Abstract
A case of an epulis in an 8-month-old boy is reported. The tumor was localized on the mandibular alveolar ridge and, according to the parents, was a recurrence of a congenital tumor excised when the boy was 2 months old. Microscopically, it was composed of many spindled or ovoid cells with vesicular nuclei and non-granular eosinophilic cytoplasm, and covered by acanthotic parakeratinized squamous epithelium with broad rete pegs. Immunohistochemically, the spindled and ovoid cells were intensely positive for vimentin and neuron-specific enolase, and negative for S-100 protein. The final diagnosis was spindle cell epulis. The possible correlation of this lesion with congenital granular cell epulis is discussed.
Keywords: Congenital, Epulis, Newborn, Oral tumors
Introduction
The congenital granular cell epulis (CGCE) or congenital epulis is a rare benign tumor arising on the anterior alveolar mucosa of newborns [1, 2]. It is 9–10 times more common in females than males, and 2–3 times more common in the maxilla than the mandible. Clinically, it presents as a solitary, lobular and pedunculated tumor, with smooth surface and normal color. It is usually asymptomatic and rarely interferes with respiration or feeding.
Microscopically, CGCE is composed of sheets of large to medium-sized, round to polyhedral cells that have a discrete cellular membrane, abundant granular cytoplasm, and a single darkly staining nucleus with an eccentrically placed nucleolus [1, 3, 4]. The covering epithelium is thin and attenuated, without rete pegs, and rests of odontogenic epithelium may be found among the granular cells. Immunohistochemically, granular cells are positive for vimentin and, in most cases, for neuron-specific enolase (NSE), but negative for S-100 protein [2, 4]. Odontogenic epithelium, fibroblasts, histiocytes, smooth muscle, nerve-related cells, endothelial cells, pericytes, myofibroblasts, and undifferentiated mesenchymal cells have been implicated in the pathogenesis of CGCE, which, however, remains undefined [4].
In previous reports, elongated, fusiform, or spindled cells were identified in the stroma of CGCE [3–14]. These cells had occasional granular cytoplasm, [5–8], but were predominantly non-granular fibroblastic cells [4, 6, 9–14], localized between [4, 9] or around [10, 12] the granular cells, or around small blood vessels [9, 11]. Some authors described them as interstitial cells [4, 9–12]. However, their origin and association with granular cells is not clear.
We report a case of a recurrent epulis in an 8-month-old boy exclusively composed of spindled and ovoid non-granular cells that had a CGCE immunophenotype.
Case Report
The tumor was localized on the mandibular alveolar ridge and, according to the parents, was a recurrence of a congenital tumor excised when the boy was 2 months old, without being submitted to microscopic examination. He was otherwise healthy and was born with normal delivery after an uneventful pregnancy. The tumor did not interfere with respiration or breastfeeding. It was pedunculated, with a smooth surface and normal color (Fig. 1). After conservative excision, wound healing was uneventful and no recurrence had been reported 1 year after treatment.
Fig. 1.
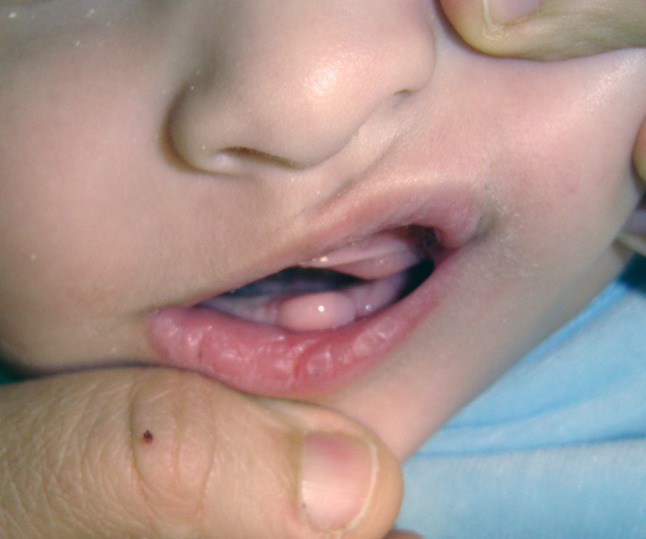
Pedunculated tumor on the mandibular alveolar ridge
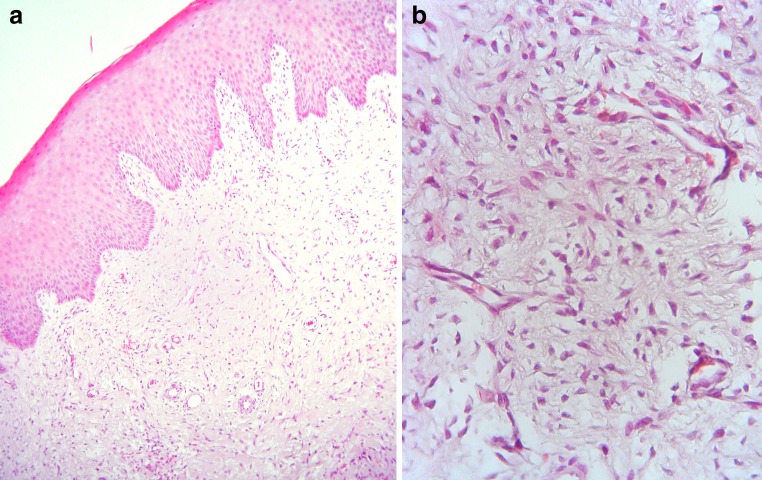
Multiple 5 μm-thick formalin-fixed, paraffin-embedded tissue sections showed a mass of loose vascular connective tissue covered by acanthotic parakeratinized squamous epithelium with broad rete pegs (Fig. 2a). In the connective tissue, many spindled or ovoid cells with vesicular nuclei and non-granular eosinophilic cytoplasm were seen (Fig. 2b). The cells did not show a certain arrangement and were widely spaced. No typical granular cells were identified after studying multiple sections. Mild focal lymphocytic infiltration was present. Those features were consistent with a congenital fibrous epulis.
Fig. 2.
a Low-power view shows mass of loose vascular connective tissue covered by acanthotic parakeratinized squamous epithelium with broad rete pegs. b High-power view shows many spindled or ovoid cells with vesicular nuclei and scant, non-granular eosinophilic cytoplasm (hematoxylin and eosin stain, original magnifications a ×100 and b ×400)
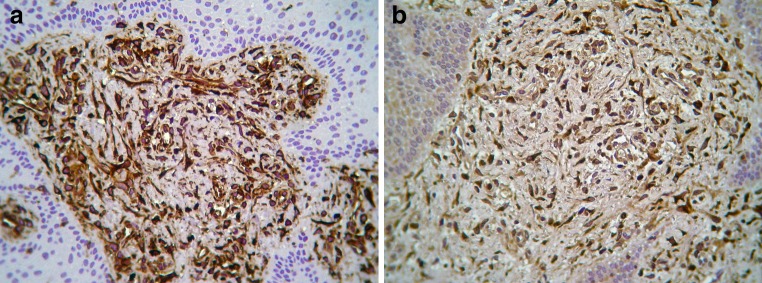
Streptavidin-biotin-peroxidase immunohistochemistry was performed with the Ventana BenchMark XT fully automated slide preparation system (Ventana Medical Systems Inc., Tucson, AZ) and the iView DAB detection kit (Ventana). Clone, dilution and manufacturer of primary antibodies are shown in Table 1. Appropriate positive controls were used according to the instructions of the antibodies’ manufacturers, while in negative controls primary antibodies were substituted with a non-immune serum of the same specificity. The spindled and ovoid cells were intensely positive for vimentin (Fig. 3a) and NSE (Fig. 3b), and negative for pan-keratin, S-100 protein, carcinoembryonic antigen (CEA), glial fibrilliary acidic protein (GFAP), smooth muscle actin, muscle specific actin (HHF35), desmin, CD68, and CD31. Smooth muscle actin, muscle specific actin (HHF35) and CD31 revealed a complex network of vascular channels.
Table 1.
Clone, dilution, and manufacturer of primary antibodies used in the present case
| Antibody | Clone | Dilution | Manufacturer |
|---|---|---|---|
| Vimentin | V9 | 1:100 | DAKO |
| Neuron-specific enolase | BBS/NC/VI-H14 | 1:150 | DAKO |
| Pan-keratin | AE1/AE3 | 1:50 | DAKO |
| S-100 protein | 4C4.9 | 1:200 | ZYTOMEDa |
| Carcinoembryonic antigen (CEA) | A0115 | 1:40 | DAKO |
| Glial fibrilliary acidic protein (GFAP) | 6F2 | 1:100 | DAKO |
| Alpha smooth muscle actin | NCL-SMA | 1:50 | NOVOCASTRAb |
| Muscle specific actin | HHF35 | Ready to use | DAKO |
| Desmin | D33 | 1:100 | DAKO |
| CD68 | KP-1 | 1:100 | DAKO |
| CD31 | JC70A | 1:40 | DAKO |
aZytomed Systems GmbH, Berlin, Germany
bNovocastra™, Leica Biosystems Newcastle Ltd, Newcastle Upon Tyne, UK
Fig. 3.
Immunohistocehmistry shows intense positivity for a vimentin and b neuron-specific enolase (avidin-biotin-peroxidase, original magnification ×400)
The final diagnosis was spindle cell epulis with CGCE immunophenotype.
Discussion
Elongated, fusiform, or spindled cells have been noticed in previous cases of CGCE, in particular, near the tumors’ margins [3–13, 15], but those cells were scanty and did not comprise the main cell population. Their ultrastructural features were consistent with fibroblasts or histiocytes [13]. Their immunohistochemical phenotype was variable, as they were reported to be positive for S-100 protein [4, 9–12], CD68, and occasionally calretinin [4]; positive [10, 12] or negative [4] for vimentin; and positive [10] or negative for NSE [4, 12]. In addition, they were found to be negative for neurofilaments, GFAP, Leu-7, and myelin basic protein [10]. Based on those findings, it was suggested that they were Langerhans cells or histiocytes [11], “developing neural tissue” [9], or cells with phagolysosomes [4]. Those divergent immunohistochemical findings may be explained by the fact that under the descriptive terms “elongated”, “fusiform”, and “spindled” cells several different cell populations are included, such as fibroblasts, histiocytes, perineural cells, and perivascular cells, among others.
The congenital epulis presented herein had the following unusual microscopic features: (1) it was composed of spindled and ovoid cells without granular cytoplasm; (2) was devoid of typical granular cells; and (3) the overlying epithelium was acanthotic with broad rete pegs. Although these features were more consistent with a congenital fibrous epulis [14], the immunophenotype was identical to that of the granular cells of CGCE [4]. In addition, the spindled cells did not express markers of histiocytes, perineural cells, or muscle cells.
Some authors [8, 9, 13, 15, 16] have described transformation or transition of elongated, fusiform, or spindled cells to granular cells. Back in 1953, Bauer and Bauer [15] suggested that spindled cells were fibroblasts from the subepithelial connective tissue, endoneurium, or perineurium of the subepithelial nerves, or from the tooth germ and described CGCE as “granular cell fibroblastoma”. Vered et al. [4] thought that spindled cells represent “an earlier stage” in development of CGCE, but Takahashi et al. [10] considered them to represent a different cell population from granular cells. It would be tempting to suggest that the tumor presented herein is the initial stage in the development of a CGCE, but as Vered et al. [4] accurately stated, “reaching a meaningful conclusion from a single lesion… is invariably difficult.”
This is also true for the unique biologic behavior of the present lesion, which was reported to have recurred, since CGCE does not recur even after incomplete excision and may even spontaneously regress [1, 2]. However, the tumor excised from the same area when the infant was 2 months old was not submitted to microscopic examination, therefore, its relation to the present lesion is speculative.
Acknowledgments
The expert technical assistance of Mr. Georgios Babaliaris is acknowledged.
References
- 1.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and maxillofacial pathology. 3. St. Louis: Saunders; 2009. [Google Scholar]
- 2.Waal I. World Health Organization classification of tumours. Pathology and genetic of head and neck tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 3.Childers EL, Fanburg-Smith JC. Congenital epulis of the newborn: 10 new cases of a rare oral tumor. Ann Diagn Pathol. 2011;15(3):157–161. doi: 10.1016/j.anndiagpath.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Vered M, Dobriyan A, Buchner A. Congenital granular cell epulis presents an immunohistochemical profile that distinguishes it from the granular cell tumor of the adult. Virchows Arch. 2009;454(3):303–310. doi: 10.1007/s00428-009-0733-y. [DOI] [PubMed] [Google Scholar]
- 5.Mellor WC, Stockdale CR. Congenital epulis of the newborn; report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1957;10(11):1219–1226. doi: 10.1016/0030-4220(57)90078-6. [DOI] [PubMed] [Google Scholar]
- 6.Damm DD, Cibull ML, Geissler RH, Neville BW, Bowden CM, Lehmann JE. Investigation into the histogenesis of congenital epulis of the newborn. Oral Surg Oral Med Oral Pathol. 1993;76(2):205–212. doi: 10.1016/0030-4220(93)90206-J. [DOI] [PubMed] [Google Scholar]
- 7.Henefer EP, Abaza NA, Anderson SP. Congenital granular-cell epulis. Report of a case. Oral Surg Oral Med Oral Pathol. 1979;47(6):515–518. doi: 10.1016/0030-4220(79)90273-1. [DOI] [PubMed] [Google Scholar]
- 8.Koppang HS. Congenital gingival granular-cell myoblastoma. Oral Surg Oral Med Oral Pathol. 1972;34(1):98–100. doi: 10.1016/0030-4220(72)90277-0. [DOI] [PubMed] [Google Scholar]
- 9.Lifshitz MS, Flotte TJ, Greco MA. Congenital granular cell epulis. Immunohistochemical and ultrastructural observations. Cancer. 1984;53(9):1845–1848. doi: 10.1002/1097-0142(19840501)53:9<1845::AID-CNCR2820530908>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi H, Fujita S, Satoh H, Okabe H. Immunohistochemical study of congenital gingival granular cell tumor (congenital epulis) J Oral Pathol Med. 1990;19(10):492–496. doi: 10.1111/j.1600-0714.1990.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 11.Monteil RA, Loubiere R, Charbit Y, Gillet JY. Gingival granular cell tumor of the newborn: immunoperoxidase investigation with anti-S-100 antiserum. Oral Surg Oral Med Oral Pathol. 1987;64(1):78–81. doi: 10.1016/0030-4220(87)90120-4. [DOI] [PubMed] [Google Scholar]
- 12.Bilen BT, Alaybeyoglu N, Arslan A, Turkmen E, Aslan S, Celik M. Obstructive congenital gingival granular cell tumour. Int J Pediatr Otorhinolaryngol. 2004;68(12):1567–1571. doi: 10.1016/j.ijporl.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Lack EE, Perez-Atayde AR, McGill TJ, Vawter GF. Gingival granular cell tumor of the newborn (congenital “epulis”): ultrastructural observations relating to histogenesis. Hum Pathol. 1982;13(7):686–689. doi: 10.1016/S0046-8177(82)80018-X. [DOI] [PubMed] [Google Scholar]
- 14.Inan M, Yalcin O, Pul M. Congenital fibrous epulis in the infant. Yonsei Med J. 2002;43(5):675–677. doi: 10.3349/ymj.2002.43.5.675. [DOI] [PubMed] [Google Scholar]
- 15.Bauer WH, Bauer JD. The so-called congenital epulis. Oral Surg Oral Med Oral Pathol. 1953;6(9):1065–1071. doi: 10.1016/0030-4220(53)90219-9. [DOI] [PubMed] [Google Scholar]
- 16.Bhaskar SN, Akamine R. Congenital epulis (congenital granular cell fibroblastoma); report of a case. Oral Surg Oral Med Oral Pathol. 1955;8(5):517–523. doi: 10.1016/0030-4220(55)90083-9. [DOI] [PubMed] [Google Scholar]