Abstract
This study examined sleep, sleepiness, and daytime performance in 68 children with autism, 57 children with intellectual disability (ID), and 69 typically developing preschool children. Children in the autism and ID groups had poorer daytime performance and behaviors than the typically developing children. Children in the ID group also were significantly sleepier than children in both the autism and typically developing groups. These significant differences persisted over 6 months. Actigraph-defined sleep behaviors and problems did not relate to daytime sleepiness or daytime performance and behaviors for the children with autism or the typically developing group. For the ID group, longer night awakenings and lower sleep efficiency predicted more daytime sleepiness. For each group, parent-report sleep problems were associated with more daytime sleepiness and more behavior problems.
Keywords: autism, intellectual disability, behavior, sleep
Studies report that 40%–80% of children with autism spectrum disorders compared with 10%–30% of typically developing children have associated sleep problems (Cortesi, Giannotti, Ivanenko, & Johnson, 2010; Goldman et al., 2009; Johnson, Giannotti, & Cortesi, 2009; Krakowiak, Goodlin-Jones, Hertz-Picciotto, Croen, & Hansen, 2008; Polimeni, Richdale, & Francis, 2005; Quine, 2001; Richdale & Schreck, 2009; Souders et al., 2009). Moreover, children with disrupted sleep present with daytime behavior problems (Buckhalt, El-Sheikh, Keller, & Kelly, 2009; Foldvary-Schaefer & Malow, 2011; Gregory, Eley, O’Connor, & Plomin, 2004; Lavigne et al., 1999; Malow & McGrew, 2008; Wiggs & Stores, 1996). For example, in a parent-report study of elementary school-age children with autism, fewer hours of sleep were associated with more social skill deficits, more repetitive behaviors, and greater irritability (Schreck, Mulick, & Smith, 2004). Similarly, more severe sleep problems in children with intellectual disability were correlated with more daytime behavior problems, such as aggression (Didden, Korzilius, van Aperlo, van Overloop, & de Vries, 2002). In typically developing children, sleep problems are also associated with daytime behavior problems in both preschool-age (Hall, Scher, Zaidman-Zait, Espezel, & Warnock, 2011) and school-age children (Buckhalt et al., 2009; El-Sheikh, Kelly, Buckhalt, & Hinnant, 2010; Keller & El-Sheikh, 2010). In sum, sleep problems or inadequate sleep from variable causes (e.g., obstructive sleep apnea or family schedule disruption) are associated with a wide range of behavioral problems (Beebe, 2011).
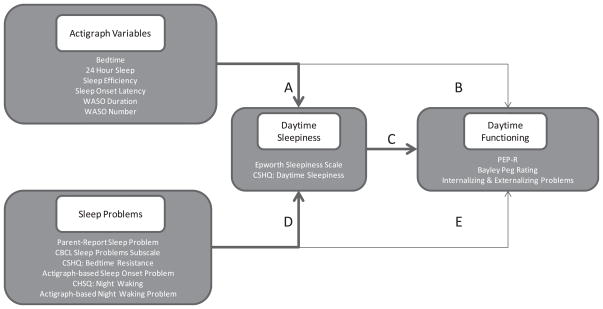
Although it is clear that sleep disruptions or problems affect daytime performance and behavior, it is not clear how daytime sleepiness affects the process. The present study tested a novel model in which daytime sleepiness is proposed to mediate the relationship between nighttime sleep disruption and daytime performance. The study focuses on children of preschool age and incorporates multiple measures of sleep behaviors and sleep problems to examine the relationships between sleep, sleepiness, and daytime behaviors in three developmentally distinct comparison populations. As illustrated in Figure 1, this study hypothesized that sleep disruption would lead to daytime sleepiness, which in turn would negatively impact daytime behavior and performance. The University of California, Davis (UC Davis), Institutional Review Board approved the protocol, and all parents signed informed consents.
Figure 1.
A conceptual model of pathways from sleep to daytime functioning that was tested in the study. Bold arrows present the hypothesized mediation paths. WASO = wake after sleep onset; CSHQ = Children’s Sleep Habits Questionnaire; PEP-R = Psychoeducational Profile—Revised; CBCL = Child Behavior Checklist.
Method
Participants
Three groups of children were enrolled: children with autism, children with intellectual disability without autism (the ID group), and typically developing children. Subjects were recruited to the project from several sources: the UC Davis M.I.N.D. Institute’s research recruitment registry, advertisements in local newspapers, and word of mouth. Families were recruited to a study “to learn more about sleep and waking patterns” and not to a study about sleep problems. Only one child per family was enrolled. Typically developing children were included only if they had no siblings with autism or other neurodevelopmental disorders. Exclusion criteria for all children included the presence of a chronic medical illness or current or prior treatment for a sleep disorder. Two children in the ID group who had been diagnosed with a seizure disorder that was well controlled by anticonvulsant medication were included. None of the other participants were on any medication. The ages of children at enrollment ranged from 24 to 66 months (M = 44.4 months, SD = 11.1 months). Of the 194 children (autism = 68, ID = 57, typical development = 69) recorded at Time 1, 179 children were studied at Time 2, and 173 children completed all three recording sessions, accounting for a 89% completion rate. Families who dropped from the study did so for various reasons that included moving from the area, schedules that were too busy, or households that were too hectic. No family dropped because of an adverse event resulting from the study. There were no significant differences in gender, ethnicity, or diagnosis between those who completed the study and those who did not.
Children with a neurodevelopmental disorder underwent an initial diagnostic evaluation consisting of the Autism Diagnostic Observation Scale (ADOS; Lord, Rutter, DiLavore, & Risi, 1999); the Mullen Scales of Early Learning (MSEL; Mullen, 1995), a test of cognitive ability; and the Vineland Adaptive Behavior Scales (VABS; Sparrow, Balla, & Cicchetti, 1984), a test of adaptive functioning. The Autism Diagnostic Interview—Revised (ADI-R; Rutter, Le Couteur, & Lord, 2003) was completed with parents of children in the group with autism. Only children who met diagnostic criteria for autistic disorder using the ADOS cutoff and who met criteria on all domains of the ADI-R were included as subjects in the autism group. The ADOS and ADI-R are gold standard measures and were administered by certified practitioners. Children in the ID group scored below the autism spectrum disorder (ASD) cutoff score on the ADOS and below 70 on the MSEL Early Learning Composite (ELC). Typically developing children were not given the ADOS but completed the VABS and MSEL with ELC scores above 75.
Group characteristics at enrollment are presented in Table 1. The typically developing group was on average 6 months younger (M = 40.9 months) than the children in the autism (M = 46.8 months) and ID (M = 45.7 months) groups at initial enrollment (both ps < .05). Both age and developmental status impact maturation of sleep–wake organization in young children; however, it is not clear in populations with neurodevelopmental disorders which of these two factors has more impact. In an attempt to match children on their maturational age, we intentionally recruited typically developing children who were slightly younger in chronologic age. Chronologic age was used as a covariate in all statistical analyses; therefore, all models are adjusted to control for the imbalance of age across groups. Mothers of children in the ID group were significantly more likely to be single, and fewer had graduated from college (p < .05). Children in the two neurodevelopmentally disordered groups were well matched on the MSEL ELC and VABS Adaptive Behavior Composite (ABC) scores and differed significantly from the typically developing children on these scales. See Table 1.
Table 1.
Characteristics of Children and Families by Diagnostic Group at Time 1
| Variable | Autism (n = 68) | Intellectual disability (n = 57) | Typically developing (n = 69) |
|---|---|---|---|
| Gender (% male) | 81 | 74 | 70 |
| Age at baseline (months) | |||
| Mean (SD) | 47 (10) | 46 (12) | 41 (11)* |
| Range | 28–68 | 24–70 | 24–62 |
| Ethnicity (% Caucasian) | 59 | 47 | 70 |
| Mullen ELC | |||
| Mean (SD) | 60 (18) | 55 (7) | 101 (17)* |
| Range | 49–140 | 49–74 | 72–148 |
| Vineland ABC | |||
| Mean (SD) | 62 (11) | 62 (12) | 98 (17)* |
| Range | 41–87 | 31–104 | 65–151 |
| Education (% college graduates) | 64 | 39* | 72 |
| Marital status (% married) | 86 | 79* | 94 |
| Attrition (% dropout) | 9 | 12 | 12 |
Note. Mullen ELC = Early Learning Composite on the Mullen Scales of Early Learning; ABC = Adaptive Behavior Composite on the Vineland Adaptive Behavior Scales.
p < .05.
Assessment of Sleep
Each child’s sleep patterns were measured via actigraphy and a parent-completed sleep diary and recorded over 7 consecutive 24-hr periods, except when bathing. Weeklong recordings were completed on three occasions: Time 1, at enrollment; Time 2, at 3 months after enrollment; and Time 3, at 6 months after enrollment. The actigraph, a Mini Mitter Actiwatch (AW64; Mini Mitter, Oregon), was embedded in a foam pad, secured by a Velcro strap on the child’s nondominant ankle. Actigraph data were analyzed with the manufacturer’s algorithm set at medium sensitivity (Mini Mitter). A secondary laboratory “smoothing” filter recoded isolated, single-minute actigraph waking epochs after sleep onset as sleep epochs, a procedure that significantly improved reliability (Sitnick, Goodlin-Jones, & Anders, 2008).
Actigraph sleep variables included bedtime and naptime (recorded clock time from the diary), 24-hr sleep (sum of nighttime sleep duration plus nap duration in minutes), sleep efficiency (time in bed asleep divided by the total time in bed), sleep onset latency time (number of minutes from bedtime to sleep start time), wake after sleep onset (WASO) duration (total minutes awake after sleep onset), and WASO number (the number of nighttime awakenings after sleep onset). Nap durations and frequencies were also obtained from the actigraph. Naps were defined as any sleep that took place outside of the child’s nighttime sleep, roughly between 7 a.m. and 7 p.m., and confirmed on the parent-report sleep diary.
Defining Problem Sleep
The presence of a sleep problem during each of the three recording weeks was defined in three ways: global sleep problems, bedtime resistance or sleep onset problems, and night awakening problems. For each of the sleep problem definitions, two indices were used (summarized in Table 2). For global sleep problems, parents were asked whether, in their opinion, their child currently had a sleep problem (yes/no). Additionally, each parent completed the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2000), and from this questionnaire, the empirically based Sleep Problems subscale was extracted, a procedure commonly done in studies of typically developing children and children with disabilities. For bedtime resistance and sleep onset and night awakening problems, parents completed the Children’s Sleep Habits Questionnaire (CSHQ; Owens, Spirito, & McGuinn, 2000), from which the Bedtime Resistance and Night Awakening subscales were used. The actigraph-derived variables of WASO duration, WASO number, and sleep onset latency were used to determine either a night waking or sleep onset behavioral insomnia, or both, according to predetermined cut points (Anders & Dahl, 2007).
Table 2.
Sleep Problems Indices
| Index | Measure |
|---|---|
| Global sleep problems |
|
| Bedtime resistance and sleep onset problems |
|
| Night awakening sleep problems |
|
Note. Each sleep problem index was completed or computed for each recording session (at Times 1, 2, and 3).
Assessment of Daytime Sleepiness
To assess daytime sleepiness, we used two parent-report indices. First, parents completed a modified version of the Epworth Sleepiness Scale (ESS; Johns, 1991). The ESS has not been standardized for use with this age group; however, because of its ease of use and the potential for generalization to studies of older children, it was selected as an exploratory method. The ESS is an eight-item questionnaire that inquires about the likelihood of the child falling asleep in everyday situations (e.g., watching television, riding in a car, sitting after a meal). Sleep propensity is measured on a 4-point Likert scale ranging from 0 (would never sleep) to 3 (very likely to sleep). Scores on each item are totaled for a range from 0 to 24. The ESS has been used in typically developing older children (Chan et al., 2009) and children with neurodevelopmental concerns (Elkhayat et al., 2010; Joo et al., 2010). In adult studies, the ESS is correlated with the Multiple Sleep Latency Test (MSLT; Johns, 1991), but this association in young children has not been established. The ESS was completed at each of the three recording periods.
The second daytime sleepiness index was derived from the Children’s Sleep Habits Questionnaire (CSHQ; Owens et al., 2000). The Daytime Sleepiness subscale asks parents to rate whether their child wakes independently, wakes in a bad mood, has a hard time getting out of bed, seems tired, takes a long time to become alert, and falls asleep during the day while riding in a car or watching TV. Subscale scores for this sample ranged from 8 to 22 and were calculated at each assessment period. Most children presented with minimal daytime sleepiness on this scale.
Assessment of Daytime Functioning
During each actigraph recording week, children and their parents completed an hour-long laboratory battery to assess daytime performance and behavior. Children were tested on the Psychoeducational Profile—Revised (PEP-R; Schopler, Reichler, Bashford, Lansing, & Marcus, 1990), and the Bayley peg-board task (Bayley, 1969). Parents completed the CBCL for children ages 1.5 to 5 years (Achenbach & Rescorla, 2000). These measures were chosen because of their suitability for both typical and neurodevelopmentally impaired preschool-age children. The PEP-R measures several different domains of functioning, such as perception, eye–hand coordination, and fine and gross motor coordination. The PEP-R has been used with children on the autism spectrum in a pre-post design and is a good measure for demonstrating improvement in functioning (Ozonoff & Cathcart, 1998). The peg-board task, a part of the Bayley Scales of Infant Development (Bayley, 1969), assesses attentiveness and coordination. The mean peg “rate” reflects the number of correctly placed pegs per unit of time over three trials. The CBCL is a parent-report measure that includes 99 problem item responses plus descriptions of problems, disabilities, concerns, and strengths of the child. The CBCL is well validated in typically developing children (Achenbach & Rescorla, 2000) and is commonly used with populations of children who have developmental concerns (Eisenhower, Baker, & Blacher, 2005; Ivanova et al., 2010; Spratt, Salor, & Macias, 2007). For the present study, the commonly used subscales of Externalizing and Internalizing Behavior Problems were used for analyses. These subscales do not contain the CBCL sleep problem items.
Data Analysis
We conducted our statistical analyses with SAS Version 9.2 and included descriptive statistics for all categorical and continuous variables. We used random-effects regression models (Laird & Ware, 1982) to (a) estimate patterns of change in amount and timing of sleepiness and daytime behavioral and performance variables, (b) test whether diagnosis and other covariates were related to the initial level or rate of change in these variables, and (c) assess whether daytime sleepiness mediated the relationships between sleep behaviors and problems and daytime performance. This approach allowed the use of all available data for each child, while accounting for the correlated nature of the data that resulted from repeated measurements on the same individual.
The initial set of models included fixed effects for diagnosis, age at baseline, and time (in months) since baseline. A second set of models added the interaction of diagnosis with age and time. The interaction term between diagnosis and time tested whether the rate of change varied by diagnosis. Parameters that did not add significantly to the model were removed; therefore, the reported models include only significant predictors.
Another set of models included the actigraph variables as predictors and their interaction with diagnosis and time. A final set of models added terms for sleep problems and their interaction with diagnosis and time to evaluate their effect on baseline levels and change in sleepiness and daytime behavioral and performance variables. These last two sets of models included one actigraph or sleep problem predictor at a time, because of the potential associations among these variables.
To examine possible mediation effects of daytime sleepiness, we used as a prerequisite the significance of Paths B or E, respectively (cf. Figure 1; Baron & Kenny, 1986). For those actigraph and sleep problems variables that had a significant effect on daytime functioning, we proceeded to ascertain whether the daytime sleepiness variables functioned as mediators of the relationship between sleep behaviors or sleep problems and daytime functioning. Daytime sleepiness variables were thus added (one at a time) to the corresponding models predicting daytime functioning using actigraph variables and sleep problems, respectively. If both Paths A and C (or D and C) were significant and the new coefficients for the actigraph and sleep problems variables were closer to zero than those in Path B (or E), we deemed daytime sleepiness as a mediator of the relationship between actigraph and sleep problems and daytime functioning.
We used residual analyses and graphical diagnostics to check the validity of the model assumptions. We concluded from these analyses that model assumptions were adequately met.
Results
Sleep–wake variables scored from the actigraph have been described in detail elsewhere (Anders, Iosif, Schwichtenberg, Tang, & Goodlin-Jones, 2011; Schwichtenberg, Iosif, Goodlin-Jones, Tang, & Anders, 2011). Briefly summarizing the results (Table 3), we found that WASO durations were significantly elevated for children in the ID group compared with children in the autism and typically developing groups. Children in the ID group also had, on average, one more awakening per night than children in the other two groups. Children in the autism group slept significantly less during each recording week. Children in the ID group had significantly longer and more naps (and on more days per week), on average, than children in the autism group. Daily nap patterns for children in the ID group resembled the patterns of children in the chronologically younger typically developing group. No group differences were noted for bedtimes, sleep onset latency times, and sleep efficiency. Finally, as reported previously (Anders et al., 2011), children in all three diagnostic groups had significantly more within-child variability than between-group variability in all of the sleep–wake variables. Children in both neurodevelopmental groups had more within-child variability than did children in the typically developing group. See Table 3.
Table 3.
Summary of Actigraph Sleep–Wake Variables and Group Differences Over 6 Months
| Actigraph variable | Time 1 (n = 194): M ± SD (Q1 to Q3) | Time 2 (n = 179): M ± SD (Q1 to Q3) | Time 3 (n = 173): M ± SD (Q1 to Q3) |
|---|---|---|---|
| Bedtime (hr:min) | |||
| Autism | 21:01 ± 1:08 (20:18–21:31) | 21:00 ± 1:14 (20:11–21:40) | 20:47 ± 1:37 (20:06–21:44) |
| Intellectual disability | 21:08 ± 1:01 (20:36–21:41) | 21:07 ± 1:02 (20:37–21:42) | 20:59 ± 1:19 (20:24–21:33) |
| Typically developing | 20:46 ± 0:51 (20:10–21:05) | 20:55 ± 0:54 (20:21–21:17) | 20:50 ± 0:59 (20:21–21:15) |
| Sleep onset latency (min) | |||
| Autism | 39 ± 28 (23–46) | 33 ± 23 (16–44) | 36 ± 31 (17–47) |
| Intellectual disability | 42 ± 31 (24–50) | 41 ± 28 (23–54) | 42 ± 26 (26–51) |
| Typically developing | 35 ± 19 (25–41) | 36 ± 19 20–45) | 38 ± 21 (21–46) |
| WASO duration (min) | |||
| Autism | 19 ± 17 (8–25) | 17 ± 16 (6–26) | 16 ± 16 (5–23) |
| Intellectual disability | 29 ± 22 (14–36)*** | 25 ± 21 (9–34)*** | 23 ± 24 (6–38)*** |
| Typically developing | 18 ± 12 (9–24) | 18 ± 14 (7–24) | 15 ± 12 (7–19) |
| WASO number | |||
| Autism | 2.5 ± 1.7 (1.4–3.0) | 2.1 ± 1.5 (1.0–2.7) | 2.1 ± 1.7 (0.9–3.0) |
| Intellectual disability | 3.7 ± 2.4 (2.3–5.0)** | 3.3 ± 2.5 (1.5–4.3)** | 3.1 ± 2.5 (0.9–4.6)** |
| Typically developing | 3.1 ± 1.8 (1.7–4.3) | 3.2 ± 2.5 (1.4–4.0) | 2.9 ± 2.2 (1.3–4.0) |
| Sleep efficiency (%) | |||
| Autism | 91 ± 5 (90–94) | 92 ± 4 (91–96) | 92 ± 5 (90–96) |
| Intellectual disability | 90 ± 5 (86–93) | 91 ± 5 (87–95) | 91 ± 6 (88–95) |
| Typically developing | 92 ± 3 (90–95) | 92 ± 4 (89–95) | 92 ± 4 (90–95) |
| Total sleep in 24 hr (hr:min) | |||
| Autism | 10:36 ± 0:51 (10:06–11:09)*** | 10:30 ± 0:44 (9:50–11:05)*** | 10:39 ± 1:41 (9:54–10:56)*** |
| Intellectual disability | 11:06 ± 0:55 (10:21–11:46) | 10:56 ± 0:51 (10:08–11:37) | 11:06 ± 1:16 (10:15–11:26) |
| Typically developing | 11:14 ± 0:44 (10:42–11:39) | 10:58 ± 0:46 (10:23–11:32) | 10:59 ± 0:45 (10:30–11:34) |
| Nap duration (min) | |||
| Autism | 48 ± 32 (20–74)** | 36 ± 28 (13–54)** | 0:37 ± 0:31 (16–46)** |
| Intellectual disability | 58 ± 39 (25–81) | 55 ± 36 (27–81) | 0:57 ± 0:39 (22–91) |
| Typically developing | 55 ± 35 (31–69) | 51 ± 38 (16–80) | 0:51 ± 0:38 (18–78) |
| Days with naps per week | |||
| Autism | 3.6 ± 1.8 (2–4)** | 3.8 ± 2.0 (1–4)** | 2.7 ± 1.6 (2–4)** |
| Intellectual disability | 4.3 ± 1.9 (2.5–6) | 3.6 ± 1.9 (2–6) | 3.8 ± 2.0 (2–5) |
| Typically developing | 4.2 ± 1.9 (3–6) | 4.2 ± 1.9 (2–5) | 3.6 ± 2.0 (2–5) |
Note. Nap summaries are computed for the children who napped. Q1 = lower quartile (cuts off lowest 25% of data); Q3 = upper quartile (cuts off highest 25% of data); WASO = wake after sleep onset.
p < .01.
p < .001.
This report focuses on the developmental trajectories over 6 months for each of the daytime variables of interest (sleepiness, behavior problems, perception, fine motor skills, and eye–hand coordination) and on the relationships between sleep, sleepiness, and daytime performance.
Developmental Trajectories
Table 4 summarizes the daytime sleepiness (ESS and CSHQ Daytime Sleepiness subscale) and performance and behavior (CBCL, Bayley peg rate, PEP-R) variables for the three diagnostic groups at the each recording time. Table 5 presents the results of the random-effects model that predicts these variables by using diagnostic group, age, and time in the study (recording period).
Table 4.
Summary of Daytime Sleepiness, Performance, and Behavior Over 6 Months
| Variable | Time 1 (n = 194) M ± SD | Time 2 (n = 179) M ± SD | Time 3 (n = 173) M ± SD |
|---|---|---|---|
| ESS | |||
| Autism | 7.0 ± 3.8 | 6.7 ± 3.5 | 5.8 ± 3.5 |
| Intellectual disability | 7.5 ± 3.0* | 8.5 ± 3.6*** | 7.6 ± 3.7** |
| Typically developing | 6.0 ± 2.5 | 6.1 ± 2.7 | 5.7 ± 2.8 |
| CSHQ: daytime sleepiness | |||
| Autism | 12.2 ± 2.6 | 12.8 ± 2.9 | 12.3 ± 2.9 |
| Intellectual disability | 12.5 ± 2.7 | 12.5 ± 2.7 | 12.8 ± 3.1 |
| Typically developing | 11.9 ± 2.9 | 11.7 ± 2.4 | 11.6 ± 2.4 |
| CBCL: externalizing problems | |||
| Autism | 59.0 ± 10.0 | 58.0 ± 9.5 | 58.1 ± 9.4 |
| Intellectual disability | 59.4 ± 10.5 | 58.5 ± 10.8 | 59.1 ± 11.8 |
| Typically developing | 50.9 ± 10.9*** | 50.2 ± 12.7*** | 46.8 ± 11.4*** |
| CBCL: internalizing problems | |||
| Autism | 61.7 ± 8.5 | 60.1 ± 9.0 | 60.7 ± 8.7 |
| Intellectual disability | 59.6 ± 10.4 | 58.3 ± 11.2 | 59.4 ± 11.4 |
| Typically developing | 49.3 ± 10.7*** | 58.5 ± 10.8*** | 45.8 ± 11.3*** |
| Peg rate | |||
| Autism | 0.31 ± 0.17 | 0.31 ± 0.18 | 0.36 ± 0.21 |
| Intellectual disability | 0.27 ± 0.16 | 0.30 ± 0.17 | 0.31 ± 0.19 |
| Typically developing | 0.43 ± 0.19*** | 0.47 ± 0.22*** | 0.55 ± 0.26*** |
| PEP-R: perception | |||
| Autism | 9.2 ± 3.1 | 9.7 ± 3.0 | 10.1 ± 2.6 |
| Intellectual disability | 9.6 ± 3.4 | 9.8 ± 3.2 | 10.5 ± 2.8 |
| Typically developing | 11.5 ± 2.1*** | 12.0 ± 1.2*** | 12.5 ± 0.8*** |
| PEP-R: fine motor | |||
| Autism | 9.1 ± 3.1 | 9.7 ± 3.6 | 10.5 ± 2.8 |
| Intellectual disability | 8.8 ± 3.9 | 9.6 ± 3.6 | 10.1 ± 3.9 |
| Typically developing | 11.8 ± 2.6*** | 12.4 ± 2.3*** | 13.1 ± 1.9*** |
| PEP-R: eye–hand | |||
| Autism | 5.8 ± 2.8 | 6.9 ± 3.3 | 7.4 ± 3.3 |
| Intellectual disability | 5.1 ± 3.1 | 5.4 ± 3.3 | 6.7 ± 3.4 |
| Typically developing | 8.0 ± 3.2*** | 9.0 ± 3.0*** | 9.6 ± 3.0*** |
Note. ESS = Epworth Sleepiness Scale; CSHQ = Children’s Sleep Habits Questionnaire; CBCL = Child Behavior Checklist; PEP-R = Psychoeducational Profile—Revised (Perception, Fine Motor, and Eye–Hand Coordination Scale scores).
p < .05.
p < .01.
p < .001.
Table 5.
Parameter Estimates (and Standard Errors) of the Random-Effects Modelsa Assessing the Relationship of Diagnostic Group and Chronologic Age with Baseline Level and Rates of Change Over Time in Sleepiness and Performance and Behavior
| Model variable | Sleepiness and performance and behavior variable
|
|||||||
|---|---|---|---|---|---|---|---|---|
| ESS | DS | CBCL–I | CBCL–Eb | Peg rate | PEP-R P | PEP-R FM | PEP-R EH | |
| Effects on initial level of variable | ||||||||
| Intercept (TD)c | 6.08 (.30)*** | 11.83 (.28)*** | 49.96 (1.31)*** | 55.22 (2.53)*** | .48 (.02)*** | 11.66 (.13)*** | 12.22 (.18)*** | 8.77 (.21)*** |
| Autism diagnosis | .87 (.50)† | .46 (.42) | 10.73 (1.62)*** | 8.41 (1.86)*** | −.19 (.03)*** | −2.70 (.35)*** | −3.39 (.42)*** | −3.20 (.41)*** |
| Intellectual disability diagnosis | 2.12 (.50)*** | .69 (.45) | 9.42 (1.82)*** | 8.30 (1.98)*** | −.22 (.03)*** | −2.20 (.39)*** | −3.52 (.47)*** | −3.83 (.42)*** |
| Aged (at baseline) | −.12 (.06)* | .11 (.05)* | .72 (.18)*** | −.24 (.40) | .04 (.00)*** | .15 (.03)*** | .45 (.05)*** | .68 (.05)*** |
| Age × Autism | NS | ns | ns | .18 (.51) | −.03 (.01)*** | ns | −.27 (.12)* | −.40 (.11)*** |
| Age × Intellectual disability | NS | ns | ns | 1.14 (.52)* | −.03 (.01)*** | ns | −.02 (.12) | −.26 (.11)* |
| Effects on rate of change during study | ||||||||
| Timed | −.27 (.10)** | ns | −.62 (.25)* | −.69 (.24)** | .05 (.01)*** | .43 (.07)*** | .63 (.06)*** | .69 (.07)*** |
| Autism diagnosis | ns | ns | ns | ns | −.03 (.01)* | ns | ns | ns |
| Intellectual disability diagnosis | ns | ns | ns | ns | −.03 (.01)* | ns | ns | ns |
Note. The reported models contain only significant effects, except for some of the terms reflecting the interaction between diagnosis and age. Those estimates without a symbol were not significant, but the corresponding terms had to be included in the model to preserve the interpretability of the interaction with diagnosis.
ESS = Epworth Sleepiness Scale; DS = Daytime Sleepiness subscale of the Children’s Sleep Habits Questionnaire; CBCL–I = Child Behavior Checklist—Internalizing Problems subscale; CBCL–E = Child Behavior Checklist—Externalizing Problems subscale; PEP-R P = Psychoeducational Profile—Revised (PEP-R) Perception Scale; FM = Fine Motor Scale; EH = Eye–Hand Coordination Scale; TD = typically developing.
Models included and tested terms for demographic characteristics (ethnicity, mother’s age, marital status, and education).
Mother’s marital status was a significant predictor; children with unmarried mothers scored significantly higher than those with married mothers.
Age at baseline was centered at 44 months, so the intercept can be interpreted as the average response at the first recording time for a 44-month-old TD child.
Effects of age, time, and their interaction with diagnosis are reported for 3-month increments.
p < .10.
p < .05.
p < .01.
p < .001.
Daytime sleepiness
As detailed in Table 5, for the ESS, children in the ID group were significantly sleepier than children in both the typically developing (p < .001) and autism groups (p = .03) over the three occasions. There was a trend for children in the autism group to be sleepier than children in the typically developing group (p = .08). Children who were older at baseline were significantly less sleepy during the daytime than younger children (p < .05), and for all three groups, daytime sleepiness (as indexed by the ESS) decreased over the course of the study at the same rate (p < .01). Contrary to our expectations, the CSHQ Daytime Sleepiness subscale did not echo these results. Children who were older at baseline were rated as more sleepy during the day by their parents. No significant differences at baseline and no changes across time in any of the three groups emerged with the CSHQ Daytime Sleepiness subscale. In sum, children who entered the study with higher rates of daytime sleepiness continued to display more sleepiness at each of the assessment periods.
Child behavior problems
At baseline, children in the autism and ID groups were rated about 10 points higher on the CBCL Internalizing and 8 points higher on the CBCL Externalizing subscales than children in the typically developing group (all ps < .001). These significant differences persisted over the course of the study. The main effect for time was significant (p < .01) with scores for all groups decreasing over time at similar rates, across both CBCL subscales. As portrayed in Table 5, there was a significant age-at-baseline effect, with older children at baseline scoring higher on the Internalizing Behavior Problem subscale. For the Externalizing Behavior Problem subscale, this effect was present only in the ID group (p < .05).
Bayley peg rate
As illustrated in Table 4, children in both neurodevelopmentally disordered groups had significantly lower performance on the peg rate task than did children in the typically developing group on all occasions. There was also a significant interaction (p < .001; see Table 5) between diagnosis and age at baseline on this task. Whereas older children in the typically developing group placed more pegs than did younger typically developing children, this age effect was less pronounced for children in the neurodevelopmentally disordered groups. Additionally, children in the typically developing group showed significantly greater improvement over time than did children in the autism and ID groups.
Psychoeducational Profile—Revised
On the PEP-R Perception, Eye–Hand Coordination, and Fine Motor Scales, children in the typically developing group scored significantly better than children in the autism and ID groups at baseline (all ps < .001). These differences persisted over 6 months, and the performance on all three scales improved over time at similar rates for the three groups. Age at baseline was a significant predictor of performance on all three scales, with older children in all groups scoring higher. This age effect was less pronounced in the autism group for fine motor and eye–hand coordination and in the ID group for eye–hand coordination.
Relationships Between Sleep, Sleepiness, and Daytime Performance
Actigraph variables, daytime sleepiness, and performance
Table 6 summarizes the random-effects models assessing the relationship between actigraph variables and daytime sleepiness and performance. These models tested Paths A and B in Figure 1. Overall, shorter WASO durations were associated with higher PEP-R Perception (p < .001) and Eye–Hand Coordination scores (p < .01; Path B). There was a trend for WASO duration to be associated with PEP-R Fine Motor Coordination (p < .01) for all children. For children in the typically developing and autism groups, the actigraph variables related neither to daytime sleepiness (Path A) nor to any of the daytime performance and behavior indices (Path B). For children in the ID group, sleep efficiency (p < .01) and WASO duration (p < .05) related to daytime sleepiness; sleep efficiency also related to PEP-R Perception (p < .05). That is, higher sleep efficiency and shorter WASO durations were associated with less sleepiness in the ID group, and children in this group performed better when they presented with higher sleep efficiency scores. See Table 6.
Table 6.
Parameter Estimates (and Standard Errors) of the Random-Effects Models Assessing the Relationship of Actigraph and Sleep Problem Variables with Baseline Level and Rates of Change Over Time in Sleepiness and Performance and Behavior
| Variable | Sleepiness and performance and behavior variablesa
|
||||||
|---|---|---|---|---|---|---|---|
| ESS | DS | CBCL–I | CBCL–E | PEP-R P | PEP-R FM | PEP-R EH | |
| Actigraphb | Path A
|
Path B
|
|||||
| WASO durationc | −.00 (.37) | ns | ns | ns | −.53 (.15)*** | −.26 (.14)† | −.46 (.16)** |
| WASO duration × Autism | .56 (.56) | ns | ns | ns | ns | ns | ns |
| WASO duration × Intellectual disability | 1.19 (.50)* | ns | ns | ns | ns | ns | ns |
| Sleep efficiency | −.02 (.05) | ns | ns | ns | .02 (.03) | .04 (.02)* | .07 (.02)** |
| Sleep efficiency × Autism | .01 (.07) | ns | ns | ns | .06 (.05) | ns | ns |
| Sleep efficiency × Intellectual disability | −.18 (.06)** | ns | ns | ns | .09 (.04)* | ns | ns |
| Sleepiness | Path C
|
||||||
| ESS | ns | ns | ns | ns | ns | ||
| CSHQ daytime sleepiness | .99 (.28)*** | .35 (.14)* | ns | ns | .08 (.04)* | ||
| CSHQ daytime sleepiness × Autism | −.78 (.35)* | ns | ns | ns | ns | ||
| CSHQ daytime sleepiness × Intellectual disability | −.84 (.38)* | ns | ns | ns | ns | ||
| Sleep problemd | Path D
|
Path E
|
|||||
| CSHQ night awakening | ns | .14 (.07)* | .72 (.22)** | .58 (.22)** | ns | ns | ns |
| CSHQ bed resistance | .16 (.05)** | .15 (.04)*** | .54 (.14)*** | .66 (.14)*** | ns | ns | ns |
| CBCL sleep problems | .05 (.02)*** | .05 (.01)*** | .31 (.04)*** | .29 (.04)*** | ns | ns | ns |
| Parent-report global sleep problems | ns | .34 (.20)† | 1.35 (.61)* | 1.57 (.60)** | ns | ns | ns |
Note. The reported models contain only significant effects, except for some of the terms reflecting the interaction between diagnosis and actigraph variables. Those estimates without a symbol were not significant, but the corresponding terms had to be included in the model to preserve the interpretability of the interactions with diagnosis.
ESS = Epworth Sleepiness Scale; DS = Daytime Sleepiness subscale of the Children’s Sleep Habits Questionnaire (CSHQ); CBCL–I = Child Behavior Checklist (CBCL)—Internalizing Problems subscale; CBCL–E = Child Behavior Checklist—Externalizing Problems subscale; PEP-R P = Psychoeducational Profile—Revised (PEP-R) Perception Scale; FM = Fine Motor Scale; EH = Eye–Hand Coordination Scale; WASO = wake after sleep onset.
Models were also fitted for peg rate, but none of the actigraph or sleep problem measures had a significant effect on them.
Models included terms for total sleep, sleep onset latency, and numbers of awakenings, but these terms did not predict any of the interactions and were not significant.
Effects of WASO duration and its interaction with diagnosis are reported for 30-min increments.
Models included a term for actigraph-defined sleep problems, but this term was not significant in any of the models.
p < .10.
p < .05.
p < .01.
p < .001.
Sleep problems, daytime sleepiness, and performance
Table 6 also presents group differences in Pathways D and E as depicted in Figure 1. For all groups, parent reports of bedtime resistance (as indexed by the CBCL) were associated with more daytime sleepiness on both the ESS and CSHQ Daytime Sleepiness Subscales (all ps < .01; Path D). This was not true for parent “yes/no” reports of a global sleep problem. Parent reports of night awakening, bedtime resistance, and global sleep problems predicted both externalizing and internalizing behavior problems (Path E). Children with more parent-reported sleep problems had more parent-reported daytime behavior problems. There were no associations between actigraph-defined sleep problems and the ESS or CSHQ Daytime Sleepiness subscale (Path D) or daytime performance and behavior measures for any of the diagnostic groups (Path E); therefore, actigraph-defined sleep onset and night waking problems were not included in Table 6. Finally, no significant associations were noted between ESS and daytime performance and behavior in any group (Path C). For all three groups of children, higher CSHQ Daytime Sleepiness predicted higher CBCL Externalizing scores and better Eye–Hand Coordination (all ps < .05). For the CBCL Internalizing subscale, daytime sleepiness predicted higher scores but only in the typically developing children (p < .001).
Mediating role of daytime sleepiness
We tested the mediating role of daytime sleepiness in the relationship between sleep behaviors or problems and daytime functioning by assessing the magnitude of change seen in this relationship with the addition of daytime sleepiness to the model. Table 7 summarizes the results of the mediating models. Adding daytime sleepiness to the significant paths in B or E did not significantly change the estimates for the actigraph and sleep problems. Contrary to our predictions, daytime sleepiness did not account for a significant portion of the relationship between any of our indices of sleep (or sleep problems) and daytime functioning. In sum, daytime sleepiness did not mediate the relationships between sleep or sleep problems and daytime functioning but rather functioned as a distinct significant predictor of daytime performance.
Table 7.
Parameter Estimates (and Standard Errors) of the Models Assessing Whether CSHQ Daytime Sleepinessa Mediates the Relationship Between Actigraph and Sleep Problem Variables and Performance and Behavior
| Variable | Performance and behavior
|
||||
|---|---|---|---|---|---|
| CBCL–I | CBCL–E | PEP-R P | PEP-R FM | PEP-R EH | |
| Actigraphb | |||||
| WASO durationc | ns | ns | −.53 (.16)** | −.28 (.14)† | −.55 (.16)*** |
| Sleep efficiency | ns | ns | .02 (.03) | .04 (.02)† | .08 (.02)*** |
| Sleep efficiency × Autism | ns | ns | .07 (.05) | ns | ns |
| Sleep efficiency × Intellectual disability | ns | ns | .08 (.04)† | ns | ns |
| Sleep problemd | |||||
| CSHQ night awakening | .75 (.22)*** | .56 (.22)** | ns | ns | ns |
| CSHQ bed resistance | .50 (.14) *** | .63 (.14)*** | ns | ns | ns |
| CBCL sleep problems | .30 (.04)*** | .29 (.04)*** | ns | ns | ns |
| Parent-report global sleep problems | 1.23 (.62)* | 1.24 (.60)** | ns | ns | ns |
Note. The reported models contain only significant effects, except for some of the terms reflecting the interaction between diagnosis and sleep efficiency. Those estimates without a symbol were not significant, but the corresponding terms had to be included in the model to preserve the interpretability of the interactions with diagnosis.
CHSQ = Children’s Sleep Habits Questionnaire; CBCL–I = Child Behavior Checklist (CBCL)—Internalizing Problems subscale; CBCL–E = Child Behavior Checklist—Externalizing Problems subscale; PEP-R P = Psychoeducational Profile—Revised (PEP-R) Perception Scale; FM = Fine Motor Scale; EH = Eye–Hand Coordination Scale; WASO = wake after sleep onset.
Reported models are for testing CSHQ Daytime Sleepiness mediation effects, because the Epworth Sleepiness Scale did not significantly predict any of the performance and behavior problems.
Daytime sleepiness was significant in the models predicting PEP-R EH but not PEP-R P or PEP-R RM.
Effects of WASO duration and its interaction with diagnosis are reported for 30-min increments.
Daytime sleepiness was significant in all the models predicting CBCL–I and CBCL–E.
p < .10.
p < .05.
p < .01.
p < .001.
Discussion
This study used a short-term, repeated measures design and incorporated both objective and subjective assessments to examine whether disrupted sleep was associated with daytime sleepiness and disturbances in daytime performance and behavior, whether these relationships were stable over time, and how children with neurodevelopmental disorders differed from typically developing children.
In contrast to the actigraph sleep variables, for which patterns remained stable over the 6-month period (Anders et al., 2011), the trajectory of daytime sleepiness and daytime performance measures changed over time. Reduced sleepiness and improved waking performance over time were significantly related to diagnostic group, baseline chronologic age, and time. Children in both of the neurodevelopmentally disordered groups performed less well on both the PEP-R and peg rate tasks than children in the typically developing group. Performance on these measures was more related to diagnostic group than to the hypothesized sleep and sleepiness measures. Similarly, parents of these children rated daytime behaviors on the CBCL as more problematic than did parents of children in the typically developing group, again suggesting that diagnostic group was more important than the sleep and sleepiness measures. Improvement in both the behavior and performance measures was noted over time in all groups. There was less improvement, however, for children in both of the neurodevelopmentally disordered groups.
Relationships between sleep variables, sleep problems, daytime sleepiness, and daytime performance and behavior were complex. Actigraph variables and actigraph-defined sleep problems did not predict daytime sleepiness or daytime functioning, with a few notable exceptions for the children in the ID group (WASO duration and sleep efficiency predicted daytime sleepiness and PEP-R performance). Parent reports of sleep problems predicted parent reports of sleepiness and daytime behavior problems but not observed performance on the PEP-R. Daytime sleepiness on the CHSQ but not the ESS was associated with daytime behavior problems. Overall, indices measured by parent report showed more agreement than observed or physiological measures. These findings resemble those reported recently in which actigraph variables were not directly related to parent-reported mood or behavior symptoms (El-Sheikh et al., 2010).
The mediator role of daytime sleepiness was not supported by the present study. The overarching pattern of significant findings highlights daytime sleepiness as an important but distinctly different variable from nighttime sleep behaviors or problems. In preschool-age children, this may reflect their daily nap needs or that daytime sleep is more proximal to their waking activity. This finding is not unique in the field of sleep, as previous studies have highlighted the influential role of daytime sleep in young children (Hupbach, Gomez, Bootzin, & Nadel, 2009; Schwichtenberg, Anders, Volbrecht, & Poehlmann, 2011; Watamura, Donzella, Kertes, & Gunnar, 2004).
Limitations
Sleepiness in young children is difficult to measure and may not manifest in the same way as sleepiness in adults. Young children may “fight” their sleepiness and present with “fidgety-ness” and hyperactivity (Melendres, Lutz, Rubin, & Marcus, 2004) rather than lethargy and decreased motor activity as is seen in adolescences and adults. For the present study, we selected two parent-report measures. Although these have not been extensively validated in preschool-age children, they are established measures with previous applications to clinical populations. The lack of symmetry in the findings across both measures (i.e., older children scored higher on the CSHQ Daytime Sleepiness index and lower on the ESS) highlights the need for validated sleepiness indices in young children. Additionally, developmentally appropriate tests of daytime functioning that are valid for both neurodevelopmentally disordered and typically developing preschool-age children also are difficult to find. Our selection of the PEP-R, the Bayley peg-board task, and the CBCL was based on their feasibility in these groups (Ozonoff & Cathcart, 1998). Computer-based memory, attention, and reaction time tasks might demonstrate more consistent associations with sleep disruption and daytime sleepiness.
Finally, a combination of methodologies, including parent reports, structured questionnaires, and objective actigraph recordings, as used in this study, offer both unique information and potential difficulties in interpretation (Sadeh, 2008; Sitnick et al., 2008). Some measures may agree because of observer bias; other may disagree because of method variance. For example, two parent reports (CBCL and CSHQ) were significantly correlated. In contrast, when the actigraph-defined Night Waking index did not agree with the parent-report Night Waking subscale, it is likely that the child awakened without the parent’s knowledge. When using multimethod strategies, it is important to have predetermined rules for reporting and interpreting differences.
Conclusion
Consistent with previous studies, the present report provides some support for the association between sleep disruption and daytime impairment (Richdale, Francis, Gavidia-Payne, & Cotton, 2000; Wiggs, 2001). A linear pathway from actigraph variables and sleep problems to daytime sleepiness and daytime functioning, as originally hypothesized, was not robustly supported by the findings of this study. Some sleep variables (WASO duration and sleep efficiency) predicted daytime sleepiness for children in the ID group, but this was not true for children in the autism and typically developing groups. These findings are not entirely inconsistent with the reports of previous studies. For example, a recent study by Geiger, Achermann, and Jenni (2010) reported that daytime sleepiness was not associated with IQ test performance in typically developing school-age children but that select actigraph sleep variables were. Similarly, a meta-analysis of a large number of studies that examined sleep quality, sleep duration, sleepiness, and age on school performance demonstrated small overall effect sizes in older children and adolescents for each of these variables; however, causal pathways were not evident (Dewald, Meijer, Oort, Kerkhof, & Bogels, 2010).
The mediating role of daytime sleepiness remains unclear. Daytime performance and behavior continue to be difficult to measure reliably when comparing neurodevelopmentally disordered and typically developing children. Increased efforts to use more objective, laboratory-based or observer-based instruments should be encouraged.
Contributor Information
Thomas Anders, Email: tfanders@gmail.com, Department of Psychiatry and Behavioral Sciences, University of California, Davis, School of Medicine, Sacramento, CA 95817, USA.
Ana-Maria Iosif, University of California, Davis.
A. J. Schwichtenberg, University of California, Davis
Karen Tang, Notre Dame University.
Beth Goodlin-Jones, University of California, Davis.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms & profiles. Burlington: University of Vermont Research Center for Children, Youth & Families; 2000. [Google Scholar]
- Anders T, Dahl R. Classifying sleep disorders in infants and toddlers. In: Narrow WE, First MB, Sivoratka P, Regier DA, editors. Age and gender considerations in psychiatric diagnosis: A research agenda for DSM–V. Arlington, VA: American Psychiatric Association Press; 2007. pp. 215–227. [Google Scholar]
- Anders T, Iosif A, Schwichtenberg A, Tang K, Goodlin-Jones B. Six-month sleep-wake organiation and stability in pre-school age children with autism, developmental delay and typical development. Behavioral Sleep Medicine. 2011;9:1–15. doi: 10.1080/15402002.2011.557991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality & Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. New York, NY: Psychological Corporation; 1969. [Google Scholar]
- Beebe DW. Cognitive, behavioral, and functional consequences of inadequate sleep in children and adolescents. Pediatric Clinics of North America. 2011;58:649–665. doi: 10.1016/j.pcl.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckhalt JA, El-Sheikh M, Keller PS, Kelly RJ. Concurrent and longitudinal relations between children’s sleep and cognitive functioning: The moderating role of parent education. Child Development. 2009;80:875–892. doi: 10.1111/j.1467-8624.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- Chan EY, Ng DK, Chan CH, Kwok KL, Chow PY, Cheung JM, Leung SY. Modified Epworth Sleepiness Scale in Chinese children with obstructive sleep apnea: A retrospective study. Sleep Breath. 2009;13:59–63. doi: 10.1007/s11325-008-0205-7. [DOI] [PubMed] [Google Scholar]
- Cortesi F, Giannotti F, Ivanenko A, Johnson K. Sleep in children with autistic spectrum disorder. Sleep Medicine. 2010;11:659–664. doi: 10.1016/j.sleep.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bogels SM. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep Medicine Reviews. 2010;14:179–189. doi: 10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Didden R, Korzilius H, van Aperlo B, van Overloop C, de Vries M. Sleep problems and daytime problem behaviours in children with intellectual disability. Journal of Intellectual Disability Research. 2002;46(Pt. 7):537–547. doi: 10.1046/j.1365-2788.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- Eisenhower AS, Baker BL, Blacher J. Preschool children with intellectual disability: Syndrome specificity, behaviour problems, and maternal well-being. Journal of Intellectual Disability Research. 2005;49(Pt. 9):657–671. doi: 10.1111/j.1365-2788.2005.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Kelly R, Buckhalt J, Hinnant JB. Children’s sleep and adjustment over time: The role of socioeconomic context. Child Development. 2010;81:870–883. doi: 10.1111/j.1467-8624.2010.01439.x. [DOI] [PubMed] [Google Scholar]
- Elkhayat HA, Hassanein SM, Tomoum HY, Abd-Elhamid IA, Asaad T, Elwakkad AS. Melatonin and sleep-related problems in children with intractable epilepsy. Pediatric Neurology. 2010;42:249–254. doi: 10.1016/j.pediatrneurol.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Foldvary-Schaefer N, Malow B. Video recordings and video polysomnography. Handbook of Clinical Neurology. 2011;98:65–70. doi: 10.1016/B978-0-444-52006-7.00005-8. [DOI] [PubMed] [Google Scholar]
- Geiger A, Achermann P, Jenni O. Association between sleep duration and intelligence scores in healthy children. Developmental Psychology. 2010;46:949–954. doi: 10.1037/a0019679. [DOI] [PubMed] [Google Scholar]
- Goldman SE, Surdyka K, Cuevas R, Adkins K, Wang L, Malow BA. Defining the sleep phenotype in children with autism. Developmental Neuropsychology. 2009;34:560–573. doi: 10.1080/87565640903133509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, Eley TC, O’Connor TG, Plomin R. Etiologies of associations between childhood sleep and behavioral problems in a large twin sample. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43:744–751. doi: 10.1097/01.chi/0000122798.47863.a5. [DOI] [PubMed] [Google Scholar]
- Hall WA, Scher A, Zaidman-Zait A, Espezel H, Warnock F. A community-based study of sleep and behaviour problems in 12- to 36-month-old children. Childcare, Health & Development. 2011 doi: 10.1111/j.1365-2214.2011.01252.x. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Bootzin R, Nadel L. Nap-dependent learning in infants. Developmental Science. 2009;12:1007–1012. doi: 10.1111/j.1467-7687.2009.00837.x. [DOI] [PubMed] [Google Scholar]
- Ivanova MY, Achenbach TM, Rescorla LA, Harder VS, Ang RP, Bilenberg N, Verhulst FC. Preschool psychopathology reported by parents in 23 societies: Testing the seven-syndrome model of the Child Behavior Checklist for ages 1.5–5. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:1215–1224. doi: 10.1016/j.jaac.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns MW. A new method of measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Johnson KP, Giannotti F, Cortesi F. Sleep patterns in autism spectrum disorders. Child and Adolescent Psychiatry Clinics of North America. 2009;18:917–928. doi: 10.1016/j.chc.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Joo EY, Hong SB, Sohn YB, Kwak MJ, Kim SJ, Choi YO, Jin DK. Plasma adiponectin level and sleep structures in children with Prader-Willi syndrome. Journal of Sleep Research. 2010;19(1 Pt 2):248–254. doi: 10.1111/j.1365-2869.2009.00786.x. [DOI] [PubMed] [Google Scholar]
- Keller P, El-Sheikh M. Children’s emotional security and sleep: Longitudinal relations and directions of effects. Journal of Child Psychology & Psychiatry. 2010;52:64–71. doi: 10.1111/j.1469-7610.2010.02263.x. [DOI] [PubMed] [Google Scholar]
- Krakowiak P, Goodlin-Jones B, Hertz-Picciotto I, Croen LA, Hansen RL. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: A population-based study. Journal of Sleep Research. 2008;17:197–206. doi: 10.1111/j.1365-2869.2008.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lavigne JV, Arend R, Rosenbaum D, Smith A, Weissbluth M, Binns HJ, Christoffel KK. Sleep and behavior problems among preschoolers. Journal of Developmental & Behavioral Pediatrics. 1999;20:164–169. doi: 10.1097/00004703-199906000-00005. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Malow B, McGrew SG. Sleep disturbances and autism. Sleep Medicine Clinics. 2008;3:479–488. [Google Scholar]
- Melendres MC, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114:768–775. doi: 10.1542/peds.2004-0730. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning: AGS edition. Circle Pines, MN: American Guidance Services; 1995. [Google Scholar]
- Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–1051. [PubMed] [Google Scholar]
- Ozonoff S, Cathcart K. Effectiveness of a home program intervention for young children with autism. Journal of Autism & Developmental Disorders. 1998;28:25–32. doi: 10.1023/a:1026006818310. [DOI] [PubMed] [Google Scholar]
- Polimeni MA, Richdale AL, Francis AJ. A survey of sleep problems in autism, Asperger’s disorder and typically developing children. Journal of Intellectual Disability Research. 2005;49(Pt. 4):260–268. doi: 10.1111/j.1365-2788.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- Quine L. Sleep problems in primary school children: Comparison between mainstream and special school children. Childcare, Health & Development. 2001;27:201–221. doi: 10.1046/j.1365-2214.2001.00213.x. [DOI] [PubMed] [Google Scholar]
- Richdale AL, Francis A, Gavidia-Payne S, Cotton S. Stress, behaviour, and sleep problems in children with an intellectual disability. Journal of Intellectual & Developmental Disability. 2000;25:147–161. [Google Scholar]
- Richdale AL, Schreck KA. Sleep problems in autism spectrum disorders: Prevalence, nature, & possible biopsychosocial aetiologies. Sleep Medicine Reviews. 2009;13:403–411. doi: 10.1016/j.smrv.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview—Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Sadeh A. Commentary: Comparing actigraphy and parental report as measures of children’s sleep. Journal of Pediatric Psychology. 2008;33:406–407. doi: 10.1093/jpepsy/jsn018. [DOI] [PubMed] [Google Scholar]
- Schopler E, Reichler R, Bashford J, Lansing M, Marcus l. Psychoeducational Profile Revised (PEP-R) Austin TX: Pro-Ed; 1990. [Google Scholar]
- Schreck KA, Mulick JA, Smith AF. Sleep problems as possible predictors of intensified symptoms of autism. Research in Developmental Disabilities. 2004;25:57–66. doi: 10.1016/j.ridd.2003.04.007. [DOI] [PubMed] [Google Scholar]
- Schwichtenberg AJ, Anders T, Volbrecht M, Poehlmann J. Daytime sleep and parenting interactions in infants born pre-term. Developmental & Behavioral Pediatrics. 2011;32:8–17. doi: 10.1097/DBP.0b013e3181fa57e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwichtenberg AJ, Iosif A, Goodlin-Jones B, Tang K, Anders T. Daytime sleep patterns in preschool children with autism, developmental delay, and typical development. American Journal of Intellectual & Developmental Disabilities. 2011;116:142–152. doi: 10.1352/1944-7558-116.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnick SL, Goodlin-Jones BL, Anders TF. The use of actigraphy to study sleep disorders in preschoolers: Some concerns about detection of nighttime awakenings. Sleep. 2008;31:395–401. doi: 10.1093/sleep/31.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souders MC, Mason TB, Valladares O, Bucan M, Levy SE, Mandell DS, Pinto-Martin J. Sleep behaviors and sleep quality in children with autism spectrum disorders. Sleep. 2009;32:1566–1578. doi: 10.1093/sleep/32.12.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S, Balla D, Cicchetti D. Vineland Adaptive Behavior Scales (survey form) Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Spratt E, Salor C, Macias M. Assessing parenting stress in multiple samples of children with special needs. Families, Systems & Health. 2007;25:435–449. [Google Scholar]
- Watamura SE, Donzella B, Kertes D, Gunnar MR. Developmental changes in baseline cortisol activity in early childhood: Relations with napping and effortful control. Developmental Psychobiology. 2004;45:125–133. doi: 10.1002/dev.20026. [DOI] [PubMed] [Google Scholar]
- Wiggs L. Sleep problems in children with developmental disorders. Journal of the Royal Society of Medicine. 2001;94:177–179. doi: 10.1177/014107680109400406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs L, Stores G. Severe sleep disturbance and daytime challenging behaviour in children with sever learning disabilities. Journal of Intellectual Disability Research. 1996;40:518–528. doi: 10.1046/j.1365-2788.1996.799799.x. [DOI] [PubMed] [Google Scholar]