Abstract
Beta-amyloid (Aβ) is a histopathological hallmark of Alzheimer’s disease dementia, but high levels of Aβ in the brain can also be found in a substantial proportion of nondemented subjects. Here we investigated which 2-year rate of brain and cognitive changes are present in nondemented subjects with high and low Aβ levels, as assessed with cerebrospinal fluid and molecular positron emission tomography (PET)–based biomarkers of Aβ. In subjects with mild cognitive impairment, increased brain Aβ levels were associated with significantly faster cognitive decline, progression of gray matter atrophy within temporal and parietal brain regions, and a trend for a faster decline in parietal Fludeoxyglucose (FDG)-PET metabolism. Changes in gray matter and FDG-PET mediated the association between Aβ and cognitive decline. In contrast, elderly cognitively healthy controls (HC) with high Aβ levels showed only a faster medial temporal lobe and precuneus volume decline compared with HC with low Aβ. In conclusion, the current results suggest not only that both functional and volumetric brain changes are associated with high Aβ years before the onset of dementia but also that HC with substantial Aβ levels show higher Aβ pathology resistance, lack other pathologies that condition neurotoxic effects of Aβ, or accumulated Aβ for a shorter time period.
Keywords: Aβ, FDG-PET, MCI, PIB-PET
Introduction
Alzheimer’s disease (AD) dementia is a syndrome that can be caused by AD. A central role in the development of pathological events in the course of AD dementia has been attributed to the increased production and deposition of beta-amyloid (Aβ1–42). According to the amyloid cascade hypothesis, increased levels of Aβ1–42 in the brain resulting from a disturbed balance between production and clearance of brain Aβ is one of the earliest pathological developments of AD, entailing neurotoxic processes that eventually lead to neuronal dysfunction and cognitive decline (Hardy and Selkoe 2002). Postmortem studies have shown that AD pathologies including Aβ and neurofibrillary changes are already present to a substantial amount in subjects who are cognitively normal or have mild cognitive impairment (MCI) (Braak and Braak 1991; Bennett et al. 2005). Results from studies using the Aβ-binding tracer “Pittsburgh Compound-B” positron emission tomography (PIB-PET) (Klunk et al. 2004) or cerebral spinal fluid (CSF) measure of Aβ in living subjects suggest that about 10 – 50% of elderly non-demented subjects show substantial Aβ levels that are comparable to those found in patients with AD dementia (Mintun et al. 2006; Aizenstein et al. 2008; Jagust et al. 2009; Shaw et al. 2009). It is a major remaining question whether the abnormal Aβ levels as measured by such biochemical and neuroimaging based biomarkers are associated with faster decline in cognition and brain volume and function in nondemented elderly subjects.
A growing number of studies have investigated Aβ-related brain and cognitive changes at the predementia stage. In MCI, both global PIB-PET binding and CSF Aβ1–42 concentration predicted global cognition and episodic memory (Hansson et al. 2006; Pike et al. 2007; Forsberg et al. 2008, 2010; Shaw et al. 2009; for review, see Ewers et al. 2011). In the presymptomatic phase, even though a substantial portion of elderly healthy control (HC) subjects showed high levels of Aβ in the brain, an association between brain Aβ and cognitive performance could not be consistently established across studies. Results from cross-sectional studies in HC subjects suggest an association between lower baseline CSF Aβ levels (i.e., higher brain Aβ levels) and lower cognitive performance (Stomrud et al. 2010; Rolstad et al. 2011). Some longitudinal studies reported a faster decline in association with elevated brain levels of Aβ as measured by PIB-PET or CSF (Gustafson et al. 2007; Pike et al. 2007; Stomrud et al. 2007, 2009; Villemagne et al. 2008; Mormino et al. 2009; Rentz et al. 2010; Resnic et al. 2010). However, among these studies, presence of such an association was dependent upon specific cognitive domains (Rolstad et al. 2011), presence of subjective memory impairment (Rami et al. 2011), low cognitive reserve (Rentz et al. 2010), or significant cognitive decline (Villemagne et al. 2008), or could not be detected (Chételat et al. 2010). In one study, the association between CSF Aβ and clinical progression could only be demonstrated when Aβ was expressed in proportion to CSF tau biomarkers (Fagan et al. 2007).
A recent study in MCI found that an association between PIB-PET and episodic memory impairment was mediated by hippocampus atrophy (Mormino et al. 2009). Thus, a first step in predicting cognitive decline in nondemented subjects may be to assess the extent of structural and functional brain decline in association with Aβ pathology.
In AD, studies showed an association between global PIB-PET values and brain atrophy (Archer et al. 2006), especially within the hippocampus (Frisoni et al. 2009). Consistent with those cross-sectional findings in AD, increased brain Aβ levels in subjects with MCI were found to be associated with reduced hippocampus volume (Schuff et al. 2009) and increased ventricular expansion (Jack, Lowe, et al. 2008). In elderly HC subjects, CSF or PIB-PET-based measures of Aβ were not found to be associated with hippocampus volume or ventricular expansion (Jack et al. 2009; Schuff et al. 2009; Apostolova et al. 2010; Driscoll et al. 2010). However, a recent 1-year longitudinal study detected gray matter volume decline in several brain areas in HC when restricting the sample to those with abnormally high levels of global brain PIB-PET (Fjell et al. 2010). That study left the question open whether abnormally high brain Aβ levels are associated with abnormally faster gray matter decline when compared with subjects with low brain levels of Aβ. Results from Schott et al. (2010) demonstrated that subjects with abnormally reduced CSF-Aβ showed faster decline in global gray matter and hippocampus volume. However, whether abnormally higher levels of brain Aβ in nondemented subjects are associated with pathologically reduced regional gray matter throughout multiple key brain regions typically afflicted in patients with AD dementia is currently unclear. Furthermore, a functional neuroimaging study including Fludeoxyglucose (FDG)-PET showed that although PIB-PET levels were correlated with brain activity, abnormally high PIB-PET was not associated with abnormal FDG-PET metabolism in MCI (Cohen et al. 2009). So far, no study has yet established whether abnormally high levels of Aβ are associated with both abnormally faster decline in functional and structural brain changes in HC and MCI and whether such brain changes are predictive of cognitive decline in both HC and MCI.
Here, we assessed in HC and MCI subjects the association between PIB-PET or CSF assessed Aβ-levels on the one hand and the 2-year rate of change in cognition, regional gray matter volume, and brain metabolism on the other hand. We first established abnormal Aβ-levels on the basis of a bimodal frequency distribution of global PIB-PET levels, dichotomizing subjects into groups of low and high Aβ-levels. The major aims of the study were to test whether 1) abnormal high levels of brain Aβ are associated with faster decline in global cognition, episodic memory, region of interest (ROI) assessed gray matter volume, and FDG-PET brain metabolism in HC and MCI subjects; 2) global PIB-PET score predict clinical progression from MCI to AD; and 3) any Aβ-related differences in the rate of cognitive change are mediated by regional structural or functional brain changes.
Materials and Methods
Subjects
The study included 465 subjects of which 124 were elderly cognitively HC subjects, 229 subjects were diagnosed with amnestic MCI and 112 subjects had probable AD, recruited within the North American multicenter Alzheimer’s Disease Neuroimaging Initiative (ADNI, for database, see www.loni.ucla.edu/ADNI). ADNI was launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration, private pharmaceutical companies, and nonprofit organizations, as a $60 million, 5-year public-private partnership. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early Alzheimer's disease (AD). The initial goal of ADNI was to recruit 800 adults, ages 55 to 90, to participate in the research—approximately 200 cognitively normal older individuals to be followed for 3 years, 400 people with MCI to be followed for 3 years, and 200 people with early AD to be followed for 2 years. For up-to-date information, see www.adni-info.org. The current sample was restricted to those subjects who had either a PIB-PET assessment or a CSF-Aβ1–42 measurement. Within this subset, PIB-PET was available in 103 subjects including 19 HC, 65 MCI, and 19 AD subjects. The CSF-Aβ1–42 concentration was assessed in a total of 116 HC, 199 MCI, and 102 AD subjects (see Fig. 1 for further information on subjects and data inclusion). Within 55 subjects, both CSF Ab1--42 and PIB-PET were assessed. The observation interval covered 2 years, where neuropsychological assessment, FDG-PET scanning, and MRI acquisition was conducted at baseline, 6, 12, and 24 month. All collected data are freely accessible online to researchers (http://www.loni.ucla.edu/ADNI). General inclusion criteria included an age between 55 and 90 years, a modified Hachinski score ≤4, education of at least 6 grade level, and stable treatment of at least 4 weeks in case of treatment with permitted medication (for full list, see http://www.adni-info.org, Procedures Manual). The diagnosis of AD was made according to the NINCDS-ADRDA criteria (McKhann et al. 1984). Inclusion criteria for AD encompassed subjective memory complaint, memory impairment as assessed by an education adjusted score on delayed recall of a single paragraph recall from the Wechsler Logical Memory II Subscale as follows: 0–7 years of education, ≤2; for 8–15 years, ≤4; for 16 years or more, ≤8, a Mini Mental State Exam (MMSE) score between 20 and 26, and a clinical dementia rating (CDR) score of 0.5 or 1. For the diagnosis of amnestic MCI, the subjects had to show subjective memory impairment and objective memory impairment identical to that for AD, a CDR of 0.5 including the memory box score of 0.5 or greater, and a MMSE score between 24 and 30, with unimpaired general cognitive ability and functional performance such that they did not meet criteria for dementia. HC had to show normal performance on the Logical Memory II Subscale adjusted for education as follows: 0–7 years, ≥3, 8–15 years, ≥5; 16 or more years, ≥9, and absence of significant impairment on cognitive function or activities of daily living (Ewers et al. 2010).
Figure 1.
Flow chart of subjects included in the current study.
CSF Measurement
All CSF samples collected at the different centers were shipped on dry ice to the Penn ADNI Biomarker Core Laboratory at the University of Pennsylvania, Philadelphia, for storage at −80°C until further analysis at the laboratory. More details on data collection of the CSF samples can be found at http://www.adni-info.org, under “ADNI study procedures.” The CSF concentration of Aβ1–42, t-tau, and p-tau181 were measured in the baseline CSF samples using the multiplex xMAP Luminex platform (Lumnix Corp, Austin, TX) at the Penn ADNI Biomarker Core Laboratory. For detailed description, see Shaw et al. (2009).
PIB-PET, FDG-PET, MRI Acquisition, and ROI Measurement
All MRI data were acquired on 1.5-T MRI scanners using a volumetric T1-weighted sequences to map brain structures, optimized for the different scanners as indicated at http://www.loni.ucla.edu/ADNI/Research/Cores/index (Jack, Bernstein, et al. 2008). Freesurfer software version 4.5 (Dale et al. 1999; Fischl et al. 1999) was employed to measure longitudinal changes in regional brain volumes. Briefly, the image-processing pipeline using FreeSurfer consisted of five stages: an affine registration with Talairach space, an initial volumetric labeling, bias field correction, nonlinear alignment to the Talairach space, and a final labeling of the volume. The fully automated labeling of volumes is achieved by warping a population based brain atlas to the target brain and by maximizing an a posteriori probability of the labels given specific constraints. A full description of the FreeSurfer processing steps can be found in (Fischl et al. 2002). The procedures have been extensively validated.
MRI-volume ROIs were selected based on the previous meta-analyses on MRI gray matter volume measures that were most predictive of AD, including the hippocampus, middle temporal gyrus, superior temporal gyrus, amygdala, parahippocampus, entorhinal cortex, inferior parietal lobe, precuneus, and thalamus (Schroeter et al. 2009).
PET data were acquired on multiple instruments of varying resolution. PIB scans were collected as 4 × 5 min frames beginning 50 min after injection of tracer. FDG scans were collected as 6 × 5 min frames beginning 30 min after injection of approximately 5 mCi of tracer. Attenuation correction was performed either via transmission scan or computer tomography. Images were uploaded to the Laboratory of Neuroimaging where they were processed to provide standard orientation, voxel size, and resolution.
FDG-PET ROIs were constructed based on a meta-analysis of the location of FDG-PET changes in the brain that are typically affected in AD as described previously (Jagust et al. 2009; Landau et al. 2009). FDG uptake was normalized to a reference region composed of the pons and cerebellum and measured in the target ROIs that included bilateral angular gyrus, posterior cingulate/precuneus, and inferior temporal cortex as described previously (Jagust et al. 2009). PIB-PET uptake was normalized to the cerebellum to generate maps of the PIB-PET score used for further statistical analysis. Target ROIs were drawn on a structural MRI template from a single 79-year-old MCI subject scanned at the University of Pittsburgh. This image was deemed an “average” older subject with typical atrophy and ventricular size. Each subject’s PIB-PET score map was coregistered to the individual MRI with SPM5 that was normalized to the MCI template with SPM5 and permitted the transformation of the subject’s PIB-PET to the template space. ROIs in which PIB uptake is known to predominate were averaged in left and right hemispheres and comprised of prefrontal, lateral temporal, anterior cingulate gyrus, parietal and posterior cingulate/precuneus. Further information is available at the ADNI webpage (http://www.loni.ucla.edu/ADNI/).
Neuropsychological Tests
Global cognitive ability was assessed with the neuropsychological test battery Alzheimer's Disease Assessment Scale—cognitive section (ADAS-cog) (Rosen et al. 1984). The ADAS-cog score is the total score on a number of tests on learning and memory, language production, language comprehension, constructional praxis, ideational praxis, and orientation (see ADNI procedures manual for details at http://www.adni-info.org/Scientists/ProceduresManuals.aspx). A higher score on ADAS-cog scores indicates lower cognitive performance.
Episodic memory was assessed with the Rey Auditory Verbal Learning Test (RAVLT), using the score on the 30-min delayed recall of a list of 15 words that had been repeatedly presented and recalled during the learning phase of 5 verbal presentations of the list (Rey 1964). The test score corresponds to the number of words recalled on the 30-min delayed test. For details on the administration and scoring, see the “Procedures Manual” (http://www.adni-info.org/Scientists/ProceduresManuals.aspx).
Statistics
Calculating the PIB-PET Signature
The probability density of the mean PIB-PET score showed a distinct bimodal distribution. The PIB-PET score with the lowest density separating the 2 distributions was determined to dichotomize the sample into the group with low and high PIB-PET values. Since the PIB-PET score was highly correlated with CSF Aβ1–42 concentration (Fagan et al. 2006) and Apolipoprotein E (ApoE) genotype (ApoE ϵ4 carrier vs. ApoE ϵ4 noncarrier, see Results for details), CSF Aβ1–42 and ApoE genotype were used to impute the PIB-PET score in those subjects for whom PIB-PET scans were not available (see also Weigand et al. 2011). Based on the imputed PIB-PET (iPIB-PET) score or—where PIB-PET scan was available—based on the global PIB-PET score, the sample was dichotomized using the same PIB-PET cutoff value as derived in the first step based on the original scans, separating the entire sample into those whose scores surpassed the cutoff threshold (iPIB-PET(+)) and those whose score fell below the threshold (iPIB-PET(−)). The agreement between the dichotomous PIB-PET status derived from actual scans and the imputed PIB-PET score was >96% (see Results section), showing a high reliability of the current approach of imputing the PIB-PET status based upon CSF Aβ1–42 values. The binary iPIB-PET status was used as a predictor in the subsequent analysis.
Predicting Change of Cognition, Regional Gray Matter Volume, and FDG-PET by Brain Aβ Levels
Univariate mixed-effects regression analyses were computed for the prediction of rate of change in ADAS-cog, RAVLT delayed recall, and each ROI of mean gray matter volume and mean FDG-PET activity by iPIB-PET status. Specifically, rates of change were determined for each measure using mixed-effects regression models according to:
Here Yij represents the variable measured at time j in subject i. The terms β and B are the respective coefficients of fixed and random effects at baseline and over time, and e indicates errors. In order to assess whether MCI subjects show faster iPIB-PET associated decline compared with HC subjects, PIB-PET rates (modeled as fixed effects of change) were modulated by diagnosis according to:
where Dx designates diagnostic group. In order to test whether a significant association between PIB-PET and cognitive change is mediated by change in gray matter volume or FDG-PET, the mixed-effect regression analyses on the prediction of ADAS-cog by iPIB-PET status was repeated, adjusting this time for change in gray matter volume or FDG-PET. A 95% confidence interval (95% CI) based on bootstrap percentiles for the reduction (mediation) of the coefficient for iPIB-PET was estimated using 1000 bootstrap samples. A reduction of the coefficient that was significantly larger than zero can be interpreted such that the particular ROI measure was a mediator of the association between PIB-PET status and decline in ADAS-cog scores. All mediation analyses were done in the MCI group only, since PIB-PET showed only in this group a significant association with MRI-assessed and FDG-PET–assessed brain changes.
Predicting Conversion from MCI to AD
The prediction of conversion from MCI to AD by iPIB-PET status was tested using interval-censored Weibull regression. Plots of residuals were used to identify the Weibull distribution as an appropriate distribution for the conversion times. Due to a fixed schedule of follow-up visits, the exact conversion times were unknown, requiring both left and right censoring. Longitudinal cognitive and MRI data were fitted using linear mixed-effects models with both a random intercept and slope, assuming an autoregressive correlation structure. Covariate adjustments were made for variables that were associated with both iPIB-PET and the outcome (gender, age, and education were assessed but were not found to be associated with predictors and outcome and therefore not included as covariates): Regression models to assess the effect of iPIB-PET status on longitudinal MRI and FDG-PET changes were controlled for ApoE genotype, ADAS-cog, intracranial volume, and the time interval between baseline assessment of brain changes and assessment of CSF or PIB-PET. For assessing the effect of iPIB-PET status on cognitive changes, the models needed to be controlled for ApoE genotype effect and time interval between baseline assessment of dependent variable and assessment of CSF or PIB-PET. Focused tests for group comparisons included Wilcoxon rank-sum test for continuous variables (e.g., age, education, MMSE, etc.) and Fisher’s exact test for categorical variables (e.g., gender, binary ApoE ϵ4 carrier status). For all analyses, model assumptions were assessed using plots of residuals. P value adjustment for multiple-comparison associated accumulation of Type I error probability was done via Holm’s method (Holm 1979), setting the significance threshold at α = 0.05. All analyses were computed with the R software library R-2.11 freely available at http://cran.r-project.org/.
Results
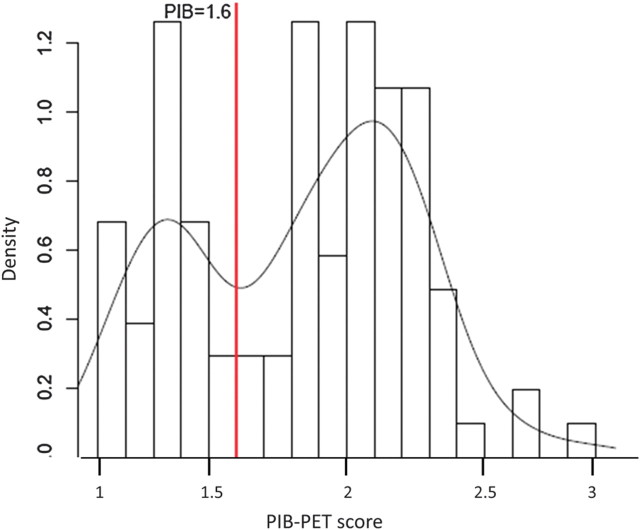
PIB-PET scans were available in 19 HC, 65 MCI, and 19 AD subjects. We tested in a first step, whether PIB-PET scores averaged across core ROI regions (Jagust et al. 2009) can be segregated into 2 clearly distinguishable groups (Jack, Lowe, et al. 2008). A density plot of the PIB-PET scores showed a bimodal distribution among all subjects (Fig. 2). The point that best separated the two distributions into PIB-PET(+) and PIB-PET(−) was 1.6, as determined by the minimum density value of the PIB-PET scores lying between the 2 modes.
Figure 2.
Frequency plot of average PIB-PET scores and a fitted smoothed curve of the distribution. The cutoff point of 1.6 (red vertical line) was derived to optimally separate the data of the bimodal distribution into PIB-PET(+) and PIB-PET(−) groups.
Applying the cutoff value of 1.6, we found that iPIB-PET(+) status was present in 89.6% (n = 17 out of n = 19) of the AD subjects, 69.8% (n = 43 out of n = 63) of the MCI subjects, and 47.9% (n = 9 out of n = 19) of the HC. A significantly higher proportion of ApoE ϵ4 carriers occurred within the PIB-PET(+) group (66.7 %) compared to the PIB-PET(−) group (17.6 %, P < 0.001, Fig. 2). CSF-Aβ1–42 concentration and ApoE genotype were used to impute PIB-PET scores in the entire sample. When applying the cutoff value of 1.6 in the sample of subjects with both PIB-PET measures and CSF-Aβ1–42 (n = 55), the CSF-based binary classification overlapped in 96.4% of the cases with the iPIB-PET–based classification, suggesting high accuracy of the binary categorization based upon the iPIB-PET values. Among all subjects, iPIB-PET(+) was present in 92.0% (n = 103 out of n = 112) of the AD subjects, 72.5% (n = 166 out of n = 229) of the MCI subjects, and 41.1% (n = 51 out of n = 124) of the HC (for demographic and biomarker values, see Table 1).
Table 1.
Descriptive demographics and basic measures for diagnostic groups and iPIB-PET classification
| Diagnosis | iPIB-PET status | Sample size | Age (SD) in years | Gender (f/m) | MMSE (SD) | ApoE genotype (ϵ4−/ϵ4+) | Education (SD) in years | CSF Aβ1–42, pg/ml | CSF total tau, pg/ml | CSF p-tau, pg/ml |
| HC | iPIB-PET(−) | 73 | 75.6 (5.4) | 38/35 | 29.0 (1.1) | 69/4** | 15.5 (SD = 2.8) | 244.7 (27.6)** | 62.0 (23.0)* | 20.5 (8.0)* |
| iPIB-PET(+) | 51 | 76.4 (5.1) | 21/30 | 29.2 (1.1) | 27/24 | 16.0 (3.0) | 152.0 (27.6) | 79.5 (37.6) | 31.0 (19.1) | |
| MCI | iPIB-PET(−) | 63 | 74.8 (8.2) | 15/48 | 27.3 (1.7) | 25/11** | 15.7 (3.0) | 244.1 (26.9)** | 62.6 (23)** | 20.0 (7.6)** |
| iPIB-PET(+) | 166 | 74.4 (7.4) | 63/103 | 26.8 (1.8) | 55/111 | 15.9 (2.9) | 136.4 (26.0) | 116.5 (62.8) | 40.5 (17.5) | |
| AD | All subjects pooled | 112 | 74.9 (8.1) | 47/65 | 23.6 (1.9) | 36/76 | 15.1 | 142.5 (39.6) | 121.5 (57.5) | 102.4 (19.8) |
Note: f, female, m, male; **P ≤ 0.001 and *P ≤0.01 for comparison with iPIB-PET(+) within diagnostic group.
iPIB-PET Signature as a Predictor of Longitudinal Changes of Cognition in HC and MCI
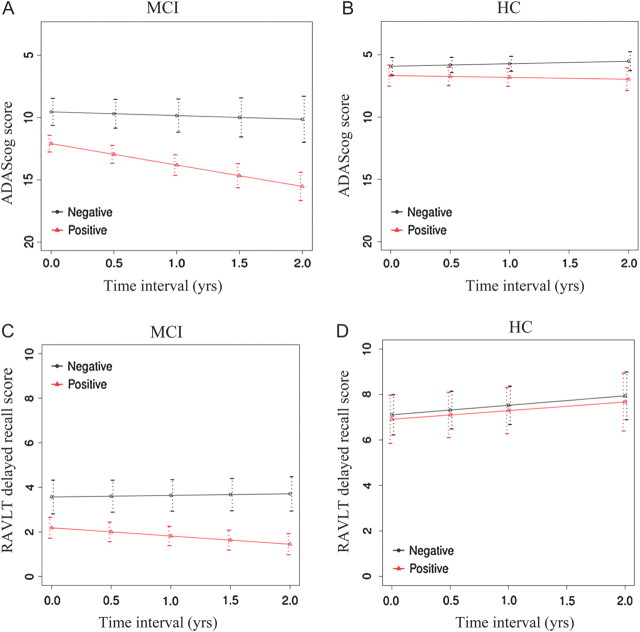
The rate of change in global cognitive ability as measured by ADAS-cog score was significantly different between iPIB-PET(+) and iPIB-PET(−), depending upon diagnosis (B = 1.12 ADAS-cog/year, standard error [SE] = 0.49, P = 0.02). In MCI subjects, the PIB-PET(−) group had a predicted annual rate of change of B = 0.3 ADAS-cog/year (SE = 0.4), and the PIB-PET(+) group a rate of change of B = 1.72 ADAS-cog/year (SE = 0.4). The PIB-PET(+) subjects showed significantly faster worsening on ADAS-cog test compared with the PIB-PET(−) group in MCI (B = 1.42 ADAS-cog/year, SE = 0.42, P < 0.001, Fig. 3A). In HC subjects, the regression coefficient of mean annual rate of change in ADAS-cog score was B = −0.2 ADAS-cog/year (SE = 0.17) in the PIB-PET(−) group and B = 0.14 ADAS-cog/year (SE = 0.17) in the PIB-PET(−) group, which was not statistically significant between the iPIP-PET groups (B = 0.34 ADAS-cog/year, SE = 0.27, P = 0.21, Fig. 3B).
Figure 3.
Regression plot of estimated longitudinal decline in the ADAS-cog (A and B) and delayed RAVLT scores (C and D) over the follow-up period (years) for iPIB-PET(+) subjects (red line) versus iPIB-PET(−) subjects (black line) in MCI (A and C) and HC (B and D). The difference in the rate of decline between iPIB-PET groups was statistically significant for measures, except for ADAS-cog in the HC group (see Results).
For the change in AVLT delayed free recall in MCI, the predicted annual rate of change was B = −0.07 AVLT/year (SE = 0.14) in PIB-PET(−) subjects and B = −0.37 AVLT/year (SE = 0.14) in PIB-PET(+) subjects. The PIB-PET(+) subjects showed a significantly faster decline in free recall than PIB-PET(−) subjects in MCI (B = −0.44, SE = 17, P = 0.01, Fig. 3C). In HC subjects, the regression coefficient of annual rate of change of free recall score was B = 0.41 AVLT/year (SE = 0.2) in iPIB-PET(−) subjects and B = 0.37 AVLT/year (SE = 0.2) in iPIB-PET(+) subjects, where the difference in annual rate of change between the iPIB-PET groups was not statistically significant (B = 0.04 AVLT/year, SE = 0.31, P = 0.91, Fig. 3D).
Prediction of Conversion from MCI to AD by iPIB-PET Status
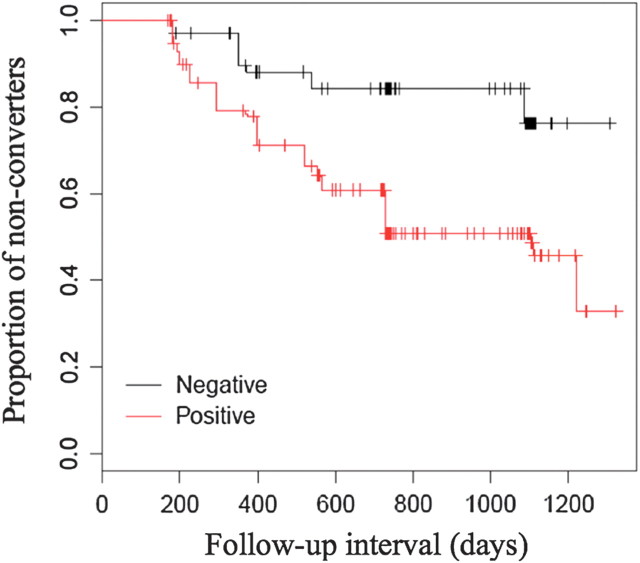
PIB-PET(+) was associated with a significantly accelerated conversion rate from MCI to AD over a 2-year follow-up interval. The odds ratio was 4.8 (95% CI = 2.1, 4.8). Within that time period, 67 out of 83 (80.7%) of the iPIB-PET(+) subjects progressed from MCI to AD, but only 8 out of 48 (16.6%) MCI subjects with a iPIB-PET(−) status converted to AD (Fig. 4).
Figure 4.
Survival plot for conversion from MCI to AD for iPIB-PET(+) shown in red and iPIB-PET(−) shown in black.
Association between iPIB-PET and Longitudinal Regional Gray Matter Volume Changes in HC and MCI
In MCI subjects, the iPIB-PET(+) group showed compared with iPIB-PET(−) a faster decline in all ROIs including the precuneus, inferior parietal lobe, middle and superior temporal gyrus, and medial temporal lobe structures such as the hippocampus, parahippocampus, entorhinal cortex, and amygdala, except for the thalamus (for details, see Fig. 5 and Table 2). The iPIB-PET effect on the rates of change was controlled for ApoE genotype, age, intracranial volume (ICV), baseline ADAS-cog score, and time between baseline MRI assessment and PIB-PET scan. In HC subject, iPIB-PET(+) showed a significantly faster decline within the amygdala, but this was no longer significant after adjusting for Type I error accumulation (Table 2). When compared with HC, subjects with MCI showed a significantly larger effect of iPIB-PET on the annual rate of volume decline for the inferior parietal lobe, entorhinal cortex, parahippocampus, and middle temporal gyrus, inferior parietal lobe, and a trend for the precuneus (see Table 2, last column).
Figure 5.
Regression plot of estimated longitudinal decline of gray matter volume in MCI subjects for the inferior parietal cortex (A), entorhinal cortex (B), parahippocampus (C), and middle temporal gyrus (D). The iPIB-PET(+)–associated acceleration in the decline of each volume was significantly larger in MCI than in HC subjects in whom the iPIB-PET–associated difference was not significant (not shown). Note that the scale of the plots varies due to the different sizes of the brain structures.
Table 2.
Rate of ROI specific gray matter volume change: results of the mixed-effect analysis for the comparison between iPIB-PET(+) and iPIB-PET(−) in MCI and HC
| ROI | Diagnosis | iPIB-PET group | % Annual change in volume | Δ In % annual change between iPIB-PET(−) versus iPIB-PET(+) in volume | B (SE) of Δ in annual volume change between iPIB-PET(−) versus iPIB-PET(+) in mm2/year | P value for interaction iPIB-PET × diagnosis in rate of volume change |
| Hippocampus | HC | PIB(−) | −0.9 | −0.5 | −32.1 (20.9) | ns |
| PIB(+) | −1.4 | |||||
| MCI | PIB(−) | −1.5 | −1.5 | −81.4 (18.2)** | ||
| PIB(+) | −3.0 | |||||
| Entorhinal cortex | HC | PIB(−) | −1.0 | −0.1 | 0.2 (15.4) | <0.001 |
| PIB(+) | −1.1 | |||||
| MCI | PIB(−) | −1.1 | −2.8 | −88.9 (15.7)** | ||
| PIB(+) | −3.9 | |||||
| Parahippocampus | HC | PIB(−) | −1.0 | −0.1 | −4.1 (14.6) | <0.05 |
| PIB(+) | −1.1 | |||||
| MCI | PIB(−) | −1.2 | −1.6 | −58.7 (15.3)** | ||
| PIB(+) | −2.8 | |||||
| Amygdala | HC | PIB(−) | 0.4 | −1.9 | −38.6 (14.7)** | ns |
| PIB(+) | −1.3 | |||||
| MCI | PIB(−) | −0.9 | −1.7 | −29.9 (911.7)* | ||
| PIB(+) | −2.6 | |||||
| Middle temporal gyrus | HC | PIB(−) | −1.0 | −0.5 | −92.6 (62.0) | <0.01 |
| PIB(+) | −1.4 | |||||
| MCI | PIB(−) | −0.8 | −2.4 | −424 (71.9)*** | ||
| PIB(+) | −3.1 | |||||
| Superior temporal gyrus | HC | PIB(−) | −0.9 | −0.3 | −67.0 (51.7) | ns |
| PIB(+) | −1.2 | |||||
| MCI | PIB(−) | −0.9 | −1.3 | −241.8 (63.2)** | ||
| PIB(+) | −2.2 | |||||
| Inferior parietal lobe | HC | PIB(−) | −1.0 | −0.3 | −75.7 (83.6) | <0.05 |
| PIB(+) | −1.3 | |||||
| MCI | PIB(−) | −0.6 | −1.7 | −358.5 (88.1)*** | ||
| PIB(+) | −2.3 | |||||
| Precuneus | HC | PIB(−) | −0.8 | −0.5 | −74.4 (49.8) | ns (P = 0.06) |
| PIB(+) | −1.3 | |||||
| MCI | PIB(−) | −0.6 | −1.4 | −221.4 (958.2)*** | ||
| PIB(+) | −2.0 | |||||
| Thalamus | HC | PIB(−) | −0.7 | −0.1 | −23.0 (51.3) | ns |
| PIB(+) | −0.8 | |||||
| MCI | PIB(−) | −0.7 | −0.9 | −115.3 (SE = 44)** | ||
| PIB(+) | −1.6 |
Note: B, regression coefficient; SE, standard error of regression coefficient; ns, not significant. P value for comparison of rates of changes between iPIB-PET(+) versus iPIB-PET(−) in each diagnostic group: *<0.05, **<0.01, ***<0.001.
Association between iPIB-PET and FDG-PET Changes in HC and MCI
MCI subjects showed a trend toward a significantly faster decline associated with iPIB-PET(+) compared with iPIB-PET(−) within the posterior cingulate gyrus (B = −0.02, SE = 0.01, P = 0.05) and the inferior/middle temporal gyrus (B = −0.03, SE = 0.02, P = 0.07), but not in the angular gyrus or frontal orbital cortex (for diagnosis-specific and iPIB-PET–specific rates of decline, see Table 3). Conversely, in HC subjects no iPIB-PET effect on the rate of change of FDG-PET was observed in any of the ROIs.
Table 3.
Rate of FDG-PET change: results of the mixed-effect analysis for the comparison between iPIB-PET(+) and iPIB-PET(−) in MCI and HC
| ROI | Diagnosis | iPIB-PET group | % Annual change in iPIB-PET score per year | Δ In % Annual change between iPIB-PET(−) versus iPIB-PET(+) in iPIB-PET score per year | B (SE) of Δ in annual volume change between iPIB-PET(−) versus iPIB-PET(+) in iPIB-PET score per year |
| Inferior/middle temporal gyrus | HC | PIB(−) | 0.2 | −0.1 | Greater than −0.01 (0.02) |
| PIB(+) | 0.1 | ||||
| MCI | PIB(−) | −0.8 | −2.3 | −0.03 (0.02) | |
| PIB(+) | −2.1 | ||||
| Angular gyrus | HC | PIB(−) | −1.0 | 0.1 | 0.02 (0.03) |
| PIB(+) | 0.0 | ||||
| MCI | PIB(−) | −1.1 | −1.1 | −0.02 (0.02) | |
| PIB(+) | −2.1 | ||||
| Posterior cingulum | HC | PIB(−) | −0.5 | 0.3 | <0.01 (0.01) |
| PIB(+) | −0.2 | ||||
| MCI | PIB(−) | −0.8 | −1.5 | −0.02 (0.01) | |
| PIB(+) | −2.3 | ||||
| Frontal orbital | HC | PIB(−) | −0.6 | 0.6 | 0.01 (0.01) |
| PIB(+) | 0.0 | ||||
| MCI | PIB(−) | −1.3 | −0.4 | Greater than −0.01 (0.01) | |
| PIB(+) | −1.7 |
Association of iPIB-PET and Longitudional Changes, Using the Alternative iPIB-PET Cut off Values in HC
While the cutoff point of 1.6 determined in the current study is consistent with a previous study (Pike et al. 2007), other studies have also reported a cutoff point of 1.5 (Jack, Lowe, et al. 2008; Schott et al. 2010) or 1.41 (Kadir et al. 2010). In order to determine whether the PIB-PET cutoff point makes a difference when assessing PIB-PET related longitudinal brain and cognitive changes, we recomputed the analyses above with alternative cutoff points previously reported in the literature. For the cutoff point of 1.5, the previous result pattern on all measures remained the same except for regional volume differences: The iPIB-PET(+) group showed compared with the iPIB-PET(−) group a faster volume decline in the hippocampus (% annual change = −1.4 vs. −0.8, respectively, group difference: P = 0.02) and the precuneus (% annual change P = −1.4 vs. −0.7 respectively, group difference: P = 0.04). Furthermore, the faster decline in the amygdala volume in the PIB-PET(+) group of the HC subjects remained significant (% annual change = −1.2 vs. 0.6, respectively, group difference: P < 0.01). The iPIB-PET–related group differences in the hippocampus, precuneus, and amygdala was also significant when tested with a lower PIB-PET cutoff point of 1.41.
Indirect Effect of iPIB-PET on Cognitive Decline via Brain Changes
Since iPIB-PET was associated with both cognitive decline and brain changes in MCI subjects, we tested the hypothesis that iPIB-PET is predictive of cognitive changes through its association with brain atrophy and decline in brain metabolism in MCI (Mormino et al. 2009). For the selection of potential mediators, we restricted the analysis to those brain regions that showed a relatively strong association with iPIB-PET based upon the P value, that is the precuneus and inferior parietal volume for MRI volume and the posterior cingulate for FDG-PET. The effect size (i.e., regression coefficient) of the association between iPIB-PET and rate of decline in ADAS-cog, was significantly reduced by adding the rate of change of inferior parietal volume (bootstrapped 95% CI of reduction of regression coefficient = 0.14, 0.82) or precuneus (95% CI = 0.12, 0.79). The association between iPIB-PET status and decline in ADAS-cog was no longer significant when the rate of change of precuneus volume was included, indicating that the precuneus volume mediated the association between iPIB-PET and ADAS-cog decline. For rates of change of FDG-PET in the posterior cingulate gyrus, the regression coefficient of iPIB-PET for changes in ADAS-cog was significantly reduced by the metabolism within the posterior cingulate gyrus (95% CI = 0.17, 0.98).
Discussion
The main findings of the current study were as follows: First, iPIB-PET(+) status in MCI was associated with faster decline in cognition and regional brain atrophy within all AD predilection ROIs tested except for the thalamus and showed a trend toward faster decline in brain metabolism when compared with iPIB-PET(−). No differences between the iPIB-PET groups in HC subjects were detected in any of the cognitive and FDG-PET measures. Difference between iPIB-PET groups in the rate of change in gray matter volume of HC subjects were restricted to medial temporal lobe structures and the precuneus and were dependent upon the iPIB-PET cutoff. Secondly, MCI subjects with iPIB-PET(+) status showed a significantly higher risk to progress to AD than MCI subjects with iPIB-PET(−) status. Thirdly, the association between iPIB-PET status and deterioration of global cognitive ability in MCI was mediated by changes in regional gray matter volume of the precuneus and posterior cingulate gyrus and metabolism in the posterior cingulate gyrus.
Our first result was that MCI subjects with higher brain Aβ levels as estimated by iPIB-PET(+) status showed faster decline in global cognition and episodic memory when compared to iPIB-PET(−). These results are in keeping with previous results which showed an association between higher global PIB-PET uptake and faster cognitive decline in MCI (Pike et al. 2007; Forsberg et al. 2010). In contrast to MCI, the HC subjects did not show a difference in cognitive decline between the iPIP-PET(+) and iPIB-PET(−), even though the PIB-PET(+) groups in both the HC and MCI subjects showed an elevation of global PIB-PET to a similar extent. The absence of a significant association between global PIB-PET and cognitive decline is consistent with previous cross-sectional reports of a lack of an association between cognitive ability and CSF-Aβ1–42 (Fagan et al. 2007) or PIB-PET (Jack, Lowe, et al. 2008) in elderly HC subjects. Two other studies reported an association between PIB-PET and cognition in elderly HC subjects only when selected on the basis of low cognitive reserve (measured by IQ score) (Rentz et al. 2010) or presence of significant cognitive decline over 6–10 years of follow-up (Villemagne et al. 2008) but not in the whole groups of elderly HC subjects assessed in these studies. However, other studies did report a significant association, including a cross-sectional study (Pike et al. 2007) and longitudinal studies that assessed the cognitive changes during up to 19 years preceding the PIB-PET scan (Gustafson et al. 2007; Storandt et al. 2009; Resnick et al. 2010). In the current study, we directly compared subjects groups dichotomized into iPIB-PET(+) and iPIB-PET(−) subjects, detecting no significant differences in the rate of change in cognition, even when applying different cutoff values ranging from 1.41 to 1.6. These results do not however preclude that subjects with abnormally elevated PIB-PET values show subtle increase in cognitive decline in correlation with higher PIB-PET values. Prolonged follow-up will show whether those HC subjects with high Aβ levels progress faster cognitive decline and are more likely to develop AD (Resnick et al. 2010).
For regional brain volume changes, we demonstrated that MCI subjects with iPIB-PET(+) status showed faster decline in several core brain regions that are typically affected by atrophy at the stage of AD dementia. These results extend previous studies showing an association between elevated brain Aβ levels (as measured by PIB-PET binding or CSF Aβ1–42) and smaller hippocampus volume or ventricular expansion (Jack, Lowe, et al. 2008; Jack et al. 2009; Mormino et al. 2009; Schuff et al. 2009). The current results, however, are somewhat at odds with a recent joint voxel-based study of PIB-PET and structural MRI, which did not find any cross-sectional association between regional PIB-PET uptake and MRI volume loss in MCI (also not in AD) (Chételat et al. 2010). Discrepancies between the findings may be explained by the use of a longitudinal vs. cross-sectional study design and the use of global vs. voxel-based PIB_PET predictors.
In elderly HC subjects, PIB-PET–related changes in gray matter volume were very restricted. Previous correlational studies reported a lack of an association between global PIB-PET binding and the annual rate of ventricular expansion over 1.5 years (Jack et al. 2009) or between CSF Aβ1–42 concentration and regional gray matter volume decline over 1-year follow-up in HC subjects (Leow et al. 2009; Schuff et al. 2009). A recent study reported that changes in regional brain volume over 10 years preceding PIB-PET scan was not associated with global iPIB-PET uptake (Driscoll et al. 2010). The authors interpreted their findings in the way that the HC subjects may not had yet reached abnormally high Aβ1–42 to a sufficient degree. Such an interpretation is supported by recent findings of an association between baseline CSF Aβ1–42 concentration and the 1-year change in regional brain volume that was detected only in elderly HC subjects with abnormally high brain Aβ levels but not in subjects with low brain Aβ levels (Fjell et al. 2010). The results of the current study corroborate that abnormally high levels of Aβ are associated with gray matter changes restricted to the temporoparietal network in HC subjects. This result is also consistent with a previous cross-sectional studies that found decreased volume within the hippocampus, temporal neocortex, cingulate (and a trend within the precuneus and parahippocampus) within the PIB-PET(+) group in elderly HC (Fagan et al. 2009; Storandt et al. 2009; Schott et al. 2010). Interestingly, in the current study, no significant PIB-PET group differences were detected when a PIB-PET cutoff of 1.6 was applied but only at lower levels of 1.41 and 1.5. The finding of significant faster decline in medial temporal lobe structures in the iPIB-PIB(+) group when using the lower cutoff values is consistent with a previous finding of more rapid 1-year decline in the hippocampus and whole-brain volume in HC subjects with CSF Aβ <192 pg/mL assessed within ADNI (Schott et al. 2010). Thus, it is possible that a lower PIB-PET cutoff value allows for a more sensitive detection of PIB-PET–related abnormalities in gray matter decline in the HC subjects, which is plausible since brain Aβ levels may be less advanced in HC when compared with MCI. Nevertheless, even at the high PIB-PET cutoff value of 1.6, almost half of the HC subjects showed abnormal PIB-PET levels that were also present in AD subjects, yet the brain changes were much less dramatic than those observed in MCI. Thus, elevated brain Aβ levels alone are unlikely to explain typical AD dementia-associated patterns of brain atrophy, and additional factors downstream or independent of the Aβ cascade may be necessary to cause the devastating brain atrophy in AD (see discussion below).
For functional brain changes assessed by FDG-PET, we found weak associations with iPIB-PET in subjects with MCI, that is, only a trend of an association between iPIB-PET status and the rate of change in FDG-PET activity in the posterior cingulate was observed. No association was observed in HC. A recent voxel-based cross-sectional study found no differences in resting state FDG-PET between PIB-PET(−) and PIB-PET(+) status in HC or MCI (Cohen et al. 2009). However, positive correlations between PIB-PET and FDG-PET were found to be widely distributed in the brain of MCI subjects and, to a spatially more confined extent, HC subjects. This result pattern was interpreted in favor of cognitive or brain reserve, that is subjects with elevated brain metabolism can accumulate more brain Aβ before progressing to dementia (Cohen et al. 2009). Alternatively, the absence of hypometabolism may result from an Aβ-related increase in activity and neuronal excitability and may herald pending neuronal degeneration (Palop et al. 2007; Buckner et al. 2009). However, in the current study, no hyperactivity in PIB-PET(+) subjects in HC or MCI was observed, rather there was a tendency toward accelerated PIB-PET(+)–associated decline. Although we did not find a significant PIB-PET(+)–associated faster rate of metabolic decline in the HC group, we cannot exclude that had we studied a larger sample or used an extended observation interval, a faster rate of decline may have been detected in elderly HC subjects.
Another major finding of the current study was that decline of gray matter volume within the precuneus and inferior parietal lobe and FDG-PET metabolism within the posterior cingulate gyrus mediated the association between iPIB-PET and global cognitive decline in MCI. These results extend previous cross-sectional results of the mediation of the association between global PIB-PET scores and episodic memory by hippocampus volume (Mormino et al. 2009). The precuneus is known to be associated with episodic memory function (Maddock et al. 2001) and has been found to show significantly faster gray matter volume loss in MCI subjects who converted to AD than MCI stable subjects (Chetela et al. 2005). It has recently been proposed that the precuneus is a hub in resting state and memory-task–related neuronal networks and may be especially vulnerable to Aβ pathology (Buckner et al. 2009; Sperling et al. 2009). Our results on regional gray matter and FDG-PET change as a mediating factor in cognitive decline supports the notion that the posterior parietal volume may be vulnerable to Aβ pathology at an early stage in the course of AD dementia and is predictive of cognitive impairment.
Compared with MCI, the limited extent of Aβ-associated brain changes over a 2-year time interval in HC is striking. Several factors may account for this observation. It is possible that the accumulation of brain Aβ in the MCI subjects began at an earlier age, and thus the MCI subjects would be further downstream of the Aβ-related pathophysiological cascade. The MCI PIB-PET(+) group did show increased CSF p-tau levels compared with the HC PIB-PET(+) group, suggesting that they may had increased neurofibrillary pathology in addition to elevated Aβ levels (Buerger et al. 2006). Secondly, cognitive reserve may modulate the association between brain levels of Aβ and cognitive or cerebral changes (Scarmeas and Stern 2004). However, education was not found to be significantly different between any of the PIB-PET groups in the HC or MCI subjects in the current study, so that we cannot confirm such a possibility on the basis of the current data. Thirdly, other factors such as vascular disease (Nicoll et al. 2004; Roy and Rauk 2005) may modulate vulnerability to Aβ-related changes and will need to be assessed in future studies.
Our study has several limitations. It could be argued that dichotomizing iPIB-PET into iPIB-PET(+) and iPIB-PET(−) groups may be an oversimplification of a continuous distribution of iPIB-PET scores and may render the approach less sensitive to the detection of Aβ1–42-related pathology. Note that the global PIB-PET scores showed a distinct bimodal density distribution of mean PIB-PET values consistent with findings of previous studies (Buckner et al. 2005; Jack, Lowe, et al. 2008), which rendered an approach of correlation PIB-PET scores across these different populations inadequate. De Meyer et al. (2010) used a mixture model approach to detect distinct subgroups based on CSF markers in the ADNI data set, reporting independent of diagnosis 2 homogenous groups of high and low CSF Aβ, which suggested the existence of 2 distinct subgroups similar to our findings based on PIB-PET data. In quantitative terms, there is also a converging tendency toward a common cutoff value. De Meyer et al. (2010) reported an optimal cutoff value of 182 pg/mL. Based on our imputation model of PIB-PET, the CSF cutoff value that corresponds to the PIB-PET cutoff of 1.6 corresponds to the CSF level of 195 pg/mL in ApoE ϵ4 carriers that is close to the one reported by De Meyer et al. (2010) and the cutoff value of CSF Aβ = 192 pg/ml reported by Shaw et al. (2009) in a sample of living HC subjects and postmortem confirmed AD cases. Furthermore, our PIB-PET cutoff value of 1.6 based on the bimodal distribution is remarkably consistent with those PIB-PET cutoff values applied in previous studies using different methods (Mintun et al. 2006; Pike et al. 2007; Rabinovici et al. 2007; Aizenstein et al. 2008; Jagust et al. 2009), including global PIB-PET cutoffs of 1.5 (Jack, Lowe, et al. 2008) or 1.6 (Pike et al. 2007), providing evidence for a convergence onto a consistent PIB-PET cutoff point across different statistical methods. The generation of binary global PIB-PET categories may reflect a biological reality of Aβ1–42 levels within the brain in elderly subjects, considering reports on postmortem findings in subjects who were found to either have Aβ1–42 deposition or who were almost totally free of it at the preclinical stage of AD (Price et al. 1991). Furthermore, such a categorization may bear a clinical diagnostic utility, as suggested by the high proportion of PIB-PET(+) in AD of >90% compared with much lower proportion in HC subjects (Mintun et al. 2006; Pike et al. 2007; Aizenstein et al. 2008; Jack, Lowe, et al. 2008).
Other limitations ought to be mentioned. We used ROIs rather than a more regionally unbiased approach such as a voxel-by-voxel group comparison of brain changes. Therefore, we may have missed additional associations outside the ROIs. Finally, because we imputed PIB-PET measures for several subjects who had no PIB-PET data, our results are only an approximation. However, as we used binary categories of Aβ quantification, this procedure allowed still a highly reliable and valid categorization with an accuracy of over 90%, consistent with a previous finding comparing CSF-Aβ1–42– and PIB-PET–based categorization (Jagust et al. 2009).
In conclusion, the current study demonstrated that increased global levels of Aβ1–42 show utility in the prediction of cognitive decline and brain changes at the preclinical stage of AD. However, the mere presence of Aβ1–42 alone does not seem to be predictive of acceleration of decline in a uniform way but may vary depending upon clinical symptoms present. Future studies are needed to find factors that mediate Aβ1–42-related toxicity.
Funding
Merck, Avid, the National Institutes of Health, the DOD, and the Veterans Affairs to M.W.W; Elan/Wyeth Alzheimer’s Immunotherapy Program North American Advisory Board, Alzheimer’s Association, Forest, University of California, Davis, Tel-Aviv University Medical School, Colloquium Paris, Ipsen, Wenner-Gren Foundations, Social Security Administration, Korean Neurological Association, National Institutes of Health, Washington University at St Louis, Banner Alzheimer’s Institute, Clinical Trials on Alzheimer’s Disease, Veterans Affairs Central Office, Beijing Institute of Geriatrics, Innogenetics, New York University, NeuroVigil, Inc., CHRU-Hopital Roger Salengro, Siemens, AstraZeneca, Geneva University Hospitals, Lilly, University of California, San Diego—ADNI, Paris University, Institut Catala de Neurociencies Aplicades, University of New Mexico School of Medicine, Ipsen, Clinical Trials on Alzheimer’s Disease, Pfizer, AD PD meeting, Paul Sabatier University, and Novartis to M.W.W. for travel; Alzheimer's Disease Neuroimaging Initiative (National Institutes of Health Grant U01 AG024904) for data collection and sharing; National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co., Inc., Novartis AG, Pfizer Inc, F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., as well as nonprofit partners the Alzheimer's Association and Alzheimer's Drug Discovery Foundation, with participation from the US Food and Drug Administration to ADNI; Foundation for the National Institutes of Health (www.fnih.org) to ADNI for private sector contributions; National Institutes of Health grants P30 AG010129, K01 AG030514, and the Dana Foundation.
Acknowledgments
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu/ADNI). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. ADNI investigators include (complete listing available at http://www.loni.ucla.edu/ADNI/Collaboration/ADNI_Manuscript_Citations.pdf). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuroimaging at the University of California, Los Angeles. Conflict of Interest : Dr Weiner has been on advisory boards for Lilly, Araclon, and Institut Catala de Neurociencies Aplicades, Gulf War Veterans Illnesses Advisory Committee, VACO, Biogen Idec, Elan/Wyeth Alzheimer’s Immunotherapy Program North American Advisory Board, Novartis Misfolded Protein Scientific Advisory Board Meeting, Banner Alzheimer’s Institute Alzheimer’s Prevention Initiative Advisory Board Meeting, and the Research Advisory Committee on Gulf War Veterans’ Illnesses. He has been a consultant for Elan/Wyeth, Novartis, Forest, Ipsen, Daiichi Sankyo, Inc., Astra Zeneca, Araclon, Medivation/Pfizer, TauRx Therapeutics LTD, Bayer Healthcare, Biogen Idec, Exonhit Therapeutics, SA, Servier, and Synarc. Dr Weiner holds stock options with Synarc and Elan. He has been on the Editorial Advisory Boards of the Journals Alzheimer's and Dementia and MRI. He has received Honoraria from American Academy of Neurology, Ipsen, NeuroVigil, Inc., and Insitut Catala de Neurociencies Aplicades. Organizations contributing to the Foundation for National Institutes of Health and thus to the National Institute on Aging funded Alzheimer’s Disease Neuroimaging Initiative are Abbott, Alzheimer's Association, Alzheimer's Drug Discovery Foundation, Anonymous Foundation, AstraZeneca, Bayer Healthcare, BioClinica, Inc. (ADNI 2), Bristol-Myers Squibb, Cure Alzheimer's Fund, Eisai, Elan, Gene Network Sciences, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson & Johnson, Eli Lilly & Company, Medpace, Merck, Novartis, Pfizer Inc, Roche , Schering Plough, Synarc, and Wyeth.
References
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Hwang KS, Andrawis JP, Green AE, Babakchanian S, Morra JH, Cummings JL, Toga AW, Trojanowski JQ, Shaw LM, et al. 3D PIB and CSF biomarker associations with hippocampal atrophy in ADNI subjects. Neurobiol Aging. 2010;31:1284–1303. doi: 10.1016/j.neurobiolaging.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer HA, Edison P, Brooks DJ, Barnes J, Frost C, Yeatman T, Fox NC, Rossor MN. Amyloid load and cerebral atrophy in Alzheimer's disease: an 11C-PIB positron emission tomography study. Ann Neurol. 2006;60:145–147. doi: 10.1002/ana.20889. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger K, Ewers M, Pirttila T, Zinkowski R, Alafuzoff I, Teipel SJ, DeBernardis J, Kerkman D, McCulloch C, Soininen H, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Landeau B, Eustache F, Mezenge F, Viader F, de la Sayette V, Desgranges B, Baron JC. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27:934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Chételat G, Villemagne VL, Bourgeat P, Pike KE, Jones G, Ames D, Ellis KA, Szoeke C, Martins RN, O'Keefe GJ, et al. Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol. 2010;67:317–324. doi: 10.1002/ana.21955. [DOI] [PubMed] [Google Scholar]
- Cohen AD, Price JC, Weissfeld LA, James J, Rosario BL, Bi W, Nebes RD, Saxton JA, Snitz BE, Aizenstein HA, et al. Basal Cerebral metabolism may modulate the cognitive effects of A{beta} in mild cognitive impairment: an example of brain reserve. J Neurosci. 2009;29:14770–14778. doi: 10.1523/JNEUROSCI.3669-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, Coart E, Hansson O, Minthon L, Zetterberg H, et al. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67:949–956. doi: 10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Zhou Y, An Y, Sojkova J, Davatzikos C, Kraut MA, Ye W, Ferrucci L, Mathis CA, Klunk WE, et al. Lack of association between (11)C-PiB and longitudinal brain atrophy in non-demented older individuals. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2009.12.008. doi: 10.1016/j.neurobiolaging.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer's disease dementia. Trends Neurosci. 2011;34:430–442. doi: 10.1016/j.tins.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M, Walsh C, Trojanowski JQ, Shaw LM, Petersen RC, Jack CR, Jr, Feldman HH, Bokde AL, Alexander GE, Scheltens P, et al. Prediction of conversion from mild cognitive impairment to Alzheimer's disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.10.019. doi: 10.1016/j.neurobiolaging.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, Holtzman DM. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Blennow K, Brewer JB, Dale AM the Alzheimer's Disease Neuroimaging Initiative. Brain atrophy in healthy aging is related to CSF levels of Aβ1-42. Cereb Cortex. 2010;20:2069–2079. doi: 10.1093/cercor/bhp279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg A, Almkvist O, Engler H, Wall A, Langstrom B, Nordberg A. High PIB retention in Alzheimer's disease is an early event with complex relationship with CSF biomarkers and functional parameters. Curr Alzheimer Res. 2010;7:56–66. doi: 10.2174/156720510790274446. [DOI] [PubMed] [Google Scholar]
- Forsberg A, Engler H, Almkvist O, Blomquist G, Hagman G, Wall A, Ringheim A, Långström B, Nordberg A. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2008;29:1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Lorenzi M, Caroli A, Kemppainen N, Nagren K, Rinne JO. In vivo mapping of amyloid toxicity in Alzheimer disease. Neurology. 2009;72:1504–1511. doi: 10.1212/WNL.0b013e3181a2e896. [DOI] [PubMed] [Google Scholar]
- Gustafson DR, Skoog I, Rosengren L, Zetterberg H, Blennow K. Cerebrospinal fluid beta-amyloid 1-42 concentration may predict cognitive decline in older women. J Neurol Neurosurg Psychiatry. 2007;78:461–464. doi: 10.1136/jnnp.2006.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, J LW, Ward C, et al. The Alzheimer's disease neuroimaging initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, Knopman DS, Boeve BF, Klunk WE, Mathis CA, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, Foster NL, Petersen RC, Weiner MW, Price JC, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadir A, Almkvist O, Forsberg A, Wall A, Engler H, Langstrom B, Nordberg A. Dynamic changes in PET amyloid and FDG imaging at different stages of Alzheimer's disease. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.06.015. doi: 10.1016/j.neurobiolaging.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, Weiner MW, Jagust WJ. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2009;32:1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leow AD, Yanovsky I, Parikshak N, Hua X, Lee S, Toga AW, Jack CR, Jr, Bernstein MA, Britson PJ, Gunter JL, et al. Alzheimer's disease neuroimaging initiative: a one-year follow up study using tensor-based morphometry correlating degenerative rates, biomarkers and cognition. Neuroimage. 2009;45:645–655. doi: 10.1016/j.neuroimage.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JA, Yamada M, Frackowiak J, Mazur-Kolecka B, Weller RO. Cerebral amyloid angiopathy plays a direct role in the pathogenesis of Alzheimer's disease. Pro-CAA position statement. Neurobiol Aging. 2004;25:584–589. doi: 10.1016/j.neurobiolaging.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Furst AJ, O'Neil JP, Racine CA, Mormino EC, Baker SL, Chetty S, Patel P, Pagliaro TA, et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007;68:1205–1212. doi: 10.1212/01.wnl.0000259035.98480.ed. [DOI] [PubMed] [Google Scholar]
- Rami L, Fortea J, Bosch B, Sole-Padulles C, Llado A, Iranzo A, Sanchez-Valle R, Molinuevo JL. Cerebrospinal fluid biomarkers and memory present distinct associations along the continuum from healthy subjects to AD patients. J Alzheimers Dis. 2011;23:319–326. doi: 10.3233/JAD-2010-101422. [DOI] [PubMed] [Google Scholar]
- Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, Sperling RA, Johnson KA. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67:353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Sojkova J, Zhou Y, An Y, Ye W, Holt DP, Dannals RF, Mathis CA, Klunk WE, Ferrucci L, et al Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74:807–815. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L'examen clinique en psychologie. Paris (France): Press Universitaires de France; 1964. [Google Scholar]
- Rolstad S, Berg AI, Bjerke M, Blennow K, Johansson B, Zetterberg H, Wallin A. Amyloid-beta42 is associated with cognitive impairment in healthy elderly and subjective cognitive impairment. J Alzheimers Dis. 2011;26:135–142. doi: 10.3233/JAD-2011-110038. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- Roy S, Rauk A. Alzheimer's disease and the ‘ABSENT’ hypothesis: mechanism for amyloid beta endothelial and neuronal toxicity. Med Hypoth. 2005;65:123–137. doi: 10.1016/j.mehy.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y. Cognitive reserve: implications for diagnosis and prevention of Alzheimer's disease. Curr Neurol Neurosci Rep. 2004;4:374–380. doi: 10.1007/s11910-004-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott JM, Bartlett JW, Fox NC, Barnes J. Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal fluid Abeta1-42. Ann Neurol. 2010;68:825–834. doi: 10.1002/ana.22315. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Stein T, Maslowski N, Neumann J. Neural correlates of Alzheimer's disease and mild cognitive impairment: a systematic and quantitative meta-analysis involving 1351 patients. Neuroimage. 2009;47:1196–1206. doi: 10.1016/j.neuroimage.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Woerner N, Boreta L, Kornfield T, Shaw LM, Trojanowski JQ, Thompson PM, Jack CR, Jr, Weiner MW the Alzheimer's Disease Neuroimaging I. MRI of hippocampal volume loss in early Alzheimer's disease in relation to ApoE genotype and biomarkers. Brain. 2009;132:1067–1077. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, et al Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, et al Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stomrud E, Hansson O, Blennow K, Minthon L, Londos E. Cerebrospinal fluid biomarkers predict decline in subjective cognitive function over 3 years in healthy elderly. Dement Geriatr Cogn Disord. 2007;24:118–124. doi: 10.1159/000105017. [DOI] [PubMed] [Google Scholar]
- Stomrud E, Hansson O, Zetterberg H, Blennow K, Minthon L, Londos E. Correlation of longitudinal cerebrospinal fluid biomarkers with cognitive decline in healthy older adults. Arch Neurol. 2010;67:217–223. doi: 10.1001/archneurol.2009.316. [DOI] [PubMed] [Google Scholar]
- Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Pike KE, Darby D, Maruff P, Savage G, Ng S, Ackermann U, Cowie TF, Currie J, Chan SG, et al Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer's disease. Neuropsychologia. 2008;46:1688–1697. doi: 10.1016/j.neuropsychologia.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Weigand SD, Vemuri P, Wiste HJ, Senjem ML, Pankratz VS, Aisen PS, Weiner MW, Petersen RC, Shaw LM, Trojanowski JQ, et al Transforming cerebrospinal fluid Abeta42 measures into calculated Pittsburgh Compound B units of brain Abeta amyloid. Alzheimers Dement. 2011;7:133–141. doi: 10.1016/j.jalz.2010.08.230. [DOI] [PMC free article] [PubMed] [Google Scholar]