Summary
Mixed lineage leukemia 5 (MLL5) is a versatile nuclear protein associated with many cellular events. We have shown previously that phosphorylation of MLL5 by Cdk1 is required for mitotic entry. In this paper, the function of MLL5 in mitotic regulation is further explored. SiRNA-mediated downregulation of MLL5 caused improper chromosome alignment at metaphase and resulted in failure of DNA segregation and cytokinesis. Mechanistic studies revealed that the chromosomal passenger complex (CPC), which plays a key role in chromosomal bi-orientation, was delocalized from the inner centromere region because of proteasome-mediated degradation in MLL5-depleted cells. Biochemical analyses further demonstrated that the central domain of MLL5 interacted with the C-terminus of Borealin, and the interaction is essential to maintain the stability of Borealin. Moreover, the mitotic defects in MLL5-depleted cells were rescued by overexpression of FLAG-MLL5, but not by a FLAG-MLL5 mutant that did not contain the central domain. Collectively, our results suggest that MLL5 functionally interacts with Borealin, facilitates the expression of CPC, and hence contributes to mitotic fidelity and genomic integrity.
Key words: Chromosomal passenger complex, Genomic integrity, MLL5
Introduction
Mitosis is a highly regulated process that ensures the accurate segregation of duplicated chromosomes to the daughter cells. Defects in mitotic fidelity cause DNA segregation errors and result in chromosome instability, which plays a causal role in tumorigenesis and relapse (Baker et al., 2009; Sotillo et al., 2010). High-throughput genomic profiling study reveals that most human cancers are aneuploid and chromosomally unstable (Beroukhim et al., 2010). Human chromosomal passenger complex (CPC) is a critical regulator of chromosome segregation during mitosis. It comprises the Aurora-B kinase and three non-enzymatic regulatory subunits, Survivin, Borealin and INCENP (Gassmann et al., 2004; Ruchaud et al., 2007). The expression of CPC is cell cycle dependent, with minimal expression in G1/S, and gradually increases during G2 phase and peaks at M phase (Honda et al., 2003; Terada et al., 1998; Zhao et al., 2000). CPC initially localizes to the chromosome arms to control the chromosome structure and organization, subsequently concentrates on the inner centromeres to promote the kinetochore-microtubule (MT) interactions (Ruchaud et al., 2007). At anaphase and telophase, CPC transfers to the spindle midzone, the equatorial cortex and to the midbody, where it is essential for the cytoplasmic division. One of the key events orchestrated by CPC is the correction of kinetochore-MT attachment errors, which is dependent on the kinase activity of Aurora-B by promoting the turnover of improper kinetochore-MT attachments (Vader et al., 2006). Within the CPC, the four subunits interact extensively, and the interactions not only regulate the centromere targeting of the complex, but also maintain the stability of CPC. Depletion of any member of the CPC by RNA interference delocalizes the complex, renders it unstable and disrupts mitotic progression (Honda et al., 2003; Klein et al., 2006).
The human Mixed lineage leukemia 5 (MLL5) gene is located on the chromosome 7q22, which is commonly deleted in acute myeloid leukemia (AML) and therapy-induced leukemia (Emerling et al., 2002). In AML patients, high MLL5 expression is associated with high overall survival and relapse-free survival, demonstrating the prognostic importance and the therapeutic potential of MLL5 in AML (Damm et al., 2011). MLL5 is involved in many aspects of cellular physiology, including hematopoietic stem cell homeostasis and epigenetic regulation. Genetic analysis of Mll5 deficiency in mice reveals that Mll5 might be dispensable for embryonic development, but plays critical roles in adult hematopoiesis (Heuser et al., 2009; Madan et al., 2009; Zhang et al., 2009). The epigenetic regulatory role of MLL5 is conveyed by the study where an isoform of MLL5 acts as histone methyltransferase and facilitates the retinoic-acid-induced granulopoiesis in human promyelocytes (Fujiki et al., 2009). The importance of MLL5 for cell cycle regulation has also been demonstrated. Overexpression of MLL5 induces cell cycle arrest at G1 phase (Deng et al., 2004), and downregulation of MLL5 by RNA interference results in both a G1/S and a G2/M phase arrest (Cheng et al., 2008). In addition, the completion of S phase progression is hastened in MLL5-depleted myoblasts (Sebastian et al., 2009). Recently, it has been shown that the phosphorylation of MLL5 by Cdk1 is required for mitotic entry (Liu et al., 2010). In this study, we demonstrate that MLL5 functionally interacts with the CPC component Borealin, and serves as a molecular chaperone for the stabilization of Borealin prior entry into mitosis.
Results
Depletion of MLL5 induces chromosomal instability
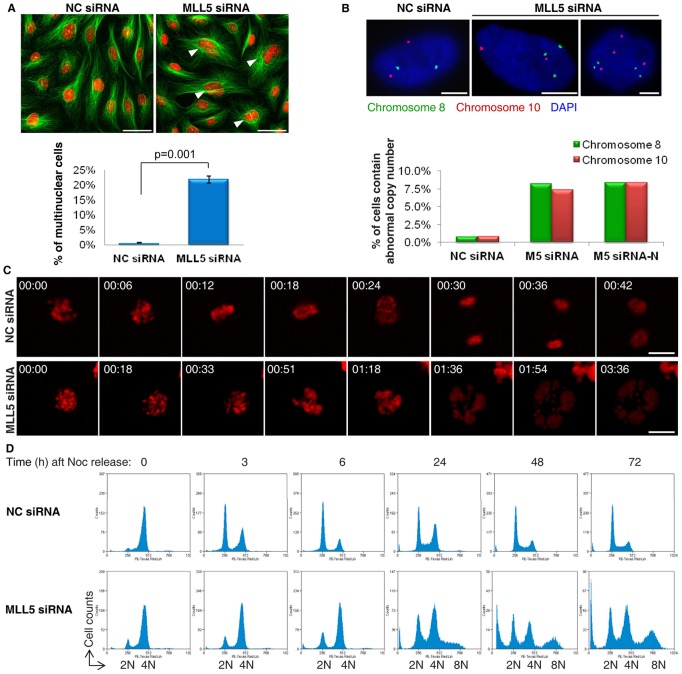
MLL5 has been shown to regulate G1/S transition, S phase progression and mitotic entry; however, the role of MLL5 in mitosis remains elusive. We initiated this study based on the interesting observation that multinuclear cells accounted for 20% of total MLL5-depleted cells after transfection of MLL5-siRNA for 48 h (Fig. 1A). Considering that a large portion (∼75%) of MLL5-depleted cells are actually arrested in G1/S phase (2N) with approximately only 25% of the population having the chance to undergo mitosis (4N) (Cheng et al., 2008), the percentage of multinuclear cells present in MLL5-siRNA-treated cells suggests that the majority (∼80%) of MLL5-depleted cells that entered mitosis experienced abnormal cell division. To determine the influence of MLL5 on chromosomal stability, we depleted MLL5 using two different siRNAs [MLL5-siRNA, which was targeted to an mRNA sequence starting 6807 bp downstream of the transcription start site (3′-UTR), and MLL5-siRNA-N, which was targeted to an mRNA sequence starting 1063 bp downstream of the transcription start site] and performed fluorescence in situ hybridization (FISH) to monitor the chromosome numbers in human diploid lung fibroblast WI-38 cells. The cells were transfected with siRNA every other day to maintain MLL5 at a low level. Cells were harvested at day 7 for FISH analysis using centromere-specific probes for chromosomes 8 and 10. As shown in Fig. 1B, 8.3% of MLL5-siRNA-treated cells exhibited abnormal numbers of chromosome 8, and 7.3% showed abnormal numbers of chromosome 10 (Fig. 1B). In contrast, only 0.8% of NC-siRNA-treated cells were found to contain abnormal copies of chromosomes 8 and 10. A similar result was obtained in MLL5-siRNA-N-transfected cells. In summary, there is a significant increase (∼10-fold) in the percentage of aneuploid cells after MLL5 knockdown, suggesting that loss of MLL5 causes the mis-segregation of chromosomes and leads to genomic instability.
Fig. 1.
Depletion of MLL5 by RNA interference causes genomic instability. (A) The average percentage of multinuclear cells in Negative Control-siRNA (NC-siRNA) and MLL5-siRNA-treated U2OS cells (P = 0.001, paired Student's t-test). Tubulin was stained green and nuclei were stained red. Arrowheads denotes multinuclear cells. Scale bars: 20 µm. (B) The cells were transfected with NC-siRNA, MLL5-siRNA or MLL5-siRNA-N every other day and harvested at day 7 for FISH. The percentage of cells exhibiting abnormal numbers of chromosomes 8 and 10 was scored (n>100 cells per sample). Scale bars: 5 µm. (C) The mitotic progression of U2OS cells expressing H2B-mCherry was monitored by live-cell imaging. Scale bars: 10 µm. (D) Cell cycle analyses showed that MLL5-depleted cells remained at 4N DNA content after releasing from nocodazole, and polyploid populations gradually increased. Noc, nocodazole.
To understand why the cells exited mitosis without proper DNA segregation, the mitotic progression of NC- and MLL5-siRNA-treated cells was recorded using fluorescence video microscopy (Fig. 1C; supplementary material Movies 1, 2). The majority of control cells completed mitosis within 1 h, but MLL5-depleted cells failed to align their chromosomes on the metaphase plate and exited mitosis without chromosome segregation and cytokinesis. By 1.5 h, the DNA in MLL5-depleted cells showed signs of de-condensation, and multinuclear cells had formed. Time-dependent cell cycle analysis confirmed that MLL5-depleted cells failed in DNA segregation and generated chromosomally unstable polyploid cells (Fig. 1D). Double staining for a mitotic cell marker and propidium iodide at 6 h after mitotic release further confirmed that MLL5-depleted cells exited mitosis despite failing to segregate their chromosomes (supplementary material Fig. S1A). Because the mitotic checkpoint and anaphase-promoting complex/cyclosome (APC/C) are key players in the segregation of sister chromatids and cytokinesis (Pines, 2006), the function of the mitotic checkpoint and the activity of APC/C were examined in MLL5-depleted mitotic cells. Upon nocodazole treatment, the mitotic checkpoint was successfully activated as revealed by the kinetochore localization of MAD2 (supplementary material Fig. S1B). After mitotic release, the rate of cyclin B1 degradation was only slightly decreased in MLL5-depleted cells (supplementary material Fig. S1C). These data imply that both the activation of the mitotic checkpoint and the function of APC/C were likely unaffected by MLL5 depletion.
Depletion of MLL5 affects the correction of kinetochore-MT attachment errors
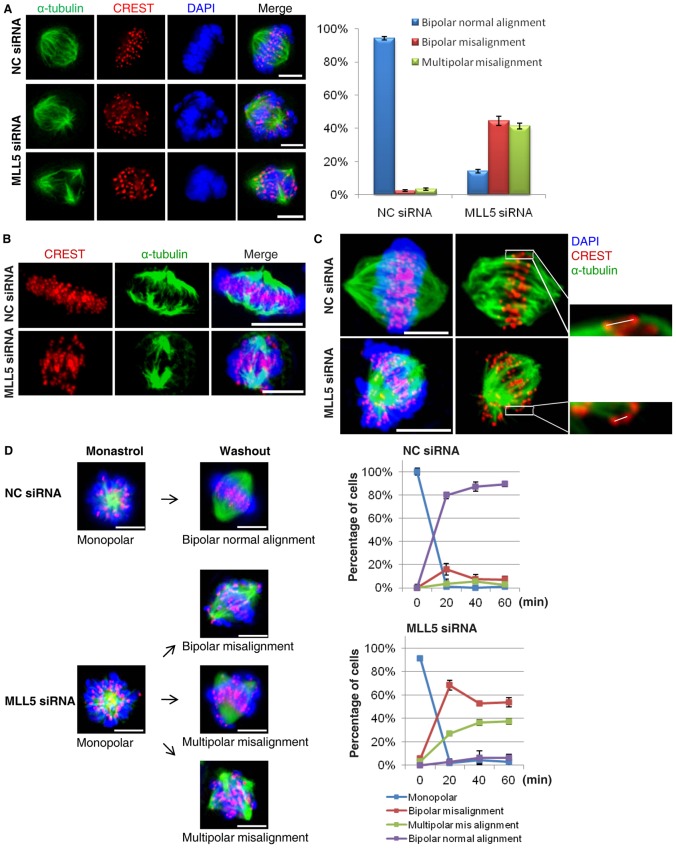
The data described above indicate that MLL5-depleted cells fail to properly align chromosomes at metaphase and thus exit from mitosis without DNA segregation and cytokinesis. To further characterize the chromosome bi-orientation process in MLL5-depleted cells, we performed indirect immunofluorescence staining on metaphase-arrested cells. The results showed that 93.4% of control cells achieved full chromosome alignment, whereas only 16.1% of MLL5-depleted cells exhibited bi-polar chromosome alignment (Fig. 2A). The same results were obtained when live MLL5-depleted cells were imaged; these cells failed to properly align chromosomes even at 2 h after nocodazole release (supplementary material Fig. S2, Movies 3, 4).
Fig. 2.
MLL5-depleted cells fail to align chromosomes on metaplate because of impaired function of the kinetochore-MT attachment error-correcting machinery. (A) Metaphase-arrested cells were categorized into three groups: bipolar normal alignment, bipolar misalignment and multipolar misalignment. Experiments were repeated three times and the average percentage is presented. Scale bars: 5 µm. (B) The kinetochore-MT capture and attachment process were examined in a cold-induced MT depolymerization assay. Kinetochore fibers were stained with α-tubulin antibody and kinetochores were labeled with CREST. Scale bars: 5 µm. (C) The average inter-kinetochore distances (for sister kinetochores) were measured, as shown, in control cells (n = 70) and MLL5-depleted cells (n = 84). Scale bars: 5 µm. (D) A time-course study of the chromosome alignment process was conducted in NC- and MLL5-depleted cells after monastrol release. Cells were categorized into four groups: monopolar, bipolar misalignment, multipolar misalignment and bipolar normal alignment, and the percentages for each group at each time point after monastrol release are presented (n = 100 cells per sample). Scale bars: 5 µm.
Chromosome misalignment can arise from several mechanistic defects in kinetochore-MT interactions, including defective kinetochore-MT attachment and impaired function of the error-correcting machinery. We first examined kinetochore-MT attachment in MLL5-depleted cells in an MT-depolymerization assay; however, there was no significant difference between control and MLL5-depleted cells with respect to density of kinetochore fibers, suggesting that depletion of MLL5 did not appear to affect kinetochore capture and MT attachment (Fig. 2B). Next, we analyzed the generation of tension between sister kinetochores. Knockdown of MLL5 resulted in a decrease in the average inter-kinetochore distance of 34.4% (NC-siRNA: 0.93±0.04 µm, MLL5-siRNA: 0.61±0.03 µm); this result reflects impaired tension across the sister kinetochores, an indication of erroneous kinetochore-MT attachment (Fig. 2C). Thus, we speculated that the function of the kinetochore-MT attachment error-correcting machinery may be affected by MLL5 depletion. To test this possibility, an Eg-5 inhibitor (monastrol) washout experiment was conducted (Fig. 2D). Treatment of cells with monastrol resulted in the accumulation of mitotic cells with syntelically attached sister kinetochore pairs. Upon removal of the drug, syntelic attachment was converted to amphitelic attachment. Sixty minutes after monastrol release, 89.3% of control cells had achieved chromosome bi-orientation. In contrast, only 6.4% of MLL5-depleted cells had completed chromosome bi-orientation and 90.9% of these contained mis-aligned chromosomes. These data suggest that the function of the kinetochore-MT attachment error-correcting machinery is compromised in MLL5-depleted cells.
MLL5 maintains the stability of CPC through a functional interaction with Borealin
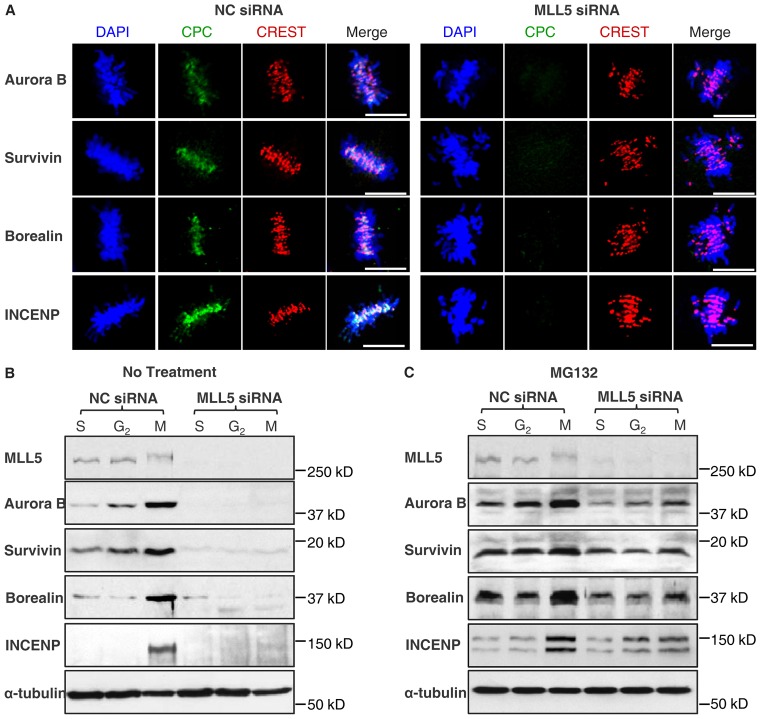
Correction of aberrant kinetochore-MT attachment is highly dependent on CPC, which selectively destabilizes incorrect kinetochore-MT interactions, providing a new opportunity for chromosome bi-orientation. To investigate whether depletion of MLL5 has any effect on CPC's function, the localization of CPC at metaphase in MLL5-depleted cells was examined. Control cells (NC-siRNA treated) typically display inner centromere localization of CPC at metaphase; strikingly, in MLL5-depleted cells, none of the subunits of CPC was detectable (Fig. 3A). Because the expression of CPC is cell cycle dependent, we proceeded to compare the expression of CPC in S, G2 and M phases in NC- and MLL5-siRNA-treated cells. Quantitative RT-PCR showed that the mRNA levels of CPC components after MLL5 knockdown were relatively unchanged compared with NC-knockdown cells (<1.5-fold changes; supplementary material Fig. S3). However, western blotting revealed a drastic loss of expression of CPC throughout S, G2 and M phases in MLL5-depleted cells (Fig. 3B). Furthermore, addition of the proteasome inhibitor MG132 to MLL5-depleted cells substantially restored the expression of all CPC components (Fig. 3C). Taken together, our data suggest that MLL5 protects the CPC from proteasome-mediated degradation prior to mitotic entry.
Fig. 3.
CPC is downregulated in MLL5-depleted cells as a result of proteasome-mediated degradation. (A) CPC was delocalized in MLL5-depleted cells. Scale bars: 10 µm. (B) NC-siRNA- and MLL5-siRNA-siRNA-transfected cells were synchronized to S, G2 or M phase. The expression of Aurora-B, Survivin, Borealin and INCENP was analyzed by western blotting. Tubulin served as a loading control. (C) Blocking of the proteasome pathway by MG132 was able to rescue the expression of all CPC components.
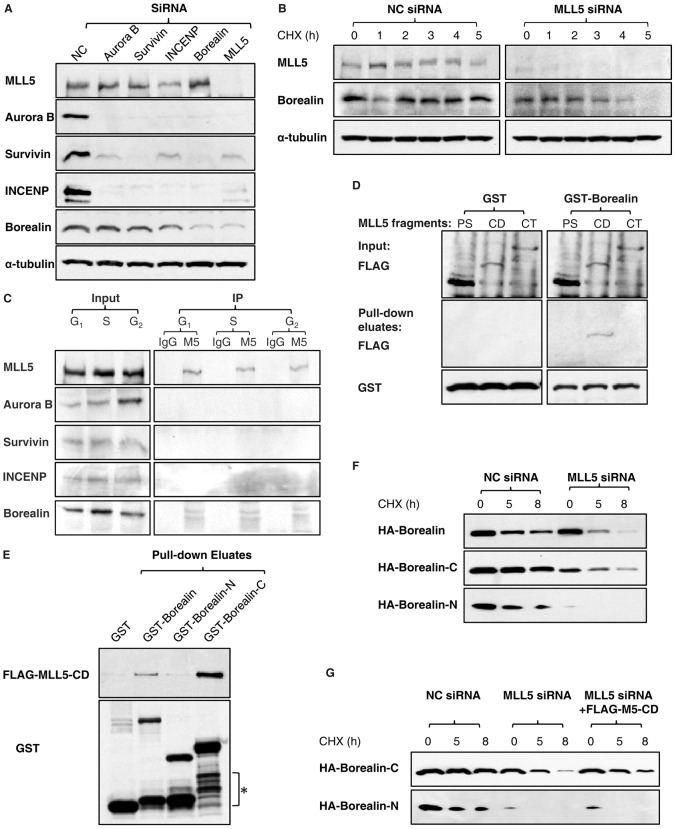
Studies have shown that the localization and/or stability of CPC subunits are inter-dependent (Honda et al., 2003; Klein et al., 2006); however, systematic analysis on the effects of knocking down each of the subunits on the protein expression in mitotic cells is lacking. Here, we knocked down Aurora B, Survivin, INCENP, Borealin and MLL5 individually by siRNA and examined the expression of other proteins by western blotting (Fig. 4A). The expression of Borealin was similar in Aurora B-, Survivin- and INCENP- siRNA-treated cells; however, Borealin-siRNA treatment resulted in degradation of all CPC components. Interestingly, MLL5-siRNA transfection mimicked the effects of Borealin-siRNA treatment, and we observed a downregulation of Borealin in MLL5-siRNA-transfected cells that was comparable to that induced by Borealin-siRNA treatment. From this, we hypothesized that the downregulation of CPC components in MLL5-depleted cells is possibly caused by degradation of Borealin, which leads to destabilization of Aurora B, Survivin and INCENP. Indeed, studies have shown that Borealin forms higher order oligomers in its isolated form, and directly interacts with Survivin to form a binary subcomplex that is competent to interact with INCENP (Bourhis et al., 2009; Zhou et al., 2009). Therefore, it is likely that Borealin serves as a basic structural unit for CPC assembly, and that its stability is affected by MLL5 depletion. To test this possibility, the stability of Borealin in control and MLL5-depleted cells was compared. Because Borealin was barely detectable after MLL5 knockdown, cells were pretreated with MG132 for 4 h to restore the level of Borealin, and subsequently incubated in cycloheximide-containing medium to block de novo protein synthesis. As shown in Fig. 4B, Borealin showed a decreased half-life in MLL5-depleted cells compared with control cells, suggesting that MLL5 indeed acts to stabilize Borealin.
Fig. 4.
MLL5 interacts with Borealin and maintains the stability of Borealin. (A) U2OS cells were transfected with CPC-siRNA or MLL5-siRNA and synchronized to M phase with nocodazole. The protein expression levels of Aurora-B, Survivin, Borealin, INCENP and MLL5 were analyzed by western blotting. Tubulin served as a loading control. (B) The stability of Borealin in NC-siRNA- and MLL5-siRNA-treated cells was compared by western blotting. CHX, cycloheximide. (C) A co-immunoprecipitation study revealed an association between MLL5 and Borealin. (D) A direct interaction between the central domain of MLL5 and the C-terminus of Borealin was established in in vitro binding assays. PS: N-terminal region containing PHD (plant homeodomain) and SET[Su(var)3-9, enhancer-of-zeste and trithorax] domain, aa 1-572; CD: central domain, aa 562-1150; CT: C-terminal domain, aa 1113-1858. (E) A GST pull-down assay demonstrated that the C-terminus of Borealin was required for the interaction with MLL5. The asterisk denotes the degradation of GST-Borealin and its mutants during induction. GST-Borealin-N: aa 1-120; GST-Borealin-C: aa 121-280. (F) The stabilities of HA-Borealin, HA–Borealin-C and HA–Borealin-N were compared in NC-siRNA- and MLL5-siRNA-treated cells. (G) Overexpression of FLAG–MLL5-CD significantly stabilized HA–Borealin-C in MLL5-depleted cells, but failed to rescue the degradation of HA–Borealin-N.
We previously reported that MLL5 localizes to the chromatin compartment during interphase but dissociates from chromatin upon phosphorylation at mitotic entry (Liu et al., 2010). On the other hand, Borealin localizes to the nucleus in interphase and is recruited to inner centromeres at metaphase (Rodriguez et al., 2006). Therefore, we hypothesized that MLL5 may interact with Borealin, and that interaction (if any) is likely to occur during interphase. Indeed, Borealin was found to co-immunoprecipitate with MLL5 throughout G1, S and G2 phases (Fig. 4C); however, Aurora-B, Survivin and INCENP were undetectable in the MLL5 immunoprecipitates. To gain more insights into the interaction between MLL5 and Borealin, a pull-down assay was performed using recombinant GST-Borealin and in vitro translated fragments of MLL5. The central domain (CD) of MLL5 was found to efficiently interact with Borealin (Fig. 4D). Similarly, GST-tagged Borealin fragments (GST–Borealin-N, aa 1-120; GST–Borealin-C, aa 121-280) were constructed and used to determine which region of Borealin interacts with the central domain of MLL5 in an in vitro pull-down assay. Our results showed that the C-terminus of Borealin was responsible for the interaction (Fig. 4E). The effects of MLL5 on the stability of Borealin were further investigated by comparing the stability of Borealin mutants in NC- and MLL5-siRNA-treated cells (Fig. 4F). The stabilities of HA-Borealin and HA–Borealin-C were significantly decreased in the absence of MLL5; interestingly, the N-terminal mutant of Borealin, which does not interacts with MLL5, was barely expressed. Moreover, exogenous overexpression of FLAG–MLL5-CD successfully stabilized the HA–Borealin-C in MLL5-depleted cells, but failed to rescue the degradation of HA–Borealin-N (Fig. 4G), suggesting that the interaction between MLL5 and Borealin protects Borealin from proteasome-mediated degradation. Taken together, the protein interaction and stability studies suggest that MLL5 may serve as a molecular chaperone for Borealin in the nucleus and contribute to the accumulation of Borealin prior to entry into mitosis.
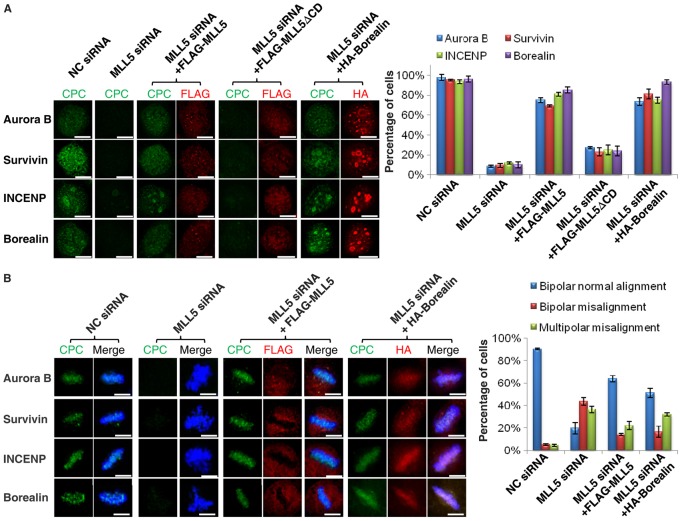
To further evaluate the effect of the association between MLL5 and Borealin on the stability of the CPC, rescue experiments were conducted in MLL5-depleted cells by ectopic overexpression of wild-type MLL5 (FLAG-MLL5), MLL5 lacking the central domain (FLAG-MLL5ΔCD) and Borealin (HA-Borealin). Because MLL5 interacts with Borealin during interphase, we first examined the expression of the CPC at G2 phase. Briefly, endogenous MLL5 was knocked down using siRNA targeting the 3′ untranslated region, which did not affect the exogenous expression of FLAG-MLL5 or FLAG-MLL5ΔCD. Sixteen hours after siRNA transfection, FLAG-MLL5, FLAG-MLL5ΔCD and HA-Borealin were introduced into the cells for 8 h, followed by treatment with RO-3306 for another 16 h. The expression and localization of CPC were examined by immunofluorescence staining, and results are shown in Fig. 5A. CPC displayed nuclear localization in control cells (NC-siRNA), but its expression was lost upon MLL5 knockdown (MLL5-siRNA). After FLAG-MLL5 overexpression, CPC expression was successfully restored in ∼80% of MLL5-knockdown cells. In contrast, overexpression of the MLL5 mutant (FLAG-MLL5ΔCD), which does not contain the domain required for the interaction with Borealin, failed to rescue the expression of CPC. These data confirm our hypothesis that the interaction between MLL5 and Borealin facilitates the accumulation of CPC components in the nucleus before mitotic entry. In addition, the successful restoration of the CPC in MLL5-depleted cells by overexpression of HA-Borealin suggests a causal relationship between the destabilization of CPC and the loss of Borealin expression.
Fig. 5.
The expression of CPC and chromosome alignment in MLL5-siRNA-treated cells can be rescued by overexpression of FLAG-MLL5. (A) The lack of expression of CPC at G2 caused by MLL5 knockdown was rescued by overexpression of FLAG-MLL5 or HA-Borealin but not FLAG-MLL5ΔCD. The efficiency of the rescue was assessed and the percentages of cells expressing CPC are presented in the bar chart (n = 60 cells per sample). (B) The CPC localization and chromosome alignment defects observed in MLL5-siRNA-treated cells were rescued by FLAG-MLL5 and HA-Borealin overexpression. The rescue experiments were repeated three times, and the average percentages of cells (normal chromosome alignment, bipolar misalignment and multipolar misalignment) were determined (n = 100 cells per sample). Scale bars: 10 µm.
Next we asked whether the function of CPC in chromosome bi-orientation in MLL5-siRNA-treated cells could be rescued by exogenous overexpression of MLL5 or Borealin. In short, 16 h after MLL5-siRNA transfection, FLAG-MLL5 and HA-Borealin were introduced into the cells for 8 h, followed by treatment with nocodazole for another 16 h. Mitotic cells were collected and released in fresh medium containing MG132 for 1 h to allow for chromosome alignment at metaphase. The chromosome alignment and the localization of CPC were examined by immunofluorescence staining, and results are shown in Fig. 5B. In control metaphase cells, CPC displayed typical inner centromere localization (first panel). After MLL5 depletion, mitotic chromosomes failed to align at the metaphase plate and the expression of CPC was no longer detectable (second panel). Importantly, after overexpression of FLAG-MLL5 or HA-Borealin, the defects in chromosome alignment and centromeric localization of CPC were substantially restored (third and fourth panels). The efficiency of the rescue was also assessed quantitatively, and the percentage of mitotic cells ith normal chromosome alignment was found to increase from 19.8% to 63.9% after exogenous overexpression of FLAG-MLL5 in MLL5-siRNA-treated cells. Similarly, HA-Borealin overexpression increased the percentage of cells with normal chromosome alignment to almost 50% (Fig. 5B). The successful functional restoration of the CPC by exogenous overexpression of FLAG-MLL5 or HA-Borealin indicates that MLL5 plays a role in facilitating the expression of CPC through a functional interaction with Borealin.
Discussion
Studies on the molecular functions of CPC provide important mechanistic insights into the mitotic regulation, ranging from kinetochore-microtubule interactions to chromatid cohesion to cytokinesis, and constitute one of the most dynamic areas of ongoing research on cell cycle. In this study, we revealed a novel mechanism explaining how the stability of CPC can be modulated by MLL5. Borealin interacts with MLL5 directly and the interaction plays a critical role in maintaining its stability. In MLL5 knockdown cells, the stability of Borealin is severely compromised, which causes the destabilization of Aurora B, Survivin and INCENP. This claim could be challenged by a previous study which showed that Borealin-siRNA causes the instability of Survivin but not of Aurora B and INCENP (Gassmann et al., 2004). However, Honda and his colleagues demonstrated that Survivin-siRNA treatment resulted in a substantial reduction in the expression of Aurora B and INCENP (Honda et al., 2003). In light of the effects of Survivin on the stability of Aurora B and INCENP, it is likely that the destabilization of Borealin in MLL5 knockdown cells causes the degradation of Aurora B and INCENP directly or indirectly through the destabilization of Survivin. The contradictory observation obtained by us and Gassmann's group could be due to cell-type specificity or western blotting sensitivity. Nonetheless, our data clearly suggest that MLL5-siRNA treatment results in down-regulation of Borealin, which in turn leads to the destruction of Aurora B, Survivin and INCENP. Moreover, the recruitment of CPC to the centromere region of chromatin has been suggested to be mediated by the interaction between Borealin and centromere adaptor Shugoshin (Kelly et al., 2010; Tsukahara et al., 2010; Wang et al., 2010). Thus it is likely that MLL5 not only functions as a molecular chaperone for Borealin in interphase, but also brings Borealin and its chromatin targets into close proximity, thus allowing the recruitment of CPC to the centromere region.
Eukaryotic cells have a sophisticated system for regulating the protein turnover, in which proteins are attached with polyubiquitin chains and directed to the proteasome for degradation (Glickman and Ciechanover, 2002). This system removes mis-folded proteins and also gets rid of native proteins for regulatory purposes. The mammalian 70-kDa and 90-kDa heat-shock protein (HSP70 and HSP90) families, which are molecular chaperones required for the conformational maturation and/or stability of a range of proteins, cooperate closely with the ubiquitin-proteasome system to orchestrate the proper cell cycle progression and successful completion of mitosis (Burrows et al., 2004; Young et al., 2004). In particular, systematic study has uncovered the diverse cellular functions of the HSP90, including involvement of HSP90 in various secretory pathways, cellular transport, cell cycle, cytokinesis and meiosis (McClellan et al., 2007). The CPC component Survivin has been shown to interact with HSP90 and disruption of the interaction destabilizes Survivin, results in mitotic defects and mitochondrial-dependent apoptosis (Fortugno et al., 2003). Similarly, Polo-like kinase is also under the HSP90 molecular chaperone regulation, in which SGT1 serves as a co-chaperone of HSP90 in stabilizing the kinase, allowing normal centrosome maturation, entry and progression through mitosis (de Cárcer et al., 2001; Martins et al., 2009). Mutations in SGT1 severely compromise the organization and function of the mitotic apparatus (Martins et al., 2009). These studies further prompt us to speculate that the role of MLL5 in Borealin stability regulation might be like a molecular co-chaperone, where it cooperates with the chaperone proteins to maintain the stability of the CPC, and facilitates the proper mitotic progression. Therefore, a potential cooperation between MLL5 and HSP90 in the control of mitosis might be an exciting possibility and deserves further investigation.
The post-translational regulation of CPC has been investigated intensively. The kinase Aurora-B is ubiquitinated and degraded at the end of mitosis by the APC/C pathway (Nguyen et al., 2005; Stewart and Fang, 2005), and the involvement of the ubiquitin-proteasome pathway in the regulation of Survivin has also been reported (Zhao et al., 2000). In addition, SUMOylation and phosphorylation modification also modulate the function of the CPC. Borealin protein is recently discovered as a target of SUMO in early mitosis, and phosphorylation of Borealin by Mps1 controls Aurora-B activity and chromosome alignment (Jelluma et al., 2008; Klein et al., 2009). In this study, we demonstrated that depletion of MLL5 causes the downregulation of Borealin by proteasome-mediated degradation, which leads to the destruction of CPC. Although the specific E3 ubiquitin ligase which is responsible for substrate recognition has yet to be determined, our study reveals the presence of a multilayered regulatory network to modulate the expression and stability of the CPC.
In this study, we found that multipolar mitosis accounts for ∼40% of the mitotic cells lacking of MLL5, which could be explained by the downregulation of Borealin, since the involvement of Borealin in spindle integrity has already been reported (Gassmann et al., 2004). However, we also noted that the multipolar misalignment in MLL5-depleted cells were substantially rescued by MLL5 overexpression, but not by Borealin overexpression (Fig. 5B), suggesting that MLL5 may possess CPC-independent function in regulating mitotic spindle integrity. The origin of the multipolar mitosis is very diverse, which can arise from overamplification of centrosomes, interference with microtubule dynamics or spindle integrity defects (Oshimori et al., 2006; Tsou and Stearns, 2006; Yang et al., 2008). In fact, growing volume of evidence has shown that mutations of certain oncogenes and tumor suppressors disrupt the numeral and functional integrity of centrosomes and directly result in chromosome instability (Fukasawa, 2007). Moreover, chromatin remodeling proteins [Chromodomain helicase DNA binding protein 3 and 4 (CHD3 and CHD4)] have also been demonstrated to play a role in regulating the centrosome integrity by modulating the centrosomal anchoring of pericentrin/γ-tubulin (Sillibourne et al., 2007). Therefore finding the mechanism by which MLL5 regulates the integrity of mitotic spindles may further our understandings on this protein and help to build a picture of the mitotic signaling of MLL5.
In conclusion, our study reveals a novel mechanism underlying the mitotic regulatory function of MLL5, and provides compelling evidence that MLL5 plays a role in the maintenance of genomic stability by modulating the stability of the chromosomal passenger complex.
Materials and Methods
Cell culture and synchronization
Cell culture conditions for U2OS, HEK293T, and human fetal lung fibroblast WI-38 were described previously (Cheng et al., 2011). WI-38 and 293T cells were used for FISH and overexpression studies, respectively. The rest of the experiments were all carried out in U2OS cells. U2OS cells were synchronized to G1 phase by double thymidine (2 mM) block. S phase U2OS cells were obtained by 4 h release from G1 arrest. G2 and M phase cells were arrested by incubation with RO-3306 (10 µM) and nocodazole (100 ng/ml), respectively for 16 h. Metaphase-arrested U2OS cells were achieved by releasing from nocodazole and incubating with MG132 (10 µM) for 1 h.
Reagents and antibodies
Thymidine, nocodazole, monastrol, propidium iodide and RNase A were purchased from Sigma-Aldrich. RO-3306 and MG132 were purchased from Calbiochem. 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI), and FluorSave reagent for indirect immunofluorescence were from Invitrogen and Merck. Rabbit anti-MLL5 antibody was described previously (Cheng et al., 2008). Primary antibodies used for western blotting or immunofluorescence were anti-α-tubulin (Sigma-Aldrich), human autoantibody against centromere (CREST) (ImmunoVision), anti-Aurora-B (BD Transduction Laboratories), anti-INCENP (Cell Signaling Technology). Antibodies against cyclin B1, Actin, Survivin, and Borealin were purchased from Santa Cruz. Horseradish peroxidase conjugated secondary antibodies for western blotting were donkey anti-rabbit (Thermo Scientific), goat anti-mouse (GE Healthcare), and donkey anti-goat (Santa Cruz). Secondary antibodies used for immunofluorescence were purchased from Invitrogen: Alexa Fluor® 488 chicken anti-rabbit, Alexa Fluor® 568 goat anti-mouse and Alexa Fluor® 594 goat anti-human.
Cloning and stable cell line selection
Human histone H2B mRNA sequence was fused with mCherry sequence and cloned into pcDNA3.1 in frame with HindIII and BamHI sites. The pcDNA-mCherry-H2B plasmid was transfected into U2OS cells by calcium phosphate method, and stable clones expressing H2B-mCherry were selected by neomycin resistance (400 µg/ml).
Transfection, cell cycle analysis and FISH
U2OS and 293T cells were seeded in antibiotic-free medium 1 day before transfection. DNA plasmids were transfected using calcium phosphate method, and siRNA duplexes were transfected using Lipofectamine RNAiMAX (Invitrogen). Cells were fixed with 70% ethanol on ice for 2 h, and stained in propidium iodide solution (20 µg/ml propidium iodide, 100 µg/ml RNase A and 0.1% Triton X-100). Cell cycle progression was analyzed by Dako Cytomation (Dako CyAn ADP, Glostrup, Denmark) and data were analyzed using Summit software version 4.3 (Beckman Coulter, Fullerton, CA, USA). For FISH study, cells were harvested, fixed and prepared for hybridization according to the protocol by Padilla-Nash et al. (Padilla-Nash et al., 2006). Aquarius alpha satellite probes for chromosomes 8 and 10 (Cytocell) were hybridized according to the manufacturer's instructions.
In vitro protein interaction studies
The in vitro transcription/translation experiment was performed using the TnT®Quick coupled transcription/translation systems (Promega) using 2 µg of pEF6-FLAG-MLL5-PS, CD or CT plasmid DNA. Expression of GST, GST-Borealin, GST–Borealin-N and GST–Borealin-C was induced by 0.5 mM IPTG in Escherichia coli (BL21) at 18°C for 16 h. After induction, 50 ml of bacterial culture was collected and lysed in 10 ml of lysis buffer (100 mM NaCl, 50 mM Tris, 1% Triton X-100, pH 8.0) supplemented with protease inhibitor cocktail (Sigma-Aldrich). GST fusion proteins were purified using glutathione S-Sepharose beads (Amersham Biosciences). The pull-down assay was carried out using GST fusion protein beads and in vitro translated fragments of MLL5 or FLAG–MLL5-CD. Procedures for total cell lysate preparation and immunoprecipitation were described previously (Liu et al., 2010).
Indirect immunofluorescence, live-cell imaging and confocal microscopy
Indirect immunofluorescence staining was performed same as previously described (Liu et al., 2010). For live cell imaging, cells were cultured on a glass-bottom 35-mm culture dish (World Precision Instruments) and assembled in a stage-top incubator supplemented with 5% CO2. Sequences of images were acquired every 3 min for 3 h using a 20× 0.75 NA objective on the Olympus inverted fluorescence microscope (Olympus, Japan). Images were analyzed using Image-Pro Plus software (Media Cybernetics, Bethesda, MD, USA). For inter-kinetochore distance measurement, standard confocal microscopy was carried out on an Olympus FV1000 (Olympus, Japan), using an UPLAPO 100× 1.35 NA objective, equipped with excitation lasers with 405 nm, 473 nm and 559 nm wavelengths. The confocal microscope was operated by Olympus fluoview ver.1.7c software. For each cell, a stack of images with 0.46 µm step size in the z-direction was obtained. Images were analyzed using ImageJ software, and sister kinetochores were identified by their presence in the same plane of focus. A single line was drawn between the brightest pixels of each kinetochore pair and the length of the line was recorded.
Statistical analysis
All statistical analyses were performed using Microsoft Office Excel 2007. Results are expressed as means ± s.d. of at least three independent experiments. Statistical significance was calculated using Student's t-tests.
Supplementary Material
Acknowledgments
We are grateful to Dr Cynthia Y. He and Dr Yih-Cherng Liou for valuable suggestions.
Footnotes
Funding
This work was supported by an Academic Research Fund Tier 1 grant [grant number R-183-000-286-112 to L.W.D]; a Ministry of Education Academic Research Fund Tier 2 grant [grant number R-183-000-195-112 to L.W.D.]; the National Medical Research Council [grant number R-183-000-293-213 to L.W.D.]; and research scholarships from Ministry of Education, Singapore [grant number R-183-000-195-112 to J.L. and F.C.].
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.110411/-/DC1
References
- Baker D. J., Jin F., Jeganathan K. B., van Deursen J. M. (2009). Whole chromosome instability caused by Bub1 insufficiency drives tumorigenesis through tumor suppressor gene loss of heterozygosity. Cancer Cell 16, 475–486 10.1016/j.ccr.2009.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beroukhim R., Mermel C. H., Porter D., Wei G., Raychaudhuri S., Donovan J., Barretina J., Boehm J. S., Dobson J., Urashima M.et al. (2010). The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905 10.1038/nature08822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourhis E., Lingel A., Phung Q., Fairbrother W. J., Cochran A. G. (2009). Phosphorylation of a borealin dimerization domain is required for proper chromosome segregation. Biochemistry 48, 6783–6793 10.1021/bi900530v [DOI] [PubMed] [Google Scholar]
- Burrows F., Zhang H., Kamal A. (2004). Hsp90 activation and cell cycle regulation. Cell Cycle 3, 1530–1536 10.4161/cc.3.12.1277 [DOI] [PubMed] [Google Scholar]
- Cheng F., Liu J., Zhou S. H., Wang X. N., Chew J. F., Deng L. W. (2008). RNA interference against mixed lineage leukemia 5 resulted in cell cycle arrest. Int. J. Biochem. Cell Biol. 40, 2472–2481 10.1016/j.biocel.2008.04.012 [DOI] [PubMed] [Google Scholar]
- Cheng F., Liu J., Teh C., Chong S. W., Korzh V., Jiang Y. J., Deng L. W. (2011). Camptothecin-induced downregulation of MLL5 contributes to the activation of tumor suppressor p53. Oncogene 30, 3599–3611 [DOI] [PubMed] [Google Scholar]
- Damm F., Oberacker T., Thol F., Surdziel E., Wagner K., Chaturvedi A., Morgan M., Bomm K., Göhring G., Lübbert M.et al. (2011). Prognostic importance of histone methyltransferase MLL5 expression in acute myeloid leukemia. J. Clin. Oncol. 29, 682–689 10.1200/JCO.2010.31.1118 [DOI] [PubMed] [Google Scholar]
- de Cárcer G., do Carmo Avides M., Lallena M. J., Glover D. M., González C. (2001). Requirement of Hsp90 for centrosomal function reflects its regulation of Polo kinase stability. EMBO J. 20, 2878–2884 10.1093/emboj/20.11.2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L. W., Chiu I., Strominger J. L. (2004). MLL 5 protein forms intranuclear foci, and overexpression inhibits cell cycle progression. Proc. Natl. Acad. Sci. USA 101, 757–762 10.1073/pnas.2036345100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerling B. M., Bonifas J., Kratz C. P., Donovan S., Taylor B. R., Green E. D., Le Beau M. M., Shannon K. M. (2002). MLL5, a homolog of Drosophila trithorax located within a segment of chromosome band 7q22 implicated in myeloid leukemia. Oncogene 21, 4849–4854 10.1038/sj.onc.1205615 [DOI] [PubMed] [Google Scholar]
- Fortugno P., Beltrami E., Plescia J., Fontana J., Pradhan D., Marchisio P. C., Sessa W. C., Altieri D. C. (2003). Regulation of survivin function by Hsp90. Proc. Natl. Acad. Sci. USA 100, 13791–13796 10.1073/pnas.2434345100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki R., Chikanishi T., Hashiba W., Ito H., Takada I., Roeder R. G., Kitagawa H., Kato S. (2009). GlcNAcylation of a histone methyltransferase in retinoic-acid-induced granulopoiesis. Nature 459, 455–459 10.1038/nature07954 [DOI] [PubMed] [Google Scholar]
- Fukasawa K. (2007). Oncogenes and tumour suppressors take on centrosomes. Nat. Rev. Cancer 7, 911–924 10.1038/nrc2249 [DOI] [PubMed] [Google Scholar]
- Gassmann R., Carvalho A., Henzing A. J., Ruchaud S., Hudson D. F., Honda R., Nigg E. A., Gerloff D. L., Earnshaw W. C. (2004). Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J. Cell Biol. 166, 179–191 10.1083/jcb.200404001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman M. H., Ciechanover A. (2002). The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 82, 373–428 [DOI] [PubMed] [Google Scholar]
- Heuser M., Yap D. B., Leung M., de Algara T. R., Tafech A., McKinney S., Dixon J., Thresher R., Colledge B., Carlton M.et al. (2009). Loss of MLL5 results in pleiotropic hematopoietic defects, reduced neutrophil immune function, and extreme sensitivity to DNA demethylation. Blood 113, 1432–1443 10.1182/blood-2008-06-162263 [DOI] [PubMed] [Google Scholar]
- Honda R., Körner R., Nigg E. A. (2003). Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol. Biol. Cell 14, 3325–3341 10.1091/mbc.E02-11-0769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelluma N., Brenkman A. B., van den Broek N. J., Cruijsen C. W., van Osch M. H., Lens S. M., Medema R. H., Kops G. J. (2008). Mps1 phosphorylates Borealin to control Aurora B activity and chromosome alignment. Cell 132, 233–246 10.1016/j.cell.2007.11.046 [DOI] [PubMed] [Google Scholar]
- Kelly A. E., Ghenoiu C., Xue J. Z., Zierhut C., Kimura H., Funabiki H. (2010). Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science 330, 235–239 10.1126/science.1189505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U. R., Nigg E. A., Gruneberg U. (2006). Centromere targeting of the chromosomal passenger complex requires a ternary subcomplex of Borealin, Survivin, and the N-terminal domain of INCENP. Mol. Biol. Cell 17, 2547–2558 10.1091/mbc.E05-12-1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U. R., Haindl M., Nigg E. A., Muller S. (2009). RanBP2 and SENP3 function in a mitotic SUMO2/3 conjugation-deconjugation cycle on Borealin. Mol. Biol. Cell 20, 410–418 10.1091/mbc.E08-05-0511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wang X. N., Cheng F., Liou Y. C., Deng L. W. (2010). Phosphorylation of mixed lineage leukemia 5 by CDC2 affects its cellular distribution and is required for mitotic entry. J. Biol. Chem. 285, 20904–20914 10.1074/jbc.M109.098558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan V., Madan B., Brykczynska U., Zilbermann F., Hogeveen K., Döhner K., Döhner H., Weber O., Blum C., Rodewald H. R.et al. (2009). Impaired function of primitive hematopoietic cells in mice lacking the Mixed-Lineage-Leukemia homolog MLL5. Blood 113, 1444–1454 10.1182/blood-2008-02-142638 [DOI] [PubMed] [Google Scholar]
- Martins T., Maia A. F., Steffensen S., Sunkel C. E. (2009). Sgt1, a co-chaperone of Hsp90 stabilizes Polo and is required for centrosome organization. EMBO J. 28, 234–247 10.1038/emboj.2008.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan A. J., Xia Y., Deutschbauer A. M., Davis R. W., Gerstein M., Frydman J. (2007). Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approaches. Cell 131, 121–135 10.1016/j.cell.2007.07.036 [DOI] [PubMed] [Google Scholar]
- Nguyen H. G., Chinnappan D., Urano T., Ravid K. (2005). Mechanism of Aurora-B degradation and its dependency on intact KEN and A-boxes: identification of an aneuploidy-promoting property. Mol. Cell. Biol. 25, 4977–4992 10.1128/MCB.25.12.4977-4992.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshimori N., Ohsugi M., Yamamoto T. (2006). The Plk1 target Kizuna stabilizes mitotic centrosomes to ensure spindle bipolarity. Nat. Cell Biol. 8, 1095–1101 10.1038/ncb1474 [DOI] [PubMed] [Google Scholar]
- Padilla–Nash H. M., Barenboim–Stapleton L., Difilippantonio M. J., Ried T. (2007). Spectral karyotyping analysis of human and mouse chromosomes. Nat. Protoc. 1, 3129–3142 10.1038/nprot.2006.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J. (2006). Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol. 16, 55–63 10.1016/j.tcb.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Rodriguez J. A., Lens S. M., Span S. W., Vader G., Medema R. H., Kruyt F. A., Giaccone G. (2006). Subcellular localization and nucleocytoplasmic transport of the chromosomal passenger proteins before nuclear envelope breakdown. Oncogene 25, 4867–4879 10.1038/sj.onc.1209499 [DOI] [PubMed] [Google Scholar]
- Ruchaud S., Carmena M., Earnshaw W. C. (2007). Chromosomal passengers: conducting cell division. Nat. Rev. Mol. Cell Biol. 8, 798–812 10.1038/nrm2257 [DOI] [PubMed] [Google Scholar]
- Sebastian S., Sreenivas P., Sambasivan R., Cheedipudi S., Kandalla P., Pavlath G. K., Dhawan J. (2009). MLL5, a trithorax homolog, indirectly regulates H3K4 methylation, represses cyclin A2 expression, and promotes myogenic differentiation. Proc. Natl. Acad. Sci. USA 106, 4719–4724 10.1073/pnas.0807136106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillibourne J. E., Delaval B., Redick S., Sinha M., Doxsey S. J. (2007). Chromatin remodeling proteins interact with pericentrin to regulate centrosome integrity. Mol. Biol. Cell 18, 3667–3680 10.1091/mbc.E06-07-0604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillo R., Schvartzman J. M., Socci N. D., Benezra R. (2010). Mad2-induced chromosome instability leads to lung tumour relapse after oncogene withdrawal. Nature 464, 436–440 10.1038/nature08803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S., Fang G. (2005). Destruction box-dependent degradation of aurora B is mediated by the anaphase-promoting complex/cyclosome and Cdh1. Cancer Res. 65, 8730–8735 10.1158/0008-5472.CAN-05-1500 [DOI] [PubMed] [Google Scholar]
- Terada Y., Tatsuka M., Suzuki F., Yasuda Y., Fujita S., Otsu M. (1998). AIM-1: a mammalian midbody-associated protein required for cytokinesis. EMBO J. 17, 667–676 10.1093/emboj/17.3.667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou M. F., Stearns T. (2006). Mechanism limiting centrosome duplication to once per cell cycle. Nature 442, 947–951 10.1038/nature04985 [DOI] [PubMed] [Google Scholar]
- Tsukahara T., Tanno Y., Watanabe Y. (2010). Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature 467, 719–723 10.1038/nature09390 [DOI] [PubMed] [Google Scholar]
- Vader G., Medema R. H., Lens S. M. (2006). The chromosomal passenger complex: guiding Aurora-B through mitosis. J. Cell Biol. 173, 833–837 10.1083/jcb.200604032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Dai J., Daum J. R., Niedzialkowska E., Banerjee B., Stukenberg P. T., Gorbsky G. J., Higgins J. M. (2010). Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science 330, 231–235 10.1126/science.1189435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Liu X., Yin Y., Fukuda M. N., Zhou J. (2008). Tastin is required for bipolar spindle assembly and centrosome integrity during mitosis. FASEB J. 22, 1960–1972 10.1096/fj.07-081463 [DOI] [PubMed] [Google Scholar]
- Young J. C., Agashe V. R., Siegers K., Hartl F. U. (2004). Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell Biol. 5, 781–791 10.1038/nrm1492 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wong J., Klinger M., Tran M. T., Shannon K. M., Killeen N. (2009). MLL5 contributes to hematopoietic stem cell fitness and homeostasis. Blood 113, 1455–1463 10.1182/blood-2008-05-159905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Tenev T., Martins L. M., Downward J., Lemoine N. R. (2000). The ubiquitin-proteasome pathway regulates survivin degradation in a cell cycle-dependent manner. J. Cell Sci. 113, 4363–4371 [DOI] [PubMed] [Google Scholar]
- Zhou L., Li J., George R., Ruchaud S., Zhou H. G., Ladbury J. E., Earnshaw W. C., Yuan X. (2009). Effects of full-length borealin on the composition and protein-protein interaction activity of a binary chromosomal passenger complex. Biochemistry 48, 1156–1161 10.1021/bi801298j [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.