Abstract
Nasopharyngeal carcinomas of the undifferentiated or lymphoepithelial type are most commonly seen in South East Asians. Identical tumors have also been described at a variety of other sites including lung, skin and salivary gland and have been referred to by a number of names including lymphoepithelial carcinoma (LEC). LECs of major salivary gland are extremely rare. They are particularly common amongst the Inuit populations of the arctic region including Greenland (Denmark), Canada and Alaska, as well as South East Asians. Within the Inuit group, this tumor represents the majority of all salivary gland carcinomas. Amongst primary LEC of major salivary gland, most cases reported in the literature have represented typical nasopharynx-like tumors. Variants of Epstein–Barr Virus (EBV) associated LEC have not been described previously, to the best of our knowledge. In this report, we describe 4 EBV-associated major salivary gland LECs with prominent basaloid morphology, which represent 22 % of a cohort of 18 salivary LECs from an Inuit population in Greenland. The features described in these cases raise a differential diagnosis of other basaloid tumors, particularly in light of the salivary gland location. A basaloid variant of LEC in major salivary gland should be recognized, especially in highly prone populations, to avoid misdiagnosis of other more common salivary tumors.
Keywords: EBV, Lymphoepithelial-like, Undifferentiated, Carcinoma, Basaloid
Introduction
Nasopharyngeal carcinomas of the undifferentiated or lymphoepithelial carcinoma (LEC) type are most commonly observed in South East Asian, and Inuit populations, as well as descendents from these populations living in the West [1–3]. They are rarely seen in Caucasians. Identical tumors have also been described at a variety of other sites including lung, skin and salivary gland and have been referred to by a number of names including LEC or “lymphoepithelial-like” carcinoma. The former term is currently adopted for salivary gland in the World Health Organization “blue book” [1]. LEC are rare and represent <1 % of all major salivary gland malignancies. They are particularly common amongst the Inuit populations of the arctic region including Greenland (Denmark), Canada and Alaska, as well as South East Asians [1, 4–8]. Amongst the Inuit, this Epstein–Barr Virus (EBV)-associated tumor is the most common malignant salivary gland carcinoma representing 92 % of all cases [4]. This tumor may occur in any salivary gland location, although the parotid represents the most common site [5].
LECs in major salivary gland usually show similar features to the more common primary nasopharynx cancer and therefore a metastasis must be excluded. They generally show a lymphoid rich tumor composed of sheets and nests of large vesicular cells with prominent nucleoli and syncytial cytoplasm. Nasopharyngeal “undifferentiated” carcinoma can show variable features including spindling, squamous, and hybrid features and occasional EBV positive basaloid squamous cell carcinomas of nasopharynx have also been described [1]. To the best of our knowledge, variants of EBV-associated LEC of the major salivary gland have not been described previously. In this report, we highlight 4 major salivary gland LECs with prominent basaloid morphology, gleaned from a cohort of 18 salivary LECs from an Inuit population in Greenland, and discuss the differential diagnostic pitfalls associated with their unusual morphologies.
Materials and Methods
Greenland is part of the Danish Kingdom and approximately 90 % of the total population (56,600 inhabitants, 2011) is of Inuit descent. All pathology and most of the treatments for head and neck cancer in Greenlandic patients are performed at Rigshospitalet in Denmark. As part of an ongoing study, salivary gland carcinomas in individuals born in Greenland and diagnosed between 1988 and 2011 were identified and the material was retrieved. These all represented patients treated in Denmark who were from the Inuit population of Greenland. A total of 18 cases of undifferentiated carcinomas were identified and 4 of these cases (22 %) with unusual basaloid morphology represent the basis of this report. All 4 cases had morphology reminiscent of other basaloid tumors, and therefore represent diagnostic pitfalls in salivary gland. All cases had H&E sections re-cut from blocks of primary tumor for review as well as confirmation of EBV positivity with EBV-encoded RNA (EBER) testing. Briefly, the surgical specimens were originally fixed in 10 % neutral buffered formalin and processed routinely. Hematoxylin and eosin stains were performed on 3–4 μ thick sections of formalin fixed paraffin-embedded tissue. In situ hybridization for the presence of EBV encoded small RNAs (EBER) was performed using a pre-diluted probe (Ventana systems) following standard protocols.
Results
The clinical and pathological findings are summarized in Table 1. The patients were 2 males and 2 females with an age range of 43–70 years old (average 54.3 years). The tumors arose in parotid (×2) and submandibular gland (×2). None showed a concurrent nasopharynx malignancy. All 4 patients presented with a mass and were treated with primary resection, and in all but one patient (case 4) they were followed by adjuvant radiotherapy. After treatment in Denmark, Inuit patients in general return to Greenland and systematic clinical follow-up is often not possible [9]. Follow-up was however available in 2 cases (cases 1 and 2) and showed no signs of recurrence in these patients, 1 and 6 years after treatment, respectively. Case 3 was a recent case and so no follow-up is available yet and case 4 was lost to follow up entirely.
Table 1.
Clinical and pathological findings in 4 cases of LEC with basaloid morphology
| Case | Age and gender | Site | Perineural invasion | Dominant pattern | Basaloid percentage (%) | Treatment | Follow up (months) |
|---|---|---|---|---|---|---|---|
| 1 | 60F | Submandibular | + | Sclerosing BCC like | 90 | Surgery + radiation | NED 1 year |
| 2 | 43F | Submandibular | + | Basaloid SCC | 80 | Surgery + radiation | NED 6 years |
| 3 | 44M | Parotid | − | Sclerosing BCC like | 20 | Surgery + radiation | N/A |
| 4 | 70M | Parotid | − | Basal cell adenoma or lymphadenoma like | 90 | Surgery alone | N/A |
M male, F female, BCC basal cell carcinoma, SCC squamous cell carcinoma, NED no evidence of disease, N/A not available
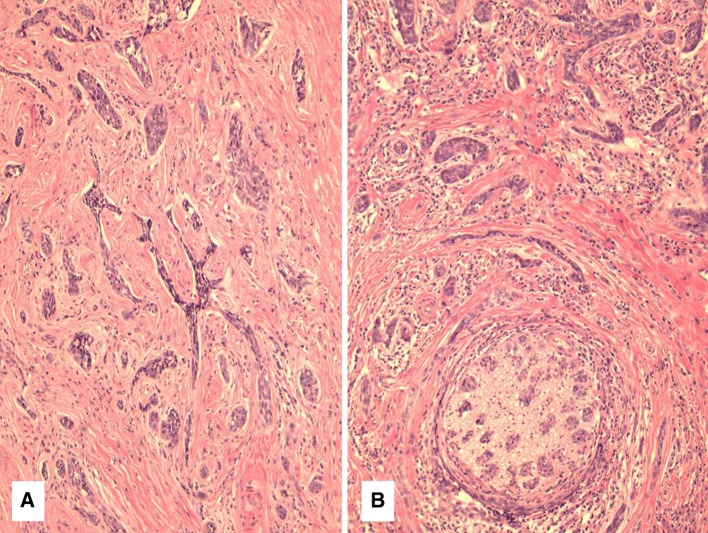
The 4 tumors showed a variety of basaloid features that were not seen in the remainder of the 18 cases of primary major salivary gland EBV-associated LEC. These basaloid features were usually associated with lymphocyte depletion. Case 1 showed a combination of small basaloid nests as well as “syringoma-like” cords of cells, invading and surrounding submandibular gland tissue (Fig. 1a). This pattern represented almost the entire tumor; however there were focal areas typical of LEC with lymphoid stroma, syncytial nests and vesicular chromatin as well. The areas with the basaloid growth pattern were somewhat reminiscent of a sclerosing basal cell carcinoma (BCC), but there was no involvement of skin or subcutaneous tissues. These areas were highly sclerotic with only focal lymphocyte infiltration. Focal clear cell change was noted as well. There was extensive perineural and even intraneural invasion by these small basaloid nests (Fig. 1b).
Fig. 1.
a Case 1 showing cords and nests of basaloid cells infiltrating a fibrous stroma. b Perineural invasion was seen in 2 cases including case 1 with prominent perineural and intraneural invasion
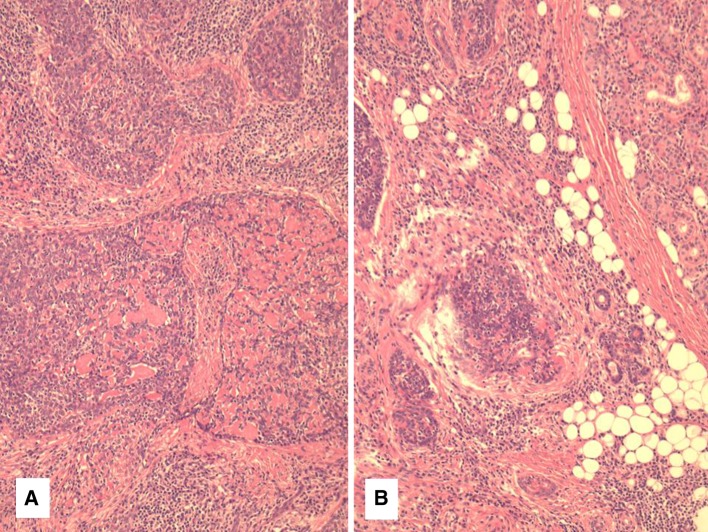
Case 2 was also a submandibular gland tumor which was composed of large round nests of a basaloid neoplasm with extensive hyalinized basement membrane-like material (Fig. 2a). This imparted a cribriform like pattern and was identical to basaloid squamous cell carcinoma in mucosal sites (Fig. 2b). Epithelioid non-necrotizing granulomas were seen adjacent to many nests of the tumor. The tumor surrounded normal salivary elements and showed perineural invasion as well. This was most evident in the more sclerotic areas, as in case 1. Approximately 20 % of the tumor showed more typical features of LEC.
Fig. 2.
Case 2 showing basaloid squamous cell carcinoma with basement membrane like material imparting a cribriform pattern. Infiltration of atrophic submandibular gland elements can be seen in b
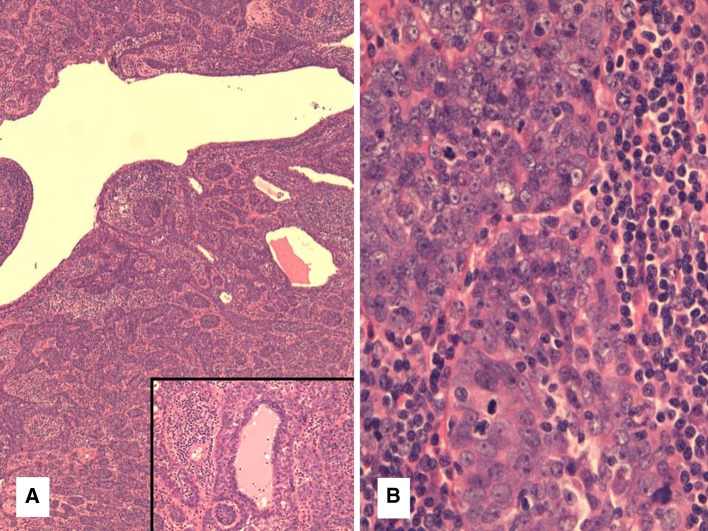
Case 3 was a parotid tumor similar to case 1 although it contained about 80 % typical LEC with lymphoid stroma and nests and sheets of cells with syncytial cytoplasm and vesicular nuclei. The remaining 20 % of tumor was made up of smaller basaloid nests and cords of tumor cells with a sclerotic stroma with more minimal lymphocytic infiltration. No perineural invasion was identified. Finally, case 4 showed the most unusual pattern with a relatively circumscribed but unencapsulated tumor with a cystic, nested and trabecular architecture reminiscent of a basal cell adenoma (Fig. 3a). However it also contained a lymphoid background somewhat similar to a lymphadenoma. The lymphoid tissue showed follicles with germinal center formation intermixed with the epithelial elements. The epithelial component showed squamous pearls but no keratinization. Occasional lumina were also noted (Fig. 3a inset). On higher power, the nuclei were large, atypical and vesicular and there were numerous mitotic figures (8 per 10 HPFs) (Fig. 3b), in keeping with a malignant neoplasm.
Fig. 3.
a Case 4 had a low power appearance similar to a basal cell adenoma or lymphadenoma with small nests and interconnecting trabecular structures. Inset focal lumina were noted in this case as well. b High power of case 4 showing vesicular chromatin, atypia and mitotic figures more reminiscent of typical “undifferentiated (lymphoepithelial)” carcinoma. This was seen at least focally in all 4 tumors
Immunohistochemical stains accompanying the case showed evidence of epithelial (pankeratin) and squamous differentiation (p63), when performed in cases 2 and 3, respectively. These were not performed on the remaining cases as the tumors showed obvious morphological evidence of epithelial and squamous differentiation. All four tumors showed strong and diffuse nuclear expression with EBER, which was absent in the normal surrounding tissues (Fig. 4). The EBER was also negative in the associated non-necrotizing granulomas of case 2 and the lymphoid tissue in all cases.
Fig. 4.
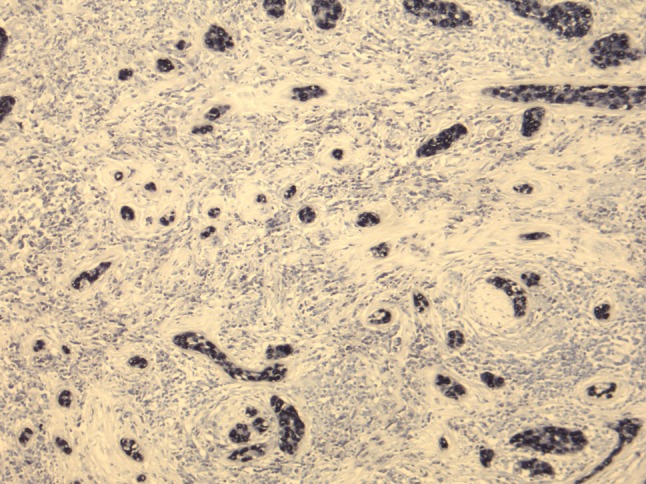
EBER was diffusely positive in all 4 cases. Case 1 is shown here
Discussion
Lymphoepithelial carcinomas are rare malignancies of major salivary gland that are similar to their nasopharyngeal counterparts. They are particularly common amongst the Inuit populations of the arctic region including Greenland (Denmark), Canada and Alaska as well as South East Asians [1, 4–8]. Amongst the Inuit, this EBV associated tumor is the most common malignant salivary gland carcinoma [4]. This tumor may occur in any salivary gland location, although the parotid represents the most common site [5]. In general, the prognosis is poor for these patients [1, 4]. Although there have been rare reports of association with Sjögren’s or other autoimmune disease [10], as well as pre-existing sialadenitis or familial association [1], most cases arise de novo in predisposed populations. In Inuit populations, the existence of a very high familial risk of both nasopharyngeal and salivary gland carcinomas, indicate shared environmental or genetic risk factors [11]. LEC in major salivary gland is identical to its nasopharyngeal counterpart in morphology and therefore a primary nasopharynx cancer must be excluded in all cases. It is usually a lymphoid rich tumor composed of sheets and nests of large cells with syncytial cytoplasm and prominent nucleoli. Squamous differentiation can also be seen, as can spindle cells, clear cell change and in situ like growth [1].
Each of the 4 cases described herein could potentially cause diagnostic difficulty owing to the unusual basaloid morphology associated with lymphocyte depletion. These changes represented 22 % of the larger cohort of undifferentiated salivary gland carcinomas/LEC and are therefore not rare, at least in this Greenland Inuit population. They represent features that may not raise the suspicion of EBV in a non-endemic region, although each case showed at least focal features typical of “undifferentiated carcinoma” of the nasopharynx as well as a lymphoid component. The lymphoid tissue diminished in the sclerotic regions with basaloid morphology further causing difficulty in the diagnosis. All were confirmed to be EBV associated with strongly positive EBER staining. Cases 1 and 3 showed features similar to a sclerosing BCC with small basaloid nests, cords and angulated “syringoma-like” nests set in a sclerotic background. There was prominent perineural and intraneural invasion in case 1. Sclerosing BCC or syringomatous carcinomas could be ruled out by the absence of skin or subcutaneous tissue involvement and positivity for EBV. EBV has not been shown to be a factor in cutaneous carcinomas previously [12]. This is a rather important differential diagnosis as BCC secondarily invading parotid is relatively common. Sometimes in our experience, no skin involvement is seen in recurrent tumors and no history of a previous BCC being “shaved off” is given, further complicating the diagnosis.
Case 2 of this series showed a typical basaloid squamous cell carcinoma with a cribriform pattern containing basement membrane like material. It was strongly EBER positive and also showed focal more typical LEC features with lymphoid tissue. This is the first reported case of pure EBV-associated basaloid SCC in the submandibular salivary gland, to our knowledge. It showed non-necrotizing granulomas, which has been described previously [1]. Variants of EBV associated carcinoma in the nasopharynx have rarely been described and include basaloid squamous cell carcinoma [1]. According to the WHO, there have been about a half dozen reported basaloid squamous cell carcinomas of nasopharynx making this a very rare variant. Of these, 3 of 4 cases tested, which all represented Asian patients, were EBER positive and therefore EBV associated [1, 13, 14]. However, an oropharyngeal or laryngeal primary should also be excluded, as it would represent a more common site of origin for this type of morphology and HPV would represent a more common etiology than EBV. The EBER positivity and lymphoid component also help to exclude an adenoid cystic carcinoma (AdCC) or membranous basal cell adenoma/adenocarcinoma (dermal analog tumor) with a cribriform pattern and also makes a metastasis from a mucosal SCC less likely. In addition to EBV positivity, the lack of angulated hyperchromatic nuclei and presence of focal squamous pearls makes an AdCC highly unlikely.
Occasional cases of other more unusual major salivary gland EBV-positive LECs with a “myoepithelial” or “epi-myoepithelial” component have been reported previously and referred to as hybrid tumors [15, 16]. These may represent a similar phenomenon to some of the features described in this report, particularly with respect to case 4 which showed lumina formation and features similar to a basal cell adenoma or lymphadenoma. This case was somewhat circumscribed appearing and had small basaloid nests, interconnecting trabecular structures, squamous pearls and a background of lymphoid tissue. However, unlike lymphadenomas and basal cell adenomas, there was marked atypia, vesicular chromatin and mitotic activity, similar to typical LEC nuclear morphology in the nasopharynx. There was also EBV positivity, which is consistently absent in lymphadenoma [17]. A “lymphadenocarcinoma” or “basal cell adenocarcinoma ex lymphadenoma” are also possibilities; however these tumors have not been extensively studied for EBV, to our knowledge.
Most of these features can be seen in a variety of salivary gland tumors and are not specific to EBV-associated LEC. In fact they are more common in these potential mimics. The clues to the diagnosis mainly rest in the ethnicity and at least focal lymphoid background. EBV testing by in situ hybridization (EBER) should be considered in this context. One large study by Saku et al. [5] showed that EBV was not present in any other type of carcinoma in a series of major salivary gland malignancies from Asia and Russia. Interestingly they separated their LEC into those with large nests and lymphoid tissue and those with smaller nests and lymphocyte depletion. This latter group may contain cases such as the 4 tumors described herein and may suggest that this variant of LEC is more common than recognized in the literature.
The remaining question is whether these 4 unusual cases represent the same tumor as typical LEC, a variant of LEC or other tumors entirely that just happen to be associated with EBV. This latter possibility cannot be eliminated completely. Although it is possible that Inuit are predisposed to EBV associated tumors in general, studies of EBV-associated gastric carcinoma have found similar frequencies in Inuit and Caucasians [18], speaking against a general susceptability to tumor formation due to EBV. It is more likely that the cases described herein are just rare variant morphologies of one tumor type that Inuits are known to be susceptible to. It is noteworthy that all 4 cases showed the same vesicular nuclei, atypia and mitotic figures with at least focal areas resembling typical LEC. In that context, it is likely that the areas of basaloid morphology with lymphocyte depletion represent a form of differentiation or maturation as apposed to a hybrid tumor. It is also likely that the cause of this differentiation is different in each of these tumors as they did not share identical basaloid features. This latter point also argues against this basaloid morphology representing a uniform variant of LEC. The title a “spectrum of basaloid morphology” was specifically used to avoid confusing this for a novel variant and to offer a range of possible basaloid morphology that may impact on a differential diagnosis or cause diagnostic confusion in these cases.
In summary we have presented unusual basaloid morphology in a subset of major salivary gland EBV-associated LEC in an endemic predisposed population. All showed features reminiscent of other non-EBV associated tumors and therefore would represent significant pitfalls in diagnosis.
References
- 1.Tsang WYW, Kuo TT, Chan JKC. Lymphoepithelial carcinoma. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 251–252. [Google Scholar]
- 2.Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 3.Boysen T, Friborg J, Andersen A, Poulsen GN, Wohlfahrt J, Melbye M. The Inuit cancer pattern—the influence of migration. Int J Cancer. 2008;122(11):2568–2572. doi: 10.1002/ijc.23367. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen NH, Mikkelsen F, Hansen JP. Incidence of salivary gland neoplasms in Greenland with special reference to an anaplastic carcinoma. Acta Pathol Microbiol Scand A. 1978;86(2):185–193. doi: 10.1111/j.1699-0463.1978.tb02030.x. [DOI] [PubMed] [Google Scholar]
- 5.Saku T, Cheng J, Jen KY, Tokunaga M, Li J, Zhang W, Liu A, Wu L, Lu Y, Zhou Z, Li Y, Li R, Ouyang J, Yang L, Yu S, Lou T, Wang S, Lin D, Rao H, Lin H, Sderk P, Chen Z, Chen Z, Cai C, Kim H, Hong S. Epstein–Barr virus infected lymphoepithelial carcinomas of the salivary gland in the Russia-Asia area: a clinicopathologic study of 160 cases. Arkh Patol. 2003;65(2):35–39. [PubMed] [Google Scholar]
- 6.Kuo T, Hsueh C. Lymphoepithelioma-like salivary gland carcinoma in Taiwan: a clinicopathological study of nine cases demonstrating a strong association with Epstein–Barr virus. Histopathology. 1997;31(1):75–82. doi: 10.1046/j.1365-2559.1997.5830814.x. [DOI] [PubMed] [Google Scholar]
- 7.Tsai CC, Chen CL, Hsu HC. Expression of Epstein–Barr virus in carcinomas of major salivary glands: a strong association with lymphoepithelioma-like carcinoma. Hum Pathol. 1996;27(3):258–262. doi: 10.1016/S0046-8177(96)90066-0. [DOI] [PubMed] [Google Scholar]
- 8.Leung SY, Chung LP, Yuen ST, Ho CM, Wong MP, Chan SY. Lymphoepithelial carcinoma of the salivary gland: in situ detection of Epstein–Barr virus. J Clin Pathol. 1995;48(11):1022–1027. doi: 10.1136/jcp.48.11.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen RG, Friborg J, Rosborg J, Specht L, Brofeldt S, Hamilton Therkildsen M, Homøe P. Survival of head and neck cancer in Greenland. Int J Circumpolar Health. 2010;69(4):373–382. doi: 10.3402/ijch.v69i4.17673. [DOI] [PubMed] [Google Scholar]
- 10.Saqui-Salces M, Martinez-Benitez B, Gamboa-Dominguez A. EBV + lymphoepithelial carcinoma of the parotid gland in Mexican Mestizo patients with chronic autoimmune diseases. Pathol Oncol Res. 2006;12(1):41–45. doi: 10.1007/BF02893430. [DOI] [PubMed] [Google Scholar]
- 11.Friborg J, Wohlfahrt J, Koch A, Storm H, Olsen OR, Melbye M. Cancer susceptibility in nasopharyngeal carcinoma families—a population-based cohort study. Cancer Res. 2005;65(18):8567–8572. doi: 10.1158/0008-5472.CAN-04-4208. [DOI] [PubMed] [Google Scholar]
- 12.Huston BM, Maia DM. Absence of latent Epstein–Barr virus in cutaneous squamoproliferative lesions after solid organ transplantation. Mod Pathol. 1997;10(12):1188–1193. [PubMed] [Google Scholar]
- 13.Wan SK, Chan JK, Lau WH, Yip TT. Basaloid-squamous carcinoma of the nasopharynx. An Epstein–Barr virus-associated neoplasm compared with morphologically identical tumors occurring in other sites. Cancer. 1995;76(10):1689–1693. doi: 10.1002/1097-0142(19951115)76:10<1689::AID-CNCR2820761003>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Weiss LM, Movahed LA, Butler AE, Swanson SA, Frierson HF, Jr, Cooper PH, Colby TV, Mills SE. Analysis of lymphoepithelioma and lymphoepithelioma-like carcinomas for Epstein–Barr viral genomes by in situ hybridization. Am J Surg Pathol. 1989;13(8):625–631. doi: 10.1097/00000478-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Herbst H, Niedobitek G. Sporadic EBV-associated lymphoepithelial salivary gland carcinoma with EBV-positive low-grade myoepithelial component. Virchows Arch. 2006;448(5):648–654. doi: 10.1007/s00428-005-0109-x. [DOI] [PubMed] [Google Scholar]
- 16.Piana S, Damiani S, Asioli S, Magrini E, Barbieri W, Cavazza A. Epstein–Barr-positive lymphoepithelial carcinoma and epi-myoepithelial cell carcinoma of the parotid gland: a hitherto unreported example of hybrid tumour. Virchows Arch. 2004;445(4):425–428. doi: 10.1007/s00428-004-1075-4. [DOI] [PubMed] [Google Scholar]
- 17.Seethala RR, Thompson LD, Gnepp DR, Barnes EL, Skalova A, Montone K, Kane S, Lewis JS, Jr, Solomon LW, Simpson RH, Khan A, Prasad ML. Lymphadenoma of the salivary gland: clinicopathological and immunohistochemical analysis of 33 tumors. Mod Pathol. 2012;25(1):26–35. doi: 10.1038/modpathol.2011.135. [DOI] [PubMed] [Google Scholar]
- 18.Boysen T, Mohammadi M, Melbye M, Hamilton-Dutoit S, Vainer B, Hansen AV, Wohlfahrt J, Friborg J. EBV-associated gastric carcinoma in high- and low-incidence areas for nasopharyngeal carcinoma. Br J Cancer. 2009;101(3):530–533. doi: 10.1038/sj.bjc.6605168. [DOI] [PMC free article] [PubMed] [Google Scholar]