Abstract
Sinonasal mucosal melanomas (SNMM) of the head and neck regions are rare and aggressive malignancies. Although they can affect patients of any ethnicity, they are more numerous in Chinese patients. The diagnosis and treatment of these tumors can be challenging. Recent studies have reported that Sox10 is a sensitive melanocytic marker for cutaneous melanoma (Nonaka et al. in Am J Surg Pathol 32:1291–1298, 2008). In addition, a CD117 (c-kit) gene mutation has been identified in cutaneous melanomas, indicating that there may be potential therapeutic benefits of tyrosine kinase inhibitors, such as Imatinib. The purpose of this study was to detect and test the immunohistochemical expression of Sox10 and c-kit in mucosal melanomas (MM) arising in the nasal cavities of Chinese patients. Twenty eight patients with mucosal melanomas of the nasal cavity were treated in two major hospitals in China. All cases had been locally diagnosed as primary SNMM. We confirmed all diagnoses with positive immunohistochemical stains for S100 and HMB-45. Additionally, automated immunohistochemistry was performed using a goat polyclonal Sox10 antibody and a monoclonal c-kit antibody counterstained using a standard avidin–biotin complex method. Immunohistochemical positive expression of Sox10 was defined by nuclear stain; and positivity for c-kit resulted in a distinct membranous staining. The extent of nuclear positivity for Sox10 and membranous stain for c-kit was graded by 4 board certified pathologists as follows: 1+, 1–25 % of positive tumor cells; 2+, 25–50 %; 3+, 50–75 %; and 4+, ≥75 %. Sox10 nuclear expression was found in all cases (100 %), with 4+ staining in 26 out of 28 cases (92.8 %) and 3+ staining in two cases with (7.1 %). The overall positivity for S100 staining was 23 out of 28 (82.1 %), with 1+ staining in 10 cases, 2+ staining in 6 cases, 3+ staining in 7 cases, and no staining in 5 cases. The sensitivity and intensity of Sox10 immunohistochemistry were both higher than with S100 immunohistochemistry. Immunopositivity of membranous stain for c-kit (CD117) was seen in 24 out of 28 cases (85.7 %), including 6 tumors that were 4+, eight that were 3+, six that were 2+, and four that showed 1+ staining. Our results demonstrate that Sox10 is a sensitive marker for SNMM and it may possess diagnostic value in addition to that of S100 protein. The expression of c-kit in the majority of MMs suggests that it may be useful in the assessment of these tumors for potential treatment with tyrosine kinase inhibitors.
Keywords: Sinonasal mucosal melanomas, Immunohistochemistry, Sox10, c-kit, Tyrosine kinase inhibitor
Introduction
Primary sinonasal mucosal melanomas (SNMM) are rare malignancies, accounting for 0.3–2 % malignant melanomas and about 4 % of head and neck melanomas [2]. The biologic behavior of these tumors is not only worse than their cutaneous counterparts, but they are often diagnosed at a later stage because of anatomical limitations [3–5]. For example, patients with SNMM may present with epistaxis due to obstruction of nasal cavities as their first symptom. Furthermore, SNMMs tend to metastasize earlier and relapse more frequently [2, 6, 7].
The correct diagnosis of SNMM may be challenging due to the notorious variability of tumor morphology and architectural and cytological growth patterns, especially in the absence of melanin pigment. SNMM can mimic any other type of malignant neoplasm. The usual differential diagnosis of SNMM includes poorly differentiated sinonasal malignancies, such as sinonasal undifferentiated carcinoma, sinonasal small cell carcinoma and neuroendocrine tumor, olfactory neuroblastoma, rhabdomyosarcoma, and malignant lymphoma. Immunohistochemical panels are useful in their distinction. While melanocytic markers, S100 protein, HMB-45, and Melan-A have been recognized as helpful in resolving the differential diagnosis, no single marker, however, is deemed sufficiently sensitive and specific as to support a final diagnosis.
Sox10 is a newly discovered melanocytic marker [1]. It functions as an important transcription factor of the Sox group E, and plays a crucial role in the normal development of neural crest [8]. The neural crest gives rise to migratory cells that colonize a wide range of embryonic tissues and will act as stem cells for a variety of local cells including melanocytes. Sox10 cooperates with other transcription factors to direct the development and differentiation of melanocytes and is essential for the commitment of the neural crest cells into melanocytic lineages [9, 10]. Sox10 expression persists throughout the entire differentiation pathway leading to mature melanocytes; when its expression is inhibited, the pathway leads toward alternative differentiation to other cell lineages [11]. The important role of Sox10 in the differentiation of melanocytes and its persistence throughout the pathway theoretically makes it a desirable marker for the detection of neoplasms of melanocytic origin [1]. Recent studies indicate that Sox10 is a sensitive and specific marker in the diagnosis of cutaneous melanomas [11–13]. While Sox10 has also been demonstrated to be a useful marker for identification of malignant melanoma in other organs, [13, 14] the utility of its expression has not been investigated in SNMM.
c-kit (CD117), a transmembrane receptor tyrosine kinase encoded by the proto-oncogene KIT, [15] enables the binding of the phosphorylate receptors to various substrate proteins when activated by carcinogenic mutations. This, in turn, leads to the activation of the signal transduction cascades that regulate cell proliferation, apoptosis and chemotaxis [16, 17].
Recent studies have reported that c-kit positivity is present in melanocytic lesions [15]. Inhibitors of tyrosine kinase, such as Imatinib, yield a favorable clinical response in cutaneous melanomas [18, 19]. c-kit gene mutations in various cutaneous and other mucosal melanomas have been identified by RT-PCR, but the immunostaining patterns for c-kit in SNMM tissue has not as yet been described.
Our objective was to study the immunohistochemical expression of Sox10 and c-kit protein in 28 SNMMs arising in Chinese patients to determine the value of Sox10 in their diagnosis and to identify potential candidates for tyrosine inhibitor-targeted therapy.
Materials and Methods
Case Selection
With the approval of both Institutional Review Boards, 28 cases of SNMM were retrieved from the archival files of the Department of Pathology, Beijing Tong Ren Hospital, Capital Medical University (Beijing, China) and Eye, Ear, and Throat Hospital, Fu Dan University (Shanghai, China), from April 2000 to July 2009. The patients ranged from 43 to 82 years of age, with a mean of 65.2 years (Table 1). Female patients slightly predominated (M:F = 13:15). All cases had been diagnosed as primary mucosal melanomas and confirmed by immunohistochemical stains for melanocytic markers including S100 protein, HMB-45, and/or Melan-A and PNL-2.
Table 1.
The clinical data of 28 sinonasal mucosa melanomas in Chinese patients
| Case | Sex | Age | Tumor sites | Adjuvant therapy after resection | Follow up status (from April 2000 to July 2009) |
|---|---|---|---|---|---|
| 1 | M | 68 | Left turbinate and nasal cavity | DOD after 4 years 9 months | |
| 2 | M | 62 | Right nasal cavity | Chemo, Rt | NOD for 6 year 2 months |
| 3 | F | 70 | right nasal cavity and septum | With recurrent tumor, LOF | |
| 4 | M | 76 | Left turbinate | LOF | |
| 5 | F | 66 | Right maxillary and ethmoid sinus | Chemo | LOF |
| 6 | F | 82 | Right nasal cavity | LOF | |
| 7 | M | 71 | Right nasal cavity | LOF | |
| 8 | M | 57 | Right nasal cavity and ethmoid sinus | Rt | Neck metastases, DOD after 2 years |
| 9 | M | 54 | Right nasal septum | LOF | |
| 10 | F | 43 | Left nasal cavity | With recurrent tumor, LOF | |
| 11 | M | 78 | Left nasal cavity/maxillary sinus and septum | Chemo | With recurrent tumor, DOD after 2 years |
| 12 | M | 49 | Left nasal cavity | Chemo | With recurrent tumor, LOF |
| 13 | F | 68 | Right nasal cavity | Rt | With recurrent tumor, LOF |
| 14 | F | 75 | Right nasal cavity | Rt | DOD after 1 year |
| 15 | M | 77 | Right nasal cavity | Chemo | DOD after 1 year and 3 months |
| 16 | F | 47 | Right nasal cavity | Chemo | With recurrent tumor and lung metastases, DOD after 2 years and 1 month |
| 17 | F | 61 | Left maxillary sinus | Chemo | With distant metastases, alive with disease for 6 months, LOF |
| 18 | M | 68 | Left nasal cavity | Chemo, Rt | With lung metastases, LOF |
| 19 | M | 54 | Left turbinate | With neck metastases, LOF | |
| 20 | F | 48 | Left nasal cavity | Chemo | DOD after 2 years |
| 21 | F | 51 | Right ethmoid sinus | LOF | |
| 22 | M | 65 | Left nasal cavity | DOD after 9 months | |
| 23 | F | 73 | Right nasal cavity | Chemo | With multiple organ metastases, DOD after 10 months |
| 24 | F | 70 | Right nasal cavity and septum | Chemo | With multiple abdominal organ metastases, DOD after 10 months |
| 25 | F | 78 | Left nasal cavity | LOF | |
| 26 | F | 68 | Right nasal cavity | Rt | With mediastinal metastases, DOD after 1 year 5 months |
| 27 | M | 71 | Right nasal cavity | LOF | |
| 28 | F | 77 | Right nasal cavity | LOF |
Due to geography and because patients frequently switch hospitals, this follow up data was based on telephone interviews with the patient and/or the patient’s family; independent medical documentation for confirmation was not available
DOD dead of disease after initial diagnosis, Chemo chemotherapy, Rt radiation, NOD negative of disease, LOF loss of follow up
Immunohistochemistry
Blocks of formalin-fixed, paraffin-embedded tissue were retrieved from the archive and sectioned at 4 μm. Serial sections were used for immunohistochemical analysis and stained for the following antibodies: Sox10 (goat polyclonal antibody,1:100, Santa Cruz, CA, USA) and CD117 (c-kit, pre-diluted monoclonal antibody, clone 9.7, Ventana Medical Systems, Tucson, AZ, USA). Immunostaining was performed using the Ventana Benchmark XT immnostainer (Ventana Medical Systems, Tucson, AZ, USA) with the manufacturer’s deparaffinization procedure, antigen retrieval, and detection reagents. Antigen retrieval consisted in incubating the tissue sections in microwave-heated 0.01 M citrated buffer (pH 6.0) and allowing them to cool to room temperature prior to staining. After secondary antibody application, visualization of immunostaining was achieved by a standard avidin–biotin complex detection using diaminobenzidine. In some cases with a high content of melanin pigment, 3-Ethylamino carbazole (AEC) was used to avoid the interference of pigment. Slides were counterstained with hematoxylin. Mast cells were identified on each slide and used as an internal positive control for the c-kit (CD117) staining. Non-neoplastic sinonasal mucosa was used as negative controls using tissue sections incubated with isotype-matched serum without primary antibody.
Immunohistochemical expression of Sox10 was defined by nuclear staining. The c-kit (CD117) positivity was characterized by distinct membranous staining. The extent of nuclear positivity for Sox10 and membranous stain for c-kit was graded as follows: 1+, 1–25 % of tumor cells stained; 2+, 25–50 %; 3+, 50–75 %; 4+, ≥75 %. All stained slides were reviewed by 4 board–certified pathologists.
Results
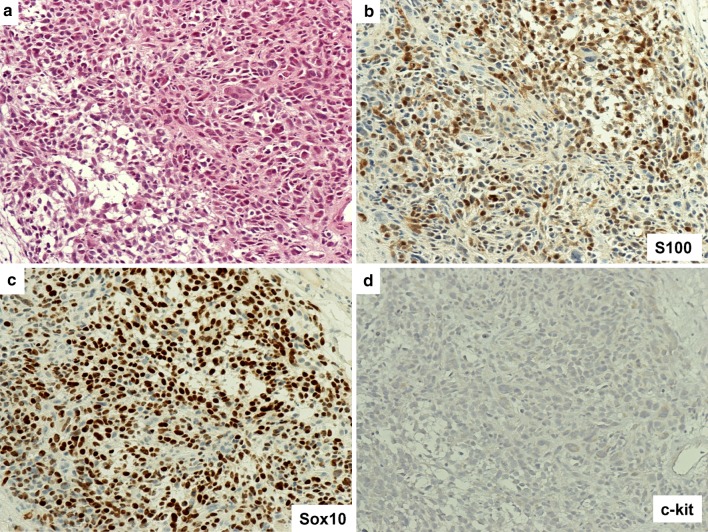
Sox10 nuclear staining was found in all cases (100 %). Specifically, 26 out of the 28 cases revealed diffuse stain 4+ (92.8 %, Figs. 1c, 2c, 3c), and 2 cases were 3+ (7.1 %).
Fig. 1.
Representative case of mucosal melanoma, conventional morphology. a Section of SNMM shows epithelioid tumor cells [Hematoxylin and Eosin (H&E)] b Immunostain for S100 staining highlights both nuclei and cytoplasm. c Sox10 nuclear stain. d Immunostain for c-kit shows negative staining. Original magnification ×200 for a–d
Fig. 2.
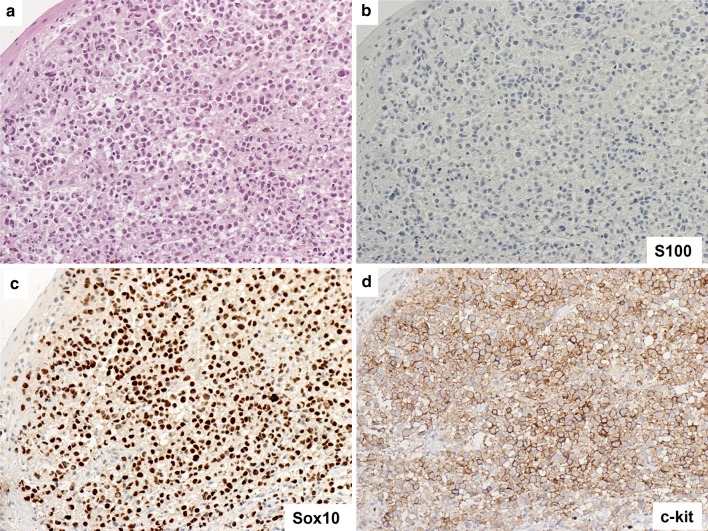
Representative case of mucosal melanoma, plasmacytoid morphology. a Overlying squamous epithelium and sub-epithelial melanoma with plasmacytoid tumor cells and occasional bi-nucleated forms (H&E). b No expression for S100. c Sox10 highlights all nuclei of the melanoma cells. d Immunostain for c-kit shows strong membranous staining (4+). Original magnification ×200 for a–d
Fig. 3.
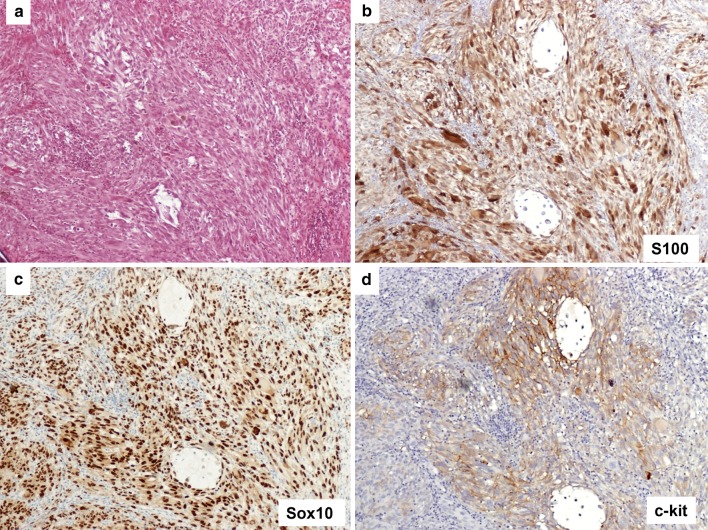
Representative case of mucosal melanoma, spindle cell morphology. a Section of melanoma composed of spindle shaped tumor cells (H&E). b Immunostain for S100 shows nuclear and cytoplasmic staining. c Sox10 highlights all nuclei of the melanoma cells. d Immunostain for c-kit shows membrane staining (3+). Original magnification ×100 for a–d
S100 staining was negative in 5 cases (Fig. 2b); 1+ in 10 cases; 2+ in 6 cases (Fig. 1b); 3+ in 7 cases (Fig. 3b); 4+ , in 0 cases. The overall positivity was 23/28 (82.1 %). Our preliminary data demonstrated that Sox10 staining is more sensitive and stronger than S100 in comparing Sox10 and S100 staining patterns.
c-kit membranous staining was seen in the cytoplasmic membranes in 24 out of 28 cases (85.7 %), including 6 tumors which were diffusely positive 4+ (Fig. 2d), 8 which were 3+ (Fig. 3d), 6 which were 2+ and 4 with focal and weak staining 1+.
Discussion
Mucosal malignant melanomas are infrequent tumors. The head and neck region accounts for approximately half of all human mucosal melanomas representing 9 % of all malignant head and neck tumors [7]. The remaining mucosal melanomas arise from the anorectal mucosa, the female genitalia, and the urinary tract [3, 5, 20]. Mucosal melanomas of the head and neck area most commonly occur in the nasal cavity, followed by the oral cavity and paranasal sinuses; they are much less common in nasopharynx, larynx and middle ear [21]. In general, sinonasal tract mucosal melanomas (SNMM) affect the nasal cavity more frequently than the paranasal sinuses, and are typically seen in patients in their 6th to 8th decades of life. There is no significant sex predilection observed in mucosal melanoma of the head and neck [19, 22–24]. In our series, 28 Chinese patients had an average age of 65.2 years (Table 1). Female patients were affected slightly more than male (M:F = 13:15).
Clinical symptoms caused by SNMM can be variable, and are usually associated with tumor obstruction of the sinuses. Patients with nasal cavity mucosal melanoma may present with epistaxis and difficulty breathing, leading to earlier discovery than those with tumors of the paranasal sinuses. Risk factors for SNMM are unclear.
The pathogenesis of SNMM is unknown. SNMMs are characterized by aggressive biological behavior, leading to a 5 years survival rate of <25 to 30 % [7, 19]. Local recurrence is common, resulting by from incomplete resection [19]. The diagnosis of primary SNMM can be difficult. The pathologist is expected to identify the presence of mucosal melanoma in situ and needs to exclude metastases. An in situ component is uncommon, being found in only 23 out of 115 cases in the series of Thompson et al. [2].
Morphologically, melanoma is a notorious mimicker, which may appear epithelioid, spindled, and even plasmacytoid. Its cells can be arranged in an organoid pattern or a solid growth pattern. Histologic diagnosis of mucosal melanoma of the head and neck can be challenging, requiring immunohistochemical stains which are less reliable than in cutaneous lesions. Melanocytic markers including S100, human melanoma black-45 (HMB-45), Melan-A, microphthalmia transcription factor (MITF), Tyrosinase and PNL-2 may be helpful in confirmation of the diagnosis [3, 23]. PNL-2 is a monoclonal antibody, directly against a formalin resistant melanocytic antigen. It has been used as an adjunctive marker in combination of S100 and HMB-45 in diagnoses of melanocytic lesions [25]. Sox10 is a novel marker that has not been examined in mucosal melanoma.
Sox10 immunoreactivity
Recent studies have reported that Sox10 is a sensitive melanocytic marker for cutaneous melanoma [1, 12, 13]. During the embryonic development, Sox10, as a transcription factor of the Sox group E, directs melanocytes derived from neural crest in their migration to skin and mucosal epithelia [8–10]. Expression of Sox10 is continuous throughout the differentiation pathway toward mature melanocytes [11, 26]. Sox10 is essential for the specification and differentiation of melanocytic lineage. The essential function of Sox10 in the specification and differentiation of the melanocytic lineage makes it a useful melanocytic marker [1, 11]. Although it has been reported that Sox10 can be highlighted in epidermal melanocytes and cutaneous melanomas [10–13], little data are available on Sox10 expression in SNMM.
We report the first study of the expression of Sox10 in SNMM. Our results showed that Sox10 was expressed in all 28 SNMM cases with diffuse and strong nuclear staining (Figs. 1c, 2c, 3c). For comparison, the expression of S100 was detected in 23 of 28 cases (1+ in 10 cases, 2+ in 6 cases and 3+ in 7 cases). Several authors reported that Sox10 had a higher sensitivity and specificity for cutaneous malignant melanoma as well as for metastatic melanoma in sentinel lymph nodes than other immunohistochemical markers such as S100 protein, HMB-45, Melan-A [1, 13, 14].
S100 protein has been one of the most frequently used screen markers for melanoma diagnosis. However, it possesses a lower specificity, and is expressed by a variety of normal cell types including reactive lesions and benign and malignant neoplasmas [15, 16]. When comparing it, S100 protein immunoreacts with fibrocytes and histiocytes, complicating the assessment of residual melanoma versus scar [12]. Additionally, the S100 protein signal is present in both nuclear and cytoplasmic locations; its utilization to evaluate the presence of melanoma in situ may not always be helpful [12]. In this study, 5 of 28 SNMM were negative for S100 staining. This phenomenon has also been reported in other studies, including negativity of 9 % of malignant melanomas in the Thompson et al. series [2] and 5 % in the Prasad et al. series [27]. Similarly, other markers also have a significant percentage of negative expression in melanomas, and the general surgical pathologist (and indeed, specialty pathologist) needs to be aware of this. In addition, S-100 is not a specific stain unique for melanoma, since other cell types also react with it. Although HMB-45 may be a more sensitive marker than S100 for immunohistochemical diagnosis of primary oral and nasal MM, [28] it is less sensitive than Sox10, which might lead to false-negative results. Sox10 yields a much higher sensitivity and specificity with distinct nuclear staining pattern which make it easier to detect, especially in lesions rich in melanin. Therefore, the use of Sox10 is more sensitive than other melanocytic markers in detecting residual, metastatic, and in situ melanoma [12–14]. Our data showed similar results to those previously reported in a study in cutaneous melanoma [1, 12, 29]. For the first time, we report that Sox10 is a useful sensitive marker for the diagnosis of SNMM (Fig. 1, 2, 3).
c-kit immunoreactivity
Since the KIT mutation was first identified in melanoma in 2004, [30] several reports have found KIT that mutations involving exon 11, 13, 17 are the most frequent sites of mutations in cutaneous melanomas. Exon 11 and K642E point mutations of exon 13 are the most important genetic alternation subsites [31–38]. Moreover, about 30 % of melanoma samples with KIT mutation have also demonstrated KIT gene amplification [32–35, 38]. Remarkably, the prevalence of KIT mutation or amplification is dramatically different in specific subtypes of melanoma. c-kit mutations and amplifications have been found in approximately 15–30 % of mucosal and acral-lentiginous melanomas [7]. It has also been reported that mucosal melanoma shows a higher frequency of KIT mutation and amplification events than acral melanoma [15, 35–39].
Torres-Cabala et al. [38] and other demonstrated significant correlation between the percentage of c-kit positive cells by immunohistochemistry and KIT mutation status in invasive acral lentiginous/mucosal melanomas [30–32, 38]. The expression of c-kit was also seen in 31 % of melanomas without detectable KIT mutation or amplification [33]. Thus, other KIT function mechanisms may account for the aberrant c-kit signaling.
C-kit protein is a transmembranous receptor tyrosine kinase. Therapy based on KIT gene targeting is based on the expression of c-kit protein. In most subtypes of melanoma, c-kit expression can be detected in immunohistochemistry including superficial spreading cutaneous melanomas (53.7 %), primary anorectal melanomas (75 %), more than 80 % metastatic melanomas and acral lentiginous/mucosal melanomas (84 %) [7, 40–43]. In our study, the expression of c-kit on SNMM was present in 24 of 28 cases (85.7 %).
Treatment of resectable SNMM remains surgical. Due to the rarity of this tumor, no clear-cut protocol has been established for those cases that are not surgically resectable. In addition to surgical resection, adjuvant radiation and/or chemotherapy, and immunotherapies (e.g. interleukins and interferon) are often required because of the difficulty in achieving negative margins [42–44]. In addition to these treatment modalities, c-kit gene targeting therapy may well represent a new promising therapeutic approach [7, 41, 45].
It is well known that Imatinib is a specific competitive inhibitor of tyrosine kinase in KIT gene mutation pathway. Clinical trials have already demonstrated promising clinical response in tumors with KIT gene mutation, including melanomas expressing the c-kit protein by immunohistochemistry, [45–47] Papaspyrou et al. have advocated therapy for mucosal melanomas of the head and neck emphasizing c-kit protein inhibiting treatment modalities for tumors carrying c-kit mutations [7].
Although the underlying relationships between KIT gene mutation and/or amplification, c-kit protein expression and Imatinib sensitivity are still controversial, the c-kit expression in our study may provide insight into the value of Imatinib in the treatment of SNMM. We found that the expression of c-kit protein in SNMM by routine immunohistochemistry is reproducible.
In summary, our results demonstrate that Sox10 is a highly sensitive and specific melanocytic marker for SNMM. If added to conventional melanocytic markers, Sox10 may have more diagnostic value than S100 protein. In addition, the expression of c-kit in the majority of SNMM by immunohistochemistry may provide useful information for the treatment by potential target therapy using tyrosine kinase inhibitors.
Acknowledgments
The authors thank Dr. M. J. Klein for his advice in the preparation of this manuscript. This study is funded by NIH/NCRR 1UL1RR029893-01.
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
Dr. Herman Yee deceased.
References
- 1.Nonaka D, Chiriboda L, Rubin BP. SOX10: a pan-schwannian and melanocytic marker. Am J Surg Pathol. 2008;32:1291–1298. doi: 10.1097/PAS.0b013e3181658c14. [DOI] [PubMed] [Google Scholar]
- 2.Thompson LD, Wieneke JA, Miettinen M. Sinonasal tract and nasopharyngeal melanomas: a clinicopathologic study of 115 cases with a proposed staging system. Am J Surg Pathol. 2003;27:594–611. doi: 10.1097/00000478-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Younes MN, Myers JN. Melanoma of the head and neck: current concepts in staging, diagnosis, and management. Surg Oncol Clin N Am. 2004;13:201–229. doi: 10.1016/S1055-3207(03)00125-X. [DOI] [PubMed] [Google Scholar]
- 4.Penel N, Mallet Y, Mirabel X, et al. Primary mucosal melanoma of head and neck: prognostic value of clear margins. Laryngoscope. 2006;116:993–995. doi: 10.1097/01.mlg.0000217236.06585.a9. [DOI] [PubMed] [Google Scholar]
- 5.Meleti M. leemans CR, de Bree R, et al. Head and neck mucosal melanoma: experience with 42 patients, with emphasis on the role of postoperative radiotherapy. Head Neck. 2008;30:1543–1551. doi: 10.1002/hed.20901. [DOI] [PubMed] [Google Scholar]
- 6.Pandey M, Mathew A, Iype EM, et al. Primary malignant mucosal melanoma of the head and neck region: pooled analysis of 60 published cases from India and review of literature. Eur J Cancer Prev. 2002;11:3–10. doi: 10.1097/00008469-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Papaspyrou G, Garbe C, Schadendorf D, et al. Mucosal melanomas of the head and neck: new aspects of the clinical outcome, molecular pathology, and treatment with c-kit inhibitors. Melanoma Res. 2011;21:475–482. doi: 10.1097/CMR.0b013e32834b58cf. [DOI] [PubMed] [Google Scholar]
- 8.Gershon TR, Oppenhermer O, Chin SS, et al. Temporally regulated neural crest transcription factors distinguish neuroectodermal tumors of varying malignancy and differentiation. Neoplasia. 2005;7:575–584. doi: 10.1593/neo.04637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potterf SB, Mollaaghababa R, Hou L, et al. Analysis of SOX10 function in neural crest-derived melanocyte development: SOX10-dependent transcriptional control of dopachrome tautomerase. Dev. Biol. 2001;237:245–257. doi: 10.1006/dbio.2001.0372. [DOI] [PubMed] [Google Scholar]
- 10.Kordes U, Cheng YC, Scotting PJ. Sox group E gene expression distinguishes different types and maturational stages of glial cells in developing chick and mouse. Brain Res Dev Brain Res. 2005;157:209–213. doi: 10.1016/j.devbrainres.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Kelsch RN. Sorting out SOX10 functions in neural crest development. BioEssays. 2006;28:788. doi: 10.1002/bies.20445. [DOI] [PubMed] [Google Scholar]
- 12.Ramos-Herberth FI, Karamchandani J, Kim J, et al. SOX10 immunostaining distinguishes desmoplastic melanoma from excision scar. J Cutan Pathol. 2001;37:944–952. doi: 10.1111/j.1600-0560.2010.01568.x. [DOI] [PubMed] [Google Scholar]
- 13.Blochin E, Nonaka D. Diagnostic value of Sox10 immunohistochemical staining for the detection of metastatic melanoma in sentinel lymph nodes. Histopathology. 2009;55:626–628. doi: 10.1111/j.1365-2559.2009.03415.x. [DOI] [PubMed] [Google Scholar]
- 14.Jennings C, Kim J. Identification of Nodal Metastases in Melanoma Using Sox-10. Am J Dermatopathol. 2011;33:474–482. doi: 10.1097/DAD.0b013e3182042893. [DOI] [PubMed] [Google Scholar]
- 15.Rivera RS, Nagatsuka H, Gunduz M, et al. c-kit protein expression correlated with activating mutations in KIT gene in oral mucosal melanoma. Virchows Arch. 2008;452:27–32. doi: 10.1007/s00428-007-0524-2. [DOI] [PubMed] [Google Scholar]
- 16.Lennartsson J, Blume-Jensen P, Hermanson M, et al. Phosphorylation of Shc by Src family kinases is necessary for stem cell factor receptor/c-kit mediated activation of Ras/MAP kinase pathway and c-fos induction. Oncogen. 1999;18:5546–5553. doi: 10.1038/sj.onc.1202929. [DOI] [PubMed] [Google Scholar]
- 17.Lin SC, Liu CL, Wang TI, et al. Clinical implication of C-kit gene mutation in patients with large gastrointestinal stromal tumors. J Gastroenterol Hepatol. 2006;21:1604–1608. doi: 10.1111/j.1440-1746.2006.04322.x. [DOI] [PubMed] [Google Scholar]
- 18.Handolias D, Hamilton AL, Salemi R, et al. Clinical responses observed with imatinib or sorafenib in melanoma patients expressing mutations in KIT. Br J Cancer. 2010;102:1219–1223. doi: 10.1038/sj.bjc.6605635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clifton N. Harrison l, Bradley PJ, Jones NS. Malignant melanoma of nasal cavity and paranasal sinuses: report 24 patients and literature review. J Laryngol Otol. 2011;125:479–485. doi: 10.1017/S0022215110002720. [DOI] [PubMed] [Google Scholar]
- 20.Mori W. Geographic pathology of malignant melanoma in Japan. In: McGovem VJ, Russell P, editors. Pigment cell mechanism in pigmentation. Basel: Karger; 1973. pp. 246–254. [Google Scholar]
- 21.Shah JP, Huvos AG, Strong EW. Mucosal melanomas of the head and neck. Am J Surg. 1977;134:531–535. doi: 10.1016/0002-9610(77)90393-2. [DOI] [PubMed] [Google Scholar]
- 22.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base Report on cutaneous and non cutaneous melanoma. A summary of 84,836 cases from the past decade. Cancer. 1998;83:1664–1678. doi: 10.1002/(SICI)1097-0142(19981015)83:8<1664::AID-CNCR23>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 23.Wagner M, Morris CG, Werning JW, et al. Mucosal melanoma of the head and neck. Am J Clin Oncol. 2008;31:43–48. doi: 10.1097/COC.0b013e318134ee88. [DOI] [PubMed] [Google Scholar]
- 24.Patrick RJ, Fenske NA, Messina JL. Primary mucosal melanoma. J Am Academy Dermatol. 2007;56:828–834. doi: 10.1016/j.jaad.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Morris LG, Wen YH, Nonaka D, et al. melanocytic marker in immunohistochemical evaluation of primary mucosal melanoma of the head and neck. Head Neck. 2008;30:771–775. doi: 10.1002/hed.20785. [DOI] [PubMed] [Google Scholar]
- 26.Jiao Z, Zhang ZG, Hornyak TJ, et al. Dopachrome tautomerase (Dct) regulates neural progenitor cell proliferation. Dev Biol. 2006;296:396–408. doi: 10.1016/j.ydbio.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Prasad ML, Jungbluth AA, Iversen K, Huvos AG, Busam KJ. Expression of melanocytic differentiation markers in malignant melanomas of the oral and sinonasal mucosa. Am J Surg Pathol. 2001;25:782–787. doi: 10.1097/00000478-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Yu CH, Chen HH, Jeng YM, et al. HMB-45 may be a more sensitive marker than S100 or Melan-A for immunohistochemical diagnosis of primary oral and nasal mucosa melanomas. J Oral Pathol Med. 2005;34:540–545. doi: 10.1111/j.1600-0714.2005.00340.x. [DOI] [PubMed] [Google Scholar]
- 29.Shin J, Vincent JG, Cuda JD, et al. Sox10 is expressed in primary melanocytic neoplasms of various histologies but not in fibrohistiocytic proliferations and histiocytoses. J Am Acad Dermatol. 2012; Feb 9. [Epub ahead of print]. PMID:22325460. [DOI] [PubMed]
- 30.Went PT, Dirnhofer S, Bundi M, et al. Prevalence of KIT expression in human tumors. J Clin Oncol. 2004;22:4514–4522. doi: 10.1200/JCO.2004.10.125. [DOI] [PubMed] [Google Scholar]
- 31.Willmore-Payne C, Holden JA, Tripp S, et al. Human malignant melanoma: detection of BRAF- and c-kit-activating mutations by high-resolution amplicon melting analysis. Hum Pathol. 2005;36:486–493. doi: 10.1016/j.humpath.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Willmore-Payne C, Holden JA, Hirshowitz S, et al. BRAF and c-kit gene copy number in mutation-positive malignant melanoma. Hum Pathol. 2006;37:520–527. doi: 10.1016/j.humpath.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Curtin JA, Busam K, Pinkel D, et al. Somatic activation of KIT in distinct subtypes of melamoma. J Clin Oncol. 2006;24:4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 34.Antonescu CR, Busam KJ, Francone TD, et al. L567P KIT mutation in anal melanomas correlates with KIT protein expression and is sensitive to specific kinase inhibition. Int J Cancer. 2007;121:257–264. doi: 10.1002/ijc.22681. [DOI] [PubMed] [Google Scholar]
- 35.Beadling C, Jacobson-Dunlop E, Hodi FS, et al. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res. 2008;14:6821–6828. doi: 10.1158/1078-0432.CCR-08-0575. [DOI] [PubMed] [Google Scholar]
- 36.Satzger I, Schaefer T, Kuettler U, et al. Analysis of c-KIT expression and KIT gene mutation in human mucosal melanomas. Br J Cancer. 2008;99:2065–2069. doi: 10.1038/sj.bjc.6604791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashida A, Takata M, Murata H, et al. Pathological activation of KIT in metastatic tumors of acral and mucosal melanomas. Int J Cancer. 2009;124:862–868. doi: 10.1002/ijc.24048. [DOI] [PubMed] [Google Scholar]
- 38.Torres-Cabala CA, Wang WL, Trent J, et al. Correlation between KIT expression and KIT mutation in melanoma: a study of 173 cases with emphasis on the acral-lentiginous/mucosal type. Mod Pathol. 2009;22:1446–1456. doi: 10.1038/modpathol.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodman SE, Davies MA. Targeting KIT in melanoma: a paradigm of molecular medicine and targeted therapeutics. Biochem Pharmacol. 2010;80:568–574. doi: 10.1016/j.bcp.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chute DJ, Cousar JB, Mills SE. Anorectal malignant melanoma: morphologic and immunohistochemical features. Am J Clin Pathol. 2006;126:93–100. doi: 10.1309/DVWLTV8FFKC3L80H. [DOI] [PubMed] [Google Scholar]
- 41.Alexis BJ, Martinez AE, Lutzky J. An immunohistochemical evaluation of c-kit (CD117) expression in malignant melanoma, and results of imatinib mesylate (Gleevec) therapy in three patients. Melanoma Res. 2005;15:283–285. doi: 10.1097/00008390-200508000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Medina JE, Ferlito A, Pellitteri PK, et al. Current management of mucosal melanoma of the head and neck. J Surg Oncol. 2003;83:116–122. doi: 10.1002/jso.10247. [DOI] [PubMed] [Google Scholar]
- 43.Patel SG, Prasad ML, Escrig M, et al. Primary mucosal malignant melanoma of the head and neck. Head Neck. 2002;24:247–257. doi: 10.1002/hed.10019. [DOI] [PubMed] [Google Scholar]
- 44.Gavriel H, McArthur G, Sizeland A, et al. Review: mucosal melanoma of the head and neck. Melanoma Res. 2011;21:257–266. doi: 10.1097/CMR.0b013e3283470ffd. [DOI] [PubMed] [Google Scholar]
- 45.Fiorentini G, Rossi S, Lanzanova G, et al. Tyrosine Kinase inhibitor imatinib mesylate as an anticancer agent for advanced ocular melanoma expressing immunohistochemical C-KIT (CD117): preliminary results of a compassionate use clinical trail. J Exp Clin Cancer Res. 2003;22:17–20. [PubMed] [Google Scholar]
- 46.Ivan D, Niveiro M, Diwan AH, et al. Analysis of protein tyrosine kinases expression in the melanoma metastases of patients treated with Imatinib Mesylate (STI571, Gleevec) J Cutan Pathol. 2006;33:280–285. doi: 10.1111/j.0303-6987.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- 47.Lefevre G, Glotin AL, Calipel A, et al. Roles of stem cell factor/c-kit and effects of Glivec/STI571 in human uveal melanoma cell tumorigenesis. J Biol Chem. 2004;279:31769–31779. doi: 10.1074/jbc.M403907200. [DOI] [PubMed] [Google Scholar]