Abstract
Solid pseudopapillary tumour (SPT) is an uncommon cystic exocrine pancreatic neoplasm. The typical patient is a female in the third decade of life presenting with pain and/or palpable mass. Classic imaging characteristics include large size, mixed solid and cystic nature, encapsulation and haemorrhage. A pancreatic mass with these features in a young adult female should raise suspicion for an SPT. Although typically a non-aggressive neoplasm with surgery curative in most cases, SPT may exhibit more aggressive features such as local invasion, metastases or recurrence in up to 20% of cases.
Solid papillary tumour (SPT) is an uncommon exocrine pancreatic neoplasm first described by Frantz [1]. It accounts for 6% of all exocrine pancreatic tumours in some series published since 2000 [2]. In our retrospective review, SPT represented 2.4% of all pancreatic specimens from 2000 to 10.
The accepted nomenclature for this tumour has varied through the years, including solid and papillary epithelial neoplasm (SPEN), as well as solid and cystic papillary epithelial neoplasm, among others. After the World Health Organization (WHO) reclassification, SPT is now classified as an epithelial tumour under the “Borderline (Uncertain Malignant Potential)” subcategory. Pathologically, SPT is classified as a “rare cystic pancreatic neoplasm”.
About 90% of cases involve females, with the mean age of patients being in their third decade of life. The most common presenting symptom is abdominal pain followed by upper abdominal mass. Mean tumour size is 6–8 cm, with the most common site being the pancreatic head (34–40%) or the pancreatic tail (24–36%). With the increased use of cross-sectional imaging, however, an increasing number of cases are being detected incidentally.
Methods and materials
After obtaining approval from the institutional review board committee, the pathology specimens database at Henry Ford Health System was queried for all patients with a diagnosis of solid pseudopapillary tumour (or equivalent term) of the pancreas from 2000 to 10. Of 376 total pancreatic specimens, 9 carried a final diagnosis of SPT. These cases were crossmatched with the radiology picture archiving and communications system (PACS). Imaging findings were correlated with gross and histopathological features.
Of the nine patients diagnosed at our institution (Table 1), all were female, with slightly more African Americans (56%) than Caucasians (44%). Average age at diagnosis was 34.7 years, but only 30.1 years if an atypical 71-year-old patient is excluded. Average tumour size in maximum dimension was 4.9 cm. There was no clear predilection for tumour location within the pancreatic head (44%), body (22%) or tail (33%). None of the patients had positive surgical margins or positive lymph nodes at resection, but one tumour did demonstrate direct extension to the spleen. No patient had tumour recurrence, with average follow-up of 40.6 months for the seven patients still followed.
Table 1. Patient and tumour characteristics.
| Patient | Race | Age (years) | Tumour sizea (cm) | Tumour location (pancreas) | Follow-up (months) |
| 1 | Caucasian | 21 | 3.1b | Head | 28 |
| 2 | African American | 33 | 4.4 | Head | 19 |
| 3 | Caucasian | 22 | 4.3 | Body | 26 |
| 4 | African American | 34 | 7.3 | Tail | 79 |
| 5 | Caucasian | 33 | 2.5 | Head | 39 |
| 6 | Caucasian | 48 | 2.0 | Body | —c |
| 7 | African American | 71 | 6.0 | Taild | 40 |
| 8 | African American | 18 | 8.9b | Tail | 53 |
| 9 | African American | 32 | 6.0 | Head | —c |
| Average | 34.7 | 4.9 | 40.6 |
aSize in maximum dimension on CT.
bSize based on pathological specimen since pre-operative CT not available.
cLost to follow-up after surgery.
dDirect extension to spleen.
Although typically a non-aggressive neoplasm, metastasis or local invasion has been reported in up to 20% of cases. The lesion is classified as solid pseudopapillary carcinoma (SPC) if malignant behaviour such as perineural invasion, angioinvasion or deep invasion of surrounding tissue is seen. Following surgery, recurrence is low, with rates of 3–7% reported. Long-term prognosis is excellent, with 5-year survival rates of 95–97%. The limited data available suggest 10-year survival may be as high as 93% [3].
Doubling time for this tumour is greater than 2 years. More than 95% of patients with SPT confined to the pancreas are cured with complete surgical excision. Malignant characteristics as described above are usually not contraindications to resection. Even when tumours are considered “non-resectable”, patients can survive longer than 10 years after surgical debulking. Resection of liver metastases can prolong survival by at least 5 years [2].
Imaging features
CT is the primary modality used to evaluate SPT. The tumour typically appears as a mixed-density lesion with solid component peripherally and cystic component more centrally. Larger tumours tend to be well-encapsulated with sharp demarcation from the normal pancreas (Figure 1). Haemorrhage can be seen as areas of high attenuation within the cystic portion of the lesion (Figures 2 and 3). On contrast-enhanced studies, the capsule and solid portion of SPT will enhance similar to normal pancreatic parenchyma in both arterial and venous phases, vs pancreatic adenocarcinoma that is hypoattenuating in the venous phase and neuroendocrine tumours that are hyperattenuating in the arterial phase [4]. A fluid-debris level has been reported in 18% of cases [5]. SPT will typically displace adjacent structures rather than invading them (Figure 4). Calcification peripherally can be seen in 30% of cases (Figure 5a) [5].
Figure 1.
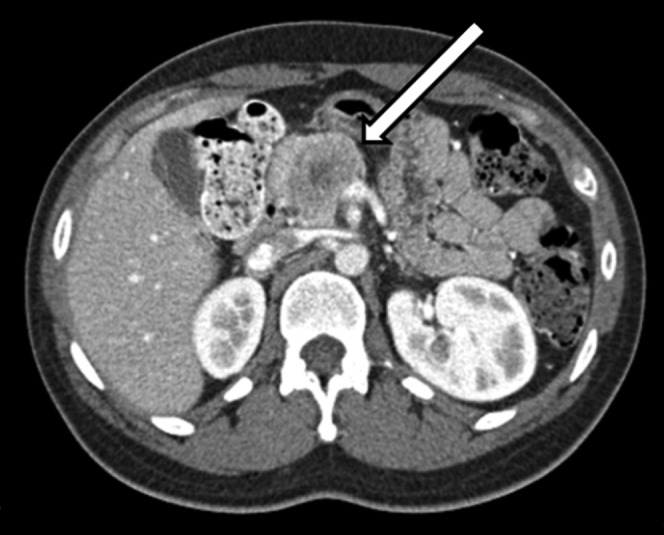
Contrast-enhanced axial CT demonstrates an encapsulated, lobulated mass (arrow) in the pancreatic head. Central area of mixed density represents haemorrhage and necrosis.
Figure 2.
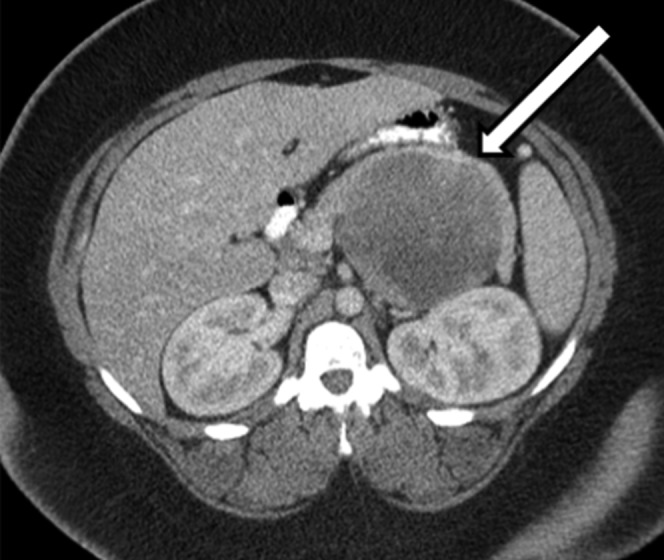
Contrast-enhanced axial CT shows a cystic lesion (arrow) in the pancreatic body with areas of high density representing haemorrhage.
Figure 3.
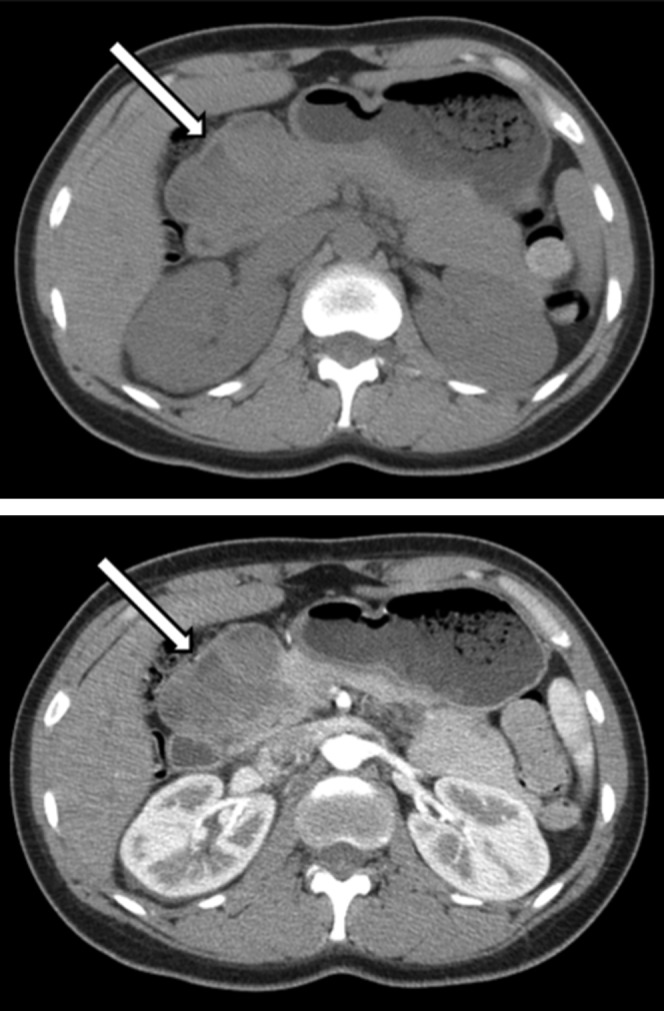
Non-contrast and post-contrast axial CT images demonstrate an encapsulated, multicystic mass (arrows) in the pancreatic head. High density within the lesion corresponds to haemorrhage.
Figure 4.
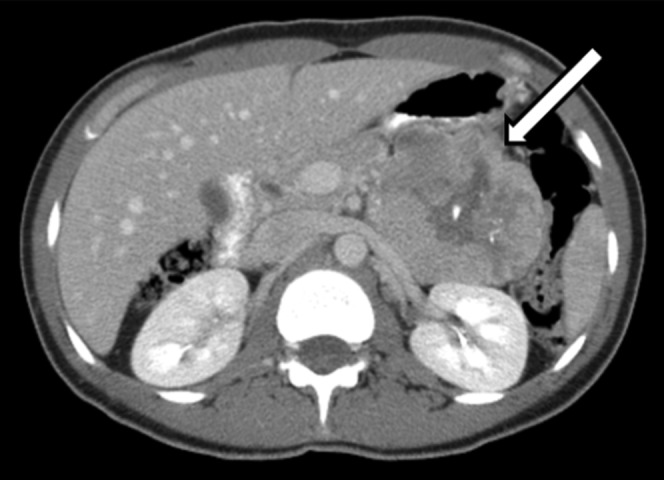
Contrast-enhanced axial CT shows a well-encapsulated lesion (arrow) in the pancreatic tail with central necrosis and areas of calcification. The lesion is displacing but not invading adjacent structures.
Figure 5.
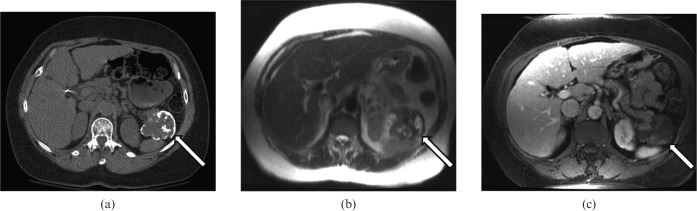
(a) Non-contrast axial CT shows a lobulated lesion (arrow) in the pancreatic tail with peripheral eggshell calcification. (b) T2 weighted axial MRI from the same patient shows areas of high and low signal intensity corresponding to cystic and solid components, respectively, with the lesion (arrow). (c) T1 weighted post-gadolinium axial MRI from the same patient demonstrates heterogeneous peripheral enhancement of the lesion (arrow).
MRI can improve accurate diagnosis of SPT. Typically, lesions have variable signal intensity on T1 weighted images, high signal intensity on T2 weighted images (Figure 5b) and a hypointense rim on both T1 and T2 weighted images. Haemorrhage can be seen as areas of high signal on T1 weighted images and low signal on T2 weighted images. In one series, 75% of cases had a haemorrhagic component [6]. On post-gadolinium images, lesions demonstrate heterogeneous peripheral enhancement (Figure 5c) or, less often, complete homogeneous enhancement during arterial phase, with progressive but incomplete enhancement during portal venous and equilibrium phases. Compared with the lesion, the surrounding capsule tends to enhance earlier and more intensely. Fluid-debris levels are seen in only a minority of cases.
On ultrasound, the tumour appears as a well-defined lesion with variable echogenicity [5]. A predominantly solid, predominantly cystic, or mixed solid and cystic mass can be seen (Figure 6). Endoscopic ultrasound with fine-needle aspiration is increasingly being used for evaluation (including 44% of the cases in our series). Appearance is similar to conventional ultrasound (Figure 7).
Figure 6.
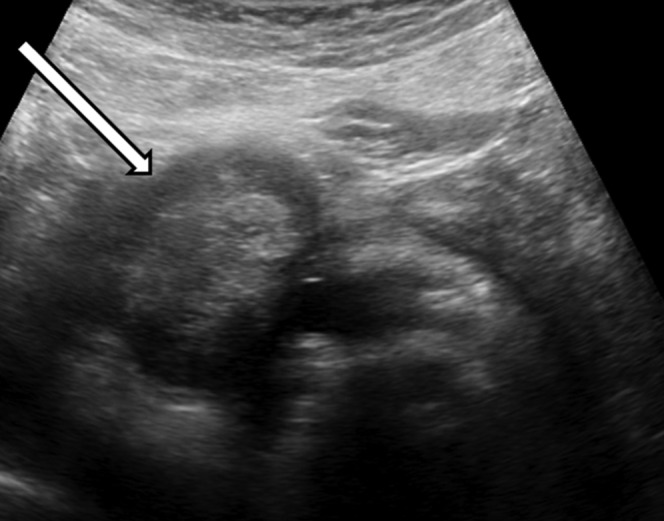
Greyscale ultrasound image from same patient as Figure 1 shows an encapsulated, lobulated mass (arrow) in the pancreatic head. Central area of mixed echogenicity represents haemorrhage and necrosis.
Figure 7.
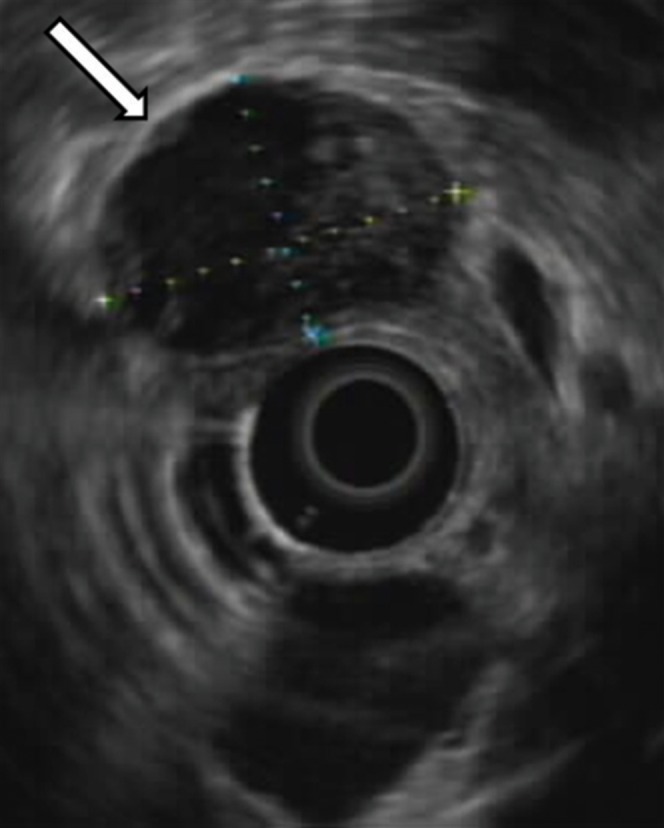
Greyscale endoscopic ultrasound image demonstrates an oval, heterogeneously hypoechoic mass (arrow) in the pancreatic head. Image courtesy of Dr Gregory Olds.
Because features of SPT overlap with other pancreatic neoplasms, misdiagnosis is common. In the study by Yu et al [7], only 24% of patients had suspicion of SPT based on imaging features prior to surgery. It can be difficult to differentiate SPT from non-hyperfunctioning islet cell tumours. Both entities can appear cystic, contain calcifications, have areas of haemorrhage and metastasise to the liver.
There have been attempts to identify imaging characteristics to help differentiate benign SPT from malignant SPC. In a case series by Chung et al [8], the only variables with statistical significance were tumour margin morphology (oval/round or smoothly lobulated in 95% of SPT vs focal/eccentric lobulation in 50% of SPC) and tumour capsule morphology (complete encapsulation or no visible capsule in 94% of SPT vs focal capsule discontinuity in 58% of SPC).
Gross pathology and histopathology
SPT is of uncertain histogenesis because it lacks clear evidence of ductal, acinar or endocrine differentiation. Smaller tumours are less likely to contain cystic components, are usually less well circumscribed and are often unencapsulated (Figure 8). Larger tumours contain a well-defined fibrous pseudocapsule and are well demarcated from adjacent pancreatic parenchyma. On the cut surface, large spongy or cystic areas of haemorrhage and necrosis alternate with regions of solid growth (Figure 9) [2,7]. Marked cystic degeneration may simulate the appearance of a pseudocyst.
Figure 8.
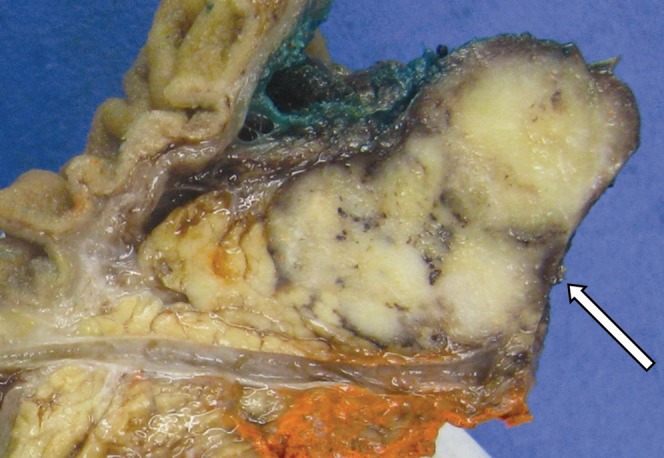
Gross pathology of a predominantly solid pseudopapillary tumour. Cut surface demonstrates homogeneous tan–grey soft tissue (arrow).
Figure 9.
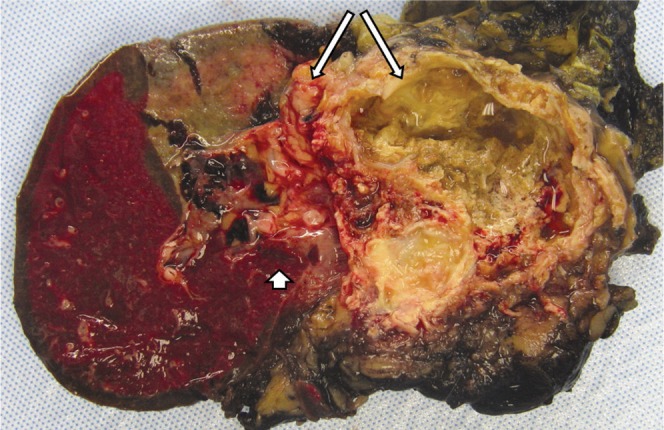
Gross pathology of a larger solid pseudopapillary tumour demonstrates cystic areas of haemorrhage and necrosis (long arrows). There was invasion into the spleen (short arrow).
Histologically, smaller tumours are composed of solid areas of uniform, bland neuroendocrine-looking epithelial cells arranged around delicate fibrovascular stalks (Figure 10). A pseudopapillary pattern is formed as tumour cells outgrow their blood supply, forming cystic spaces containing foamy macrophages, cholesterol clefts, psammoma bodies and haemorrhage (Figure 11) [3]. Venous invasion and high nuclear grade, features that portend poor prognosis, are also associated with metastases to the liver and peritoneum (seen in 10–15% of cases) [9]. Occasional cases may demonstrate elements of high-grade carcinoma [9].
Figure 10.
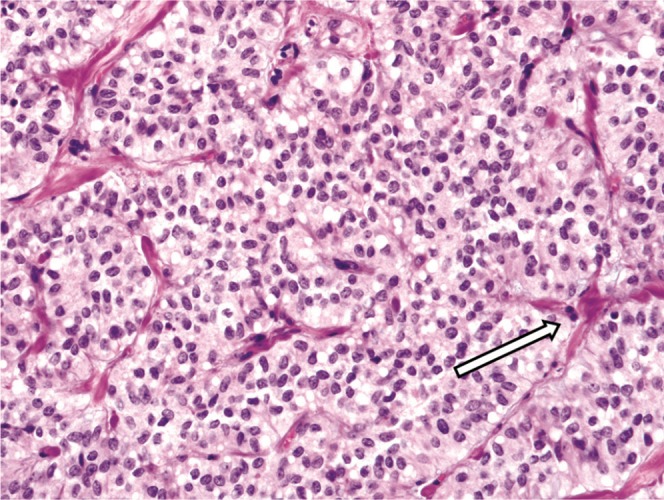
Histopathology with haematoxylin and eosin stain (40×) of a smaller, predominantly solid pseudopapillary tumour demonstrates solid nests of uniform epithelial cells around delicate fibrovascular stalks (arrow).
Figure 11.

Histopathology with haematoxylin and eosin stain (40×) of a larger solid pseudopapillary tumour demonstrates pseudopapillary structures composed of tumour cells (arrow) surrounding small central vessels.
Summary
SPT should be strongly suspected when a pancreatic mass with typical imaging findings is found in a young adult female. Classic imaging characteristics include large size, mixed solid and cystic nature, encapsulated appearance and presence of haemorrhage. SPT is typically a non-aggressive neoplasm, but up to 20% of cases demonstrate malignant characteristics. Data are emerging on imaging characteristics that could help differentiate benign SPT from malignant SPC. Surgery is curative in the vast majority of cases.
References
- 1.Frantz VK. Tumors of the pancreas. In: Atlas of tumor pathology, section VII, fascicles 27 and 28. Washington, DC: US Armed Forces Institute of Pathology; 1959 [Google Scholar]
- 2.Papavramidis T, Papavramidis S. Solid pseudopapillary tumours of the pancreas: review of 718 patients reported in english literature. J Am Coll Surg 2005;200:965–72 [DOI] [PubMed] [Google Scholar]
- 3.Adams AL, Siegal GP, Jhala NC. Solid pseudopapillary tumour of the pancreas: a review of salient clinical and pathologic features. Adv Anat Pathol 2008;15:39–45 [DOI] [PubMed] [Google Scholar]
- 4.Butte JM, Brennan MF, Gönen M, Tang LH, D'Angelica MI, Fong Y, et al. Solid pseudopapillary tumours of the pancreas: clinical features, surgical outcomes, and long-term survival in 45 consecutive patients from a single center. J Gastrointest Surg 2011;15:350–7 [DOI] [PubMed] [Google Scholar]
- 5.Buetow PC, Buck JL, Pantongrag-Brown L, Beck KG, Ros PR, Adair CF. Solid and papillary epithelial neoplasm of the pancreas: imaging pathologic correlation in 56 cases. Radiology 1996;199:707–11 [DOI] [PubMed] [Google Scholar]
- 6.Cantisani V, Mortele KJ, Levy A, Glickman JN, Ricci P, Passariello R, et al. MR imaging features of solid pseudopapillary tumour of the pancreas in adult and pediatric patients. AJR Am J Roentgenol 2003;181:395–401 [DOI] [PubMed] [Google Scholar]
- 7.Yu PF, Hu ZH, Wang XB, Guo JM, Cheng XD, Zhang YL, et al. Solid Pseudopapillary tumours of the pancreas: a review of 553 cases in Chinese literature. World J Gastroenterol 2010;16:1209–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung YE, Kim MJ, Choi JY, Lim JS, Hong HS, Kim YC, et al. Differentiation of benign and malignant pseudopapillary neoplasms of the pancreas. J Comput Assist Tomogr 2009;33:689–94 [DOI] [PubMed] [Google Scholar]
- 9.Tang LH, Aydin H, Brennan MF, Klimstra DS. Clinically aggressive solid pseudopapillary tumours of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol 2005;29:512–19 [DOI] [PubMed] [Google Scholar]