Abstract
Background
Atrial fibrillation is common among older persons. Catheter ablation is increasingly used in patients for whom medical therapy has failed.
Methods and Results
We conducted a retrospective cohort study of all fee-for-service Medicare beneficiaries 65 years or older who underwent catheter ablation for atrial fibrillation between July 1, 2007, and December 31, 2009. The main outcome measures were major complications within 30 days and mortality, heart failure, stroke, hospitalization, and repeat ablation within 1 year. A total of 15,423 patients underwent catheter ablation for atrial fibrillation. Mean age was 72 years, 41% were women, and more than 95% were white. For every 1000 procedures, there were 17 cases of hemopericardium requiring intervention, 8 cases of stroke, and 8 deaths within 30 days. Over 40% of patients required hospitalization within 1 year; however, atrial fibrillation or flutter was the primary discharge diagnosis in only 38.4% of cases. Eleven percent of patients underwent repeat ablation within 1 year. Renal impairment (hazard ratio, 2.07; 95% confidence interval, 1.66–2.58), age greater than 80 years (3.09; 2.32–4.11), and heart failure (2.54; 2.07–3.13) were major risk factors for 1-year mortality. Advanced age was a major risk factor for all adverse outcomes.
Conclusions
Major complications after catheter ablation for atrial fibrillation were associated with advanced age but were fairly infrequent. Few patients underwent repeat ablation. Randomized trials are needed to inform risk-benefit calculations for older persons with drug-refractory, symptomatic atrial fibrillation.
Keywords: Atrial Fibrillation, Catheter Ablation, Medicare, Outcomes Assessment (Health Care)
Introduction
Atrial fibrillation is the most common sustained arrhythmia in clinical practice, particularly among older patients. Prevalence doubles with each decade of life,1,2 and lifetime risk is as high as 22% to 26%.3,4 More than 2 million people 65 years and older in the United States have atrial fibrillation.5 This growing epidemic is costly6 and complicates the management of concurrent medical disorders. In addition to greater risk of stroke,7 heart failure,8,9 and cognitive impairment,10 atrial fibrillation is associated with lower quality of life.11 Patients frequently suffer from debilitating symptoms, despite attempts at rate or rhythm control. Recurrence associated with antiarrhythmic drug therapy is high, usually exceeding 50% at 1 year.12
During the past decade, catheter ablation has emerged as an increasingly common option for rhythm control among patients for whom medical therapy has failed.13 A meta-analysis of small randomized clinical trials reported that, compared with antiarrhythmic drug therapy, catheter ablation reduced rates of atrial fibrillation and cardiovascular hospitalization at 1 year.14 On the basis of these studies, guidelines recommend catheter ablation for medically refractory atrial fibrillation in patients with paroxysmal and persistent atrial fibrillation.15 However, clinical trials have enrolled relatively young patients with limited comorbidity. The objective of our analysis was to describe the use of catheter ablation for atrial fibrillation and associated outcomes in the US Medicare population.
Methods
Data Sources
We obtained inpatient, outpatient, and carrier claim files and the corresponding denominator files from the US Centers for Medicare & Medicaid Services for all Medicare beneficiaries who underwent intracardiac catheter ablation between January 1, 2007, and December 31, 2009. The inpatient files contain institutional claims for facility costs covered under Medicare Part A. The outpatient files contain outpatient provider claims covered under Medicare Part B. The carrier files contain noninstitutional provider claims for services covered under Medicare Part B. The denominator files contain beneficiary demographic characteristics, dates of death, and program eligibility and enrollment information. We limited our analyses to fee-for-service Medicare beneficiaries 65 years or older and living in the United States. The institutional review board of the Duke University Health System approved the study.
Study Population
We searched carrier claims submitted between July 1, 2007, and December 31, 2009, for reports of intracardiac catheter ablation (Healthcare Common Procedure Coding System [HCPCS] code 93651) and a primary diagnosis of atrial fibrillation (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 427.31). To improve specificity, we excluded patients who underwent atrioventricular node ablation (HCPCS code 93650) and patients with a secondary diagnosis of anomalous atrioventricular excitation (ICD-9-CM code 426.7) or paroxysmal supraventricular tachycardia (427.0) on the catheter ablation claim. For patients with multiple claims for catheter ablation, we defined the earliest as the index procedure. To assess comorbid conditions, we required continuous enrollment in fee-for-service Medicare for 6 months before the index procedure, thus the minimum age was 65.5 years. We matched index carrier claims to inpatient or outpatient institutional claims and retained Medicare hospital identifiers and service dates.
Demographic characteristics included age, sex, race, and state of residence. We used self-reported race categories “black” and “white” and combined all other categories as “other.” On the basis of state of residence, we grouped beneficiaries into 4 geographic regions. For clinical characteristics, we used previously validated coding algorithms to search claims in the 180 days before the index procedure for evidence of cancer, cerebrovascular disease, chronic obstructive pulmonary disease, dementia, diabetes mellitus, heart failure, hypertension, ischemic heart disease, peripheral vascular disease, renal disease, stroke or transient ischemic attack (TIA), and valvular heart disease. Specifically, we used the coding algorithm described by Tirschwell and Longstreth for stroke or TIA.16 For all other conditions, we used the coding algorithms described by Birman-Deych et al17 and Quan et al.18 We used the approach of Gage et al19 to define the CHADS2 score.
Outcomes
We assessed outcomes up to 1 year after the index procedure. All-cause mortality was based on death dates in the Medicare denominator files. Other outcomes included stroke or TIA,16 pericardial effusion or cardiac tamponade (ICD-9-CM code 423.0 or 423.3 on an inpatient claim, or HCPCS code 33010 on a carrier claim), myocardial infarction (ICD-9-CM code 410.x1 as primary diagnosis on an inpatient claim), heart failure (428.x, 402.x1, 404.x1, or 404.x3 as primary diagnosis on an inpatient claim), and vascular complications requiring surgery (using the algorithm described by Ellis et al20). When calculating rates of all-cause hospitalization, we excluded inpatient claims for the index procedure, interhospital transfers, and admissions for rehabilitation. We categorized the reasons for the first new hospitalization according to a primary diagnosis of atrial fibrillation or atrial flutter (ICD-9-CM code 427.31 or 427.32), other cardiac diagnoses (390.x-398.x, 402.x, or 404.x-429.x),21 or other noncardiac diagnoses. Identification of repeat catheter ablation was the same as for the index procedure, except that the service date was more than 30 days after the index procedure.
Statistical Analysis
We present frequencies with percentages for categorical variables and means with standard deviations for continuous variables. We report use of catheter ablation as a count of all index procedures between July 1, 2007, and December 31, 2009, overall and stratified by age, sex, and race.
We report unadjusted outcome rates overall and stratified by age, sex, and race. Thirty-day outcomes included mortality, stroke or TIA, pericardial effusion, myocardial infarction, and vascular complications requiring intervention. One-year outcomes included mortality, stroke or TIA, heart failure, hospitalization, and repeat ablation. We used Kaplan-Meier methods to estimate unadjusted outcomes. In a sensitivity analysis, we examined 30-day unadjusted outcomes among beneficiaries with no diagnosis of atrial flutter in the 6 months preceding the index catheter ablation. We examined unadjusted and adjusted associations between outcomes and patient characteristics using Cox proportional hazards models. In multivariable analysis, we adjusted for age, sex, race, geographic region, and comorbid conditions. Analyses of unadjusted and adjusted rates of stroke or TIA and heart failure included only patients who had no such diagnosis in the 6 months before the index procedure. We censored data for patients if they enrolled in Medicare managed care and administratively at the end of follow-up (December 31, 2009). For outcomes other than mortality, we censored data at the time of death. We used robust standard errors to account for hospital-level clustering. The 2-sided significance level was .05. We used SAS version 9.2 (SAS Institute Inc, Cary, North Carolina) for all analyses.
Results
Between July 2007 and December 2009, 15,423 patients underwent intracardiac catheter ablation for atrial fibrillation (Table 1). Mean age was 72 years (SD, 5 years); more than 70% of the patients were younger than 75 years. Approximately 40% of the patients were women, more than 95% were white, and more than two-thirds lived in the South or West. The procedures were performed at 808 sites in the United States. Hypertension (78%) and ischemic heart disease (51%) were prevalent comorbid conditions; debilitating conditions like dementia (0.6%) and stroke (6.4%) were relatively rare.
Table 1.
Characteristics of Medicare Beneficiaries Undergoing Catheter Ablation for Atrial Fibrillation, July 2007–December 2009
| Characteristic | Beneficiaries (N = 15 423) |
|---|---|
| Age, mean (SD), y | 72.0 (5.3) |
| Age group, No. (%) | |
| 65–69 y | 6036 (39.1) |
| 70–74 y | 4956 (32.1) |
| 75–79 y | 2519 (16.3) |
| ≥ 80 y | 1912 (12.4) |
| Female, No. (%) | 6265 (40.6) |
| Race, No. (%) | |
| Black | 278 (1.8) |
| White | 14 822 (96.1) |
| Other | 323 (2.1) |
| US geographic region, No. (%) | |
| Midwest | 3237 (21.0) |
| Northeast | 1950 (12.6) |
| South | 6417 (41.6) |
| West | 3819 (24.8) |
| Comorbid conditions, No. (%) | |
| Cancer | 1756 (11.4) |
| Cerebrovascular disease | 2086 (13.5) |
| Chronic obstructive pulmonary disease | 3793 (24.6) |
| Dementia | 90 (0.6) |
| Diabetes mellitus | 3618 (23.5) |
| Heart failure | 3980 (25.8) |
| Hypertension | 12 072 (78.3) |
| Ischemic heart disease | 7935 (51.4) |
| Peripheral vascular disease | 2534 (16.4) |
| Renal disease | 1257 (8.2) |
| Stroke or transient ischemic attack | 992 (6.4) |
| Valvular heart disease | 6032 (39.1) |
| CHADS2 score, No. (%) | |
| 0 | 1948 (12.6) |
| 1 | 5550 (36.0) |
| ≥ 2 | 7925 (51.4) |
Adverse outcomes at 30 days were rare (Table 2). A total of 120 patients died, for a rate of 8 deaths per 1000 beneficiaries. Mortality was lower among patients younger than 75 years (0.5% vs. 1.4%), but there was no difference between men and women (0.8% for both). Similarly, the rate of stroke increased with advancing age but was consistent between men and women. Myocardial infarction (0.3%) and vascular complications (0.5%) occurred rarely. Pericardial effusion or cardiac tamponade requiring pericardiocentesis was the most common adverse event (17 per 1000 beneficiaries). Women developed pericardial effusion more often than men (2.2% vs 1.4%; Supplemental Tables 1 and 2). New hospitalizations within 30 days occurred in 12.3% of patients. Within 90 days, this rate increased to 20.3%. After multivariable adjustment, several factors were associated 30-day mortality, including advancing age, chronic obstructive pulmonary disease, diabetes mellitus, heart failure, and renal disease (Supplemental Table 2). When we excluded patients with atrial flutter in the previous 6 months, rates of adverse outcomes at 30 days were similar to those in the full cohort (Supplemental Table 3).
Table 2.
Cumulative Incidence of 30-Day Outcomes of Medicare Beneficiaries Undergoing Catheter Ablation for Atrial Fibrillation, July 2007–December 2009
| Outcome | No. of Beneficiaries (Rate per 100 Beneficiaries) | ||||||
|---|---|---|---|---|---|---|---|
| Overall | Age Group | Sex | |||||
| 65–69 y | 70–74 y | 75–79 y | ≥ 80 y | Male | Female | ||
| Death | 120 (0.8) | 30 (0.5) | 27 (0.6) | 28 (1.1) | 35 (1.8) | 68 (0.8) | 52 (0.8) |
| Myocardial infarction | 48 (0.3) | —† | —† | 12 (0.5) | 22 (1.2) | 30 (0.3) | 18 (0.3) |
| Pericardial effusion | 264 (1.7) | 101 (1.7) | 88 (1.8) | 47 (1.9) | 28 (1.5) | 127 (1.4) | 137 (2.2) |
| Stroke or transient ischemic attack* | 108 (0.8) | 35 (0.6) | 35 (0.8) | 22 (1.0) | 16 (0.9) | 66 (0.8) | 42 (0.7) |
| Vascular complication requiring surgery | 76 (0.5) | 26 (0.4) | 28 (0.6) | —† | 12 (0.6) | 38 (0.4) | 38 (0.6) |
Calculated in the subset of patients with no stroke or transient ischemic attack reported on any claim in the previous 6 months.
No cell size < 11 may be displayed, according to the data use agreement with the Centers for Medicare & Medicaid Services.
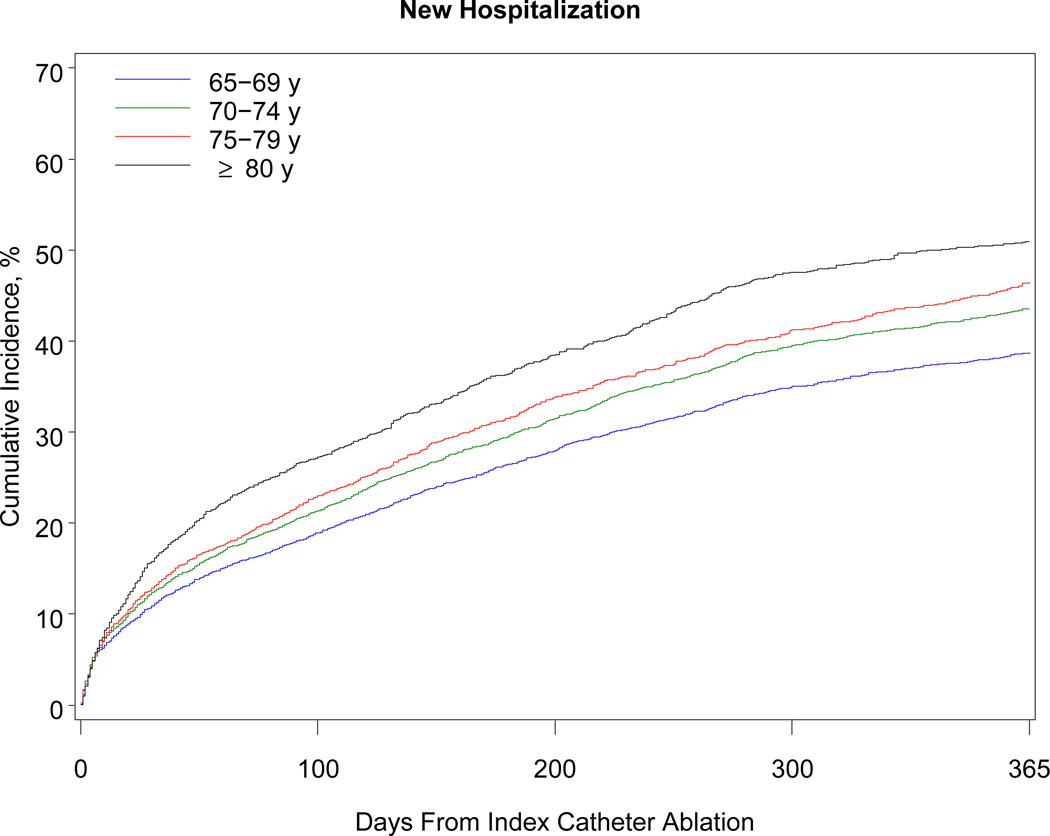
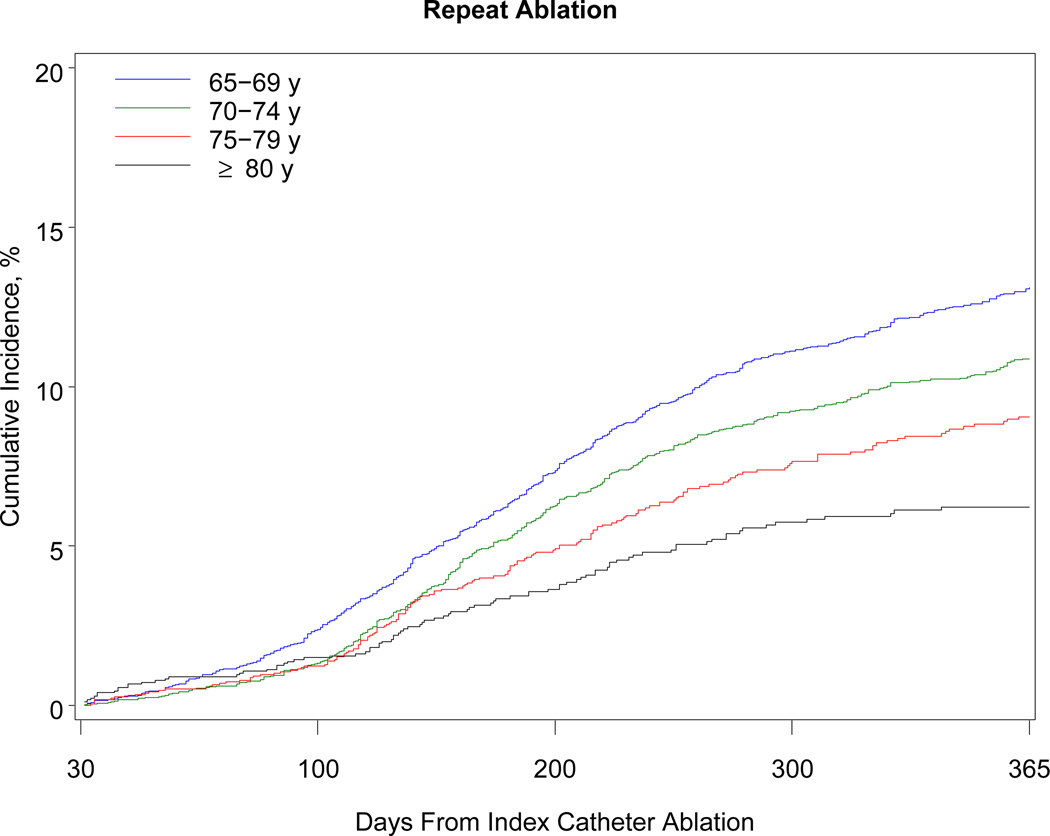
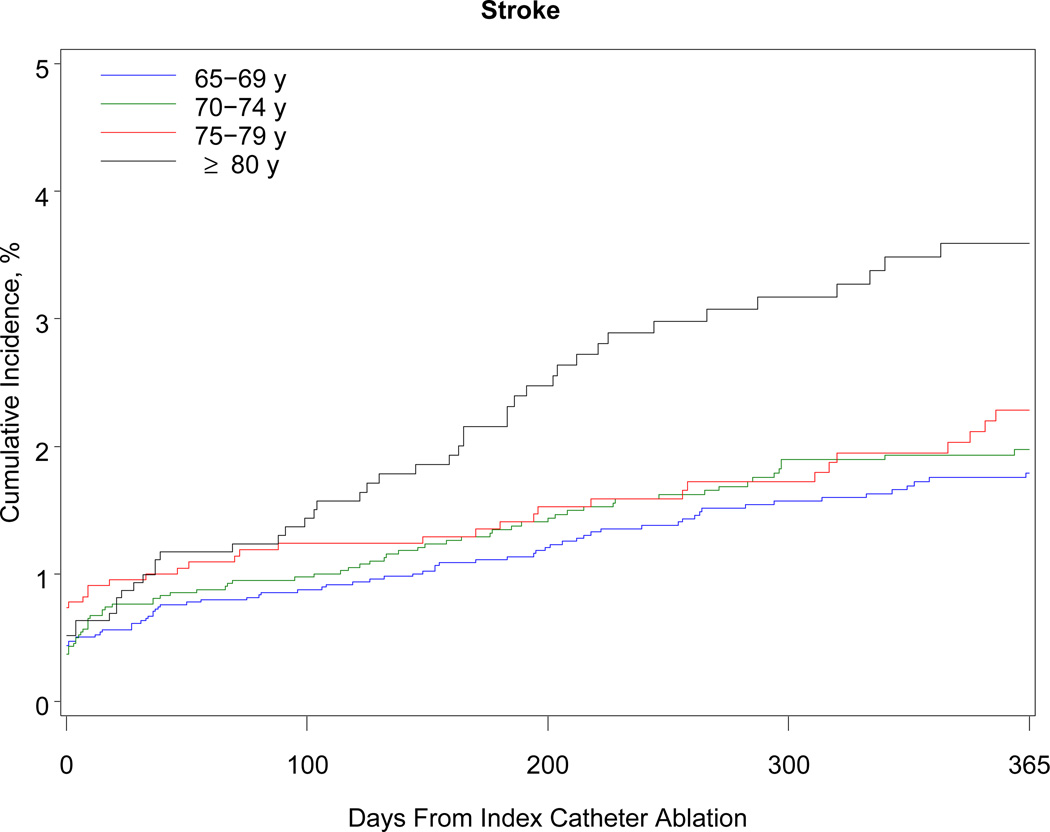
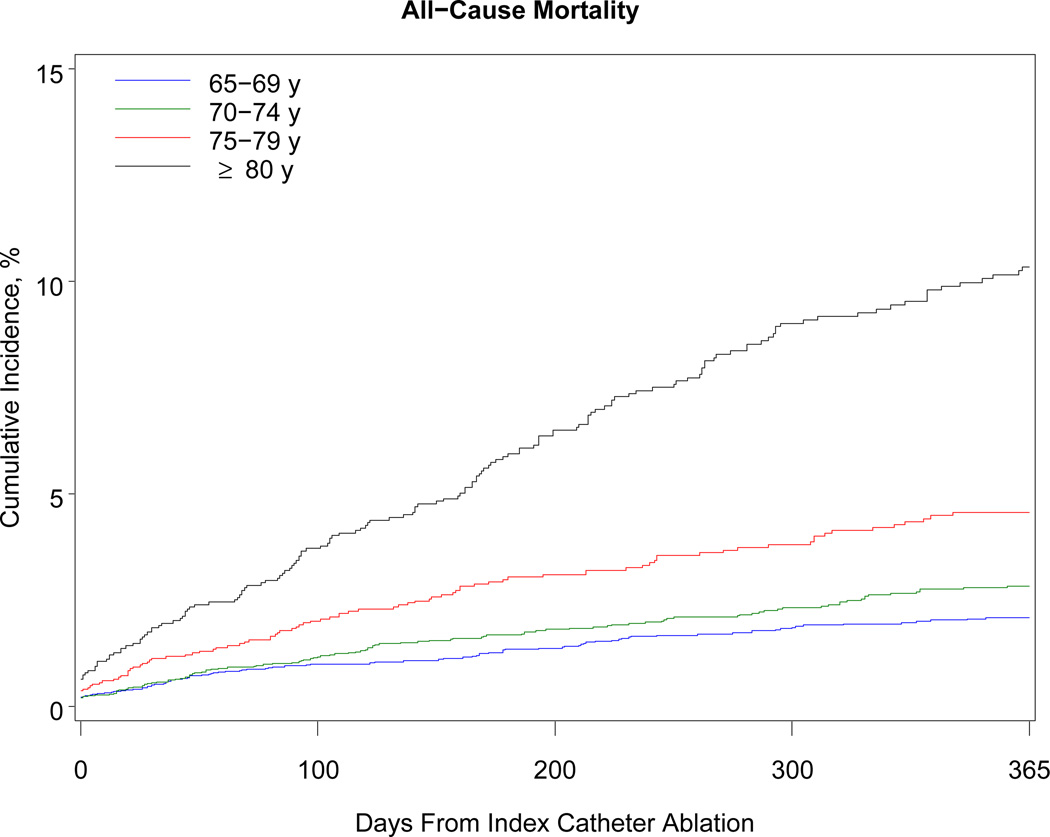
Table 3 shows the 1-year outcomes. Within 1 year, 468 patients died (38 per 1000 beneficiaries). The association with mortality was strongly related to advancing age: patients older than 80 years had a risk of death almost 5 times higher than patients 69 years or younger (10.3% vs 2.1%). Although the number of black beneficiaries in the cohort was small (n = 278), black patients were 3 times more likely than white patients to die within 1 year. Women developed heart failure more often than men. More than 40% of patients required hospitalization within the year after the procedure. Hospitalization for atrial fibrillation or atrial flutter was the primary discharge diagnosis in 38% of cases, other cardiovascular reasons in 19%, and noncardiac reasons in 43%. Almost 11% of patients underwent a repeat ablation procedure. Patients 69 years or younger were more than twice as likely as patients older than 80 years to undergo repeat ablation (13.1% vs 6.2%). The Figure shows the cumulative incidence of hospitalization, repeat ablation, stroke, and all-cause mortality.
Table 3.
Cumulative Incidence of 1-Year Outcomes of Medicare Beneficiaries Undergoing Catheter Ablation for Atrial Fibrillation, July 2007–December 2009
| Outcome | No. of Beneficiaries (Rate per 100 Beneficiaries) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Age Group | Sex | Race | |||||||
| 65–69 y | 70–74 y | 75–79 y | ≥ 80 y | Male | Female | Black | White | Other | ||
| Death | 468 (3.8) | 103 (2.1) | 113 (2.8) | 93 (4.6) | 159 (10.3) | 270 (3.7) | 198 (3.9) | 24 (11.8) | 432 (3.6) | 12 (4.8) |
| Heart failure* | 252 (2.5) | 71 (1.7) | 76 (2.3) | 46 (3.1) | 59 (5.8) | 119 (2.0) | 133 (3.3) | —‡ | 237 (2.5) | —‡ |
| Stroke/TIA† | 255 (2.1) | 85 (1.8) | 77 (2.0) | 43 (2.3) | 50 (3.6) | 139 (1.9) | 116 (2.5) | —‡ | 241 (2.1) | —‡ |
| Hospitalization | 5504 (43.0) | 1936 (38.7) | 1790 (43.5) | 961 (46.4) | 817 (51.0) | 3056 (40.3) | 2448 (47.1) | 112 (49.2) | 5281 (43.0) | 111 (40.5) |
| Repeat ablation | 1237 (10.9) | 584 (13.1) | 399 (10.9) | 165 (9.1) | 89 (6.2) | 761 (11.3) | 476 (10.4) | 12 (5.9) | 1206 (11.0) | 19 (8.1) |
Abbreviation: TIA, transient ischemic attack.
Calculated in the subset of patients with no heart failure reported on any claim in the previous 6 months.
Calculated in the subset of patients with no stroke or transient ischemic attack reported on any claim in the previous 6 months.
No cell size < 11 may be displayed, according to the data use agreement with the Centers for Medicare & Medicaid Services.
Figure.
One-Year Cumulative Incidence of Hospitalization, Repeat Ablation, Stroke or Transient Ischemic Attack, and All-Cause Mortality Among Medicare Beneficiaries Undergoing Catheter Ablation for Atrial Fibrillation, Stratified by Age, 2007–2009. Note: The scale on the y-axis differs for each graph according to the range of rates observed for the outcome.
In the multivariable analysis of 1-year outcomes, advanced age was a major risk factor for all adverse outcomes of interest (Table 4). Women were predisposed to heart failure (hazard ratio, 1.53; 95% confidence interval, 1.18–1.99), hospitalization (1.24; 1.17–1.30), and stroke or TIA (1.22; 0.97–1.54). Black race was associated with greater risks of death (1.79; 1.15–2.77) and heart failure (2.77; 1.48–5.18). Comorbid conditions particularly predicted death and readmission. Renal impairment (2.07; 1.66–2.58), age greater than 80 years (3.09; 2.32–4.11), and heart failure (2.54; 2.07,–3.13) were notable risk factors for 1-year mortality. Comorbid conditions, including cardiopulmonary disease and renal disease, were associated with hospitalization but not repeat ablation.
Table 4.
Multivariable Predictors of 1-Year Outcomes of Medicare Beneficiaries Undergoing Catheter Ablation for Atrial Fibrillation, July 2007–December 2009*
| Variable | Hazard Ratio (95% Confidence Interval) | ||||
|---|---|---|---|---|---|
| Death | Stroke/TIA† | Heart Failure† | Hospitalization | Repeat Ablation | |
| Age group | |||||
| 65–69 y | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] |
| 70–74 y | 1.20 (0.91–1.59) | 1.09 (0.78–1.51) | 1.28 (0.94–1.74) | 1.11 (1.03–1.19)§ | 0.82 (0.72–0.92)§ |
| 75–79 y | 1.68 (1.26–2.23)‖ | 1.17 (0.77–1.76) | 1.61 (1.09–2.38)‡ | 1.12 (1.03–1.21)§ | 0.68 (0.55–0.83)‖ |
| ≥ 80 y | 3.09 (2.32–4.11)‖ | 1.69 (1.12–2.57)‡ | 3.15 (2.17–4.58)‖ | 1.22 (1.11–1.34)‖ | 0.49 (0.36–0.67)‖ |
| Female | 1.06 (0.88–1.28) | 1.22 (0.97–1.54) | 1.53 (1.18–1.99)§ | 1.24 (1.17–1.30)‖ | 0.95 (0.84–1.08) |
| Race | |||||
| Black | 1.79 (1.15–2.77)‡ | 1.73 (0.91–3.28) | 2.77 (1.48–5.18)§ | 1.02 (0.86–1.20) | 0.62 (0.35–1.10) |
| White | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] |
| Other | 1.15 (0.66–2.02) | 1.01 (0.43–2.36) | 1.06 (0.47–2.39) | 1.00 (0.82–1.23) | 0.70 (0.44–1.12) |
| US geographic region | |||||
| Midwest | 1.01 (0.74–1.38) | 1.13 (0.77–1.67) | 1.07 (0.70–1.65) | 1.17 (1.05–1.29)§ | 0.73 (0.56–0.94)‡ |
| Northeast | 0.94 (0.64–1.37) | 1.33 (0.83–2.14) | 1.11 (0.73–1.70) | 1.14 (1.01–1.29)‡ | 0.76 (0.55–1.03) |
| South | 1.37 (1.05–1.78)‡ | 1.50 (1.05–2.13)‡ | 1.19 (0.85–1.66) | 1.14 (1.03–1.28)‡ | 0.87 (0.65–1.16) |
| West | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] |
| Comorbid conditions | |||||
| Cancer | 1.31 (1.03–1.68)‡ | 1.22 (0.89–1.68) | 0.91 (0.60–1.37) | 1.09 (1.00–1.19)‡ | 1.02 (0.86–1.21) |
| Cerebrovascular disease | 1.03 (0.75–1.42) | 1.19 (0.79–1.81) | 1.40 (0.92–2.12) | 1.02 (0.91–1.13) | 0.91 (0.73–1.12) |
| Chronic obstructive pulmonary disease | 1.83 (1.49–2.25)‖ | 1.18 (0.89–1.56) | 1.27 (0.92–1.73) | 1.25 (1.18–1.33)‖ | 0.92 (0.79–1.07) |
| Dementia | 1.62 (0.92–2.83) | 1.68 (0.39–7.25) | 1.65 (0.40–6.77) | 1.51 (1.14–1.99)§ | 1.00 (0.39–2.56) |
| Diabetes mellitus | 1.27 (1.03–1.57)‡ | 1.11 (0.84–1.46) | 1.35 (1.02–1.78)‡ | 1.14 (1.07–1.21)‖ | 0.92 (0.80–1.06) |
| Heart failure | 2.54 (2.07–3.13)‖ | 1.50 (1.11–2.02)§ | —¶ | 1.30 (1.22–1.39)‖ | 1.00 (0.85–1.18) |
| Hypertension | 0.84 (0.66–1.08) | 1.01 (0.72–1.40) | 1.27 (0.89–1.81) | 1.14 (1.06–1.23)‖ | 0.92 (0.80–1.06) |
| Ischemic heart disease | 1.14 (0.92–1.41) | 0.98 (0.76–1.26) | 1.17 (0.91–1.52) | 1.16 (1.09–1.23)‖ | 0.96 (0.84–1.09) |
| Peripheral vascular disease | 1.32 (1.07–1.64)‡ | 1.08 (0.80–1.47) | 0.82 (0.57–1.17) | 1.10 (1.03–1.18)§ | 0.92 (0.77–1.09) |
| Renal disease | 2.07 (1.66–2.58)‖ | 1.17 (0.72–1.90) | 1.90 (1.29–2.78)§ | 1.32 (1.21–1.44)‖ | 0.95 (0.76–1.18) |
| Stroke or transient ischemic attack | 0.97 (0.64–1.46) | —¶ | 0.84 (0.47–1.50) | 1.10 (0.96–1.27) | 1.09 (0.80–1.47) |
| Valvular heart disease | 0.98 (0.80–1.21) | 0.78 (0.61–0.99)‡ | 1.26 (0.99–1.61) | 1.04 (0.98–1.10) | 1.03 (0.91–1.16) |
Abbreviation: TIA, transient ischemic attack.
The multivariable models for death, hospitalization, and repeat ablation include all variables listed in Table 1. The model for stroke/TIA includes all variables except prior stroke or transient ischemic attack. The model for heart failure includes all variables except prior heart failure. The reference group for each dichotomous comorbid condition variable is the absence of the condition.
The stroke/TIA analysis was limited to the subset of patients with no heart failure reported on any claim in the previous 6 months. The heart failure analysis was limited to the subset of patients with no heart failure reported on any claim in the previous 6 months.
P < .05
P < .01
P < .001
No cell containing data for a sample size < 11 may be displayed, according to the data use agreement with the Centers for Medicare & Medicaid Services.
Discussion
We analyzed outcomes of more than 15,000 Medicare beneficiaries who underwent catheter ablation for atrial fibrillation. Our findings suggest that complication rates associated with catheter ablation in older patients are low but increase with advancing age. Moreover, hospitalization is common after catheter ablation. Approximately 40% of patients were hospitalized within 1 year after the procedure. Renal impairment, advanced age, and heart failure were risk factors for 1-year mortality.
For every 1000 catheter ablation procedures, there were approximately 8 deaths (0.8%), 8 cases of stroke (0.8%), and 17 cases of pericardial effusion or tamponade (1.7%) within 30 days. Our analysis included all fee-for-service Medicare beneficiaries who underwent catheter ablation for atrial fibrillation. A recent analysis of catheter ablation in 4156 patients in California reported a 2.4% risk of stroke, a 2.5% risk of cardiac perforation or tamponade, and a 30-day risk of death of approximately 0.2%.22 An international survey of electrophysiologists performing catheter ablation in 16,309 patients at 85 sites reported a 0.15% periprocedural risk of death, a 0.23% risk of stroke, and a 1.31% risk of cardiac tamponade.23
Previous studies have reached contradictory conclusions about the likelihood of complications after catheter ablation in older patients. Although some studies have found similar complication rates across age categories,24,25 others have suggested increased risks.22,26 In our study, complication rates were higher among patients 70 years and older. For example, the 30-day risk of death was 0.5% among patients 69 years or younger, compared with 1.8% among patients 80 years and older. We found unexpectedly that the risk of complications was similar among men and women, though women had a greater risk of pericardial effusion. Women in other studies experienced higher rates of vascular complications after interventional procedures, including catheter ablation.27,28 Catheter ablation performed in the South was associated with a higher risk of major complications, perhaps because of higher prevalence of comorbid conditions in the region.29
Our findings provide some reassurance regarding the safety of catheter ablation in older patients. Given that 1-year mortality among unselected Medicare beneficiaries with incident atrial fibrillation is approximately 25%,5 the 1-year mortality rate of 3.8% we observed is relatively low in comparison. Considering further that one-quarter of the patients in our study had chronic obstructive pulmonary disease and another quarter had heart failure, the mortality rate was remarkably low.
Data from randomized controlled trials suggest that there is a significantly lower risk of cardiovascular hospitalization after catheter ablation, compared with antiarrhythmic drug therapy.14 Other information about the frequency of cardiovascular or all-cause hospitalizations is sparse, especially for older patients. In our cohort, new hospitalizations occurred in 12% of patients at 30 days, 20% at 90 days, and 43% at 1 year. However, less than 40% of hospitalizations within 1 year were associated with a primary diagnosis of atrial fibrillation or atrial flutter. Also, few hospitalizations were for major cardiovascular events such as stroke (2.1%) and heart failure (2.5%). These findings are similar to results of a recent analysis of data from California showing hospitalization rates of 9% at 30 days and 39% at 1 year in a younger population.22 Acknowledging that the major cardiovascular hospitalization rates in our analysis occurred among patients who did not have events in the previous 6 months, the rates we observed are comparable to rates in other Medicare postprocedure data. We found a 43% hospitalization rate after catheter ablation; however, among all fee-for-service Medicare beneficiaries, hospitalization within 1 year after any surgical procedure is 47%.30 Consistent with other recent findings in the Medicare population, we found significant regional variation in all-cause hospitalization.31 Factors contributing to hospitalization after catheter ablation require further investigation.
In our study, the cumulative incidence of stroke after catheter ablation was 0.8% at 30 days and 2.1% at 1 year. Prospective studies are needed to evaluate the long-term risk of stroke and the need for anticoagulation after ablation. Current guidelines recommend long-term anticoagulation therapy after catheter ablation in in patients with atrial fibrillation if they are older or at higher risk for cerebrovascular events.32 Our findings provide indirect observational support for this recommendation.
The rate of repeat ablation within 1 year was 17% in clinical trials and reached 20% to 40% in observational studies.14,33,34 In our study, the rate of repeat ablation more than 30 days after the index procedure was approximately 10%. This low rate in an older, higher-risk population may be explained by patients’ reluctance to undergo repeat procedures, physician reluctance to perform them, relatively lower burden of symptoms in older compared to younger patients,35 and the higher frequency of continued antiarrhythmic therapy in older patients after catheter ablation.25 Additional research is needed to determine whether symptom relief and postprocedure improvement in quality of life are similar between younger and older patients.
Older patients who undergo catheter ablation for atrial fibrillation differ from patients in clinical trials and unselected older adults with atrial fibrillation. The Medicare population has greater comorbidity than recent clinical trial samples.14 For example, our study population was older (72 vs 56 years), had higher rates of previous stroke or TIA (6.4% vs 1.9%), and had a higher prevalence of diabetes mellitus (23.5% vs 9.5%) than patients with paroxysmal atrial fibrillation in the ThermoCool trial.36 Compared with an age- and sex-standardized population of otherwise unselected Medicare beneficiaries with prevalent atrial fibrillation, those undergoing catheter ablation had less debilitating comorbid conditions such as dementia, heart failure, and stroke.5 These differences may reflect careful patient selection by referring physicians or electrophysiologists to optimize the effectiveness and safety of catheter ablation in older patients. Although less frequent when compared with the overall Medicare population with prevalent atrial fibrillation, a quarter of the patients who underwent catheter ablation had heart failure. Catheter ablation in the context of heart failure was only recently included in treatment guidelines.15 Outcomes of these patients in particular requires further investigation.
The primary goal of catheter ablation for atrial fibrillation is to improve symptoms and quality of life. Accordingly, identification of patients at risk for poor outcomes (including death) is paramount. We identified several factors associated with adverse outcomes. Age greater than 80 years, renal impairment, and heart failure were strongly associated with 1-year mortality. Patients older than 80 years were also more likely to experience stroke, heart failure, and hospitalization within 1 year. Thus, patients older than 80 years should be counseled regarding the higher risk for complications.
A critical question remains unanswered: How do outcomes of older patients who undergo catheter ablation for atrial fibrillation compare with outcomes of older patients with atrial fibrillation who, although eligible, do not undergo catheter ablation? The appropriate comparison group—patients eligible for catheter ablation who do not undergo the procedure—cannot be identified with claims data alone. Pragmatic randomized clinical trials, like the Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation trial (clinicaltrials.gov registry number NCT00911508), will address both the efficacy and the net clinical benefit of catheter ablation, including its impact on incident stroke and mortality. However, the effectiveness of catheter ablation in routine clinical practice will be more challenging to address. Even a nationwide registry would be ill-equipped to address the question, because registries do not include comparable patients who are eligible for but do not receive the therapy.
Our study has some limitations. First, no procedure code indicates catheter ablation for atrial fibrillation specifically. We combined nonspecific codes for catheter ablation with diagnosis codes for atrial fibrillation to identify the procedures of interest. Our approach is comparable to previously published coding algorithms.20,37 Second, claims data do not distinguish between types of atrial fibrillation. Outcomes after catheter ablation are influenced by the type of atrial fibrillation, and guideline recommendations are strongest for patients with paroxysmal and medically refractory atrial fibrillation.15 Moreover, it is possible that patients with atrial flutter and atrial fibrillation underwent right-sided ablation of atrial flutter with concomitant miscoding of atrial fibrillation as a primary diagnosis. This miscoding may have led to an underestimate of complication rates, though a sensitivity analysis excluding patients with atrial flutter yielded similar results. Third, no data on pre- or postprocedure drug therapy were available. Fourth, billing codes for complications, such as hemopericardium and vascular complications requiring surgery, are specific but lack sensitivity. Therefore, we may have underestimated the rates of these complications. Fifth, the study population included few patients from racial and ethnic minority groups; thus, we had limited power to examine factors associated with outcomes by race and ethnicity. Finally, the generalizability of our findings to younger patients and those enrolled in Medicare managed care plans is unclear.
Conclusion
Medicare beneficiaries who underwent catheter ablation for atrial fibrillation were older and had greater comorbidity than participants in clinical trials. Major complications after catheter ablation for atrial fibrillation were relatively infrequent. Randomized trials are needed to assess efficacy of catheter ablation for atrial fibrillation in older adults and to better inform risk-benefit calculations for older patients with drug-refractory, symptomatic atrial fibrillation.
Supplementary Material
Acknowledgments
Damon M. Seils, MA, Duke University, provided editorial assistance and prepared the manuscript. Mr Seils did not receive compensation for his assistance apart from his employment at the institution where the study was conducted.
Funding/Support: This work was supported by grants R01HL102214, R01HL068986, R01HL092577, R01HL104156, K24HL105780, and RC1HL101056 from the National Heart, Lung, and Blood Institute and grant R21DA027021 from the National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Institute on Drug Abuse, or the National Institutes of Health.
Disclosures: Dr Piccini reported receiving research grant funding > $10,000 from Janssen Pharmaceuticals; and receiving consultant/advisory board income from Forest Laboratories (< $10,000), Johnson & Johnson (> $10,000), Medtronic (< $10,000), and Titan Pharmaceuticals (< $10,000). Dr Daubert reported receiving research grant funding (> $10,000) from Biosense Webster, Biotronik, Gilead, and Medtronic; receiving honoraria from Biotronik, Boston Scientific, Medtronic, Sorin, and St. Jude; serving as an expert witness for a patient with sudden cardiac death due to coronary artery disease; having ownership interest (> $10,000) in Biosense Webster inherited by his wife/children in a generation-skipping trust for the children; and receiving consultant/advisory board income (< $10,000) from Premier, Inc. Dr Hernandez reported receiving research grant funding from Johnson & Johnson. Dr Curtis reported receiving research grant funding from Johnson & Johnson. Drs Piccini, Hernandez, and Curtis have made available online detailed listings of financial disclosures (http://www.dcri.duke.edu/aboutus/ conflict-of-interest/). No other authors reported disclosures.
References
- 1.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 2.Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D’Agostino RB. Secular trends in the prevalence of atrial fibrillation: the Framingham Study. Am Heart J. 1996;131:790–795. doi: 10.1016/s0002-8703(96)90288-4. [DOI] [PubMed] [Google Scholar]
- 3.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D’Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 5.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 7.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 8.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 9.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna W, Seward JB, Iwasaka T, Tsang TS. Incidence and mortality risk of congestive heart failure in atrial fibrillation patients: a community-based study over two decades. Eur Heart J. 2006;27:936–941. doi: 10.1093/eurheartj/ehi694. [DOI] [PubMed] [Google Scholar]
- 10.Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study. The Rotterdam Study. Stroke. 1997;28:316–321. doi: 10.1161/01.str.28.2.316. [DOI] [PubMed] [Google Scholar]
- 11.Dorian P, Jung W, Newman D, Paquette M, Wood K, Ayers GM, Camm J, Akhtar M, Luderitz B. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol. 2000;36:1303–1309. doi: 10.1016/s0735-1097(00)00886-x. [DOI] [PubMed] [Google Scholar]
- 12.AFFIRM First Antiarrhythmic Drug Substudy Investigators. Maintenance of sinus rhythm in patients with atrial fibrillation: an AFFIRM substudy of the first antiarrhythmic drug. J Am Coll Cardiol. 2003;42:20–29. doi: 10.1016/s0735-1097(03)00559-x. [DOI] [PubMed] [Google Scholar]
- 13.Wazni O, Wilkoff B, Saliba W. Catheter ablation for atrial fibrillation. N Engl J Med. 2011;365:2296–2304. doi: 10.1056/NEJMct1109977. [DOI] [PubMed] [Google Scholar]
- 14.Piccini JP, Lopes RD, Kong MH, Hasselblad V, Jackson K, Al-Khatib SM. Pulmonary vein isolation for the maintenance of sinus rhythm in patients with atrial fibrillation: a meta-analysis of randomized, controlled trials. Circ Arrhythm Electrophysiol. 2009;2:626–633. doi: 10.1161/CIRCEP.109.856633. [DOI] [PubMed] [Google Scholar]
- 15.Wann LS, Curtis AB, Ellenbogen KA, Estes NA, 3rd, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM. 2011 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines . J Am Coll Cardiol. 2011;57:1330–1337. doi: 10.1016/j.jacc.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 17.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 18.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 19.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 20.Ellis ER, Culler SD, Simon AW, Reynolds MR. Trends in utilization and complications of catheter ablation for atrial fibrillation in Medicare beneficiaries. Heart Rhythm. 2009;6:1267–1273. doi: 10.1016/j.hrthm.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 22.Shah RU, Freeman JV, Shilane D, Wang PJ, Go AS, Hlatky MA. Procedural complications, rehospitalizations, and repeat procedures after catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2012;59:143–149. doi: 10.1016/j.jacc.2011.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–38. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 24.Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, Anderson JL, Lappe DL, Muhlestein JB, Nelson J, Day JD. Long-term clinical efficacy and risk of catheter ablation for atrial fibrillation in octogenarians. Pacing Clin Electrophysiol. 2010;33:146–152. doi: 10.1111/j.1540-8159.2009.02604.x. [DOI] [PubMed] [Google Scholar]
- 25.Zado E, Callans DJ, Riley M, Hutchinson M, Garcia F, Bala R, Lin D, Cooper J, Verdino R, Russo AM, Dixit S, Gerstenfeld E, Marchlinski FE. Long-term clinical efficacy and risk of catheter ablation for atrial fibrillation in the elderly . J Cardiovasc Electrophysiol. 2008;19:621–626. doi: 10.1111/j.1540-8167.2008.01183.x. [DOI] [PubMed] [Google Scholar]
- 26.Spragg DD, Dalal D, Cheema A, Scherr D, Chilukuri K, Cheng A, Henrikson CA, Marine JE, Berger RD, Dong J, Calkins H. Complications of catheter ablation for atrial fibrillation: incidence and predictors. J Cardiovasc Electrophysiol. 2008;19:627–631. doi: 10.1111/j.1540-8167.2008.01181.x. [DOI] [PubMed] [Google Scholar]
- 27.Hoyt H, Bhonsale A, Chilukuri K, Alhumaid F, Needleman M, Edwards D, Govil A, Nazarian S, Cheng A, Henrikson CA, Sinha S, Marine JE, Berger R, Calkins H, Spragg DD. Complications arising from catheter ablation of atrial fibrillation: temporal trends and predictors. Heart Rhythm. 2011;8:1869–1874. doi: 10.1016/j.hrthm.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 28.Lansky AJ, Pietras C, Costa RA, Tsuchiya Y, Brodie BR, Cox DA, Aymong ED, Stuckey TD, Garcia E, Tcheng JE, Mehran R, Negoita M, Fahy M, Cristea E, Turco M, Leon MB, Grines CL, Stone GW. Gender differences in outcomes after primary angioplasty versus primary stenting with and without abciximab for acute myocardial infarction: results of the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. Circulation. 2005;111:1611–1618. doi: 10.1161/01.CIR.0000160362.55803.40. [DOI] [PubMed] [Google Scholar]
- 29.Cushman M, Cantrell RA, McClure LA, Howard G, Prineas RJ, Moy CS, Temple EM, Howard VJ. Estimated 10-year stroke risk by region and race in the United States: geographic and racial differences in stroke risk. Ann Neurol. 2008;64:507–513. doi: 10.1002/ana.21493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 31.Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365:2287–2295. doi: 10.1056/NEJMsa1101942. [DOI] [PubMed] [Google Scholar]
- 32.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632.e21–696.e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 33.Kobza R, Hindricks G, Tanner H, Schirdewahn P, Dorszewski A, Piorkowski C, Gerds-Li JH, Kottkamp H. Late recurrent arrhythmias after ablation of atrial fibrillation: Incidence, mechanisms, and treatment. Heart Rhythm. 2004;1:676–683. doi: 10.1016/j.hrthm.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Steven D, Rostock T, Lutomsky B, Klemm H, Servatius H, Drewitz I, Friedrichs K, Ventura R, Meinertz T, Willems S. What is the real atrial fibrillation burden after catheter ablation of atrial fibrillation? A prospective rhythm analysis in pacemaker patients with continuous atrial monitoring. Eur Heart J. 2008;29:1037–1042. doi: 10.1093/eurheartj/ehn024. [DOI] [PubMed] [Google Scholar]
- 35.Disertori M, Lombardi F, Barlera S, Maggioni AP, Favero C, Franzosi MG, Lucci D, Staszewsky L, Fabbri G, Quintarelli S, Bianconi L, Latini R. Clinical characteristics of patients with asymptomatic recurrences of atrial fibrillation in the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico-Atrial Fibrillation (GISSI-AF) trial. Am Heart J. 2011;162:382–389. doi: 10.1016/j.ahj.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, Macle L, Daoud EG, Calkins H, Hall B, Reddy V, Augello G, Reynolds MR, Vinekar C, Liu CY, Berry SM, Berry DA ThermoCool AF Trial Investigators. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–340. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 37.Ladapo JA, David G, Gunnarsson CL, Hao SC, White SA, March JL, Reynolds MR. Healthcare utilization and expenditures in patients with atrial fibrillation treated with catheter ablation. J Cardiovasc Electrophysiol. 2012;23:1–8. doi: 10.1111/j.1540-8167.2011.02130.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.