Abstract
Objectives
Positron emission tomography with CT (PET/CT) scanning is increasingly being used in head and neck cancer to assess response after radical concomitant chemoradiotherapy. The purpose of this study was to assess the use of PET/CT following chemoradiotherapy at our institution.
Methods
All patients receiving radical chemoradiotherapy for head and neck cancer over a 9-year period were retrospectively identified. Outcome data including local control and overall survival were collected for all patients. The negative predictive value of PET/CT for local recurrence was calculated. Of those with a reported positive PET/CT scan the maximum standardised uptake values were compared with the incidence of local recurrence.
Results
92 patients were identified having a post-treatment PET/CT from a total of 301 patients receiving radical concomitant chemoradiotherapy. Median time from completion of chemoradiotherapy to PET/CT scan was 3 (range 2–8) months. Median follow-up in surviving patients was 19 and 25 months in the PET/CT and non-PET/CT groups, respectively. The negative predictive value for local recurrence was 91.8%. The median maximum standardised uptake values were 10.2 (range 3.1–33) and 6.89 (range 3.1–30) in those with local recurrence and with no local recurrence, respectively.
Conclusions
Post-chemoradiotherapy PET/CT may aid subsequent management decisions. Patients with a negative PET/CT scan after radical chemoradiotherapy have a 91.8% chance of remaining free of local recurrence 19 months post-treatment. A higher maximum standardised uptake value on the post-chemoradiotherapy PET/CT may predict subsequent local recurrence and warrants further investigation.
Advances in knowledge
Post-chemoradiotherapy PET/CT imaging aids subsequent management decisions.
A large population of patients with head and neck squamous cell carcinomas are currently treated with radical concomitant chemoradiotherapy (CRT) in preference to surgery to achieve similar cure rates with less morbidity. There remains significant controversy and variation in practice over what constitutes optimal management of the post-CRT neck. Some centres offer all patients with node-positive disease a neck dissection, whereas others are now using positron emission tomography (PET) CT imaging to assess the post-CRT neck with a view to avoiding neck dissection in those who have had a complete radiological response.
There is evidence that some patients being treated with definitive CRT will benefit from a post-CRT selective neck dissection through early resection of residual disease [1]. However, a large proportion of patients will have a complete pathological response post CRT [2], and if these patients can be accurately identified radiologically, unnecessary neck dissections may be avoided.
The 2-[18F]-fluoro-2-deoxy-d-glucose (FDG) standardised uptake value (SUV) is not well studied as a marker for assessing response and risk of recurrence after radical CRT. Pre-CRT mean FDG-SUV levels have previously been considered as a possible predictor of response, with conflicting results: 1 prospective study of 88 patients has shown that an increasing pre-treatment mean FDG-SUV of the primary tumour was associated with decreased disease-free survival [3]. Conversely, a similar study involving 77 patients has failed to show that the mean FDG-SUV is predictive of response to CRT [4]. A meta-analysis considering the role of PET/CT in assessing prognosis for head and neck cancer has recently been published. This confirms that FDG uptake, as measured by the SUV, is valuable for predicting long-term survival in head and neck cancer. High FDG uptake may be useful for identifying patients requiring more aggressive treatment [5]. Difficulties regarding the interpretation of FDG uptake post-CRT have been well documented as a result of potential false positives due to post-treatment inflammation or infection [6].
The purpose of this study was to assess the role of PET/CT following CRT at our institution in predicting local recurrence. The use of the maximum FDG-SUV (SUVmax) on the post-CRT PET/CT as a predictor of subsequent local recurrence was also evaluated.
Methods and materials
All patients receiving concomitant CRT with radical intent for squamous cell carcinoma of the head and neck at Queen Elizabeth Hospital, Birmingham, between November 2002 and March 2011 were retrospectively identified from an existing radiotherapy database. Tumour characteristics were obtained by reviewing the relevant medical notes, histology reports and radiology reports. The use of PET/CT imaging in this cohort was evaluated, and all patients receiving a PET/CT following completion of CRT to assess response were identified. PET/CT images were acquired 60 min post injection of 18F-FDG on a GE discovery ST 8-slice scanner after a 6-h fast. Image processing used three-dimensional (3D) iterative reconstruction. CT images were acquired according to a standard protocol with helical acquisition, 140 kV; 80 mA; 3.75 mm slice thickness; rotation time of 0.8 s; pitch ratio of 1.675:1 and displayed field of view (DFOV) of 50 cm. No intravenous or oral contrast was used. Both CT and PET scans extended from the top of the skull to the upper thighs. A fasting blood glucose was checked on all patients, and the procedure was only performed if the blood glucose level was ≤10 mmol l−1. A positive PET/CT was defined according to local policy as a SUVmax of more than 3.0.
Site of uptake and the maximum FDG-SUVs (SUVmax) on these PET/CT images were reviewed alongside any locoregional recurrence. Baseline tumour characteristics and treatment regimen delivered were reviewed. We included patients treated with concomitant carboplatin chemotherapy at a dose of AUC4 (area under the curve: which refers to a method of dosing chemotherapy related to the concentration of the drug available within the bloodstream for a certain period of time), given either once or twice during radiotherapy, or those receiving weekly concomitant cetuximab. Patients treated for nasopharyngeal cancer and those with non-squamous pathology were excluded. Radical radiotherapy was given using a conformal technique or intensity-modulated radiotherapy (IMRT); treating to a radical dose of 55 Gy in 20 fractions, 2.75 Gy per fraction to the primary site and involved nodal regions. Prophylactic neck nodal levels were treated to a dose of 41.25 Gy in 15 fractions, 2.75 Gy per fraction. Patients treated with neoadjuvant chemotherapy prior to definitive CRT were included in the study. Patients were followed up weekly following CRT until the acute toxicity had resolved. Follow-up was then carried out at regular intervals under the surgical teams for clinical assessment to exclude recurrence.
The study was registered as an audit with the relevant hospital clinical governance committee (reference number: CA4-03121-10).
Outcomes
The primary outcomes of the study were the positive and negative predictive values of PET/CT imaging in all patients offered PET/CT imaging to assess response post-definitive CRT. All PET/CT scans were reported by expert local radiologists and were reviewed at the time of scanning in our local multidisciplinary team meeting. For the study these reports were reviewed by the authors and a “positive” PET was included if any uptake (SUVmax ≥3) within the head and neck region was reported, even if it were felt to be caused by probable inflammation rather than malignancy.
Secondary outcomes included local recurrence, as determined through histological analysis, and overall survival rates in all patients and the subgroups of those either undergoing or not undergoing a post-CRT PET/CT. These were compared using the log rank test. Survival time was taken as the time to death of any cause or censored at the last known follow-up. The SUVmax was calculated within the FDG uptake using a volume of interest, using a proprietary GE Advantage Workstation. The SUVmax on the post-CRT PET/CT was correlated with any local recurrence to assess whether this might be used as a predictor for recurrence.
Results
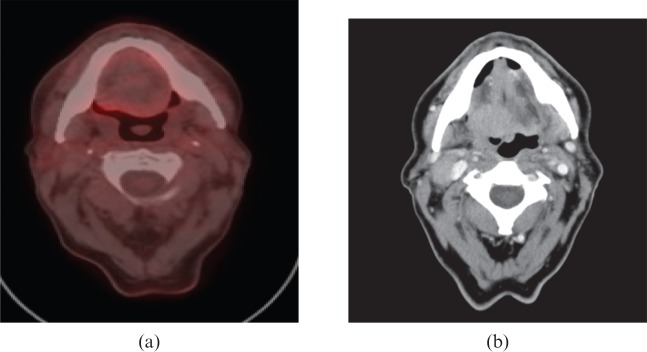
301 patients were identified receiving radical intent CRT with either concomitant carboplatin or cetuximab between November 2002 and March 2011; of these, 92 had a post-CRT PET/CT scan performed. Patients were selected on an individual case basis for post-CRT PET/CT imaging at the local multidisciplinary team meeting with the aim of minimising unnecessary neck dissections. Although there was no strict protocol, typical clinical scenarios were low-volume primary disease and non-bulky but multiple nodes (T1–T2N2) to see whether neck dissection could be avoided; bulky primary disease with involved neck nodes (T3–T4N2–N3) to see whether the primary was controlled before dissecting the neck. A static PET/CT imaging service was introduced at our institution in 2005. Prior to this, a mobile service had been available from 2002. At the time of analysis, of the total 301 patients, 69 had died and 232 were still alive; of the 92 in the PET/CT group, 17 had died and 75 were still alive and of the 209 in the no PET/CT group, 52 had died and 157 were still alive. Median follow-up in surviving patients was 21 months in all patients, 19 months in the PET/CT group and 25 months in the non-PET/CT group. The median time from the end of treatment to PET/CT scan was 3 (range 2–8) months. Baseline characteristics are shown in Table 1. Figure 1 shows an example of a negative post-CRT PET/CT image, compared to the baseline corresponding pre-CRT CT image. Figure 2 shows an example of a positive post-CRT PET/CT image, compared to the baseline corresponding pre-CRT CT image.
Table 1. Baseline characteristics.
| Characteristic | All patients (n=301) | PET/CT (n=92) | No PET/CT (n=209) |
| Median age (range) (years) | 59 (31–85) | 57 (35–77) | 60 (31–85) |
| Female | 67 (22.3%) | 22 (23.9%) | 45 (21.5%) |
| Male | 234 (77.7%) | 70 (76.1%) | 164 (78.5%) |
| Tumour site | |||
| Oral cavity | 4 (1.3%) | 0 | 4 (1.9%) |
| Oropharynx | 194 (64.5%) | 68 (74.0%) | 126 (60.3%) |
| Larynx | 75 (24.9%) | 12 (13.0%) | 63 (30.1%) |
| Hypopharynx | 28 (9.3%) | 12 (13.0%) | 16 (7.7%) |
| T stage | |||
| Tx | 2 (0.7%) | 0 | 2 (1.0%) |
| T1 | 30 (10.0%) | 7 (7.6%) | 23 (11.0%) |
| T2 | 90 (29.9%) | 24 (26.1%) | 66 (31.6%) |
| T3 | 94 (31.2%) | 25 (27.2%) | 69 (33.0%) |
| T4 | 85 (28.2%) | 36 (39.1%) | 49 (23.4%) |
| N stage | |||
| N0 | 101 (33.6%) | 15 (16.3%) | 86 (41.1%) |
| N1 | 46 (15.3%) | 14 (15.2%) | 32 (15.3%) |
| N2A | 22 (7.3%) | 11 (12.0%) | 11 (5.3%) |
| N2B | 82 (27.2%) | 30 (32.6%) | 52 (24.9%) |
| N2C | 34 (11.3%) | 18 (19.6%) | 16 (7.7%) |
| N3 | 16 (5.3%) | 4 (4.3%) | 12 (5.7%) |
| Neoadjuvant chemotherapy | |||
| Yes | 53 (17.6%) | 39 (42.4%) | 14 (6.7%) |
| No | 248 (82.4%) | 53 (57.6%) | 195 (93.3%) |
| Concurrent chemotherapy | |||
| Carboplatin (2 cycles) | 209 (69.4%) | 65 (70.7%) | 144 (68.9%) |
| Carboplatin (1 cycle) | 56 (18.6%) | 15 (16.3%) | 41 (19.6%) |
| Cetuximab | 36 (11.9%) | 12 (13.0%) | 24 (11.5%) |
PET, positron emission tomography.
Figure 1.
An example of a “negative” post-chemoradiotherapy (CRT) positron emission tomography/CT scan (a) compared with the pre-CRT CT scan (b).
Figure 2.
An example of a “positive” post-chemoradiotherapy (CRT) positron emission tomography/CT scan (a) compared with the pre-CRT CT scan (b).
Primary outcome
Of the 92 patients undergoing a PET/CT, 31 had a locally reported positive PET/CT scan which showed an FDG SUVmax uptake ≥3 in one or more areas. 61 patients had locally reported negative PET/CT scans with no area on the images having an FDG SUVmax uptake ≥3. Of the 31 showing uptake, 17 developed locoregional recurrence confirmed on biopsy. In comparison, just 5 of 61 in the group with no FDG uptake developed locoregional recurrence confirmed on biopsy (Table 2). The negative predictive value (NPV) was 91.8% and the positive predictive value (PPV) was 54.8%. The sensitivity and specificity of the PET/CT within all patients undergoing imaging were 77% and 80%, respectively.
Table 2. Local recurrence within the PET/CT group (%).
| Uptake on PET/CT | LR | No LR | Total |
| Positive | 17 | 14 | 31 |
| Negative | 5 | 56 | 61 |
| Total | 22 | 70 | 92 |
LR, local recurrence; PET, positron emission tomography.
In those having a PET/CT less than 3 months after CRT, there was a 50% false-positive rate with 2 patients of the 4 with positive PET/CT scans not developing local recurrence. This compares with a 44.4% false-positive rate in those having their PET/CT scan more than 3 months after CRT, with 12 patients of the 27 with positive PET/CT scans not developing local recurrence.
Secondary outcomes
Locoregional recurrence occurred in 22 of all the 92 patients scanned, of whom 17 had shown uptake on post-CRT PET/CT. 2-year local control rates were significantly worse in the PET/CT group than in the non-PET/CT group [68.75% (95% CI 57.3–80.2%) vs 82.25% (95% CI 76.2–88.2%), p=0.02]. No significant difference was seen in the 2 year overall survival rates between the PET/CT group and non-PET/CT groups [78.6% (95% CI 68.5–88.7%) vs 78.97% (95% CI 72.7–85.3%), p=0.66].
The sites of local uptake on the PET/CT scans were as follows (Table 3): 16 within the primary tumour alone; 11 within the neck and 4 locally but at a different site. Of the 16 with uptake in the primary site, 10 had local recurrence with a median time to recurrence from the end of CRT of 8.5 (3–30) months. Of those with uptake in the neck nodes: one had previously had a pre-CRT neck dissection; seven had a post-CRT neck dissection and three did not have a neck dissection. Reasons for not undertaking a neck dissection in these cases included two patients who were too unwell for surgery, and the other who had a negative fine-needle aspirate after PET scan review and suspected false positive. This patient did however develop local recurrence within the primary site 8 months later. Six out of the eight neck dissections performed revealed histologically confirmed metastatic squamous cell carcinoma, including the pre-CRT neck dissection.
Table 3. Positive PET–SUV uptake and subsequent local recurrence (n=24).
| Maximum FDG-SUV | Site of FDG-SUV uptake | Local recurrence | Site of local recurrence | Time to LR from completion of RT (months) |
| 33 | Primary | Yes | Nodes (and lung) | 17 |
| 30 | Other (nasopharynx—original primary oropharynx) | No | – | – |
| 21.9 | Primary | Yes | Primary | 8 |
| 19.5 | Primary | Yes | Primary | 6 |
| 14.6 | Primary | Yes | Primary (and lung) | 10 |
| 13.3 | Primary | Yes | Primary and nodes | 4 |
| 12.1 | Primary | Yes | Primary | 4 |
| 11.1 | Other (larynx—original primary oropharynx) | Yes | Primary and nodes | 19 |
| 11.0 | Nodes | Yes | Nodes | 7 |
| 10.2 | Primary | Yes | Primary and nodes | 3 |
| 7.3 | Primary | No | – | – |
| 6.7 | Primary | No | – | – |
| 6.6 | Other (nasopharynx—original primary oropharynx) | No | – | – |
| 5.5 | Primary | Yes | Primary | 12 |
| 5.3 | Primary | Yes | Primary | 30 |
| 5 | Primary | No | – | – |
| 4.9 | Primary | No | – | – |
| 4.7 | Nodes | No | – | – |
| 4.7 | Nodes | Yes | Nodes | 4 |
| 4.6 | Nodes | No | – | – |
| 4.6 | Other (supraglottis—original primary oropharynx) | No | – | – |
| 4.4 | Primary | Yes | Primary and nodes | 9 |
| 4.3 | Primary | No | – | – |
| 3.9 | Nodes | Yes | Nodes | 8 |
| 3.9 | Primary | No | – | – |
| 3.8 | Nodes | Yes | Nodes | 3 |
| 3.5 | Nodes | No | – | – |
| 3.2 | Nodes | Yes | Nodes | 5 |
| 3.1 | Nodes | Yes | Nodes | 6 |
| 3.1 | Nodes | No | – | – |
| 3.1 | Nodes | No | – | – |
FGD, 2-[18F]-fluoro-2-deoxy-d-glucose; LR, local recurrence; RT, radiotherapy; SUV, standardised uptake value.
SUVmax in PET/CT-positive patients
31 patients had uptake within the head and neck on PET/CT imaging (Table 3). 17 of these went onto develop locoregional recurrence (either primary/nodal site). Of these the overall median of the SUVmax was 10.2 (range 3.1–33). In comparison, 14 patients remained recurrence free at the time of analysis and had a median SUVmax of 4.65 (range 3.1–30).
Of the 16 with FDG uptake within the primary site, the median SUVmax was 12.7 (range 4.4–33) in those who went on to develop local recurrence and 4.95 (range 3.9–7.3) in those who remained recurrence free at the time of analysis. 10 of the 16 (62.5) developed local recurrence. The median time to developing local recurrence was 8.5 (range 3–30) months
Of the 11 with FDG uptake within the nodal area, the median SUVmax was 3.85 (range 3.1–11) in those who went on to develop local recurrence and 3.3 (range 3.1–4.7) in those who remained recurrence free at the time of analysis. 6 of the 11 (54.5) developed local recurrence. The median time to developing local recurrence was 5 (range 3–7) months.
Four patients were reported to have FDG uptake in a different local site on the post-CRT PET/CT. The sites of uptake were two nasopharynx; one supraglottis and one glottis; all in patients treated for oropharyngeal primary tumours. Only one of these patients developed local recurrence and this occurred in both the primary oropharyngeal site and the lymph nodes.
Discussion
It is recognised that a negative PET/CT after CRT may allow omission of the neck dissection, avoiding unnecessary surgery and all the associated risks and potential morbidity [7-13]. The data from this study are consistent with prior studies in confirming this finding. Table 4 shows a comparison of the data presented here with results published elsewhere. The variation in current practice of the use of PET/CT imaging after CRT has been reviewed and recommendations for clinical practice produced [14]. These suggest that all patients with disease staged on baseline imaging/clinical assessment as N2 or worse should be offered a PET/CT scan within 10–12 weeks of completing CRT. Further management is then dependent upon the size of the residual nodes on clinical examination and those showing uptake on PET/CT imaging.
Table 4. Comparison with published studies.
| Study | Patient number (nodal stage) | Median follow-up (months) | Median time to PET/CT post chemoradiation (months) | Negative predictive value (%) | Positive predictive value (%) |
| This study | 92 (N0–N3) | 19 | 3 (range 2–8) | 91.8 | 54.8 |
| 63 (N2A–3) | 87.9 | 23.8 | |||
| Loo et al7 | 34 (N2A–2C) | 39.1 | 3 | 100 | – |
| Tan et al8 | 48 (N2A–3) | 20 | 2.5 (range 1.1–5.3) | 95 | 20 |
| Yao et al9 | 188 (N2A–3) | 36.2 | 3.8 (range 1.3–10.9) | 99 (neck) | 4371 (neck) |
| 98.7 (primary) | 32.4 (primary) | ||||
| Ong et al10 | 65 (N1–3) | 43 | 3 (range 2–6.75) | 97 | 38 |
| Abgral et al11 | 91 (N0-3) | 11.5 | 11.6 (range 7.2–16) | 100 | 77 |
| Nayak et al12 | 43 (N2A–3) | 18.1 | Within 6 months | 97 | 70 |
| Gupta et al13 | 57 (N0–2B) | 26 | 2.25 (range 1.25–7.25) | 86.7 | 58.3 |
PET, positron emission tomography.
The survival and local recurrence rates in this study are worse within the PET/CT group. This group had a worse initial prognosis and were more likely to receive neoadjuvant chemotherapy prior to definitive CRT than the non-PET/CT group. Within the PET/CT group, more patients had T4 disease at presentation than the non-PET/CT group. Similarly, the PET/CT group was much more likely to have N2c disease at presentation.
In addition, the results, despite small numbers, do suggest a trend towards a higher post-CRT FDG-SUVmax in those patients destined to develop local recurrence; hence this may have a role in predicting future locoregional recurrence. Few data currently exist regarding the role of the SUVmax in squamous cell carcinoma of the head and neck. Further evaluation of this is warranted, as this would provide further weight to the omission of the post-CRT neck dissection in patients with a negative PET/CT. This should also allow identification of those at higher risk of recurrence, thereby allowing them to be more closely monitored with the aim of earlier detection of recurrence.
The role of the pre-treatment FDG-SUVmax is well established in non-small cell lung cancer to predict prognosis, and may be a useful prognostic factor in diffuse large B-cell lymphoma [15,16]. Studies in rectal cancer have failed to establish a role for SUVmax in assessing either response or accurate prediction of recurrence following concomitant chemoradiation; however, there may be a role for the percentage of SUVmax difference (response index) between the pre-CRT and post-CRT PET/CT scans [17,18]. No established role for the SUVmax has been defined within head and neck cancer to date. Studies considering pre-treatment PET/CT imaging have failed to consistently find a prognostic role for SUVmax; however, there are some data suggesting that a higher SUVmax pre-treatment may be associated with a poorer disease-free survival [3]. The limitations of PET/CT imaging are well recognised and include false negatives due to disease with low metabolic activity; misregistration between PET and CT imaging due to patient movement; uncontrolled diabetes; lesions that are too small to be detected; and false positives due to inflammation, for example in this context post CRT if the scan is done too early following treatment.
The ideal timing for a PET/CT after CRT has yet to be established, although, most commonly, within the literature, scans 3 months post CRT are used with the hope of minimising post-treatment inflammation, maximising potential tumour cell kill after CRT and without delaying the scan for too long to allow progression of residual disease. A meta-analysis has found that the sensitivity of PET scans in predicting local recurrence after CRT is significantly higher for those performed more than 10 weeks after CRT [19], in accordance with the opinion that 12 weeks after CRT remains the optimum time to perform the PET/CT. Within the data presented here the time between CRT and PET/CT does vary, as the optimum timing had yet to be established when these patients were treated. A subgroup analysis assessing the timing of the PET/CT in this cohort does suggest, in concordance with the meta-analysis, a trend towards a higher rate of false positives if the PET/CT is done too early, i.e. less than 3 months after CRT. There is evidence that the incidence of false-positive uptake in patients imaged too early, i.e. less than 3 months after CRT, is higher. The results shown here suggest a trend towards this, but the numbers are too small to draw any firm conclusions [20].
Previous research assessing diffusion-weighted MRI pre and post CRT for head and neck tumours has shown that the change in the apparent diffusion coefficient between pre- and post-treatment scans predicts treatment response [21]. Similar research has not to date been published considering the change in SUVmax between pre- and post-CRT PET/CT scans.
Limitations of the data presented here include that patients were selected for PET/CT imaging on an individual case basis as discussed at the local multidisciplinary team meeting. Analysis of the change in FDG-SUVmax pre and post CRT was not performed as only 11 patients within the cohort had both pre-and post-CRT PET/CT imaging. Limited resources meant that baseline scans were reserved for only those in whom they were known to definitely influence management decisions, for example in the case of the unknown primary. The role of baseline PET/CT in head and neck cancer remains controversial and is not standard practice at present within the UK. In addition, further research is needed before regular reporting of FDG-SUVmax can be incorporated into routine practice.
The data from this study add to the evidence supporting the role of PET/CT when assessing response to CRT. Baseline imaging after CRT is recognised within many tumour sites as being invaluable when interpreting future scans [22]; however, for head and neck tumours there is no standard for post-treatment imaging, with some centres using CT or MR scans and some PET/CT. A different approach has considered post-RT PET/CT scans as positive, equivocal or negative based on qualitative interpretation of the PET component and correlation with the CT component to assess if there is residual anatomical abnormality. This has allowed sparing of neck dissections in some patients [23]. The UK National Cancer Research Network PET Neck Trial is now recruiting patients with a view to providing guidance to the best possible management of the post-CRT neck. While the results of this trial are awaited, uncertainty remains regarding the use of PET/CT imaging in this patient group. PET/CT scans are expensive and their routine use will need to be justified with high-quality prospective data clearly showing that they increase the success of salvage treatments in terms of patient outcome, prior to implementation in routine practice. The use of PET/CT after CRT may, however provide valuable information for patients and carers, especially if the post-CRT PET/CT does not show any FDG uptake. This may provide a higher degree of reassurance that treatment has been successful and may help to stratify follow-up such that often limited resources are concentrated on those patients who require them most.
Conclusions
Post-CRT PET/CT does aid subsequent management decisions. Patients with a negative PET/CT scan after radical CRT have a 91.8% chance of remaining free of local recurrence 19 months post treatment. A higher SUVmax on the post-CRT PET/CT may predict local recurrence and warrants further investigation.
References
- 1.Boyd TS, Harari PM, Tannehill SP, Voytovich MC, Hartig GK, Ford CN, et al. Planned post-radiotherapy neck dissection in patients with advanced head and neck cancer. Head Neck 1998;20:132–7 [DOI] [PubMed] [Google Scholar]
- 2.Langerman A, Plein C, Vokes EE, Salama JK, Haraf DJ, Blair EA, et al. Neck response to chemoradiotherapy: complete radiographic response correlates with pathologic complete response in locoregionally advanced head and neck cancer. Arch Otolaryngol Head Neck Surg 2009;135:1133–6 [DOI] [PubMed] [Google Scholar]
- 3.Higgins KA, Hoang JK, Raoch MC, Chino J, Yoo DS, Turkington TG, et al. Analysis of pretreatment FDG-PET SUV parameters in head-and-neck cancer: tumor SUV (mean) has superior prognostic value. Int J Radiat Oncol Biol Phys 2012;82:548–53 [DOI] [PubMed] [Google Scholar]
- 4.Schinagl DA, Span PN, Oyen WJ, Kaanders JH. Can FDG PET predict radiation treatment outcome in head and neck cancer? Results of a prospective study. Eur J Nucl Med Mol Imaging 2011;38:1449–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie P, Li M, Zhao H, Sun X, Fu Z, Yu J. 18F-FDG PET or PET-CT to evaluate prognosis for head and neck cancer: a meta-analysis. J Cancer Res Clin Oncol 2011;137:1085–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mori M, Tsukuda M, Horiuchi C, Matsuda H, Taguchi T, Takahashi M, et al. Efficacy of fluoro-2-deoxy-D-glucose positron emission tomography to evaluate responses to concurrent chemoradiotherapy for head and neck squamous cell carcinoma. Auris Nasus Larynx 2011;38:724–9 [DOI] [PubMed] [Google Scholar]
- 7.Loo SW, Geropantas K, Beadsmoore C, Montgomery PQ, Martin WM, Roques TW. Neck dissection can be avoided after sequential chemoradiotherapy and negative post-treatment positron emission tomography-computed tomography in N2 head and neck squamous cell carcinoma. Clin Oncol (R Coll Radiol) 2011;23:512–17 [DOI] [PubMed] [Google Scholar]
- 8.Tan A, Adelstein DJ, Rybicki LA, Saxton JP, Esclamado RM, Wood BG, et al. Ability of positron emission tomography to detect residual neck node disease in patients with head and neck squamous cell carcinoma after definitive chemoradiotherapy. Arch Otolaryngol Head Neck Surg 2007;133:435–40 [DOI] [PubMed] [Google Scholar]
- 9.Yao M, Smith RB, Hoffman HT, Funk GF, Lu M, Menda Y, et al. Clinical significance of postradiotherapy [18F]-fluorodeoxyglucose positron emission tomography imaging in management of head-and-neck cancer-a long-term outcome report. Int J Radiat Oncol Biol Phys 2009;74:9–14 [DOI] [PubMed] [Google Scholar]
- 10.Ong SC, Schöder H, Lee NY, Patel SG, Carlson D, Fury M, et al. Clinical utility of 18F-FDG PET/CT in assessing the neck after concurrent chemoradiotherapy for Locoregional advanced head and neck cancer. J Nucl Med 2008;49:532–40 [DOI] [PubMed] [Google Scholar]
- 11.Abgral R, Querellou S, Potard G, Le Roux PY, Le Duc-Pennec A, Marianovski R, et al. Does 18F-FDG PET/CT improve the detection of post treatment recurrence of head and neck squamous cell carcinoma in patients negative for disease on clinical follow-up? J Nucl Med 2009;50:24–9 [DOI] [PubMed] [Google Scholar]
- 12.Nayak JV, Walvekar RR, Andrade RS, Daamen N, Lai SY, Argiris A, et al. Deferring planned neck dissection following chemoradiation for stage IV head and neck cancer: the utility of PET-CT. Laryngoscope 2007;117:2129–34 [DOI] [PubMed] [Google Scholar]
- 13.Gupta T, Jain S, Agarwal JP, Rangarajan V, Purandare N, Ghosh-Laskar S, et al. Diagnostic performance of response assessment FDG-PET/CT in patients with head and neck squamous cell carcinoma treated with high-precision definitive (chemo)radiation. Radiother Oncol 2010;97:194–9 [DOI] [PubMed] [Google Scholar]
- 14.Schoder H, Fury M, Lee N, Kraus D. PET Monitoring of therapy response in head and neck squamous cell carcinoma. J Nucl Med 2009;50:74S–88S [DOI] [PubMed] [Google Scholar]
- 15.Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Hossein-Foucher C, et al. European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. Primary tumor standardised uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for IASLC Lung Cancer Staging Project. J Thorac Oncol 2008;3:6–12 [DOI] [PubMed] [Google Scholar]
- 16.Chihara D, Oki Y, Onoda H, Taji H, Yamamoto K, Tamaki T, et al. High maximum standard uptake value (SUVmax) on PET scan is associated with shorter survival in patients with diffuse large B cell lymphoma. Int J Hematol 2011;93:502–8 [DOI] [PubMed] [Google Scholar]
- 17.Chennupati SK, Quon A, Kamaya A, Pai RK, La T, Krakow TE, et al. Positron emission tomography for predicting pathologic response after neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Am J Clin Oncol 2012;35:334–9 E-pub ahead of print Mar 2011. [DOI] [PubMed] [Google Scholar]
- 18.Huh JW, Min JJ, Lee JH, Kim HR, Kim YJ. The predictive role of sequential FDG-PET/CT in response of locally advanced rectal cancer to neoadjuvant chemoradiation. Am J Clin Oncol 2012;35:340–4 E-pub ahead of print Mar 2011. [DOI] [PubMed] [Google Scholar]
- 19.Isles MG, McConkey C, Mehanna HM. A systematic review and meta-analysis of the role of positron emission tomography in the follow up of head and neck squamous cell carcinoma following radiotherapy or chemoradiotherapy. Clin Otolaryngol 2008;33:210–22 [DOI] [PubMed] [Google Scholar]
- 20.Paccagnella A, Ghi MG, Loreggian L, Buffoli A, Koussis H, Mione CA, et al. Concomitant chemoradiotherapy versus induction docetaxel, cisplatin and 5 fluorouracil (TPF) followed by concomitant chemoradiotherapy in locally advanced head and neck cancer: a phase II randomized study. Ann Oncol 2010;21:1515–22 [DOI] [PubMed] [Google Scholar]
- 21.Vandecaveye V, Dirix P, De Keyzer F, Op deBeeck K, Poorten VV, Hauben E, et al. Diffusion-weighted magnetic resonance imaging early after chemoradiotherapy to monitor treatment response in head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2012;82:1098–107 [DOI] [PubMed] [Google Scholar]
- 22.Sanghera P, Rampling R, Haylock B, Jefferies S, McBain C, Rees JH, et al. The concepts, diagnosis and management of early imaging changes after therapy for glioblastomas. Clin Oncol (R Coll Radiol) 2012;24:216–27 [DOI] [PubMed] [Google Scholar]
- 23.Porceddu SV, Pryor DI, Burmeister E, Burmeister BH, Poulsen MG, Foote MC, et al. Results of a prospective study of positron emission tomography-directed management of residual nodal abnormalities in node-positive head and neck cancer after definitive radiotherapy with or without systemic therapy. Head Neck 2011;33:1675–82 [DOI] [PubMed] [Google Scholar]