Abstract
For the efficient trafficking of lysosomal proteins, the cationic-dependent and -independent mannose 6-phosphate receptors and sortilin must bind cargo in the Golgi apparatus, be packaged into clathrin-coated trafficking vesicles and traffic to the endosomes. Once in the endosomes, the receptors release their cargo into the endosomal lumen and recycle back to the Golgi for another round of trafficking, a process that requires retromer. In this study, we demonstrate that palmitoylation is required for the efficient retrograde trafficking of sortilin, and the cationic-independent mannose 6-phosphate as palmitoylation-deficient receptors remain trapped in the endosomes. Importantly, we also show that palmitoylation is required for receptor interaction with retromer as nonpalmitoylated receptor did not interact with retromer. In addition, we have identified DHHC-15 as the palmitoyltransferase responsible for this modification. In summary, we have shown the functional significance of palmitoylation in lysosomal receptor sorting and trafficking.
Protein sorting from the Golgi apparatus to the endosomal/lysosomal compartment is a dynamic process involving various membrane and cytosolic proteins (1). Integral membrane proteins such as the lysosome-associated membrane proteins (LAMPs) can interact directly with the cytosolic sorting machinery such as the multimeric adaptor proteins (AP), AP-1 and AP-3 (2). However, soluble luminal proteins cannot be recognized directly by APs, so they must bind an intermediate receptor to be properly sorted (3,4). Three such trans-membrane receptors are known to function in the sorting and trafficking of proteins from the Golgi apparatus to endosomes. They are the cationic-dependent mannose 6-phosphate receptor (CD-MPR), the cationic-independent mannose 6-phosphate receptor (CI-MPR) (5) and sortilin (6). CD-MPR and CI-MPR have been shown to traffic cathepsin B (7) and other cargo, while sortilin has been shown to traffic prosaposin, GM2-activator protein (4) and acid sphingomyelinase (8). All three of these receptors bind the Golgi-localized, γ-ear-containing ADP ribosylation factor-binding proteins (GGAs) (5,6,9) and AP-1 (10,11), which have both been implicated in trafficking to the lysosomal compartment. The GGAs and AP-1 are responsible for recruiting other soluble factors to package the receptor and cargo into clathrin-coated vesicles for transport (12,13). Expression of mutant GGAs lacking their clathrin-binding motif prevented the proper sorting of sortilin and CI-MPR (4,5). The recruitment of AP-1 and GGAs to membranes is dependent on Arf1 (14,15) and the Arf1 GTP exchange factors such as brefeldin A-inhibited guanine nucleotide-exchange protein 2 (BIG2) (16) and Golgi-specific brefeldin A-resistance guanine nucleotide exchange factor 1 (GBF1) (17). Once the trafficking vesicles have reached the endosomes, the acidic environment in this compartment causes a dissociation of the cargo from its receptor. The cargo is then free to move on to the lysosomes, while the receptor is recycled back to the Golgi for another round of cargo binding and delivery (18).
The retromer complex was first identified in yeast, where it plays a role in the retrograde trafficking of the vacuole trafficking receptor, Vps10p (19). The mammalian retromer complex has recently been characterized and shown to play a role in endosome to Golgi trafficking of CI-MPR (20–22) and sortilin (10). Retromer comprises of two complexes: the cargo recognition VPS26–VPS29–VPS35 heterotrimer and a membrane-targeting heterodimer or homodimer consisting of sorting nexin 1 (SNX1) and/or SNX2 (23,24). Depletion of the retromer complex using small interfering RNA (siRNA) decreased the half-life of the receptor because of lysosomal degradation (20–22). The exact mechanism for how retromer recognizes its target proteins in specific endosomal structures and shunts these proteins from a degradative pathway to a recycling pathway back to the Golgi is unclear. Some have proposed that retromer serves as a coat molecule and that other factors are involved in sorting the various recycled receptors into retromer coats. Candidate factors could include phosphofurin acidic cluster sorting protein-1 (PACS-1) or tail-interacting protein of 47 kD (TIS47), both of which have been implicated in the retrograde trafficking of CI-MPR. However, to date, retromer is the unifying requirement for recycling back to the Golgi. In addition to the lysosomal receptors, retromer has also been implicated in the sorting of the polymeric immunoglobulin receptor (25) and wntless protein (26,27). Despite the growing list of receptors, what regulates when a receptor gets recycled through retromer versus degraded is still unknown.
It has been demonstrated that one of the receptors that retromer recycles back to the Golgi, CD-MPR (21), is palmitoylated on cysteine residues in its cytosolic tail (28). This modification is thought to occur somewhere between the plasma membrane and the endosome and has been shown to be required to prevent the accumulation of the receptor in the lysosomal compartment (29). No mechanism has ever been established as to how or why palmitoylation is required for receptor recycling. In addition, no connection between retromer recognition of CD-MPR and the addition of a palmitoyl group to CD-MPR has ever been shown. Furthermore, CD-MPR is one member of a family of lysosomal receptors. Are the other receptors also palmitoylated? Finally, nonpalmitoylated CD-MPR is degraded and it has been shown separately that depletion of retromer causes the lysosomal degradation of CI-MPR. Could palmitoylation of the lysosomal sorting receptors be a key to recruiting retromer and recycling these receptors back to the Golgi apparatus? We present evidence that in addition to CD-MPR, the other two members of the lysosomal sorting receptors (CI-MPR and sortilin) are palmitoylated, and that this modification is a prerequisite for both the interaction with retromer and recycling of the receptors back to the Golgi. In addition, we have identified DHHC-15 as a palmitoyltransferase responsible for this important post-translational modification. We propose a novel mechanism of regulation where the post-translational modification of palmitoylation serves to alter the delivery of lysosomal enzymes by controlling the recycling of the lysosomal sorting receptors.
Results
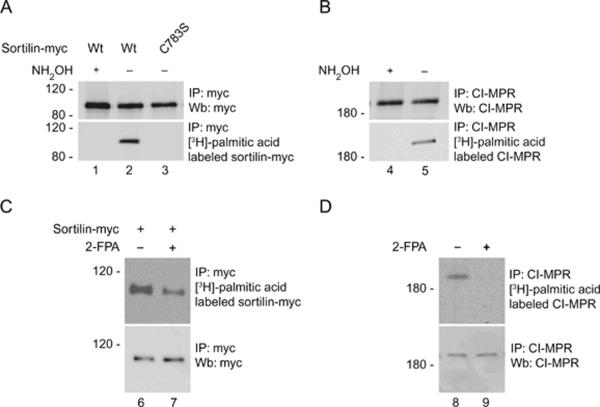
We examined the possible role of palmitoylation in trafficking of lysosomal proteins by assessing if only CD-MPR or all of the closely related receptors, CI-MPR and sortilin were also palmitoylated. Although the luminal domains of the three sorting receptors are quite divergent, their cytosolic tails contain significant functional homology. The palmitoylation of CD-MPR occurs on cysteine residues 240 and 244 of its cytosolic tail (28). CI-MPR and sortilin have cysteine residues in their respective C-terminal tails at positions 2342 and 2343 for CIMPR and position 783 for sortilin. We labeled cells with [3H]-palmitic acid and immunoprecipitated either sortilin-myc or endogenous CI-MPR from HeLa cell lysate. Following exposure of the immunoprecipitated product to film, we observed specific bands showing palmitoylated sortilin-myc (Figure 1A, lane 2, lower panel) and palmitoylated CI-MPR (Figure 1B, lane 5, lower panel). To test whether the palmitoyl chain was attached to the sorting receptors through a reversible S-palmitoylation or the more stable N-palmitoylation, we added 1 m NH2OH, a compound known to break the thioester linkage found in S-palmitoylation but not the amide linkage found in N-palmitoylation to the immunoprecipitation buffer (30). We found that the NH2OH treatment resulted in no visible palmitoylation on either sortilin-myc (Figure 1A, lane 1, lower panel) or CI-MPR (Figure 1B, lane 4, lower panel) although both proteins were well immunoprecipitated (Figure 1A,B, lane 1 and 4, upper panel). To identify the cysteine residue implicated in the palmitoylation of sortilin, we mutated cysteine 783 to a serine (sortilinC783S-myc) and tested for palmitoylation. We found that sortilinC783S-myc was not palmitoylated (Figure 1A, lanes 3, lower panel) despite the fact that it was expressed and could be immunoprecipitated with antibodies to the myc epitope (Figure 1, lanes 3, upper panel). To test the functional significance of palmitoylation on the lysosomal sorting receptors, we wanted to identify a method that would block the palmitoylation of CI-MPR and sortilin. 2-fluoropalmitic acid (2-FPA) is a cell permeable compound known to inhibit palmitoylation in cells (31). When we treated HeLa cells with 100 μm of 2-FPA, we found a significant reduction in the amount of palmitoylated sortilin (Figure 1C, upper panel, lane 7) compared with nontreated cells (Figure 1C, upper panel, lane 6), while we were not able to detect any palmitoylated CI-MPR (Figure 1D, upper panel, lane 9) in 2-FPA-treated cells compared with nontreated cells (Figure 1D, upper panel, lane 8) although the amount of receptor was equally expressed for both sortilin (Figure 1C, lower panel, lanes 6 and 7) and CI-MPR (Figure 1D, lower panel, lanes 8 and 9) in mock or 2-FPA-treated cells.
Figure 1.
CI-MPR and sortilin are palmitoylated. A) HeLa cells transfected with sortilin-myc were labeled with [3H]-palmitic acid for 4 h in the presence or not of 1 m NH2OH and immunoprecipitated with anti-myc antibody and blotted with anti-myc antibody or exposed to film for 3 weeks. B) HeLa cells were labeled with [3H]-palmitic acid for 4 h in the presence or not of 1 m NH2OH and immunoprecipitated with CI-MPR antibody and stained with CI-MPR antibody or exposed to film for 6 weeks. C) HeLa cells were prepared as in (A), but incubated in the presence of 2-FPA overnight before labeling with the [3H]-palmitic acid. D) HeLa cells were prepared as in (B), but were incubated with 2-FPA before labeling with the [3H]-palmitic acid. IP, immunoprecipitation; Wt, wild type; Wb, western blotting.
Palmitoylation is required for the retrograde sorting of CI-MPR and sortilin
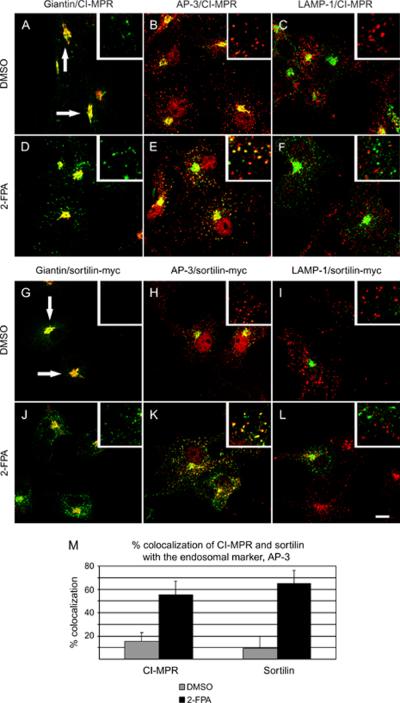
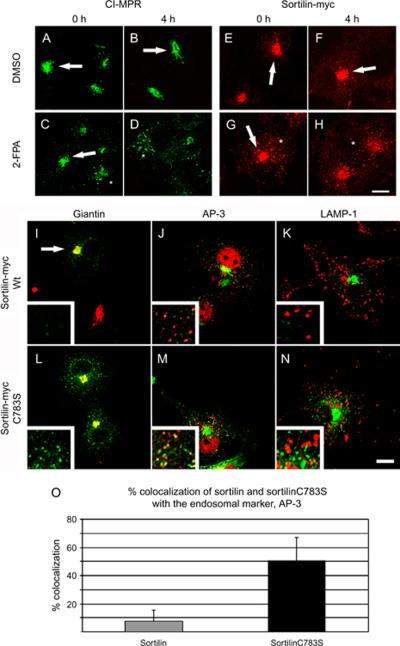
The steady-state distribution of CI-MPR and sortilin is in the Golgi apparatus although the receptors travel to and from the Golgi through the endosomal compartment (18). A small proportion of the receptor, estimated at 10%, can also be found on the cell surface (32). We asked if palmitoylation was important or not in the steady-state distribution of CI-MPR and sortilin, so we transfected sortilin-myc into COS-7 cells and treated the cells with 2-FPA. CI-MPR and sortilin-myc are usually localized to the perinuclear region at steady state with very few punctate structures (Figure 2A,G, arrows). However, following treatment with the 2-FPA, the distribution of these two proteins was significantly more punctate (Figure 2D,J, insets). This suggested that the receptors were not efficiently recycled back to the Golgi apparatus but remained trapped in some peripheral punctate compartment. In fact, in normal steady-state distribution, CI-MPR and sortilin-myc colocalize primarily with Golgi markers (Figures 2A,G, arrows) but not with the endosomal marker AP-3 (Figure 2B,H, insets) or the lysosomal marker lysosome-associated membrane protein (LAMP)-1 (Figure 2C,I, insets). However, after the treatment with 2-FPA, we could see CI-MPR and sortilin-myc were colocalizing in punctate structures that were staining positive with the endosomal marker, AP-3 (Figure 2E,K, insets). Quantification of this process showed that in dimethyl sulfoxide-treated cells, CI-MPR and sortilin-myc colocalized with AP-3 in approximately 10% of cells counted (Figure 2M, gray bars). While in 2-FPA-treated cells, close to 60% of AP-3-positive endosomes had CI-MPR and sortilin-myc staining (Figure 2M, black bars). This suggested that palmitoylation plays a significant role in the efficient retrograde trafficking of the receptors from the endosomes to the Golgi apparatus. We could not detect any localization with the lysosomal marker LAMP-1 (Figure 2F,L, insets), suggesting that the receptors were being degraded. To verify that 2-FPA was in fact blocking the retrograde transport of CI-MPR and sortilin, we treated palmitoylation-deficient cells with cycloheximide for 0 or 4 h and stained the cells with either CI-MPR or myc antibody. As cycloheximide blocks de novo protein synthesis, Golgi staining for CI-MPR and sortilin should only be found in cells that are able to efficiently recycle CI-MPR and sortilin back to the Golgi apparatus. At time 0, untreated cells had a positive staining in the Golgi apparatus for CI-MPR and sortilin and very few punctate structures (Figure 3A,E, arrows), while cells that had been treated with the 2-FPA still had some Golgi staining (Figure 3C,G, arrows) and several punctate structures (Figure 3C,G, asterisks). After a 4 -h incubation with cycloheximide, the untreated cells still had Golgi staining for CI-MPR and sortilin (Figure 3B,F, arrows), suggesting efficient recycling of the receptors, while the palmitoylation-deficient cells had predominately punctate staining (Figure 3D,H, asterisks) for CI-MPR and sortilin. As it was possible that treating cells with 2-FPA could have unforeseen side-effects not directly related to the palmitoylation state of the receptors themselves, we wanted to test the localization of mutants of sortilin that could not be palmitoylated (sortilinC783S-myc). Unlike wild-type sortilin-myc, which colocalized with the Golgi marker giantin (Figure 3I, arrow) but not with the endosomal marker AP-3 (Figure 3J, inset) or the lysosomal marker LAMP-1 (Figure 3K, inset), sortilinC783S-myc was redistributed to punctate structures (Figure 3L, inset) that colocalized with the endosomal marker AP-3 (Figure 3M, inset), but not with lysosomal protein LAMP-1 (Figure 3N, inset). Again, quantification of endosomes showed that wild-type sortilin-myc colocalized in 8% of AP-3-positive endosomes (Figure 3O, gray bars), while sortilinC783S-myc colocalized in 52% of AP-3-positive endosomes (Figure 3O, black bars). This result was in agreement with the results obtained using 2-FPA and strongly implicates palmitoylation as playing an important role in the retrograde trafficking of the lysosomal receptors.
Figure 2.
Inhibiting palmitoylation causes CI-MPR and sortilin to accumulate in endosomes. A–L) COS-7 cells were treated with dimethyl sulfoxide (DMSO) or 2-FPA and stained with CI-MPR (A–F, green) and markers (red) for the Golgi (giantin, A and D), endosomes (AP-3, B and E) or lysosomes (LAMP-1, C and F). COS-7 cells were transfected with sortilin-myc and treated with DMSO or 2-FPA for 4 h and stained with anti-myc (G–L, green) and markers (red) for the Golgi (giantin, G and J), endosomes (AP-3, H and K) or lysosomes (LAMP-1, I and L). Scale bar = 10 μm. M) Quantification of the percentage of CI-MPR and sortilin in AP-3-positive endosomes in mock-treated (gray bars) and 2-FPA-treated cells (Black bars). Data represent the average from three separate experiments.
Figure 3.
Nonpalmitoylated lysosomal sorting receptors are trapped in endosomes and do not recycle back to the Golgi apparatus. COS-7 cells were treated with DMSO (A, B, E and F) or 2-FPA (C, D, G and H) followed by 50 μg/mL of cycloheximide for either 0 (A, C, E and G) or 4 h (B, D, F and H) and stained for CI-MPR (A–D) or sortilin-myc (E–H). Arrows indicate the staining of the sorting receptors in the Golgi apparatus, while asterisks show localization to endosomes. I–N) COS-7 cells were transfected with sortilin-myc (I, J and K) or sortilinC783S-myc (palmitoylation mutant) (L, M and N) and stained with myc (green) and markers (red) of the Golgi (giantin, I and L), endosomes (AP-3, J and M) or lysosomes (LAMP-1, K and N). Scale bar = 10 μm. O) Quantification of the colocalization of sortilin (gray bar) and sortilinC783S (black bar) with the endosomal marker, AP-3. Data represent the average from three separate experiments.
Palmitoylation is required for the interaction of sortilin and CI-MPR with retromer
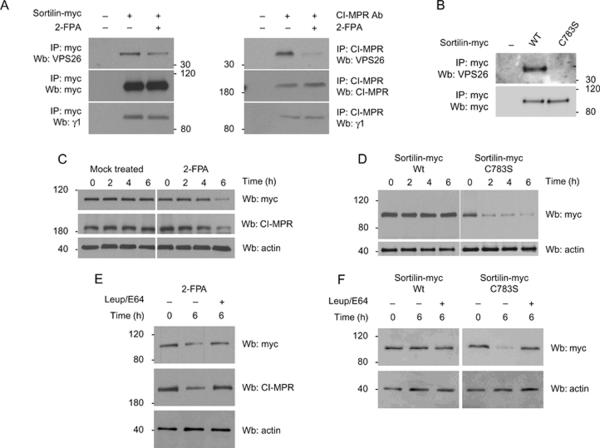
Although several proteins have been suggested to play a role in the recycling of the lysosomal receptors back to the Golgi, interaction with the retromer complex is the only unifying requirement among the whole family of receptors. Thus, we asked if palmitoylation of the lysosomal receptors affect their interaction with retromer. To test this, we transfected HeLa cells with sortilin-myc, treated the cells with the 2-FPA and immunoprecipitated with myc and CI-MPR antibodies. We found that sortilin-myc and CI-MPR interacted with retromer (VPS26) (Figure 4A, upper panel) and AP-1 (γ1 subunit) (Figure 4A, lower panel). However, in cells treated with the 2-FPA, we found less retromer binding with sortilin-myc and CI-MPR (Figure 4A, upper panel) although the binding to AP-1 was not changed (Figure 4A, lower panel). The amount of sortilin-myc and CI-MPR was similar in both the mock and 2-FPA-treated cells (Figure 4A, middle panel). To confirm that palmitoylation is directly involved in mediating the interaction between the sorting receptors and retromer, we used the palmitoylation mutant of sortilin (sortilinC783S-myc) in the co-immunoprecipitation assay. Wild-type sortilin (sortilin-myc) was able to interact with retromer (VPS26) (Figure 4B), while the palmitoylation mutant of sortilin (sortilinC783S-myc) was unable to co-precipitate retromer (Figure 4B) although both receptors were well expressed (Figure 4B).
Figure 4.
Palmitoylation is required for the interaction of the lysosomal sorting receptors with retromer. A) HeLa cells were transfected with sortilin-myc, treated with 2-FPA and immunoprecipitated with sortilin-myc or CI-MPR and blotted for the proteins indicated. B) HeLa cells were transfected with sortilin-myc or sortilinC783S-myc and immunoprecipitated with myc antibodies and blotted for the proteins indicated. C) HeLa cells were transfected with sortilin-myc and treated with the palmitoylation inhibitor 2-FPA. The cells were subsequently treated with 50 μg/mL of cycloheximide for the times indicated. D) Wild-type sortilin-myc and sortilinC783S-myc were transfected into HeLa cells and a cycloheximide treatment was performed as in (A). E) A cycloheximide treatment as in (C) was performed in the presence of the lysosomal inhibitors leupeptin and E64. F) A cycloheximide treatment was performed as in (D) in the presence of the lysosomal inhibitors leupeptin and E64. Actin served as a loading control in panels (C), (D), (E) and (F). Ab, antibody; IP, immunoprecipitation; Wt, wild type; Wb, western blotting.
Palmitoylation-deficient sortilin and CI-MPR are not recycled but are quickly degraded
Are palmitoylation-deficient receptors degraded faster because of their inability to recycle? Previous studies have shown that in cells depleted of retromer by RNA interference (RNAi), the receptors accumulate in punctate structures and pulse-chase analysis showed that CI-MPR was degraded in the lysosomes rather than being recycled back to the Golgi apparatus (20–22). As palmitoylation is required for the interaction between the sorting receptors and retromer as shown in Figure 4A,B, we tested whether or not the inhibition of palmitoylation by 2-FPA could also cause the degradation of sortilin-myc and CI-MPR. HeLa cells transfected with sortilin-myc were treated overnight with 2-FPA acid followed the next day with a treatment of cycloheximide for 0, 2, 4 or 6 h. Both sortilin-myc and CI-MPR were stable after 6 h in cells that were mock treated suggesting that the receptors were being efficiently recycled back to the Golgi apparatus and not degraded (Figure 4C). However, cells that were treated with 2-FPA had considerably less sortilin-myc and CI-MPR after 6 h, suggesting that the receptors were being degraded (Figure 4C). The staining for actin was used as a loading control. The increased rate of degradation in the absence of palmitoylation suggested that palmitoylation was an important part of the retrograde trafficking of CI-MPR and sortilin. Interestingly, the 6-h rate of degradation upon treating the cells with 2-FPA was similar to the results previously obtained upon depletion of the retromer component VPS26 (20). This result again suggests that the recognition step of the receptors by retromer was absent. To ensure that the result obtained with the 2-FPA was specific to the palmitoylation of the receptor and not to a separate unintended event; we repeated the experiment using the sortilinC783S-myc mutant. Again the mutant receptor was quickly degraded (Figure 4D), suggesting that efficient recycling was disrupted and confirming that palmitoylation of the receptors is a necessary step in its retrograde trafficking. We next asked if the degradation of the sorting receptor was because of lysosomal degradation or not. We repeated cycloheximide experiments with the 2-FPA-treated cells and the palmitoylation-deficient sortilin mutant (C783S) but this time we also included the lysosomal inhibitors leupeptin and E64. We found that sortilin and CI-MPR were degraded in the 2-FPA-treated cells after a 6-h treatment with cycloheximide but the addition of leupeptin and E64 prevented receptor degradation (Figure 4E). Similarly, the leupeptin and E64 also blocked the degradation of sortilinC783S following a 6-h incubation with cycloheximide (Figure 4F).
DHHC-15 palmitoylates CI-MPR and sortilin
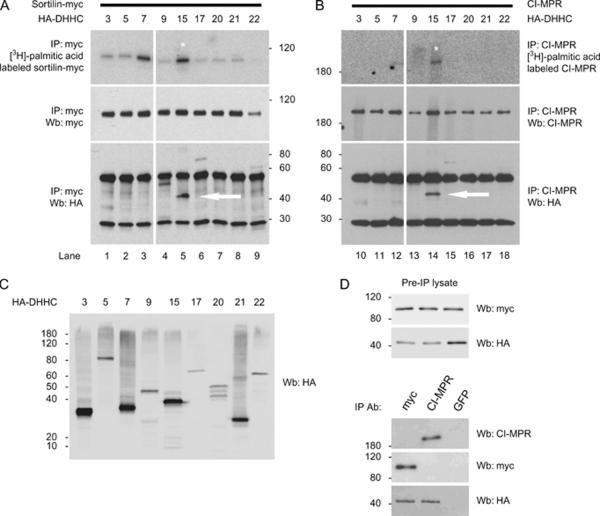
What is the enzyme responsible for palmitoylation of the lysosomal receptors? Palmitoylation is an enzymatically reversible process, so we next wanted to identify the enzyme responsible for the palmitoylation of sortilin and CI-MPR. Recently, a group of proteins have been identified as potential protein acyltransferases and have been shown to palmitoylate a variety of proteins (33). This group of proteins shares a common functional motif, aspartic acid–histidine–histidine–cysteine (DHHC) that has been shown to be the active site of the enzyme. Mutation of the cysteine residue to serine in the DHHC motif abolished the palmitoylation of endothelial nitric oxide synthase (eNOS) by DHHC-3 and DHHC-7. That same report demonstrated that the enzymes responsible for the palmitoylation of eNOS, interacted with its target and increased its palmitoylation, compared with basal levels of palmitoylation, when co-expressed in cells. We followed a similar strategy to identify the enzyme(s) responsible for the palmitoylation of sortilin and CI-MPR. First, we tested the expression of the HA-DHHC proteins by immunofluorescence microscopy. We found that the various hemagglutinin (HA) tagged-DHHC proteins were expressed in COS-7 cells (Figure S1). We also confirmed the previously published expression pattern and subcellular localization of the majority of DHHC proteins (Figure S1A–I). However, we found DHHC-20 expressed in the endoplasmic reticulum (Figure S1G) and DHHC-21 in a Golgi staining pattern (Figure S1H). Previous studies have shown that DHHC-20 and DHHC-21 were at the plasma membrane (34). It is possible that the difference in localization is because of the cell type used in our two studies [COS-7 versus human embryonic kidney cells (HEK)] or that the tag is having an effect on the localization (HA versus FLAG), although both the constructs are N-terminally tagged. We then co-transfected HeLa cells with sortilin-myc and the various HA-DHHC proteins or HA-DHHC proteins alone for CI-MPR and labeled with [3H]-palmitic acid. Cells were lysed and an immunoprecipitation performed with either myc or CI-MPR antibody. Half of the immunoprecipitation product was run and the gel dried and exposed to film to analyze which, if any, of the HA-DHHC proteins would increase the palmitoylation of sortilin and CI-MPR compared with basal levels of palmitoylation. The other half of the immunoprecipitation was run on a gel and transferred to nitrocellulose and blotted using an anti-HA antibody to evaluate the interaction of HA-DHHC proteins with sortilin-myc and CI-MPR. When we examined the function of the DHHC enzymes using the palmitoylation assay, we found that DHHC-7 and DHHC-15 increased the palmitoylation of sortilin-myc compared with basal levels of palmitoylation in the presence of the other enzymes (Figure 5A, upper panel, lanes 3 and 5). However, only DHHC-15 was able to increase the palmitoylation of CI-MPR using the same assay (Figure 5B, upper panel, lane 14). We confirmed the post-immunoprecipitation amounts of sortilin-myc (Figure 5A, middle panel) and CI-MPR (Figure 5B, middle panel) using myc and CI-MPR antibodies. As DHHC-7 and DHHC-15 were able to increase the palmitoylation of sortilin-myc and DHHC-15 was able to increase the palmitoylation of CI-MPR, we wanted to test if these enzymes interacted with the receptors. We were able to co-immunoprecipitate DHHC-9 and DHHC-15 and to a lesser extent DHHC-17 and DHHC-22 with sortilin-myc but not DHHC-7 (Figure 5A, lower panel, lanes 3, 4, 5, 6 and 9), while CI-MPR was able to bind to DHHC-15 and to a lesser extent DHHC-17 and DHHC-22 (Figure 5B, lower panel, lane 14, 15 and 18). We also confirmed the expression of the DHHC proteins by Western blotting (Figure 5C). To confirm the specificity of the interaction between the sorting receptors and DHHC-15, we performed a control experiment using lysates from cells that had been transfected with sortilin-myc and HA-DHHC-15 (Figure 5D). We immunoprecipitated the lysate using myc, CI-MPR or green fluorescent protein (GFP) antibodies and confirmed that sortilin-myc and endogenous CI-MPR were able to bind to HA-DHHC-15, while the immunoprecipitation with the random antibody (GFP) was not able to bring down either of the receptors or DHHC-15 (Figure 5D). Taken together, our data suggest that DHHC-15 is involved in the palmitoylation of sortilin and CI-MPR.
Figure 5.
DHHC-15 interacts with and palmitoylates sortilin and CI-MPR. A) HeLa cells transfected with the HA-DHHC constructs and with sortilin-myc were used to test for the change in palmitoylation and to test for interactions between sortilin and the DHHC enzymes. The HeLa cells were incubated with 500 μCi of [3H]-palmitic acid for 4 h and immunoprecipitated with an anti-myc antibody. The samples were divided equally and run on separate gels. B) Same experiment as in (A), but the immunoprecipitation was performed with an anti-CI-MPR antibody. C) HeLa cells were transfected with the HA-DHHC constructs indicated and blotted with anti-HA antibody. D) Lysates from cells transfected with sortilin-myc and HA-DHHC-15 were run on a 12% gel and stained for the antibodies indicated. The lysates were immunoprecipitated with anti-myc, CI-MPR or GFP antibodies and stained with the antibodies indicated. Ab, antibody; IP, immunoprecipitation; Wb, western blotting.
Depletion of DHHC-15 by siRNA redistributes sortilin-myc and CI-MPR to punctate structures and inhibits efficient lysosomal sorting
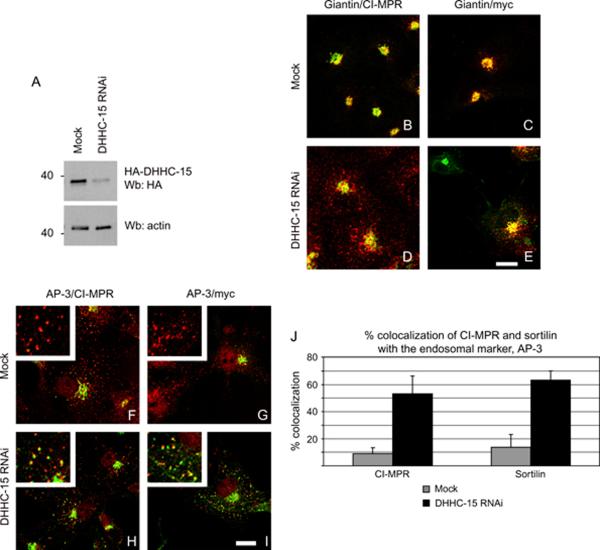
Is DHHC-15 required for the proper trafficking of the lysosomal receptors? To verify that DHHC-15 was in fact implicated in the palmitoylation of the lysosomal sorting receptors, we used an siRNA strategy to deplete cells of DHHC-15 and then observe the steady-state distribution of the receptors using immunofluorescence. We used a combination of western blotting and immunofluorescence to confirm the efficacy of the RNAi. As antibodies are not available to endogenous DHHC-15, we transfected COS-7 cells with HA-DHHC-15 and treated the cells with a mixture of 4 siRNAs. The knockdown of HA-DHHC-15 was efficient as seen by western blotting (Figure 6A). There was a decrease in the amount of HA-DHHC-15 of over 90%. In addition to detection by western blotting, we confirmed the depletion of HA-DHHC-15 by immunofluorescence in RNAi-treated cells (data not shown) compared with mock-treated cells (data not shown). As the depletion of DHHC-15 was efficient, we tested the effects of DHHC-15 depletion on the steady-state localization of CI-MPR and sortilinmyc. In mock-treated cells, CI-MPR and sortilin-myc colocalized well with the Golgi marker giantin (Figure 6B,C) but was not significantly found in punctate structures. However, when DHHC-15 was depleted by siRNA, a significant amount of CI-MPR and sortilin-myc was found in punctate structures (Figure 6D,E), suggesting an inefficient recycling of the receptors. The morphology of the Golgi apparatus did not seem to be affected by the depletion of DHHC-15 as seen by staining for giantin (Figure 6B–E). To verify the identity of the punctate structures we observed in the DHHC-15-depleted cells, we stained cells that had been depleted of DHHC-15 with AP-3. In mock-depleted cells, we found very little CI-MPR or sortilin colocalizing with AP-3 (Figure 6F,G, inserts), while in the DHHC-15-depleted cells, we found a significant increase in the amount of CI-MPR and sortilin colocalizing with AP-3 (Figure 6H,I). In fact, quantification of the AP-3-positive endosomes showed that the DHHC-15 RNAi increased the colocalization of CI-MPR with AP-3 to over 50% and sortilin to over 60% (Figure 6J, black bars) compared with 10 and 15% for CI-MPR and sortilin in mock-depleted cells (Figure 6J, gray bars).
Figure 6.
Depletion of DHHC-15 causes a redistribution of sortilin and CI-MPR. A) COS-7 cells were depleted of DHHC-15 by siRNA. A depletion of 90% was achieved. Actin was used as a loading control. B–E) COS-7 cells mock depleted (B and C) or depleted of DHHC-15 (D and E) were transfected with sortilin-myc (C and E) and stained for the Golgi marker, giantin (B–E, green) and either CI-MPR (B and D, red) or myc (C and E, red). (F–I) COS-7 cells mock depleted (F and G) or depleted of DHHC-15 (H and I) were transfected with sortilin-myc (G and I) and stained for the endosomal marker, AP-3 (F–I, red) and either CI-MPR (F and H, green) or myc (G and I, green). Scale Bar = 10 μm. J) Quantification of the percentage of CI-MPR and sortilin in AP-3-positive endosomes in mock-depleted (gray bars) and DHHC-15-depleted cells (black bars). Data represent the average from three separate experiments
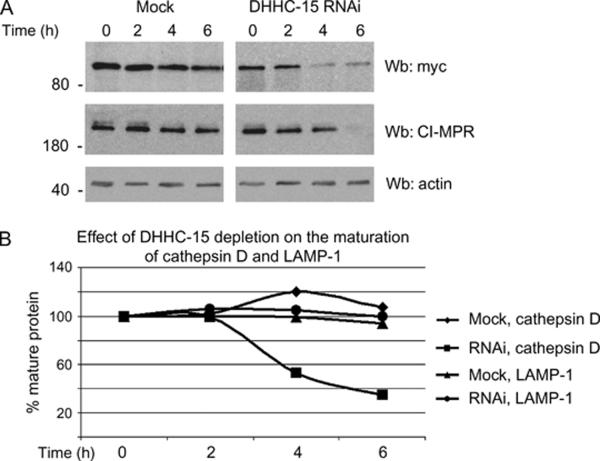
As we had previously shown that palmitoylation-deficient sortilin and CI-MPR were rapidly degraded, we tested whether the depletion of DHHC-15 also caused the degradation of sortilin-myc and CI-MPR. We found that after a 4-h incubation with cycloheximide, a significant amount of sortilin-myc and CI-MPR was degraded compared with mock-treated cells (Figure 7A, upper and middle panels), while the actin loading control remained the same in both mock and depleted cells (Figure 7A, lower panel). This result was again consistent with the half-life of the receptors observed when either depleted of the retromer component VPS26 (20), treated with 2-FPA (Figure 4C) or lacking the cysteine that is palmitoylated (Figure 4D).
Figure 7.
Depletion of DHHC-15 results in the degradation of the sortilin and CI-MPR and mis-sorting of the CIMPR cargo, cathepsin D. A) HeLa cells were depleted of DHHC-15 through siRNA and transfected with sortilin-myc. The cells were then treated with 50 μg/mL of cycloheximide for the times indicated. Actin was used as a loading control. B) Using the same cycloheximide treatment protocol as above, we followed the maturation of cathepsin D in cells depleted of DHHC-15. LAMP-1 was used as a control. Wb, western blotting.
As depleting DHHC-15 caused a redistribution of CI-MPR to punctate structures and an eventual degradation of the receptor, we wanted to test whether or not the processing of the CI-MPR cargo protein, cathepsin D, would also be affected by a lack of recycling of the receptor. Cathepsin D is synthesized as a 50 kDa protein in the Golgi, and upon the addition of a mannose 6-phosphate group, binds to its receptor, CI-MPR, that then traffics cathepsin D to lysosomes where it is processed into the mature lysosomal form of the protein. The process of maturation of cathepsin D can easily be followed by SDS-PAGE as the precursor form migrates as a 50 -kDa protein and the mature lysosomal form migrates at 30 kDa. We followed the amount of mature cathepsin D in the lysosome of HeLa cells using various cycloheximide incubation times to determine the affect, if any, of DHHC-15 depletion on the processing of cathepsin D. We found a significant decrease in the amount of mature cathepsin D (squares) in DHHC-15-depleted cells after a 6-h incubation with cycloheximide versus mock-depleted cells (diamonds) (Figure 7B), while there was no difference in the amount of the lysosomal membrane protein LAMP-1 (triangles and circles) (Figure 7B). Cells depleted of DHHC-15 do not process cathepsin D properly, a result that is similar to cells depleted of retromer, strongly supporting our hypothesis that palmitoylation and the palmitoyltransferase are required for efficient retrograde trafficking of the lysosomal sorting receptors.
How does palmitoylation regulate recycling of the lysosomal sorting receptors?
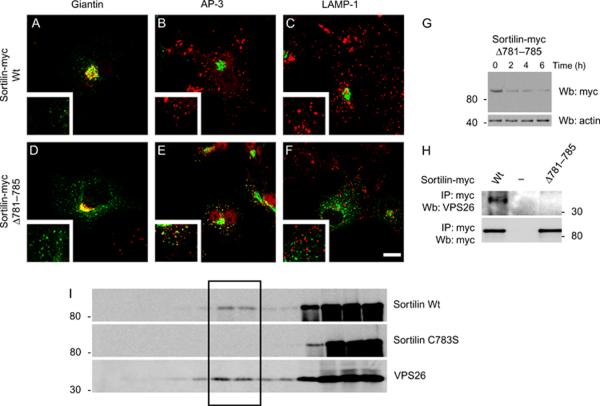
Although we demonstrated that the palmitoylation of sortilin was required for both its proper localization and its interaction with the recycling complex, retromer, it was unclear whether palmitoylation directly affects the receptor/retromer interaction. The addition of a palmitoyl group to a membrane protein has been proposed to either (i) alter the position of a binding site on the protein in relation to the membrane (28) or (ii) change the hydrophobicity of the tail so as to alter the membrane fraction where the protein can partition. To examine if palmitoylation was directly mediating the interaction with retromer through tail length, we deleted residues 781–785 (sortilinΔ781–785) that includes the palmitoylated cysteine residue. Deleting five residues would effectively shift the tail of the mutated receptor closer to the membrane such that it would be closer to the position of a wild-type receptor that had been palmitoylated. We first tested the construct for palmitoylation and as expected sortilinΔ781–785 was not palmitoylated although the protein was expressed and efficiently immunoprecipitated from HeLa cell lysate using myc antibody (data not shown). We next tested the steady-state localization of the Δ781–785 mutant. We found that the deletion mutant was also localized to punctate structures (Figure 8D) compared with the Golgi localization of wild-type sortilin (Figure 8A). In fact, much like sortilinC783S-myc, sortilinΔ781–785-myc colocalized with the endosomal marker AP-3 (Figure 8E, inset) but not the lysosomal marker LAMP-1 (Figure 8F, inset). We next tested the stability of the Δ781–785 construct over a period of 6 h. Much like sortilinC783S, sortilinΔ781–785 was degraded instead of being recycled back to the Golgi apparatus (Figure 8G) and could not bind retromer (Figure 8H). Again, suggesting that repositioning of the receptor tail in relation to the membrane is not sufficient to recycle the receptors.
Figure 8.
Palmitoylation does not seem to function by mediating tail length. AߝF) COS-7 cells were transfected with either sortilin-myc (A, B and C) or sortilinΔ781–785-myc (D, E and F) and stained with myc (green) or markers (red) of the Golgi (giantin, A and D), endosome (AP-3, B and E) or lysosomes (LAMP-1, C and F). Scale Bar = 10 μm. G) SortilinΔ781–785-myc was transfected into HeLa cells and treated as in (D). Note the rapid degradation of the sortilinΔ781–785-myc that is similar to the degradation of sortilinC783S-myc in (D). H) HeLa cells were transfected with sortilin-myc or sortilinΔ781–785-myc and immunoprecipitated with myc antibodies and blotted for the proteins indicated. I) HeLa cells were transfected with either sortilin-myc or sortilinC783S-myc, lysed and analyzed using a sucrose gradient of 42.5, 35 and 5%. Fractions of 350 μL were collected and run on a 12% gel. The blot was then stained for either myc or VPS26. IP, immunoprecipitation; Wt, wild type; Wb, western blotting.
Because the relative distance in relation to the membrane of a binding site on the tail of the receptors is not being affected by the addition of a palmitoyl group, we tested whether or not palmitoylation affected the migration of sortilin into a membrane subdomain that also contained retromer. Using a sucrose gradient, we found that wild-type sortilin was found in a membrane fraction that also contained retromer (Figure 8I, black box), while sortilinC783S was absent from that fraction (Figure 8I, black box). This suggested that palmitoylation serves to facilitate the partitioning of the lysosomal sorting receptors to an endosomal subdomain where retromer is recruited.
Discussion
Several major conclusions are supported by the data presented in this paper. First, in addition to CD-MPR, the lysosomal sorting receptors sortilin and CI-MPR are palmitoylated. Second, palmitoylation serves to shift the receptors' fate from one of degradation to one of recycling. Third, the palmitoylation of the lysosomal sorting receptors is required for the interaction with the retromer complex. Fourth, the palmitoyltransferase DHHC-15 appears to play a role in the palmitoylation of the lysosomal sorting receptors. Finally, palmitoylation facilitates recycling by allowing sortilin to move into a membrane fraction where it can now bind retromer.
Palmitoylation is a post-translational modification that has been shown to affect protein localization, protein–protein interaction and protein stability. CD-MPR has been shown to be palmitoylated and this modification was suggested to play a role in retrograde trafficking of this protein. We began by testing whether or not the other two lysosomal sorting receptors, sortilin and CI-MPR, were palmitoylated. We found that sortilin and CI-MPR were palmitoylated through a thioester bond, and we identified cysteine residue 783 of the sortilin cytosolic tail as the residue that is palmitoylated. To define the role of palmitoylation in the trafficking of the lysosomal sorting receptors, we treated COS-7 cells with the palmitoylation inhibitor 2-FPA or transfected the palmitoylation-deficient sortilin construct. In a previous study, nonpalmitoylated CD-MPR had been found in a cell fraction that contained lysosomes. However, using immunofluorescence, we were able to determine a more precise localization of the nonpalmitoylated receptors. We found a significant amount of colocalization between the nonpalmitoylated sortilin and CI-MPR and AP-3, a protein known to localize to the early endosome (35). We did not find any nonpalmitoylated receptor in lysosomes most likely because of the degradation of the receptors in this organelle. However, we were able to prevent the degradation of CI-MPR and sortilin, by using inhibitors of lysosomal function, suggesting that the ultimate destination for the nonpalmitoylated receptors are lysosomes. This data confirmed the requirements of palmitoylation in the retrograde trafficking of the lysosomal sorting receptors and were in agreement with earlier studies showing that cells depleted of retromer display a similar early endosomal localization of CI-MPR (20). To understand what step in retrograde trafficking palmitoylation was regulating, we performed co-precipitation experiments between sortilin and the retromer component VPS26. Nonpalmitoylated receptors were unable to co-precipitate VPS26, suggesting that the palmitic acid modification somehow regulates the lysosomal sorting receptors' ability to bind retromer. In support of this finding, it was recently reported that a deletion mutant of the CI-MPR tail fused to the reporter protein, CD8, that lacked the cytosolic cysteine residues failed to bind to retromer while the full-length tail did (22). It is interesting to note that while the 2-FPA treatment almost completely abolished the interaction of retromer to CI-MPR, it did not for sortilin. This probably reflects the amount of receptors found in the cells (overexpressed sortilin versus endogenous CI-MPR). As sortilin was overexpressed, a greater amount may still be palmitoylated and have the ability to bind to retromer.
Previous work had suggested that the palmitoyltransferase for CI-MPR cycled between the endosome and plasma membrane, although the identity of the enzyme was never established. We identified DHHC-15, a protein localized to the Golgi apparatus, as a palmitoyltransferase for sortilin and CI-MPR. Although other DHHC proteins did bind to the sorting receptors, they were not able to increase the palmitoylation of the receptors so they were either binding non-specifically or recognizing a binding motif but could not palmitoylate the receptors. Interestingly, DHHC-7 did increase the palmitoylation of sortilin without binding to it. One possibility is that DHHC-7 regulates the activity of DHHC-15 by palmitoylating it. However, when we depleted cells of DHHC-15, it led to the mislocalization of the lysosomal sorting receptors, faster degradation of the receptors and mistargeting of luminal lysosomal proteins. These facts strongly suggest that DHHC-15 is directly involved in the palmitoylation and hence trafficking of CI-MPR and sortilin. The interaction of AP-1 and the lysosomal sorting receptors is required to traffic the receptors out of the Golgi. This interaction is preserved in palmitoylation-deficient receptors. This suggests that palmitoylation is not required for the exit of sortilin and CI-MPR from the Golgi.
We have established that palmitoylation plays a crucial role in the retrograde trafficking of the lysosomal sorting receptors and prevents their degradation. It had been suggested, however never tested, that palmitoylation could affect the signal presentation for trafficking to the cytosolic machinery. We investigated if palmitoylation serves as a mechanism to change the distance of the sorting signal from the membrane to allow for an interaction with the recycling machinery. We deleted five amino acids from the receptor tail to mimic the distance from the membrane any putative retromer signal would move when the wild-type receptor was palmitoylated. This deletion mutant failed to interact with retromer and failed to recycle properly. This suggests that modification of the receptors acts as a facilitator that allows the lysosomal sorting receptors to migrate into a membrane subdomain where retromer could be preferentially recruited. It seems likely that the nonpalmitoylated receptor is not sorted into this subdomain, cannot interact with retromer and thus does not get recycled. This role of palmitoylation is consistent with the yeast two-hybrid experiments previously published using the lysosomal sorting receptor tails. In those experiments, palmitoylation is not required to see an interaction because the yeast two-hybrid expression systems serve to facilitate an interaction between retromer and the lysosomal sorting receptor tails by artificially placing them in the same location. This is also consistent with the role of other fatty acid modifications. For example, the addition of a palmitoylation group on ras serves to target it to the plasma membrane where it can now interact with a binding partner.
In this study, we have shown that palmitoylation is a general mechanism used by all three lysosomal sorting receptors required for retrograde transport and we have identified the enzyme responsible for this modification and its role in lysosomal trafficking. More importantly, we have shown that palmitoylation changes the interacting partners of the sorting receptors that determine the itinerary for the receptors (see model in Figure 9). This study raises the intriguing possibility that the cell uses palmitoylation of the lysosomal sorting receptors as a control for the levels of lysosomal enzymes. Although what regulates the activity of palmitoyltransferases is unclear, these results may provide a link between the metabolic state of a cell and the cell's need to increase or decrease lysosomal activity. That is, the cell could regulate levels of lysosomal enzymes by controlling the recycling of the lysosomal receptors through the activity of the palmitoyltransferase DHHC-15. Further studies will be required to understand exactly what variables affect DHHC-15 expression levels and activity.
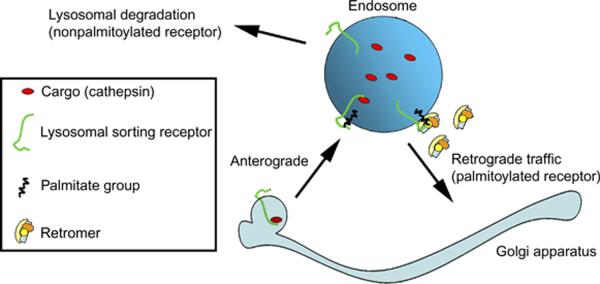
Figure 9.
Model showing the requirements of palmitoylation for the efficient retrograde (endosome to Golgi) trafficking of the lysosomal sorting receptors. Palmitoylated receptors find their way to the endosomal subdomain where retromer is recruited and can be retrograde transported back to the Golgi. Nonpalmitoylated receptors are not able to enter the membrane subdomain and are degraded in the lysosomes.
Materials and Methods
Unless otherwise noted, all reagents were purchased from Sigma-Aldrich. The following mouse monoclonal antibodies were used: CD222 antibody against CI-MPR (Serotec), MMS-150P 9E10 against the myc epitope and MMS-101P against HA (Covance Research Products). The following polyclonal antibodies were used: LAMP-1 and VPS26 antibodies (Abcam), AP-3 antibody (Santa Cruz), myc-tag antibody (Cell Signaling Technology), IM16 against cathepsin D (VWR Canlab) and PRB-114C against giantin (Covance Research Products). The sortilin-myc construct was previously described (4,10). The 2-FPA, leupeptin and E64 were purchased from Biomol.
Cell culture and immunofluorescence
Cells were grown in DMEM supplemented with 10% FBS, glutamine and penicillin/streptomycin. Transfections were performed using Lipofectamine Reagent (Invitrogen) supplemented with the Plus Reagent (Invitrogen) with 1 μg of DNA (2-cm plate) or 5 μg of DNA (10-cm plate) according to the manufacturer's instructions. Some cells were treated with 100 μm 2-FPA overnight after transfection. For routine immunofluorescence, COS-7 cells were subcultured onto round coverslips and grown overnight before transfection. Twenty-four to 48 h post-transfection, the cells were fixed with 4% paraformaldehyde in PBS for 12 min, washed twice in PBS for 5 min each and then finally incubated with the primary antibody in a solution of 0.1% saponin, 0.02% sodium azide and 0.1% BSA in PBS for 1 h. The coverslips were then incubated with the appropriate secondary antibody conjugated to either Alexa 488 or Alexa 594 (Invitrogen) diluted in the same solution and incubated for 1 h. The coverslips were then mounted onto glass slide with Fluoromount G (Fisher Scientific) and sealed with nail polish to be viewed on a Zeiss Meta 510 microscope. To quantify the changes in the distribution of CIMPR and sortilin-myc, the AP-3-positive endosomes from 33 cells from three separate experiments were counted and scored for the presence of either CI-MPR or sortilin-myc. The average was taken in each of the three groups and the standard deviation was calculated.
Cycloheximide incubation
HeLa cells were transfected as described above and treated overnight with 100 μm of 2-FPA (Figure 4C,E) then incubated with 50 μg/mL of cycloheximide for the indicated times. Some cells were also incubated with 1 mg/mL of leupeptin and 5 μg/mL of E64. The cells were then lysed in a buffer containing 150 mm NaCl, 100 mm Tris, pH 8 and 1% Triton-X-100 for 30 min. The cells were then passaged through a 23-gauge needle seven times. The lysates were centrifuged for 15 min at 800 × gto remove debris.
RNA interference
The siRNA against DHHC-15 used was a SmartPool purchased from Dharmacon and transfected into cells using Oligofectamine (Invitrogen) following the manufactures instructions. To deplete DHHC-15, we treated cells with 100 nm of the SmartPool on two consecutive days then waited another 48 h after the second treatment to perform our assays.
Co-immunoprecipitation
HeLa cells were grown to near confluence, transfected following the protocol above and then lysed as above. The lysates were then precleared using 50 μL of the protein-G- or proteinA-covered sepharose beads (GE Healthcare) followed by an overnight incubation at 4°C with a primary antibody followed by protein-G- or protein-A-covered sepharose for 2 h. The beads were spun down and the supernatants removed. The beads were then washed in PBS and run on a 4–20% gradient gel, transferred to nitrocellulose and blotted for various proteins.
Palmitoylation assay
HeLa cells were grown to near confluence as described above and transfected with sortilin-myc or sortilin-myc and the individual HA-DHHC enzymes in 2-cm plates. Twenty-four hours post-transfection, the cells were labeled with 600 μCi of [3H]-palmitic acid (Perkin-Elmer) for 4 h in regular DMEM. The lysates were immunoprecipitated following the method above with an anti-myc antibody for sortilin-myc or an antibody to CI-MPR in the presence or not of 1 m NH2OH. The immunoprecipitation product was run on a 4–20% gradient gel that was fixed, treated with amplify reagent and dried to be exposed to Kodak MS film (GE Healthcare) for 3 weeks (sortilin-myc and DHHC assay) or 6 weeks (CI-MPR).
Sucrose gradient
Two 10-cm plates grown to near confluence of HeLa cells were transfected as above with sortilin-myc or sortilinC783S-myc and subjected to a gradient analysis as previously described (36). Briefly, cells were lysed in ice-cold TNE (10 mm Tris, pH 7.5, 150 mm NaCl, 5 mm ethylenediaminetetraacetic acid) containing 1% Triton-X-100 and adjusted to 42.5% sucrose. This layer was then overlaid with 4 mL of 35% sucrose, 1 mL of 5% sucrose and the tube was then filled with TNE. The samples were then centrifuged 18 h at 180 187 ×gin a SW41Ti rotor. Thirteen fractions were collected (600 μL each) after the first 3.5 mL were removed from the top. The samples were then prepared for western blotting.
Supplementary Material
Figure S1: Expression and intracellular localization of the palmitoyltransferases. A–I) COS-7 cells were transfected with HA-tagged DHHC-3 (A), DHHC-5 (B), DHHC-7 (C), DHHC-9 (D), DHHC-15 (E), DHHC-17 (F), DHHC-20 (G), DHHC-21 (H), DHHC-22 (J) and stained with HA antibody.
Acknowledgments
This work was funded by start-up funds to S. L. from la Fondation de l'Hôpital Maisonneuve-Rosemont and by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research to P. J. M. and G. T. We would like to thank E. B. Affar, J. B. McCormick and S. Fisher-Hoch for critical reading of this manuscript. We would also like to thank Dr M. Fukata (National Institute for Longevity Sciences, Japan) for providing us with the DHHC constructs that were confirmed to have palmitoyltransferase activity.
Footnotes
Supporting Information Additional Supporting Information may be found in the online version of this article:
References
- 1.Bonifacino JS, Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol. 2003;4:409–414. doi: 10.1038/nrm1099. [DOI] [PubMed] [Google Scholar]
- 2.Robinson MS, Bonifacino JS. Adaptor-related proteins. Curr Opin Cell Biol. 2001;13:444–453. doi: 10.1016/s0955-0674(00)00235-0. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- 4.Lefrancois S, Zeng J, Hassan AJ, Canuel M, Morales CR. The lysosomal trafficking of sphingolipid activator proteins (SAPs) is mediated by sortilin. EMBO J. 2003;22:6430–6437. doi: 10.1093/emboj/cdg629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puertollano R, Aguilar RC, Gorshkova I, Crouch RJ, Bonifacino JS. Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science. 2001;292:1712–1716. doi: 10.1126/science.1060750. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen MS, Madsen P, Christensen EI, Nykjaer A, Gliemann J, Kasper D, Pohlmann R, Petersen CM. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J. 2001;20:2180–2190. doi: 10.1093/emboj/20.9.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mort JS, Buttle DJ, Cathepsin B. Int J Biochem Cell Biol. 1997;29:715–720. doi: 10.1016/s1357-2725(96)00152-5. [DOI] [PubMed] [Google Scholar]
- 8.Ni X, Morales CR. The lysosomal trafficking of acid sphingomyelinase is mediated by sortilin and mannose 6-phosphate receptor. Traffic. 2006;7:889–902. doi: 10.1111/j.1600-0854.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y, Doray B, Poussu A, Lehto VP, Kornfeld S. Binding of GGA2 to the lysosomal enzyme sorting motif of the mannose 6-phosphate receptor. Science. 2001;292:1716–1718. doi: 10.1126/science.1060896. [DOI] [PubMed] [Google Scholar]
- 10.Canuel M, Lefrancois S, Zeng J, Morales CR. AP-1 and retromer play opposite roles in the trafficking of sortilin between the Golgi apparatus and the lysosomes. Biochem Biophys Res Commun. 2008;366:724–730. doi: 10.1016/j.bbrc.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Le Borgne R, Schmidt A, Mauxion F, Griffiths G, Hoflack B. Binding of AP-1 Golgi adaptors to membranes requires phosphorylated cytoplasmic domains of the mannose 6-phosphate/insulin-like growth factor II receptor. J Biol Chem. 1993;268:22552–22556. [PubMed] [Google Scholar]
- 12.Puertollano R, Randazzo PA, Presley JF, Hartnell LM, Bonifacino JS. The GGAs promote ARF-dependent recruitment of clathrin to the TGN. Cell. 2001;105:93–102. doi: 10.1016/s0092-8674(01)00299-9. [DOI] [PubMed] [Google Scholar]
- 13.Doray B, Kornfeld S. Gamma subunit of the AP-1 adaptor complex binds clathrin: implications for cooperative binding in coated vesicle assembly. Mol Biol Cell. 2001;12:1925–1935. doi: 10.1091/mbc.12.7.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dell'Angelica EC, Puertollano R, Mullins C, Aguilar RC, Vargas JD, Hartnell LM, Bonifacino JS. GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J Cell Biol. 2000;149:81–94. doi: 10.1083/jcb.149.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Y, Traub LM, Kornfeld S. ADP-ribosylation factor 1 transiently activates high-affinity adaptor protein complex AP-1 binding sites on Golgi membranes. Mol Biol Cell. 1998;9:1323–1337. doi: 10.1091/mbc.9.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinotsuka C, Waguri S, Wakasugi M, Uchiyama Y, Nakayama K. Dominant-negative mutant of BIG2, an ARF-guanine nucleotide exchange factor, specifically affects membrane trafficking from the trans-Golgi network through inhibiting membrane association of AP-1 and GGA coat proteins. Biochem Biophys Res Commun. 2002;294:254–260. doi: 10.1016/S0006-291X(02)00456-4. [DOI] [PubMed] [Google Scholar]
- 17.Lefrancois S, McCormick PJ. The Arf GEF GBF1 is required for GGA recruitment to Golgi membranes. Traffic. 2007;8:1440–1451. doi: 10.1111/j.1600-0854.2007.00623.x. [DOI] [PubMed] [Google Scholar]
- 18.Brown WJ, Goodhouse J, Farquhar MG. Mannose-6-phosphate receptors for lysosomal enzymes cycle between the Golgi complex and endosomes. J Cell Biol. 1986;103:1235–1247. doi: 10.1083/jcb.103.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seaman MN, McCaffery JM, Emr SD. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol. 1998;142:665–681. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol. 2004;165:123–133. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seaman MN. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol. 2004;165:111–122. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seaman MN. Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J Cell Sci. 2007;120:2378–2389. doi: 10.1242/jcs.009654. [DOI] [PubMed] [Google Scholar]
- 23.Hierro A, Rojas AL, Rojas R, Murthy N, Effantin G, Kajava AV, Steven AC, Bonifacino JS, Hurley JH. Functional architecture of the retromer cargo-recognition complex. Nature. 2007;449:1063–1067. doi: 10.1038/nature06216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rojas R, Kametaka S, Haft CR, Bonifacino JS. Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors. Mol Cell Biol. 2007;27:1112–1124. doi: 10.1128/MCB.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verges M, Luton F, Gruber C, Tiemann F, Reinders LG, Huang L, Burlingame AL, Haft CR, Mostov KE. The mammalian retromer regulates transcytosis of the polymeric immunoglobulin receptor. Nat Cell Biol. 2004;6:763–769. doi: 10.1038/ncb1153. [DOI] [PubMed] [Google Scholar]
- 26.Eaton S. Retromer retrieves wntless. Dev Cell. 2008;14:4–6. doi: 10.1016/j.devcel.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Prasad BC, Clark SG. Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development. 2006;133:1757–1766. doi: 10.1242/dev.02357. [DOI] [PubMed] [Google Scholar]
- 28.Schweizer A, Kornfeld S, Rohrer J. Cysteine34 of the cytoplasmic tail of the cation-dependent mannose 6-phosphate receptor is reversibly palmitoylated and required for normal trafficking and lysosomal enzyme sorting. J Cell Biol. 1996;132:577–584. doi: 10.1083/jcb.132.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stockli J, Rohrer J. The palmitoyltransferase of the cation-dependent mannose 6-phosphate receptor cycles between the plasma membrane and endosomes. Mol Biol Cell. 2004;15:2617–2626. doi: 10.1091/mbc.E03-11-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam KK, Davey M, Sun B, Roth AF, Davis NG, Conibear E. Palmitoylation by the DHHC protein Pfa4 regulates the ER exit of Chs3. J Cell Biol. 2006;174:19–25. doi: 10.1083/jcb.200602049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeJesus G, Bizzozero OA. Effect of 2-fluoropalmitate, cerulenin and tunicamycin on the palmitoylation and intracellular translocation of myelin proteolipid protein. Neurochem Res. 2002;27:1669–1675. doi: 10.1023/a:1021643229028. [DOI] [PubMed] [Google Scholar]
- 32.Hebert E. Mannose-6-phosphate/insulin-like growth factor II receptor expression and tumor development. Biosci Rep. 2006;26:7–17. doi: 10.1007/s10540-006-9002-3. [DOI] [PubMed] [Google Scholar]
- 33.Fukata Y, Iwanaga T, Fukata M. Systematic screening for palmitoyl transferase activity of the DHHC protein family in mammalian cells. Methods. 2006;40:177–182. doi: 10.1016/j.ymeth.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Ohno Y, Kihara A, Sano T, Igarashi Y. Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim Biophys Acta. 2006;1761:474–483. doi: 10.1016/j.bbalip.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Dell'Angelica EC, Ohno H, Ooi CE, Rabinovich E, Roche KW, Bonifacino JS. AP-3: an adaptor-like protein complex with ubiquitous expression. EMBO J. 1997;16:917–928. doi: 10.1093/emboj/16.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frey T, De Maio A. Increased expression of CD14 in macrophages after inhibition of the cholesterol biosynthetic pathway by lovastatin. Mol Med. 2007;13:592–604. doi: 10.2119/2007-00054.Frey. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Expression and intracellular localization of the palmitoyltransferases. A–I) COS-7 cells were transfected with HA-tagged DHHC-3 (A), DHHC-5 (B), DHHC-7 (C), DHHC-9 (D), DHHC-15 (E), DHHC-17 (F), DHHC-20 (G), DHHC-21 (H), DHHC-22 (J) and stained with HA antibody.