Abstract
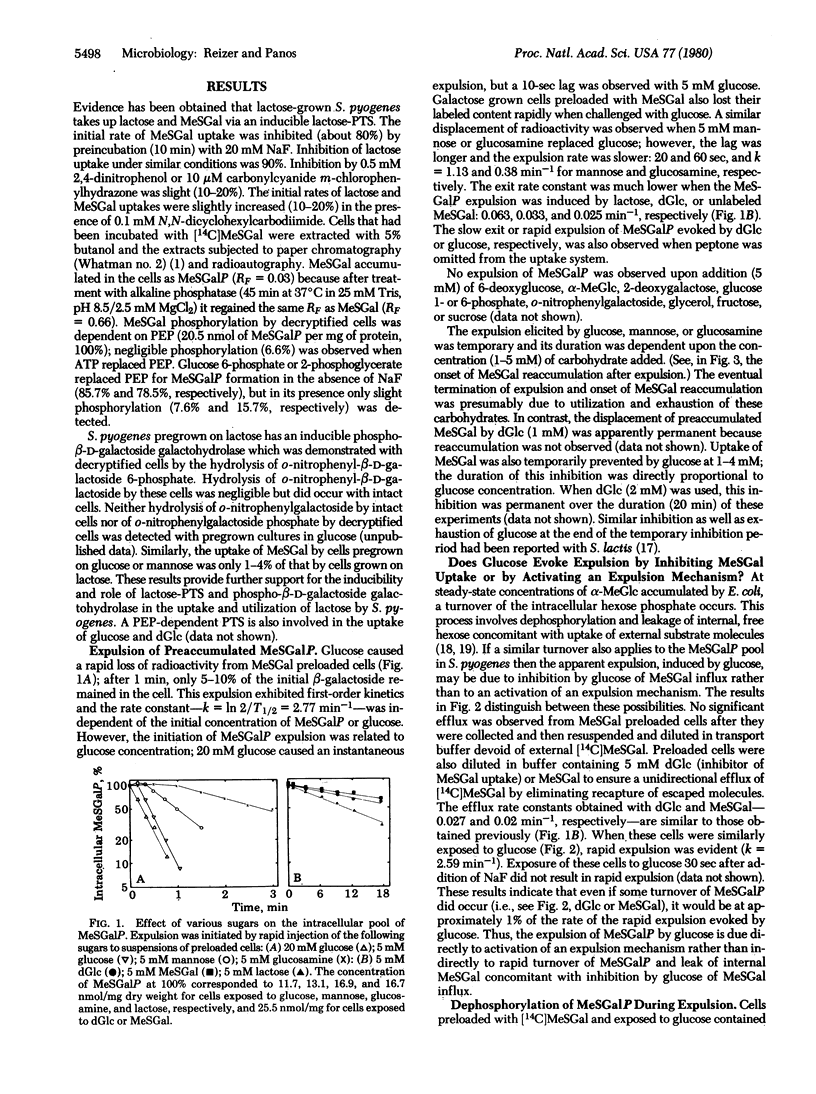
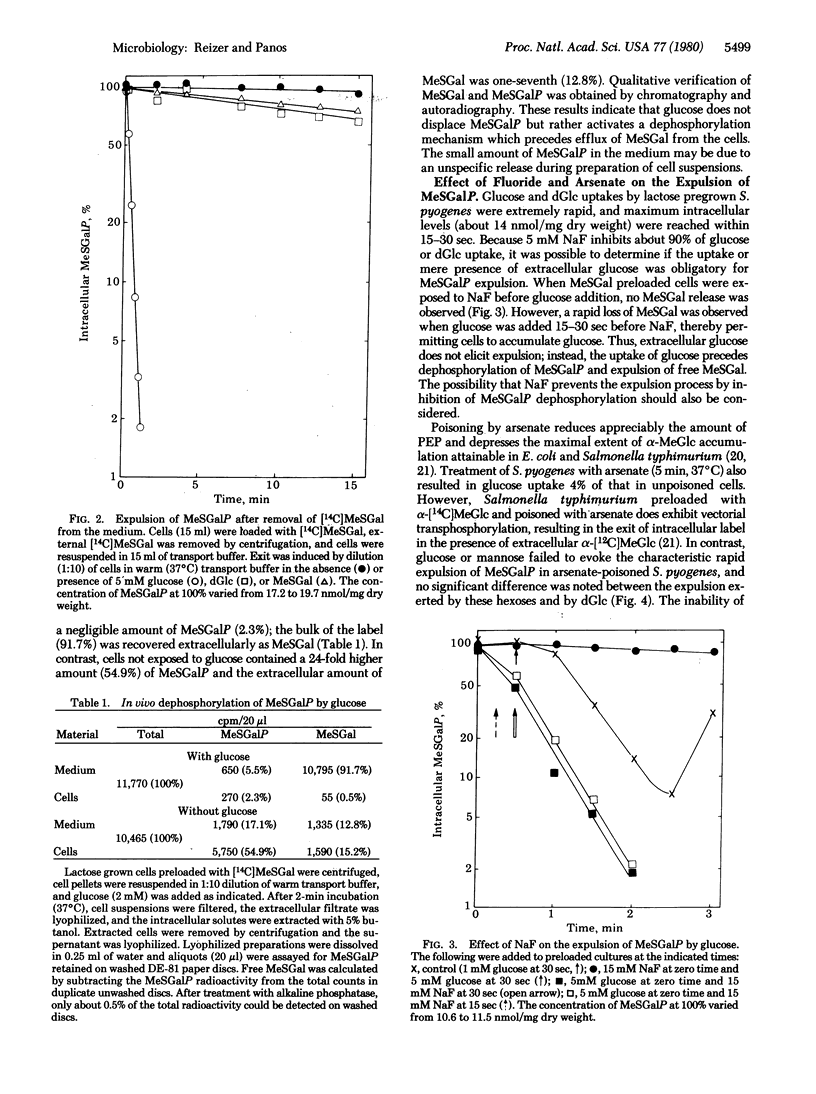
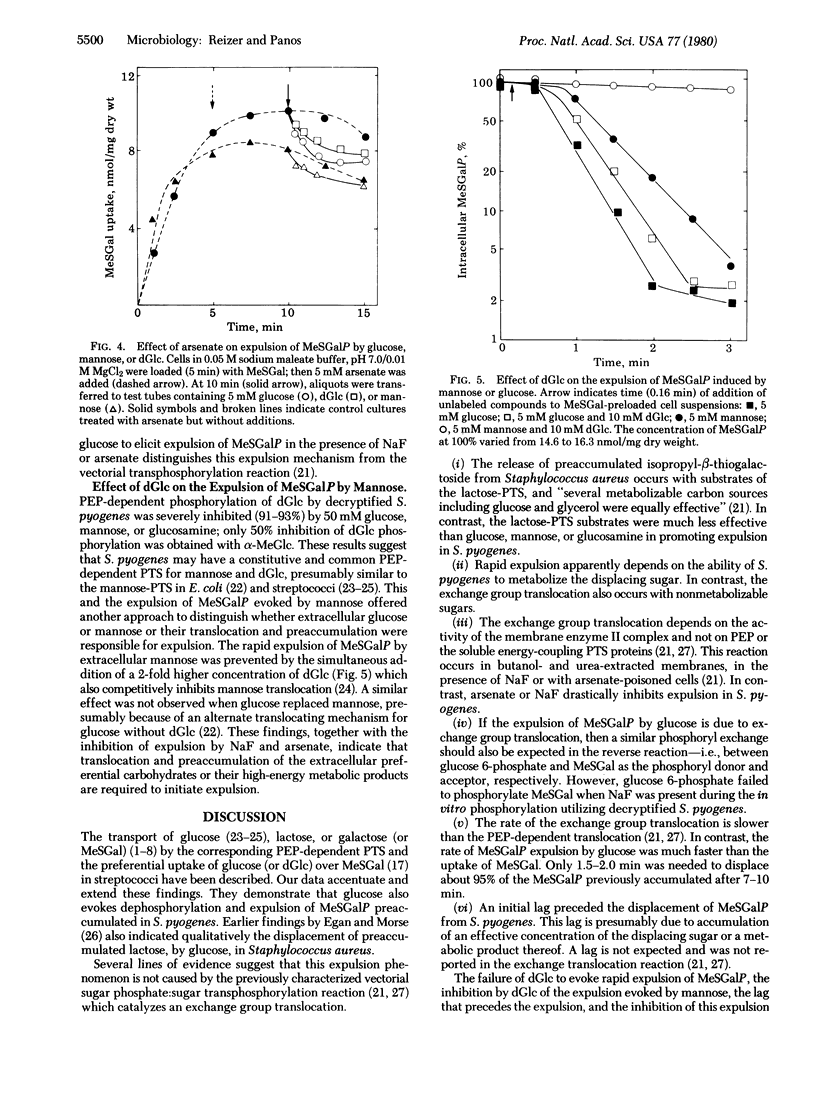
Streptococcus pyogenes pregrown on lactose took up glucose, lactose, or methyl beta-D-thiogalactopyranoside (MeSGal or TMG) by a phosphoenolpyruvate-dependent phosphotransferase system. MeSGal accumulated in the cell as MeSGal-phosphate (MeSGalP). Three effects were noted when various sugars were added to MeSGal preloaded cells: (i) no decrease in intracellular MeSGalP concentration after addition of fructose, sucrose, o-nitrophenyl-beta-D-galactoside, glycerol, 6-deoxyglucose, alpha-methyl D-glucoside, 2-deoxygalactose, glucose 1-phosphate, or glucose 6-phosphate; (ii) slow loss of preaccumulated MeSGalP evoked by lactose, 2-deoxy-D-glucose, or unlabeled MeSGal; and (iii) a short lag followed by extremely rapid expulsion of intracellular MeSGalP elicited by glucose or mannose and a slower expulsion elicited by glucosamine. The expelled compound was free MeSGal, indicating the involvement of dephosphorylation in the expulsion mechanism. Deoxyglucose inhibited the expulsion evoked by mannose, and prepoisoning of cells with fluoride or arsenate prevented the glucose-dependent expulsion. The expulsion is due to activation of an expulsion mechanism rather than to turnover of MeSGalP and leak of internal MeSGal with concomitant inhibition of MeSGal influx. The results suggest the need for phosphotransferase-dependent translocation of a preferential sugar or accumulation of the sugar catabolite for expulsion activation. The significance of the expulsion mechanism in synthesis regulation of enzymes involved in carbohydrate utilization is proposed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernlohr R. W., Haddox M. K., Goldberg N. D. Cyclic guanosine 3':5'-monophosphate in Escherichia coli and Bacillus lichenformis. J Biol Chem. 1974 Jul 10;249(13):4329–4331. [PubMed] [Google Scholar]
- Calmes R. Involvement of phosphoenolpyruvate in the catabolism of caries-conducive disaccharides by Streptococcus mutans: lactose transport. Infect Immun. 1978 Mar;19(3):934–942. doi: 10.1128/iai.19.3.934-942.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. J., Epstein W. Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol. 1975 Jun;122(3):1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. 3. Studies of the transport process. Biochim Biophys Acta. 1966 Jan 4;112(1):63–73. doi: 10.1016/s0926-6585(96)90009-6. [DOI] [PubMed] [Google Scholar]
- Haguenauer-Tsapis R., Kepes A. Unmasking of an essential thiol during function of the membrane bound enzyme II of the phosphoenolpyruvate glucose phosphotransferase system of Escherichia coli. Biochim Biophys Acta. 1977 Feb 14;465(1):118–130. doi: 10.1016/0005-2736(77)90360-1. [DOI] [PubMed] [Google Scholar]
- Haguenauer R., Kepes A. NaF inhibition of phosphorylation and dephosphorylation involved in -methyl-D glucoside transport in E. coli K 12. A pH dependant phenomenon sensitive to uncoupling agents. Biochimie. 1972;54(4):505–512. doi: 10.1016/s0300-9084(72)80235-9. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., Lo G. C. Co-induction of beta-galactosidase and the lactose-P-enolpyruvate phosphotransferase system in Streptococcus salivarius and Streptococcus mutans. J Bacteriol. 1978 Dec;136(3):900–908. doi: 10.1128/jb.136.3.900-908.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K., Röschenthaler R. beta-D-phosphogalactoside galactohydrolase of Streptococcus faecalis and the inhibition of its synthesis by glucose. Can J Microbiol. 1978 May;24(5):512–519. doi: 10.1139/m78-084. [DOI] [PubMed] [Google Scholar]
- KOCH A. L. THE ROLE OF PERMEASE IN TRANSPORT. Biochim Biophys Acta. 1964 Jan 27;79:177–200. doi: 10.1016/0926-6577(64)90050-6. [DOI] [PubMed] [Google Scholar]
- Kier L. D., Weppelman R., Ames B. N. Resolution and purification of three periplasmic phosphatases of Salmonella typhimurium. J Bacteriol. 1977 Apr;130(1):399–410. doi: 10.1128/jb.130.1.399-410.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- Koch A. L. Local and non-local interactions of fluxes mediated by the glucose and galactoside permeases of Escherichia coli. Biochim Biophys Acta. 1971 Oct 12;249(1):197–215. doi: 10.1016/0005-2736(71)90097-6. [DOI] [PubMed] [Google Scholar]
- LeBlanc D. J., Crow V. L., Lee L. N., Garon C. F. Influence of the lactose plasmid on the metabolism of galactose by Streptococcus lactis. J Bacteriol. 1979 Feb;137(2):878–884. doi: 10.1128/jb.137.2.878-884.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. P., Sowokinos J. R. Sugar phosphate phosphohydrolase. I. Substrate specificity, intracellular localization, and purification from Neisseria meningitidis. J Biol Chem. 1967 May 10;242(9):2264–2271. [PubMed] [Google Scholar]
- McKay L. L., Walter L. A., Sandine W. E., Elliker P. R. Involvement of phosphoenolpyruvate in lactose utilization by group N streptococci. J Bacteriol. 1969 Aug;99(2):603–610. doi: 10.1128/jb.99.2.603-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M. L., Hill K. L., Egan J. B., Hengstenberg W. Metabolism of lactose by Staphylococcus aureus and its genetic basis. J Bacteriol. 1968 Jun;95(6):2270–2274. doi: 10.1128/jb.95.6.2270-2274.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Cox D. F., Moczydlowski E. G. Sugar phosphate:sugar transphosphorylation coupled to exchange group translocation catalyzed by the enzyme II complexes of the phosphoenolpyruvate:sugar phosphotransferase system in membrane vesicles of Escherichia coli. J Biol Chem. 1977 Dec 25;252(24):8908–8916. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Mora W. K. Sugar phosphate: sugar transphosphorylation and exchange group translocation catalyzed by the enzyme 11 complexes of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1977 Dec 25;252(24):8899–8907. [PubMed] [Google Scholar]
- Saier M. H., Jr, Simoni R. D. Regulation of carbohydrate uptake in gram-positive bacteria. J Biol Chem. 1976 Feb 10;251(3):893–894. [PubMed] [Google Scholar]
- Schachtele C. F., Mayo J. A. Phosphoenolpyruvate-dependent glucose transport in oral streptococci. J Dent Res. 1973 Nov-Dec;52(6):1209–1215. doi: 10.1177/00220345730520060801. [DOI] [PubMed] [Google Scholar]
- Schachtele D. F., Leung W. L. Effect of sugar analogues on growth, sugar utilization, and acid production by Streptococcus mutans. J Dent Res. 1975 May-Jun;54(3):433–440. doi: 10.1177/00220345750540030301. [DOI] [PubMed] [Google Scholar]
- Thompson J. In vivo regulation of glycolysis and characterization of sugar: phosphotransferase systems in Streptococcus lactis. J Bacteriol. 1978 Nov;136(2):465–476. doi: 10.1128/jb.136.2.465-476.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Lactose metabolism in Streptococcus lactis: phosphorylation of galactose and glucose moieties in vivo. J Bacteriol. 1979 Dec;140(3):774–785. doi: 10.1128/jb.140.3.774-785.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Thomas T. D. Phosphoenolpyruvate and 2-phosphoglycerate: endogenous energy source(s) for sugar accumulation by starved cells of Streptococcus lactis. J Bacteriol. 1977 May;130(2):583–595. doi: 10.1128/jb.130.2.583-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Turner K. W., Thomas T. D. Catabolite inhibition and sequential metabolism of sugars by Streptococcus lactis. J Bacteriol. 1978 Mar;133(3):1163–1174. doi: 10.1128/jb.133.3.1163-1174.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. Inhibition of beta-galactoside transport by substrates of the glucose transport system in Escherichia coli. Biochim Biophys Acta. 1967;135(5):1030–1051. doi: 10.1016/0005-2736(67)90073-9. [DOI] [PubMed] [Google Scholar]


