0 | 100.0 | 100.0 | 15.6 | 74.7 | 9.7 |
| Zhu et al [57] | 2009 | China | 1690 | 53.6 | 57.3 | UR (6.5) | 98.0 | 0 | 14.0 | 65.0 | 21.0 |
| Yang et al [58] | 2010 | China | 160 | 70.6 | UR (63.1) | UR (3.6) | 100.0 | 0 | 18.8 | 55.0 | 26.2 |
| Abula et al [59] | 2010 | China | 215 | 74.4 | 61.0 | 21.9 | 100.0 | 100.0 | 9.6 | 57.7 | 32.7 |
| Zhao et al [60] | 2010 | China | 612 | 88.9 | 58.0 | 25.0 | 100.0 | 11.9 | 2.0 | 48.9 | 49.1 |
| Huang et al [10] | 2010 | China | 1077 | 78.1 | UR (60.2) | 19.7 | 100.0 | UR (36.7) | 5.0 | 63.2 | 31.8 |
| Wu et al [61] | 2010 | China | 262 | 81.7 | 55.2 | 22.0 | 94.3 | 100.0 | 36.6 | 48.1 | 15.3 |
| Li et al [62] | 2010 | China Japan | 763 | 68.9 | 59.0 | 7.7 | 93.4 | 11.1 | 15.4 | 61.5 | 23.1 |
| Tachimori et al [3] | 2011 | Japan | 127 | 90.6 | 62.7 | UR (19.3) | 100.0 | 100.0 | 17.3 | 52.8 | 29.9 |
| Tachimori et al [3] | 2011 | 229 | 86.9 | 62.8 | UR (64.0) | 100.0 | 100.0 | 14.4 | 46.3 | 39.3 |
UR, unreported (multiple imputation value in parentheses).
Exploration of influencing baseline characteristics
Introducing the characteristics of the studies, our meta-regression analysis showed that the differences in the mean number of resected lymph nodes, the percentage of male patients, the percentage of patients with 3FLD and the percentage of patients with middle or low TOC statistically significantly influenced the regression coefficients.
Pooled lymph node metastasis rates in different site and subgroup analysis
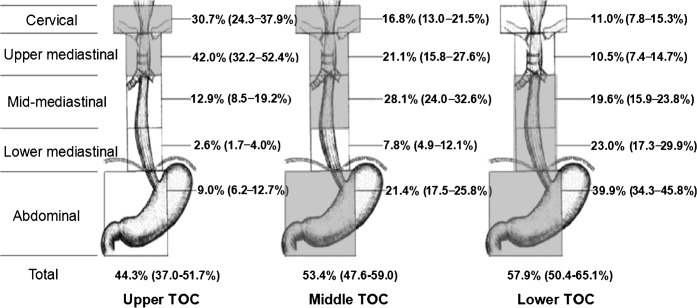
The LNMR of TOC patients in five node levels is shown in Figure 2. The commonly metastatic areas were cervical, upper and mid-mediastinal nodes in upper TOC (30.7%, 42.0% and 12.9%, respectively); cervical, upper, mid-mediastinal and abdominal nodes in mid-TOC (16.8%, 21.1%, 28.1% and 21.4%, respectively); and cervical, upper, middle and lower mediastinal and abdominal nodes in lower TOC (11.0%, 10.5%, 19.6%, 23.0% and 39.9%, respectively). The areas with LNMR higher than 15% are also marked with shadow in Figure 2. The LNMRs in the subgroups of nodes (JSED) are listed in Table 4 in descending order.
Figure 2.
Pooled lymph node metastasis rates (95% confidence interval) in five lymph node regions for thoracic oesophageal carcinoma (TOC). Areas in shadow represent high-risk nodal sites with lymph node metastasis rates >15%.
Table 4. Pooled lymph node metastasis rates (%) in lymph node subgroups defined by JSED.
| Order of rates | TOC |
Upper TOC |
Middle TOC |
Lower TOC |
||||
| Subgroup | % | Subgroup | % | Subgroup | % | Subgroup | % | |
| 1 | Paratracheal | 31.7 | Paratracheal | 43.3 | Paraoesophageal | 28.7 | Perigastric | 47.9 |
| 2 | Perigastric | 30.0 | 106recR | 35.6 | Perigastric | 26.3 | 1+2 | 42.4 |
| 3 | Paraoesophageal | 28.4 | 101 | 23.3 | 108 | 23.6 | 7 | 36.3 |
| 4 | 106recR | 24.3 | 105 | 20.5 | 106recR | 23.0 | Paraoesophageal | 34.8 |
| 5 | 7 | 22.6 | 101R | 18.5 | Paratracheal | 20.1 | 1 | 33.4 |
| 6 | 1 | 22.4 | 104 | 18.0 | 7 | 19.7 | 2 | 27.9 |
| 7 | 1+2 | 18.3 | 106recL | 17.7 | 107 | 18.6 | 3 | 27.6 |
| 8 | 107 | 16.7 | Paraoesophageal | 16.9 | 1 | 17.6 | 110 | 22.6 |
| 9 | 2 | 16.4 | 106 | 15.2 | 1+2 | 16.6 | Paratracheal | 18.2 |
| 10 | 104 | 15.7 | 106pre | 13.2 | 109 | 12.9 | 9 | 14.4 |
| 11 | 109 | 13.8 | 101L | 13.0 | 106 | 12.0 | 108 | 12.7 |
| 12 | 3 | 13.0 | 107 | 11.5 | 2 | 11.8 | 10 | 12.6 |
| 13 | 110 | 11.8 | 109 | 11.4 | 3 | 10.8 | 107 | 12.4 |
| 14 | 106recL | 11.5 | 108 | 11.2 | 110 | 10.4 | 106recR | 12.1 |
| 15 | 101 | 11.0 | 106tbL | 9.6 | 101 | 9.9 | 112 | 11.8 |
| 16 | 112 | 10.3 | Perigastric | 8.4 | 104 | 9.7 | 11 | 8.3 |
| 17 | 105 | 8.2 | 1+2 | 7.7 | 105 | 7.5 | 101 | 7.7 |
| 18 | 101R | 7.4 | 104R | 7.7 | 112 | 7.5 | 4 | 6.7 |
| 19 | 106pre | 6.5 | 110 | 6.2 | 106recL | 7.1 | 109 | 6.5 |
| 20 | 106tbL | 6.5 | 1 | 5.3 | 101R | 6.6 | 111 | 5.8 |
| 21 | 109R | 5.8 | 104L | 5.3 | 104R | 5.6 | 104 | 5.6 |
| 22 | 11 | 5.6 | 10 | 5.3 | 101L | 5.2 | 106tbL | 5.1 |
| 23 | 101L | 5.4 | 7 | 4.8 | 104L | 5.1 | 101R | 4.4 |
| 24 | 9 | 5.2 | 9 | 4.7 | 106pre | 4.9 | 109R | 4.4 |
| 25 | 104L | 4.7 | 102L | 4.3 | 11 | 4.8 | 106recL | 3.9 |
| 26 | 104R | 4.6 | 2 | 4.2 | 109L | 4.2 | 105 | 3.8 |
| 27 | 106 | 4.4 | 112 | 3.9 | 106tbL | 3.7 | 109L | 3.7 |
| 28 | 109L | 3.7 | 102R | 3.4 | 9 | 3.7 | 104R | 3.3 |
| 29 | 8 | 3.7 | 3 | 3.2 | 109R | 3.7 | 106pre | 3.2 |
| 30 | 102R | 2.9 | 109R | 3.0 | 8 | 3.2 | 101L | 3.1 |
| 31 | 102L | 2.8 | 11 | 2.7 | 102R | 2.2 | 106 | 3.0 |
| 32 | 108 | 2.0 | 109L | 2.6 | 102L | 1.3 | 104L | 3.0 |
| 33 | 111 | 1.8 | 111 | 2.5 | 111 | 1.2 | 102R | 2.0 |
| 34 | 10 | 1.8 | 8 | 1.4 | 10 | 1.0 | 102L | 1.7 |
| 35 | 4 | 0.9 | 4 | 0 | 4 | 0.6 | 8 | 0.8 |
JSED, Japanese Society for Esophageal Diseases; LNMR, lymph node metastasis rate; TOC, thoracic oesophageal cancer.
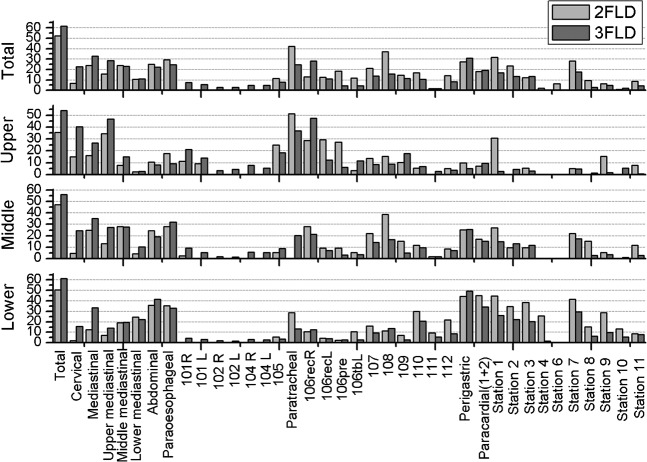
LNMRs in different sites of the TOC patients undergoing 2FLD and 3FLD are shown in Figure 3. The total LNMR of 3FLD patients was generally higher than that of 2FLD patients (61.5% vs 52.4%). This difference was especially significant in the LNMR of cervical and upper mediastinal nodes in all parts of thoracic oesophagus.
Figure 3.
Lymph node metastasis rate percentages in different sites of 2FLD and 3FLD subgroups. 2FLD, two-field lymphatic dissection; 3FLD, three-field lymphatic dissection.
Furthermore, the results of further subgroup analysis about T stage, pathological type and differentiation are shown in Table 5. First, as the T stage increased, higher pooled LNMR followed with 28.0%, 46.2%, 61.0% and 72.9% in patients with T1 to T4, respectively. Second, the pooled LNMR estimates of squamous cell carcinoma were higher than adenocarcinoma (61.4% vs 57.6%). Third, LNMRs in TOC from well to poor differentiation had a tendency to increase (37.4%, 52.8% and 67.5%, respectively), and the LNMR in other differentiation TOC was 57.7%.
Table 5. Lymph node metastasis rate percentages in T classification, histology type and differentiation subgroups.
| T classification | % LNMR | n/N | Histology type | % LNMR | n/N | Differentiation | % LNMR | n/N |
| Tis–T1 | 28.0 | 15/586 | Squamous cell carcinoma | 57.6 | 21/7879 | Well | 37.4 | 6/597 |
| T2 | 46.2 | 16/1531 | Moderate | 52.8 | 6/1474 | |||
| T3 | 61.0 | 17/3587 | Adenocarcinoma | 61.4 | 3/836 | Poor | 67.5 | 6/509 |
| T4 | 72.9 | 15/1084 | Others | 57.7 | 6/122 |
LNMR, lymph node metastasis rate; n/N, the number of studies referred to the characteristic/the total number of patients in the referred studies.
Analysis of publication bias
The publication bias was assessed through testing of funnel plot asymmetry. This analysis gives no indication of publication bias (classic fail-safe N ranged from 87 to 6086).
Discussion
Implications for radiotherapy
Generally, surgery, post-operative radiotherapy and definitive (chemo-)radiotherapy are frequently used treatments in oesophageal cancer. For patients treated with radiotherapy, accurate delivery is crucial, as inaccurate or inappropriate nodal target volumes potentially will lead to locoregional recurrence due to missed nodes within a clinical target volume (CTV) or excess toxicity due to unnecessarily large treatment volumes. Commonly, the gross tumour volume includes the primary tumour and positive lymph nodes (with diameter ≥1 cm on CT scan), while the CTV, including subclinical invasion and high-risk lymph nodes, is currently defined as 5 cm outside the tumour bed vertically and 1.5–2 cm horizontally [64]. However, global consensus is lacking on which high-risk nodal levels should be prophylactically irradiated. From the meta-analysis, it is expected to obtain useful information on how to define CTV of patients with TOC who will undergo definitive (chemo-)radiotherapy or post-operative radiotherapy.
In our present study, those sites with LNMR > 15%, an empirical cut-off value, were considered as high-risk areas and should be involved in the target volume of patients with TOC. For patients who will undergo definitive (chemo-)radiotherapy, we suggest that cervical and upper mediastinal nodes should be included in the CTV, especially lymph nodes of 101 (especially 101R), 104, 105 and 106 (especially 106 RecR) for the upper TOC. This result is in accordance with Nishimura et al's small T-shaped field radiation [16]. Tumours located in the mid-oesophagus can skip not only up to the cervical lymph nodes, but also down to the abdomen. Thus, the CTV for middle TOC should include cervical, upper, middle mediastinal and abdominal portions, especially 106 (106 recR), 107, 108, stations 1, 2 and 7 lymph nodes. As to the lower TOC from our results, the CTV should cover the middle, lower mediastinal and abdominal regions, especially including lymph nodes of 110, stations 1, 2, 3 and 7, which is consistent with the CTV definition in our institution [10]. Moreover, TOC patients of every site suffered high lymph node metastasis in paraoesophageal and paratracheal portion, from 16.9% to 34.8% and from 18.2% to 43.3%, respectively, which conformed to the nearest transferred pattern of TOC lymphatic metastasis.Thus, it is necessary to note that no matter which part of oesophagus the tumour is located in, the corresponding paraoesophageal and paratracheal nodes are considered to be covered in the CTV.
It is reported that for each part of TOC, the LNMR of cervical and upper mediastinal lymph nodes is high [8]. The anatomical complexity of the lower neck and upper mediastinum, being rich in lymphatic vessels and nerves with large blood vessels adjacent to organs, makes the exposure of lymph nodes inadequate during surgery. Therefore, completely resecting involved nodes seems to be impractical, the subclinical lesions remain, and result in lymph node metastasis and recurrence [65]. For this reason, cervical and upper mediastinal lymph nodes theoretically need to be irradiated for all TOC patients. However, our meta-analysis showed that LNMR of cervical and upper mediastinal sites for lower TOC were 11.0% and 10.5%, respectively. Although these two sites were once suggested in radiation therapy for lower TOC [65], we advocate that it be free of irradiation because of the toxicity and complications of extensive radiotherapy.
Currently, it is unclear whether the survival after 3FLD can be improved, compared with 2FLD [66], but it is becoming clear that adequate lymph node sampling is beneficial to more accurate staging [67] and lowers the recurrence rate [68]. However, 3FLD has been reported to be invasive, and has a high incidence of complications such as recurrent nerve paralysis [68]. In addition, although there is no evidence from randomised phase III trials to support the use of post-operative radiotherapy, several studies [69-71] have indicated that the locoregional control rate was significantly better for those who had undergone post-operative radiotherapy. For these patient cohorts, the following questions are raised:
Is the CTV of patients with 2FLD different from that of 3FLD?
Is it possible to narrow down the CTV of patients with 3FLD?
In our subgroup analysis results of the LNMRs of TOC patients undergoing 2FLD vs 3FLD, the higher the LNMR is in some sites, the more radical the dissection may be regarded as. For post-operative radiotherapy, we consider it is necessary to focus on some lymph node regions with subgroup differences of more than 15%. For the post-operative patients with 2FLD, cervical and 106recR lymph nodes of upper TOC should receive extra attention, as well as the cervical node for mid-TOC. For the post-operative patients with 3FLD, particular emphasis might be placed on the 106recL, 106pre and station 1 lymph nodes of upper TOC, 108 lymph nodes of mid-TOC, and paratracheal, station 1, 3, 4 and 9 nodes of lower TOC. Because these results obtained from subgroup analysis only describe nodes found at surgery of 2FLD and 3FLD, the role of radiotherapy to the areas outside the standard surgical field cannot be drawn from this work alone. Thus, its implication on the CTV definition of post-operative radiotherapy should be interpreted with caution.
When no RCTs are available, as is the case in the study, the appropriateness of performing a meta-analysis may be challenged because of the differences in baseline characteristics of each study. As with any meta-analysis of observational studies, this review comes with a number of caveats that we acknowledge. We explored the statistical heterogeneity among studies by conducting subgroup analysis or meta-regression. Regarding potential effect modifiers, the meta-regression analysis in the present study found that the differences in the mean number of resected lymph nodes, percentage of male patients, percentage of patients with 3FLD, and the percentage of patients with middle or low TOC significantly influenced the outcome (i.e. the LNMR). Of these, the percentage of middle TOC patients had a negative effect on LNMR (slope coefficient less than zero). Subgroup analysis also suggested that TOC patients with different tumour stages, pathological types, tumour locations, lymphatic dissection ranges and tumour cell differentiations had different LNMRs. Although the analysis of impact of tumour length on the LNMR of TOC patients was unable to be performed because the length data extracted from the included studies were quite non-uniform, it is noteworthy that the impact of tumour length on the LNMR of patients with TOC cannot be ignored [11,72].
Study limitations
Our study also has some limitations and the results may be misleading. First of all, among the 45 studies identified for the analysis, 41 were from Asia and 97.4% had squamous histology. These biased the data towards a population with squamous cell carcinoma in Asian countries. Second, though we conducted subgroup analysis and meta-regression, the results needed to be cautiously interpreted because statistical heterogeneity among studies was significant (I2≤90). Additionally, some subgroup analyses were based on the data of a few studies, which should be re-evaluated in further studies.
In conclusion, through the pooled data of LNMR in TOC patients in the meta-analysis, we obtained some useful information about how to define the CTV of patients with TOC who would undergo definitive (chemo-)radiotherapy or post-operative radiotherapy. However, considering the bias in the initial studies, the heterogeneity among studies and the self-limitation of meta-analysis of observational studies, more evidence is needed to define the CTV delineation for TOC. Currently, the CTV should be delineated individually by experienced oncologists according to different clinical factors influencing lymph node metastasis. In addition, the fields for radiotherapy should be guided by radiological investigations, such as endoscopic ultrasound and positron emission tomography/CT. The relationship between different CTV coverage and survival benefit is expected to be assessed in future RCTs.
Acknowledgment
We thank Dr Allen Li for his critical review of the manuscript.
Footnotes
The first two authors contributed equally to this work.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137–50 [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90 [DOI] [PubMed] [Google Scholar]
- 3.Tachimori Y, Nagai Y, Kanamori N, Hokamura N, Igaki H. Pattern of lymph node metastases of esophageal squamous cell carcinoma based on the anatomical lymphatic drainage system. Dis Esophagus 2011;24:33–8 [DOI] [PubMed] [Google Scholar]
- 4.Daly JM, Fry WA, Little AG, Winchester DP, McKee RF, Stewart AK, et al. Esophageal cancer: results of an American College of Surgeons patient care evaluation study. J Am Coll Surg 2000;190:562–72; discussion 572–3 [DOI] [PubMed] [Google Scholar]
- 5.Zieren HU, Muller JM, Jacobi CA, Pichlmaier H, Muller RP, Staar S. Adjuvant postoperative radiation therapy after curative resection of squamous cell carcinoma of the thoracic esophagus: a prospective randomized study. World J Surg 1995;19:444–9 [DOI] [PubMed] [Google Scholar]
- 6.Xiao ZF, Yang ZY, Miao YJ, Wang LH, Yin WB, Gu XZ, et al. Influence of number of metastatic lymph nodes on survival of curative resected thoracic esophageal cancer patients and value of radiotherapy: report of 549 cases. Int J Radiat Oncol Biol Phys 2005;62:82–90 [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Pan J, Zheng X, Zhu K, Li J, Chen M, et al. Number and location of positive nodes, postoperative radiotherapy, and survival after esophagectomy with three-field lymph node dissection for thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2010;82:475–82 [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa S, Kanda T, Kosugi S, Ohashi M, Suzuki T, Hatakeyama K. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg 2004;198:205–11 [DOI] [PubMed] [Google Scholar]
- 9.Li H, Zhang Y, Cai H, Xiang J. Pattern of lymph node metastases in patients with squamous cell carcinoma of the thoracic esophagus who underwent three-field lymphadenectomy. Eur Surg Res 2007;39:1–6 [DOI] [PubMed] [Google Scholar]
- 10.Huang W, Li B, Gong H, Yu J, Sun H, Zhou T, et al. Pattern of lymph node metastases and its implication in radiotherapeutic clinical target volume in patients with thoracic esophageal squamous cell carcinoma: a report of 1077 cases. Radiother Oncol 2010;95:229–33 [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Liu S, Pan J, Zheng X, Zhu K, Zhu J, et al. The pattern and prevalence of lymphatic spread in thoracic oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg 2009;36:480–6 [DOI] [PubMed] [Google Scholar]
- 12.Mei ZR, Xiang QC, Wu WJ, Jiang XM, Feng CW, Mu HD. Prospective study of prophylactic radiotherapy for postoperative esophageal carcinoma. Chin J Oncol 1997;6:188–9 [Google Scholar]
- 13.Ténière P, Hay J, Fingerhut A, Fagniez P. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter contrilled trial. French University Association for Surgical Research. Surg Gynecol Obstet 1991;173:123–30 [PubMed] [Google Scholar]
- 14.Fok M, Sham J, Choy D, Cheng S, Wong J. Postoperative radiotherapy for carcinoma of the esophagus: a prospective, randomized controlled study. Surgery 1993;113:138–47 [PubMed] [Google Scholar]
- 15.Bedard EL, Inculet RI, Malthaner RA, Brecevic E, Vincent M, Dar R. The role of surgery and postoperative chemoradiation therapy in patients with lymph node positive esophageal carcinoma. Cancer 2001;91:2423–30 [PubMed] [Google Scholar]
- 16.Nishimura Y, Ono K, Imamura M, Hiraoka M, Takahashi M, Abe M, et al. Postoperative radiation therapy for esophageal cancer. Radiat Med 1989;7:88–94 [PubMed] [Google Scholar]
- 17.Blettner M, Sauerbrei W, Schlehofer B, Scheuchenpflug T, Friedenreich C. Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int J Epidemiol 1999;28:1–9 [DOI] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12 [DOI] [PubMed] [Google Scholar]
- 19.Japanese Society for Esophageal Diseases Japanese classification of esophageal cancer, tenth edition: part II and III. Esophagus 2009;6:71–94 [Google Scholar]
- 20.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ 2001;323:101–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med 1991;10:585–98 [DOI] [PubMed] [Google Scholar]
- 22.Kawaguchi M, Fujimaki M, Maeda M, Sasaki K, Soga J. Clinico-pathological study of esophageal neoplasms–with special emphasis on lymphatic metastasis. [In Japanese.] Nihon Kyobu Geka Gakkai Zasshi 1973;21:274–5 [PubMed] [Google Scholar]
- 23.Matsubara T. Pattern of lymphatic spreading in cancer of the thoracic esophagus–analysis in cases undergoing cervical dissection. [In Japanese.] Nihon Geka Gakkai Zasshi 1992;93:377–87 [PubMed] [Google Scholar]
- 24.Kakegawa T, Yamana H, Fujita H, Shirozu G. Recent surgical treatment of thoracic esophageal carcinoma. [In Japanese.] Kyobu Geka 1991;44:1132–40 [PubMed] [Google Scholar]
- 25.Fujita H, Kakegawa T, Yamana H, Shima I, Tanaka H, Ikeda S, et al. Lymph node metastasis and recurrence in patients with a carcinoma of the thoracic esophagus who underwent three-field dissection. World J Surg 1994;18:266–72 [DOI] [PubMed] [Google Scholar]
- 26.Sayama J, Nishihira T, Hirayama K, Shineha R, Mori S. Strategic lymph node dissection for thoracic and abdominal esophageal carcinoma in relation to nodal metastasis and location of carcinoma–analysis of subgroups carcinoma location. [In Japanese.] Nihon Kyobu Geka Gakkai Zasshi 1994;42:477–85 [PubMed] [Google Scholar]
- 27.Shao C, Ye YK, Ge HZ, Wang D, Chen JS, Li XZ. Relationship between carcinoma of upper and midesophagus lymphnode metastases. (Report of 216 cases). Chin J Thoracic Cardiovascular Surg 1994;10:245–247 [Google Scholar]
- 28.Su BH, She ZL, Zhang LD, Ying MG, Zhu SK, Liu SY. Dependence of rational radical surgery of esophageal cancer on the pattern of lymph node metastasis. Chin J Clin Oncol 1994;21:508–12 [Google Scholar]
- 29.Fu JH, Lian JZ. Clinicopathological analysis of lymph node mtastasis of thoracic esophageal cancer. (A report of 1063 cases). Cancer 1996;15:283–4 [Google Scholar]
- 30.Bhansali MS, Fujita H, Kakegawa T, Yamana H, Ono T, Hikita S, et al. Pattern of recurrence after extended radical esophagectomy with three-field lymph node dissection for squamous cell carcinoma in the thoracic esophagus. World J Surg 1997;21:275–81 [DOI] [PubMed] [Google Scholar]
- 31.Nishimaki T, Suzuki T, Tanaka Y, Nakagawa S, Aizawa K, Hatakeyama K. Evaluating the rational extent of dissection in radical esophagectomy for invasive carcinoma of the thoracic esophagus. Surg Today 1997;27:3–8 [DOI] [PubMed] [Google Scholar]
- 32.Nishimaki T, Tanaka O, Suzuki T, Aizawa K, Hatakeyama K, Muto T. Patterns of lymphatic spread in thoracic esophageal cancer. Cancer 1994;74:4–11 [DOI] [PubMed] [Google Scholar]
- 33.Wang CL, Ding T, Feng SS, Zheng BX, Ma YY, Liu FC, et al. Clinical analysis of cervical and upper mediastinal lymph node metastasis of upper and mid-esophageal carcinoma. Shanxi Med J 1997;26:332–3 [Google Scholar]
- 34.She ZL, Zhu SK, Liu SY, Zhang LD. Cervical and upper-mediastinal lymphatic metastases of thoracic esophageal cancer. Chin J Clin Oncol 1998;25:530–2 [Google Scholar]
- 35.Guo ZY, Yu LC, Xu XH, Xu YL. Cervical and upper mediastinal lymph node metastasis in thoracic esophageal carcinoma. Chin J Cardiovasc Surg 1999;15:18–19 [Google Scholar]
- 36.Zheng AP, Feng LJ, Zhang WM, Han XC, Dou YX, Liu JA. Clinico-pathologic study on extra portal lymphatic metastasis in 988 cases of esophageal carcinoma. Chin J Radiat Oncol 1999;8:133–5 [Google Scholar]
- 37.Wang YG, Wang LJ, Zhang DC, Zhang RG, Zhang DW, Meng PJ. Characteristics of lymph node metastasis of squamous cell carcinoma of thoracic esophagus and its clinical significance. Chin J Oncol 2000;22:241–3 [PubMed] [Google Scholar]
- 38.Xiang JQ, Zhang YW, Ji QH, Xiang CH, Hu H, Miu LS, et al. Regularity of lymph node metastasis in 100 patients of thoracic esophageal carcinoma. China Oncol 2001;11:423–4 [Google Scholar]
- 39.Dresner SM, Lamb PJ, Bennett MK, Hayes N, Griffin SM. The pattern of metastatic lymph node dissemination from adenocarcinoma of the esophagogastric junction. Surgery 2001;129:103–9 [DOI] [PubMed] [Google Scholar]
- 40.Sato F, Shimada Y, Li Z, Kano M, Watanabe G, Maeda M, et al. Paratracheal lymph node metastasis is associated with cervical lymph node metastasis in patients with thoracic esophageal squamous cell carcinoma. Ann Surg Oncol 2002;9:65–70 [DOI] [PubMed] [Google Scholar]
- 41.An FS, Huang JQ, Chen SH. Analysis of lymph node metastases of 217 cases of thoracic esophageal carcinoma and its impact on prognosis. Chin J Cancer 2003:974–7 [PubMed] [Google Scholar]
- 42.He JK, Yu SD, Ma HT, Qin Y. Characteristics of lymph node metastasis of thoracic esophageal carcinoma and their clinical significance. Suzhou University J Med Science 2003;23:585–6 [Google Scholar]
- 43.Nakagawa S, Nishimaki T, Kosugi S, Ohashi M, Kanda T, Hatakeyama K. Cervical lymphadenectomy is beneficial for patients with carcinoma of the upper and mid-thoracic esophagus. Dis Esophagus 2003;16:4–8 [DOI] [PubMed] [Google Scholar]
- 44.Stilidi I, Davydov M, Bokhyan V, Suleymanov E. Subtotal esophagectomy with extended 2-field lymph node dissection for thoracic esophageal cancer. Eur J Cardiothorac Surg 2003;23:415–20 [DOI] [PubMed] [Google Scholar]
- 45.Li QJ, Zhang JC, Lu J, Liu P, Guo F, Li J, et al. Evaluation of esophagectomy with three-field lymphadenectomy via right thoracotomy for patient with cervical and thoracic esophageal carcinoma. The Practical J Cancer 2004;19:304–6 [Google Scholar]
- 46.Lerut T, Nafteux P, Moons J, Coosemans W, Decker G, De Leyn P, et al. Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival, and outcome: a plea for adaptation of TNM classification in upper-half esophageal carcinoma. Ann Surg 2004;240:962–72; discussion 972–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu SY, She ZL, Zhu KS. Clinical analysis of 472 patients with three-field lymphadenectomy for thoracic esophageal carcinoma. Fujian Med J 2005;27:38–40 [Google Scholar]
- 48.Feith M, Stein HJ, Siewert JR. Adenocarcinoma of the esophagogastric junction: surgical therapy based on 1602 consecutive resected patients. Surg Oncol Clin N Am 2006;15:751–64 [DOI] [PubMed] [Google Scholar]
- 49.Xu XR, Yu LC, Li F, Shi SB, Jiang ZH, Liu CX. Clinical analysis of three-field lymphadenectomy for thoracic esophageal carcinoma. J Mudanjiang Medical College 2007;28:44–6 [Google Scholar]
- 50.Sun W, Abulizi S, Gao SL. Clinical study in regularity of lymph node metastasis in 152 patients with squamous cell cancer on thoracic esophageal carcinoma. J Xinjiang Med University 2007;30:1277–9 [Google Scholar]
- 51.Fang WT, Chen WH, Chen Y, Jiang Y. Selective three-field lymphadenectomy for thoracic esophageal squamous carcinoma. Dis Esophagus 2007;20:206–11 [DOI] [PubMed] [Google Scholar]
- 52.Xue HC, Wu CR, Zhang ZB, Zhu ZH, Ma ZK, Gao J. Regulations and lymphadenectomy strategy of mediastinal and upper abdominal lymph node metastasis in thoracic esophageal carcinoma. Chin J Cancer 2007;26:1020–4 [PubMed] [Google Scholar]
- 53.Xiao ZF, Zhou ZM, Lv JM, Liang J, Ou GF, Jin J, et al. Rule of lymph node metastasis and proper target of postoperative radiotherapy for thoracic esophageal carcinoma. Chin J Radiat Oncol 2008;17:427–31 [Google Scholar]
- 54.Liu W, Hao XS, Chen Y, Li HX, Wang SJ, Wang PZ, et al. Lymph node netastases from carcinoma of the thoracic esophagus and cardia: a random sampling report of 1,526 cases. Chin J Clin Oncol 2008;35:601–5 [Google Scholar]
- 55.Wang Y, Wang YL, Wang ZM, Zhao BS, Li HC. Analysis of lymph node metastasis of thoracic esophageal cancer. Chin J Mod Drug Appl 2008;2:4–5 [Google Scholar]
- 56.Meier I, Merkel S, Papadopoulos T, Sauer R, Hohenberger W, Brunner TB. Adenocarcinoma of the esophagogastric junction: the pattern of metastatic lymph node dissemination as a rationale for elective lymphatic target volume definition. Int J Radiat Oncol Biol Phys 2008;70:1408–17 [DOI] [PubMed] [Google Scholar]
- 57.Zhu ZH, Wu CR, Xue HC, Zhang ZB, Ma ZK, Gao J, et al. Analysis of surgical therapeutic effect of esophagectomy with 2-field (thoracic and abdominal) lymph node dissection for esophageal carcinoma. China Mod Medicine 2009;16:21–3 [Google Scholar]
- 58.Yang XH, Hou YJ, Zhao HQ, Weng WJ, Chao YL, Wu W. Characteristics of lymph node metastasis of thoracic esophageal squamous cell carcinoma and its implication for postoperative radiotherapy. J Clin Medicine in Practice 2010;14:118–19 [Google Scholar]
- 59.Abula Y, Salai A, Zhang GQ, Sun W, Wang HJ, Abulizi S, et al. Influential factors to lymph node metastasis in thoracic segment esophageal squamous cell carcinomas. J Xinjiang Medical University 2010;33:415–18 [Google Scholar]
- 60.Zhao HG, Hu YJ, Mao WM, Zhou XM, Chen QX, Jiang YH. The correlation factors of thoracic or abdominal lymphatic metastasisi in thoracic esophageal squamous cell carcinoma. J Oncol 2010;16:343–6 [Google Scholar]
- 61.Wu JX, Xu SS, Zhao XD, Gong WH, Xuan HY, Cao W, et al. Extend three-field (cervical, thoracic and abdominal) lymphnode dissection for thoracic esophageal carcinoma and its clinical significance. Anhui Medical J 2010;31:468–70 [Google Scholar]
- 62.Li YM, Zhu SC, Liu ZK, Song CL, Wang YX, Wang SJ. Characteristics of the lymph node metastases and influencing factors and their value in target region delineation in postoperative radiotherapy for thoracic esophageal carcinoma. Chin J Oncol 2010;32:391–5 [PubMed] [Google Scholar]
- 63.Sharma S, Fujita H, Yamana H, Kakegawa T. Patterns of lymph node metastasis in 3-field dissection for carcinoma in the thoracic esophagus. Surg Today 1994;24:410–4 [DOI] [PubMed] [Google Scholar]
- 64.Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167–74 [DOI] [PubMed] [Google Scholar]
- 65.Cai WJ, Xin PL. Pattern of relapse in surgical treated patients with thoracic esophageal squamous cell carcinoma and its possible impact on target delineation for postoperative radiotherapy. Radiother Oncol 2010;96:104–7 [DOI] [PubMed] [Google Scholar]
- 66.Nishihira T, Hirayama K, Mori S. A prospective randomized trial of extended cervical and superior mediastinal lymphadenectomy for carcinoma of the thoracic esophagus. Am J Surg 1998;175:47–51 [DOI] [PubMed] [Google Scholar]
- 67.Rizk N, Venkatraman E, Park B, Flores R, Bains MS, Rusch V. The prognostic importance of the number of involved lymph nodes in esophageal cancer: implications for revisions of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg 2006;132:1374–81 [DOI] [PubMed] [Google Scholar]
- 68.Tachibana M, Kinugasa S, Yoshimura H, Dhar DK, Nagasue N. Extended esophagectomy with 3-field lymph node dissection for esophageal cancer. Arch Surg 2003;138:1383–9; discussion 1390 [DOI] [PubMed] [Google Scholar]
- 69.Chen G, Wang Z, Liu XY, Liu FY. Recurrence pattern of squamous cell carcinoma in the middle thoracic esophagus after modified Ivor-Lewis esophagectomy. World J Surg 2007;31:1107–14 [DOI] [PubMed] [Google Scholar]
- 70.Chen G, Wang Z, Liu XY, Liu FY. Adjuvant radiotherapy after modified Ivor-Lewis esophagectomy: can it prevent lymph node recurrence of the mid-thoracic esophageal carcinoma? Ann Thorac Surg 2009;87:1697–702 [DOI] [PubMed] [Google Scholar]
- 71.Yamamoto M, Yamashita T, Matsubara T, Kitahara T, Sekiguchi K, Furukawa M, et al. Reevaluation of postoperative radiotherapy for thoracic esophageal carcinoma. Int J Radiat Oncol Biol Phys 1997;37:75–8 [DOI] [PubMed] [Google Scholar]
- 72.Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the US: the importance of tumor length and lymph node status. Cancer 2002;95:1434–43 [DOI] [PubMed] [Google Scholar]