Abstract
Aims: The purpose of this study was to determine whether animals predisposed to prefer alcohol possess an altered acute response to alcohol on a delay discounting task relative to animals predisposed to avoid alcohol. Methods: We used rats selected to prefer or avoid alcohol to assess whether genotype moderates changes in delay discounting induced by acute ethanol exposure. Selectively bred rat lines of Sardinian alcohol-preferring (sP; n = 8) and non-preferring (sNP; n = 8) rats, and alko alcohol (AA, n = 8) and alko non-alcohol (ANA, n = 8) rats were trained in an adjusting amount task to assess delay discounting. Results: There were no significant effects of line on baseline discounting; however, both lines of alcohol-preferring rats exhibit slowed reaction times. Acute ethanol (0, 0.25, 0.5 g/kg) treatment also had no effect on delay discounting in any of the selectively bred rat lines. Conclusion: Our data indicate that in these lines of animals, alcohol preference or avoidance has no impact on delay discounting following acute ethanol exposure. It is possible that other genetic models or lines may be differentially affected by alcohol and exhibit qualitatively and quantitatively different responses in delay discounting tasks.
INTRODUCTION
A plethora of data indicate that a predisposition toward alcohol use and abuse is heritable (see recent reviews by Ducci and Goldman, 2008; Gelernter and Kranzler, 2009; Khokhar et al., 2010). Animal models of alcohol abuse exhibit a number of genetically correlated traits that accompany and contribute to excessive drinking and dependence. Data from studies using human subjects indicate that heightened impulsive behavior (delay discounting) accompanies alcohol dependence (Vuchinich and Simpson, 1998; Petry, 2001; Mitchell et al., 2005; Field et al., 2007), and it is speculated that impulse control deficits might contribute to initiation and continued alcohol use. Previous studies have found that some lines of rats (Wilhelm and Mitchell, 2008) or mice (Oberlin and Grahame, 2009) with a genetic predisposition to consume alcohol are more impulsive than animals with a genetic predisposition to avoid alcohol. However, it is not known whether the acute response to alcohol on delay discounting differs between individuals predisposed to consume alcohol (family history positive) relative to those who are family history negative. We examined the effects of acute ethanol exposure on delay discounting in rats selected to prefer or avoid alcohol.
A surprisingly small number of studies have examined the impact of alcohol on delay discounting in humans and animals, and none have examined the influence of genetics. Two studies in humans have found that acute alcohol (dose up to 0.8 g/kg) had no effect on delay discounting using a standard question-based approach (Richards et al., 1999; Reynolds et al., 2004), while a third study found a non-significant trend toward reduced impulsivity (Ortner et al., 2003). Other human studies by Reynolds et al. (2004), using an experiential delay discounting task, and Dougherty et al. (2008) using the single key impulsivity paradigm found that acute ethanol treatment caused a small increase in delay discounting. Thus, data from studies using humans appear equivocal at best. Unfortunately, results in animals are also not definitive, with Evenden and Ryan (1999) reporting no effect of ethanol on delay discounting per se, but with ethanol appearing to interfere with the ability of rats to discriminate between a large and small reward so that choice of an immediate, small reward was increased even in the absence of a delay to a larger reward. Hellemans et al. (2005) found that acute ethanol increased delay discounting, but that this effect was most apparent in rats reared in an enriched environment. Finally, Poulos et al. (1995) and Olmstead et al. (2006), using a T-maze found that acute ethanol increased the choice of an immediate, but smaller alternative. The T-maze provides an assessment of the choice between a small immediate reward and a large delayed reward similar to delay discounting, but it is unclear how much of an overlap exists between decision-making in a T-maze task and delay discounting tasks. In summary, the data regarding acute effects of ethanol on impulsive behavior are mixed in humans but on balance suggest increased impulsivity in outbred animals. The purpose of this study was to determine if the effects of alcohol on impulsive behavior are enhanced in animals predisposed to consume higher levels of alcohol and blunted in animals selected to avoid alcohol. Animals were trained in an adjusting amount delay discounting paradigm and the baseline level of delay discounting was assessed. Animals were then treated with small doses of alcohol (to avoid the known sedative effects of alcohol at higher doses (Waller et al., 1986)) and the resultant effects on delay discounting were measured.
MATERIALS AND METHODS
Subjects
Selectively bred lines of Sardinian alcohol-preferring (sP; n = 8) and -non-preferring (sNP; n = 8) rats (e.g. Colombo et al., 2006), and alko alcohol (AA, n = 8) and alko, non-alcohol (ANA, n = 8) rats (e.g. Sommer et al., 2006) were generously provided by Drs Columbo, Hytiia and Lumeng as part of an international collaboration with the Indiana Alcohol Research Center. Upon receipt, sP animals weighed 513 ± 11 g, sNP animals weighed 539 ± 11 g, AA animals weighed 398 ± 13 g and ANA animals weighed 500 ± 16 g. Rats were housed in the Department of Comparative Medicine at Oregon Health & Science University an AAALAC-approved facility (Office Laboratory Animal Welfare #A3304-01). All procedures were approved by the appropriate Institutional Animal Care and Use Committee and adhered to NIH Guidelines. To facilitate training and maintain response in behavioral tasks, 3 days prior to the start of training, animals were food restricted. Unless otherwise noted, animals were maintained at 90% of their free-feeding age-adjusted body weights with supplemental chow given following each day's test session.
Apparatus
We used eight identical (Med Associates, St. Albans, VT, USA) modular rat test chambers housed individually within melamine sound-attenuating cabinets. The chambers have been described previously (Wilhelm and Mitchell, 2008). A house light was mounted in the center of a stainless steel panel, with a response clicker mounted on the outside of this panel. Three non-retractable levers were mounted on the opposing panel directly below circular lights and above recessed nose-pokes. Thus, there were left, right and center lights, levers and nose pokes. Computer-controlled pumps were programmed to deliver variable-volume sucrose reinforcers (10% w/v) to liquid cups located in the recesses of the outer nose-pokes.
Adjusting amount training
Training has been described previously (Wilhelm and Mitchell, 2008). Briefly, Phase 1 of the training exposed subjects to a progressively delayed non-contingent reward, and a FR1 schedule on the left and right levers. When animals exhibited robust lever response (at least 60 responses within 60 min on two consecutive days), they were advanced to Phase 2 of training. In Phase 2 of training, animals were required to press the middle lever to activate the outer ‘choice’ levers. In addition, one of the outer levers was designated as the delayed lever (although no delays to reinforcement were experienced during training) and delivered a fixed 150 µl of sucrose, while the other lever became the immediate lever and delivered a variable amount of sucrose (initially 75 µl). The volume of the immediate reinforcer increased by 10% following the choice of the delayed lever, and decreased by 10% following the choice of the immediate lever. Following a choice of either immediate or delayed lever, sucrose was delivered and a variable inter trial interval period ensued. The length of the inter trial interval varied to maintain a trial length at 40 s. Animals were also introduced to forced choice trials in this training phase, whereby animals that chose either the right or left lever on two consecutive trials were forced to press the opposite lever on the subsequent trial. Sucrose reinforcers were delivered to the right or left nose pokes directly below the chosen response lever in combination with an auditory stimulus (response clicker). Sessions lasted until 60 free-choice trials occurred, or 60 min had elapsed. To complete training, rats were required to respond on at least 55 of the 60 possible free-choice trials on two consecutive sessions. Two ANA rats were unable to complete the training requirements within 25 sessions and were therefore dropped from the study. The average number of days to training completion was 28 ± 2 (AA), 38 ± 5 (ANA), 18 ± 2 (sP) and 15 ± 2 (sNP). The baseline data for examination of the acute effect of ethanol on discounting occurred on average 89 ± 2 (AA), 96 ± 4 (ANA) and 86 ± 0 (sP and sNP) days after the first day of training.
Adjusting amount delay discounting task
The adjusting amount procedure was adapted from a procedure described in Richards et al. (1997). Experimental sessions were as described in Phase 2 of training, except that a response on the delayed lever resulted in delivery of a 150 μl sucrose reinforcer delayed by 0, 2, 4, 8 or 16 s. The delay remained constant within a session but varied between sessions according to a Latin square design. Each delay was experienced at least six times, and data from occasions 2 to 6 was averaged and analyzed. Typical sessions lasted 40–45 min.
Acute ethanol effects on delay discounting
The acute effects of ethanol were assessed similarly to previous analyses of acute drug effects using the adjusting amount delay discounting procedure (Kieres et al., 2004). After animals completed the baseline discounting curves at each of the 0, 2, 4, 8 and 16 s delays described above, the delay was fixed at 4 s and 13 additional delay discounting sessions were run. This delay was chosen because it was intermediate between the 0 and 16 s indifference points, and therefore was least likely to suffer from floor or ceiling effects (Wade et al., 2000). After the single delay condition, animals were injected intraperitoneal with 0.0 (saline), 0.25 or 0.5 g/kg ethanol from a stock solution of 20% ethanol in saline on Tuesdays and Fridays of each week according to a Latin Square design and immediately placed in the experimental chambers. Discounting sessions began immediately following injections. Injections were administered in a volume of 1.6 and 3.2 ml/kg for 0.25, or 0.5 g/kg ethanol, respectively. Saline injections were administered based on the equivalent volume at the 0.5 g/kg dose. These doses were chosen based on the study done by Waller et al. (1986), which showed increases in locomotor activity following a dose of 0.25 g/kg in P rats, but not nP rats. Each dose was administered on three separate occasions. Approximately 2 weeks after completion of the sucrose preference test (described below), all animals received an injection of 0.5 g/kg ethanol. Blood samples were acquired 20 min after injection via the medial saphenous vein and the blood ethanol concentration (BEC) was determined using a gas chromatography method described previously (Rustay and Crabbe, 2004). BECs were analyzed at the 20 min time point because this is roughly the mid-point of the delay discounting session, which also roughly corresponds to the point at which animals reach their indifference point during delay discounting sessions. Some of the samples (AA n = 2, ANA n = 0, sP n = 2, sNP n = 1) contained an insufficient volume of blood to effectively assess the BEC. These samples were excluded from the analysis.
Two-bottle sucrose preference
The purpose of this experiment was to determine if any of the lines differed in consumption or preference of sucrose when offered a choice between sucrose solution and water. Differences in sucrose consumption or preference might be critical to the interpretation of the discounting data. Accordingly, subjects were maintained under food restriction as described for the discounting procedure.
On five consecutive test days, each rat was placed in a rat drinking cage for 50 min (the approximate length of a discounting session) and given access to two bottles; one containing 10% sucrose solution and the other containing water. To eliminate the potential for side bias, placement of sucrose and water bottles was alternated daily. The amount of solution consumed was determined by weighing the bottles before and after each test session. The average amount of sucrose consumed per 50 min test session was calculated in grams of sucrose/kg body weight. In addition, sucrose preference was calculated as the ratio of the amount of sucrose consumed to the total amount of fluid (water plus sucrose) consumed. Analyses were conducted on the amount of sucrose consumed and the preference ratio averaged over the final 4 sessions of testing. One ANA rat did not complete the sucrose drinking portion of the experiment due to experimenter error, and one sP rat died during the course of the study for reasons unrelated to the experiment.
Data analysis
The main dependent variable from the delay discounting task was the amount of sucrose solution delivered from the immediate alternative at the ‘indifference point’, i.e. the volume at which the immediate and delayed alternatives were selected equally often. Based on previous findings (Richards et al., 1997), rats reach the indifference point after the first 30 trials of a session, thus the median volume of sucrose associated with the immediate lever over trials 31–60 was used as an index of the subjective value of the alternate choice. Animals chose each lever with roughly equal frequency over this period (percent choice of the immediate lever over the final 30 trials: AA rats: 46 ± 1%, ANA rats: 45 ± 2%, sP rats: 47 ± 1%, sNP rats: 48 ± 1%). The median, rather than the mean, was used as a measure of central tendency because changes in the adjusting amount on successive trials were proportions of the amount on the prior trial, resulting in a skewed distribution. Hyperbolic equations were fitted to each animal's average indifference points (modified from Mazur, 1987) using Microsoft Excel 2007 (Microsoft Corp., Redmond, WA, USA):
| (1) |
where V represents the value of the adjusting reward at indifference in μl; A represents the amount of sucrose solution from the delayed alternative (150 μl); X represents the delay to receiving the reinforcer (0, 2, 4, 8 or 16 s) and the bias parameter, b, is indicative of bias, or side preference in the absence of delay (0 s delay condition) and calculated by dividing each subject's indifference point at the 0 s delay condition by 150 µl. The discount parameter (k) is a fitted parameter, and indexes the rate of discounting or overall sensitivity to delayed reinforcers. Larger values of k indicate steeper discount functions, and stronger aversion to delayed reinforcers.
For all comparisons, the AA and ANA lines were analyzed independently of the sP and sNP lines. Analysis of variance (ANOVA) and other statistical tests were carried out using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). Huynh–Feldt corrections were applied as necessary, and adjusted degrees of freedom are provided.
ANOVAs were used to examine indifference points, with LINE as a between-subject factor and DELAY as a within-subject factor. Similar ANOVAs with the additional inclusion of CHOICE as a within-subject factor were used to examine reaction times (RT; the time from the start of a trial until animal's pressed the middle lever) and choice reaction times (CHRT; the time from middle lever press until one of the outer choice levers was pressed). A t-test was used to examine line differences in k values, which were not markedly skewed in these samples, and b values. To examine ethanol effects on delay discounting, indifference points were subjected to an ANOVA with DOSE as a within- subject factor and LINE as a between-subject factor. A similar analysis was carried out on RTs and CHRTs.
RESULTS
Delay discounting in AA/ANA rats
Both AA and ANA lines exhibited a significant decrease in preference for the delayed alternative as a function of increasing delay [Fig. 1A; F(3.6, 43.4) = 65.6, P < 0.001], but there were no line [F(1, 12) = 0.45, P > 0.05] or line × delay interactions [F(3.6, 43.4) = 0.82, P > 0.05]. There were also no line differences in k values [F(1, 12) = 1.00, P > 0.05], b values [F(1, 12) = 2.56, P > 0.05] or the fit of the hyperbolic discounting function [Equation (1)] [R2 values; F(1, 12) = 2.54, P > 0.05].
Fig. 1.
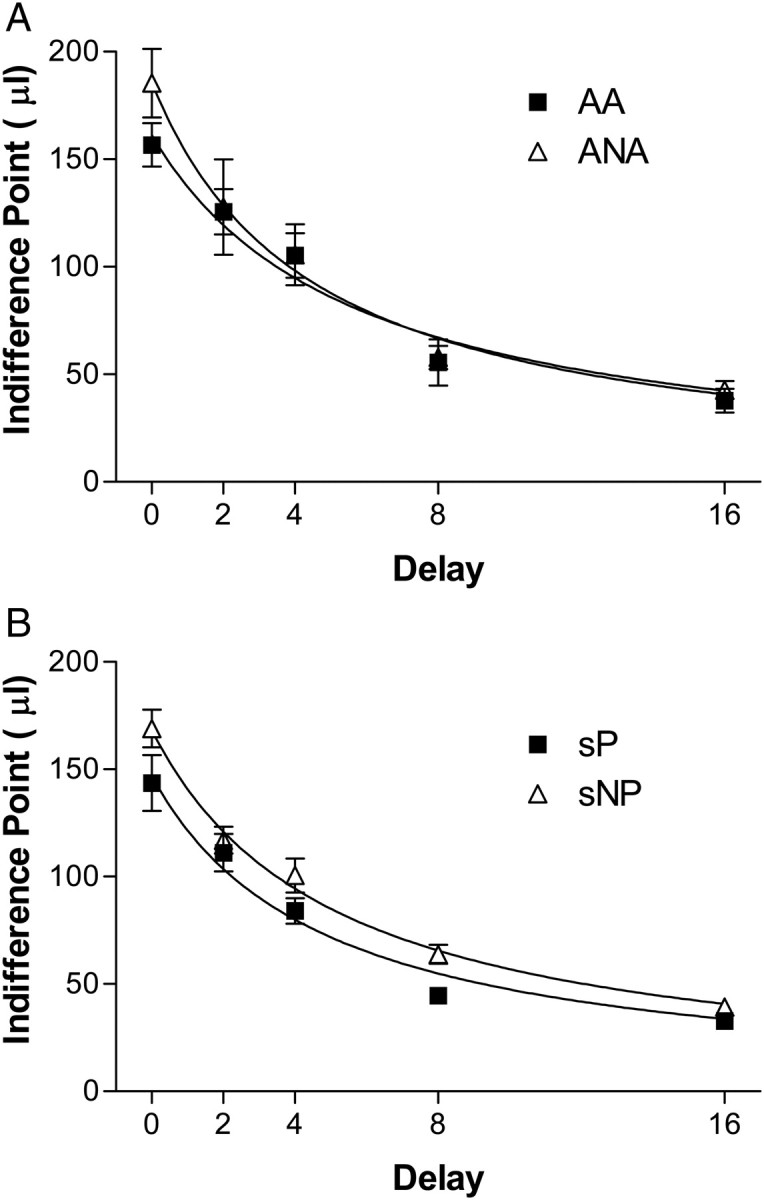
Baseline delay discounting in alcohol preferring and alcohol avoiding rat lines. Mean ± SEM indifference point for each line at each delay tested (0, 2, 4, 8 and 16 s). Data for AA and ANA rats are shown in the top (A) graph and for sP and sNP rats in the bottom (B) graph. AA n = 8, ANA n = 6, sP n = 8, sNP n = 8.
Delay discounting in sP/sNP rats
Both sP and sNP lines exhibited a significant decrease in preference for the delayed alternative as a function of increasing delay [Fig. 1B; F(3.6, 50.4) = 145.3, P < 0.001]. There were no line × delay interactions [F(3.6, 50.4) = 1.13, P > 0.05]. There was a trend for a significant line effect [F(1, 14) = 4.29, P = 0.06], with sP animals having lower indifference points than sNP animals across all delays. Lower indifference points across all delays would imply differences in bias, not sensitivity to delay. There were no line differences in k values [F(1, 14) = 0.01, P > 0.05], b values [F(1, 14) = 2.63, P > 0.05] or in the fit of the hyperbolic discounting function [Equation (1)] to the data [R2 values; F(1, 14) = 0.01, P > 0.05].
Effects of ethanol on delay discounting
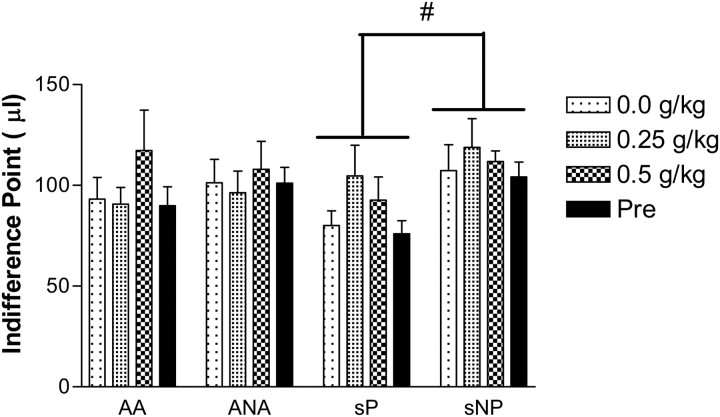
For the AA and ANA rats, an ANOVA with DOSE and LINE as factors was carried out on indifference points derived from the ethanol treatment phase of the experiment (Fig. 2). There were no significant effects or interactions for DOSE or LINE (all F's <1.72, all P's >0.05) on indifference points. BECs acquired 20 min after treatment with 0.5 g/kg ethanol showed no significant between group differences (P > 0.05; AA rats BEC = 45 ± 4 mg/dl, ANA rats BEC = 48 ± 6 mg/dl).
Fig. 2.
Effects of alcohol on delay discounting. Mean ± SEM indifference point for saline, each dose of ethanol and no injection. #P < 0.05.
For the indifference points generated by the sP and sNP rats, there was a main effect of LINE [F(1, 13) = 5.65, P < 0.05], with sP rats exhibiting lower indifference points than sNP rats (Fig. 2). This is consistent with the trend observed at baseline. There were no other significant main effects or interactions (all F's < 1.67, all P's > 0.05). Following a 20 min treatment with 0.5 g/kg ethanol, sP rats had a BEC of 30 ± 5 mg/dl, while sNP rats had a BEC of 24 ± 2 mg/dl, with no significant between group difference (P > 0.05).
Response times (baseline discounting)
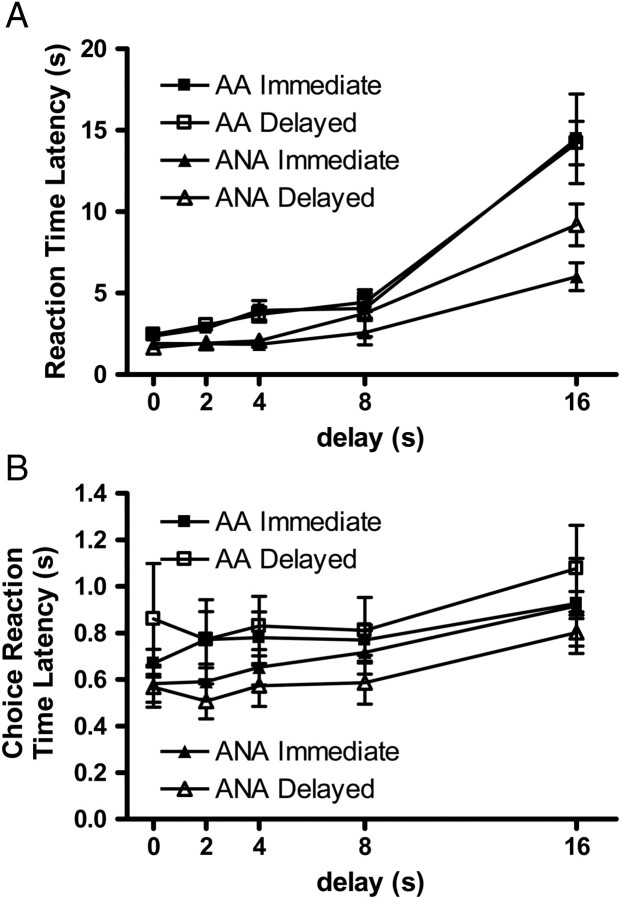
For the AA/ANA rats during baseline testing (Fig. 3), there were significant DELAY [F(2.1, 25.0) = 60.34, P < 0.001], LINE [F(1, 12) = 13.59, P < 0.01] and DELAY × LINE interaction [F(2.1, 25.0) = 7.06, P < 0.01] effects on RTs (the time from trial initiation until middle lever press). Follow-up analyses indicated that animals were slower to initiate trials as delays increased, with the bulk of the effect occurring at the 16 s time point. Overall, ANA rats were faster to respond than AA rats (average latencies: AA rats = 5.55 ± 0.59 s, ANA rats = 3.28 ± 0.37 s; excluding latencies at the 8 and 16 s delays: AA rats = 3.05 ± 0.17 s, ANA rats = 1.89 ± 0.11 s). There were also DELAY effects on CHRTs (the time from middle lever press until either of the outer levers is pressed) [F(4, 48) = 4.91, P < 0.01]. Once again, rats exhibited slower CHRTs as the delay in reinforcing receipt increased. In general, ANA rats had faster CHRTs than AA rats, however, this trend did not reach statistical significance [F(1, 12) = 3.32, P = 0.09].
Fig. 3.
Baseline reaction time (A) and choice reaction times (B) for AA and ANA rats. Mean ± SEM reaction time or choice reaction time at each delay tested.
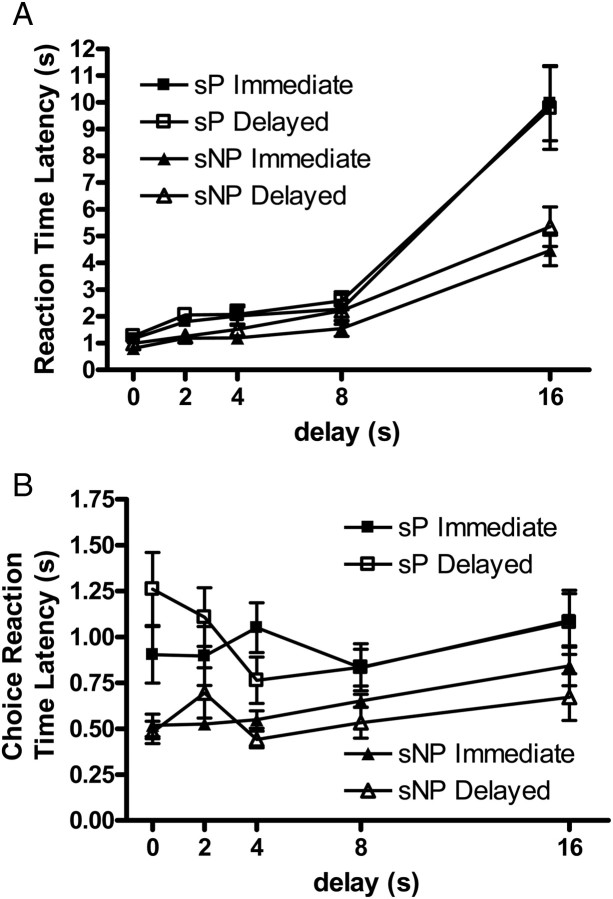
For sP and sNP rats (Fig. 4), there were DELAY [F(1.4, 19.6) = 59.58, P < 0.001], CHOICE [F(1, 14) = 5.81, P < 0.05], LINE [F(1, 14) = 9.47, P < 0.01] and DELAY × LINE [F(1.4, 19.6) = 8.52, P < 0.01] effects on RTs. RTs increased as the delayed outcome became more distant in time. In contrast to the AA and ANA rats, who showed no choice effects, sP and sNP rats were quicker to initiate trials when the immediate alternative was subsequently chosen. The sNP rats were faster to respond than sP rats (average latencies: sP rats = 3.50 ± 0.42 s, sNP rats = 2.05 ± 0.20 s; excluding latencies at the 8 and 16 s delays: sP rats = 1.73 ± 0.11 s, sNP rats = 1.16 ± 0.06 s). The DELAY × LINE interaction is the result of a large increase in RTs for the sP rats under the longest delay condition relative to a much smaller increase in RTs for the sNP rats. CHRTs also became slower as a function of DELAY [F(3.1, 43.7) = 4.32, P < 0.01], with sNP rats having quicker CHRTs than sP rats [F(1, 14) = 9.10, P < 0.01]. There was also a DELAY × CHOICE interaction [F(2.9, 40.3) = 5.47, P < 0.01], however, this effect was not systematic and will not be discussed further.
Fig. 4.
Baseline reaction time (A) and choice reaction times (B) for sP and sNP rats. Mean ± SEM reaction time or choice reaction time at each delay tested.
Effects of ethanol on response times
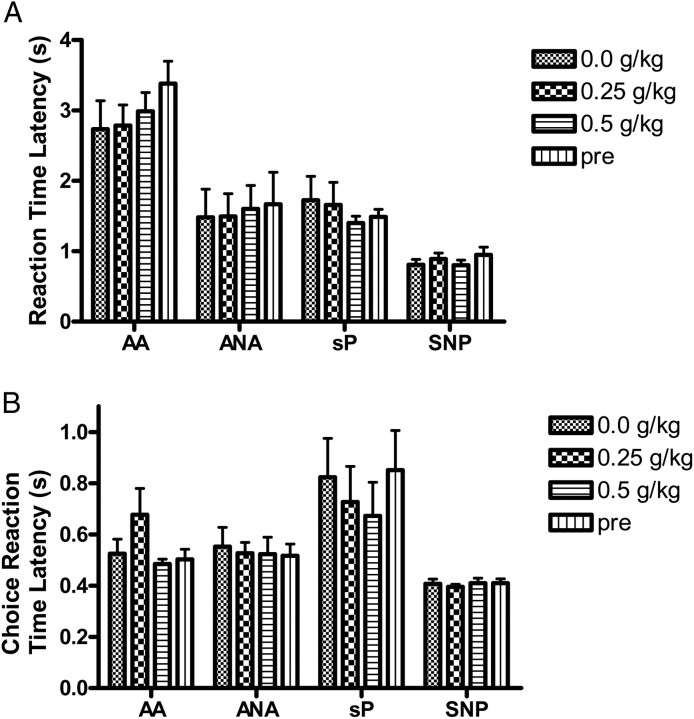
An ANOVA with DOSE and CHOICE as within subject factors and LINE as a between subject factor was carried out on RTs (Fig. 5A) and CHRTs (Fig. 5B) during the ethanol treatment portion of the experiment. ANA rats had shorter RTs than AA rats [F(1, 12) = 9.48, P < 0.05]. There were no other main effects or interactions on RTs or CHRTs, nor were there any effects associated with ethanol administration.
Fig. 5.
Effects of alcohol on reaction time (A) and choice reaction time (B). Mean ± SEM reaction time for saline, each dose of ethanol and no injection.
The sNP rats had significantly faster RTs [F(1, 14) = 11.78, P < 0.01 (Fig. 5A)] and CHRTs [F(1, 14) = 6.11, P < 0.05 (Fig. 5B)] during the ethanol treatment phase of the experiment than sP rats. No other main effects or interactions were present for either measure, nor were there any effects of ethanol administration.
Two-bottle sucrose preference
AA rats drank more sucrose than ANA rats [F(1, 11) = 5.91, P < 0.05; AA rats 122 ± 10 g/kg, ANA rats 94 ± 6 g/kg per 50 min session]. There were no other significant effects. Both lines exhibited a strong preference for the bottle containing 10% sucrose (AA rats preference = 95 ± 1%; ANA rats preference = 94 ± 1%) with no effect of line indicated [F(1, 11) = 1.82, P > 0.05].
For the sP and sNP rats, there were no significant effects or interactions. Both lines consumed similar amounts of sucrose (sP rats 138 ± 13 g/kg, sNP rats 141 ± 10 g/kg per 50 min session) and both lines exhibited a strong preference for the bottle containing 10% sucrose (sP rats preference = 96 ± 1%; sNP rats preference = 97 ± 1%) with no effect of line indicated [F(1, 11) = 1.86, P > 0.05].
DISCUSSION
The goal of this study was to determine if a genetic predisposition to prefer or avoid alcohol impacts the effect of acute ethanol treatment on delay discounting. We found no line differences in the effects of ethanol on delay discounting, which is consistent with other studies in humans (Richards et al., 1999; Ortner et al., 2003; but see also Reynolds et al., 2004) and animals (Evenden and Ryan, 1999; but see also Hellemans et al., 2005). Nevertheless, it is possible that increased power, different ethanol doses or other animal lines may yield significant effects of ethanol. The BECs we achieved in this study were moderate, however, our ethanol doses were chosen to prevent the strong sedative effects of alcohol observed above 0.5 g/kg in rats (e.g. Waller et al., 1986; unpublished observation). Our RT data support this, and indeed indicate no locomotor slowing following either ethanol dose. Interestingly, both alcohol-preferring lines had slower RTs compared with non-preferring lines, though a similar trend was not observed in a different group of selectively bred rats (Wilhelm and Mitchell, 2008). Consistent with this, children of alcoholics exhibit slower RTs in a computer-based delay discounting task (Herting et al., 2010). Similarly, using a magnetic resonance imaging-based delay discounting task, methamphetamine abusers (abstinent for 2–8 weeks) took 15% longer to respond than control subjects (Hoffman et al., 2008). It should be noted that the original study developing the adjusting amount delay discounting task found that motivation (level of water restriction) could impact RTs in this task (Richards et al., 1997). Nevertheless, the hypothesis that individuals that abuse or are prone toward abuse are slower to make difficult decisions may provide a useful tool toward identifying those individuals with the highest susceptibility of becoming alcohol or drug-dependent. Furthermore, increased difficulty with hard choices may influence other important addiction traits such as initiation and relapse.
The AA/ANA rats had BECs that were almost double the concentrations in the sP/sNP when measured 20 min after injection of a 0.5 g/kg dose. This suggests a difference in ethanol metabolism (possibly attributable in part to differences in first-pass metabolism). The maximum ethanol dose (0.5 g/kg) used was based on research with the Indiana P/NP lines (Waller et al., 1986) indicating that it was a dose that did not cause motor impairment; it is not known if a similar dose-effect is relevant in the selected lines used in the present study. This may be particularly true for the sP/sNP lines that had the lowest BEC. Therefore, we cannot rule out the possibility that a higher dose of ethanol could have effects on delay discounting in these animals.
Despite previous studies indicating significant between-group differences (Wilhelm and Mitchell, 2008; Oberlin and Grahame, 2009) in baseline levels of impulsivity between animals bred based on alcohol preference, we did not find any significant differences in delay discounting between alcohol preferring and non-preferring lines as measured by k values. Statistical analysis of baseline data also did not find significant between line differences in indifference point. However, during the ethanol treatment regimen, sP animals were found to discount the 4 s delay more so than sNP animals. This pattern was consistent with the indifference points derived from the baseline discounting sessions and appeared to be delay independent, i.e. sP rats appear to have a bias toward the immediate lever for all of the delays examined. This pattern was not mirrored in the AA and ANA lines. The concordance of behavioral differences between alcohol-preferring and non-preferring animals is not always consistent between different lines of selectively bred animals (Murphy et al., 2002). Thus, the genes that drive alcohol consumption or avoidance are likely to differ to varying degrees between selectively bred lines, with some genes contributing more strongly to the alcohol consumption phenotype than others. The gene or genes that modulate delay discounting may not be critical to high- or low-alcohol consumption, but instead are likely to be one of many factors that contribute to these phenotypes (Crabbe, 2008). Alternatively, other phenotypes associated with alcohol abuse may be more strongly associated with impulsive responding, such as intensity of cravings and sensitivity to alcohol reward.
Funding
This study was supported by grant AA017035 (C.J.W.), DA016727 (S.H.M.).
Acknowledgements
We would like to thank the NIAAA for an Alcohol Research Resource Award Supplement (R24 AA015512-03) for providing us with the AA/ANA and sP/sNP animals used in this study and Katie Curtis for her assistance with data collection and analysis.
REFERENCES
- Colombo G, Lobina C, Carai MA, et al. Phenotypic characterization of genetically selected Sardinian alcohol-preferring (sP) and –non-preferring (sNP) rats. Addict Biol. 2006;11:324–8. doi: 10.1111/j.1369-1600.2006.00031.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Review. Neurogenetic studies of alcohol addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3201–11. doi: 10.1098/rstb.2008.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Marsh-Richard DM, Hatzis ES, et al. A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug Alcohol Depend. 2008;96:111–20. doi: 10.1016/j.drugalcdep.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Goldman D. Genetic approaches to addiction: genes and alcohol. Addiction. 2008;103:1414–28. doi: 10.1111/j.1360-0443.2008.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behavior in rats VI: the effects of ethanol and selective serotonergic drugs on response choice with varying delays of a reinforcement. Psychopharmacology. 1999;146:413–21. doi: 10.1007/pl00005486. [DOI] [PubMed] [Google Scholar]
- Field M, Christiansen P, Cole J, et al. Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction. 2007;102:579–86. doi: 10.1111/j.1360-0443.2007.01743.x. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR. Genetics of alcohol dependence. Hum Genet. 2009;126:91–9. doi: 10.1007/s00439-009-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Nobrega JN, Olmstead MC. Early environmental experience alters baseline and ethanol-induced cognitive impulsivity: relationship to forebrain 5-HT(1A) receptor binding. Behav Brain Res. 2005;159:207–20. doi: 10.1016/j.bbr.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Herting MM, Schwartz D, Mitchell SH, et al. Delay discounting behavior and white matter microstructure abnormalities in youth with a family history of alcoholism. Alcohol Clin Exp Res. 2010;34:1590–602. doi: 10.1111/j.1530-0277.2010.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WF, Schwartz DL, Huckans MS, et al. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology. 2008;201:183–93. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhar JY, Ferguson CS, Zhu AZ, et al. Pharmacogenetics of drug dependence: role of gene variations in susceptibility and treatment. Annu Rev Pharmacol Toxicol. 2010;50:39–61. doi: 10.1146/annurev.pharmtox.010909.105826. [DOI] [PubMed] [Google Scholar]
- Kieres AK, Hausknecht KA, Farrar AM, et al. Effects of morphine and naltrexone on impulsive decision making in rats. Psychopharmacology. 2004;173:167–74. doi: 10.1007/s00213-003-1697-2. [DOI] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H., editors. Quantitative Analysis of Behavior: The Effects of Delay and of Intervening Events on Reinforcement Value. Vol. 5. Hillsdale, NJ,: Lawrence Erlbaum Associates; 1987. pp. 55–73. [Google Scholar]
- Mitchell JM, Fields HL, D'Esposito M, et al. Impulsive responding in alcoholics. Alcohol Clin Exp Res. 2005;29:2158–69. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol dependence. Behav Genet. 2002;32:363–88. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol Clin Exp Res. 2009;33:1294–303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Hellemans KG, Paine TA. Alcohol-induced impulsivity in rats: an effect of cue salience? Psychopharmacology. 2006;184:221–8. doi: 10.1007/s00213-005-0215-0. [DOI] [PubMed] [Google Scholar]
- Ortner CN, MacDonald TK, Olmstead MC. Alcohol intoxication reduces impulsivity in the delay-discounting paradigm. Alcohol Alcohol. 2003;38:151–6. doi: 10.1093/alcalc/agg041. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–50. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6:810–4. [PubMed] [Google Scholar]
- Reynolds B, Richards JB, de Wit H. Acute-alcohol effects on the experiential discounting task (EDT) and a question-based measure of delay discounting. Pharmacol Biochem Behav. 2004;83:194–202. doi: 10.1016/j.pbb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Richards JB, Mitchell SH, de Wit H, et al. Determination of discount functions in rats with an adjusting amount procedure. J Exp Anal Behav. 1997;67:353–66. doi: 10.1901/jeab.1997.67-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, et al. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71:121–43. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustay NR, Crabbe JC. Genetic analysis of rapid tolerance to ethanol's incoordinating effects in mice: inbred strains and artificial selection. Behav Genet. 2004;34:441–51. doi: 10.1023/B:BEGE.0000023649.60539.dd. [DOI] [PubMed] [Google Scholar]
- Sommer W, Hyytia P, Kiianmaa K. The alcohol-preferring AA and alcohol-avoiding ANA rats: neurobiology of the regulation of alcohol drinking. Addict Biol. 2006;11:289–309. doi: 10.1111/j.1369-1600.2006.00037.x. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharmacol. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharm. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Waller MB, Murphy JM, McBride WJ, et al. Effect of low dose ethanol on spontaneous motor activity in alcohol-preferring and –nonpreferring lines of rats. Pharmacol Biochem Behav. 1986;24:617–23. doi: 10.1016/0091-3057(86)90567-8. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes Brain Behav. 2008;7:705–13. doi: 10.1111/j.1601-183X.2008.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]