Abstract
Acantholytic squamous cell carcinoma is a rare variant of squamous cell carcinoma in the mucosa of upper aerodigestive tract. Histomorphologically, acantholytic squamous cell carcinoma may lose the typical features of conventional squamous cell carcinoma and mimic other epithelial or mesenchymal malignancies due to advanced acantholysis and dyskeratosis. Because of its rarity, information of prognosis, pathologic features and immunohistochemical profiles is limited. We have studied clinicopathologic features and immunohistochemical profiles of four acantholytic squamous cell carcinoma cases arising from upper aerodigestive tract. Clinical results indicate an aggressive biologic behavior. Morphologically, all tumors revealed significant acantholysis with separation of tumor cells and intratumoral spaces. The tumor cells were highly pleomorphic and growth patterns were variable. In immunohistochemical studies, all tumor cells revealed positive reactions for AE1/AE3 and p63 supporting a squamous epithelial origin. In contrast to conventional aerodigestive squamous cell carcinoma, acantholytic squamous cell carcinoma showed significant reductions of cytokeratin19, E-cadherin and concomitant up-regulation of vimentin expression. Both morphologic features and immunohistochemical profiles indicate that acantholytic squamous cell carcinoma has acquired an epithelial mesenchymal transition phenotype. However, in contrast to other solid malignant tumors, the epithelial mesenchymal transition phenotype change in acantholytic squamous cell carcinoma is not limited to the invasive front of the peripheral tumor but, rather, diffusely involves entire neoplastic lesion. In addition, because cytokeratin 19 staining is attenuated, this would be an insensitive marker for following up and/or in detecting disseminated tumor cells in cases of acantholytic squamous cell carcinoma in upper aerodigestive tract.
Keywords: Acantholytic squamous cell carcinoma, Upper aerodigestive tract, Immunohistochemical profile, Cytokeratin 19, E-cadherin, Epithelial mesenchymal transition
Introduction
Upper aerodigestive tract squamous cell carcinoma (SCC) is the most common epithelial malignancy in the head and neck region. It affects 50,000 Americans and more than 500,000 individuals worldwide. Most head and neck SCC (HNSCC) are of the conventional type. Variants of SCC, such as spindle cell SCC, papillary SCC, basaloid SCC and acantholytic SCC, may occur in head and neck region and are well recognized [1–3].
Acantholytic squamous cell carcinoma (ASCC) of the aerodigestive mucosa is a rare variant of SCC and the majority of reported cases arise from oral mucosa [4–6]. ASCC was first identified as a variant of skin SCC. It is also known as adenoid squamous carcinoma, pseudo-glandular squamous carcinoma and angiosarcoma-like squamous cell carcinoma [7]. ASCC is a SCC characterized by loss of cellular adhesion, dyskeratotic tumor cells and intratumoral pseudo-glandular spaces or lumina [7, 8]. When it occurs in skin, ASCC is associated with solar injury and a poor prognosis [9].
In head and neck mucosae, ASCC is rare. Most reported cases arise from gingiva, tongue, floor of the mouth and buccal mucosa [4, 6, 10]. Except in the lip (perhaps due to early detection and treatment), the head and neck mucosal ASCCs reported were associated with poor prognoses. Histomorphologically, ASCC may lose the typical features of SCC and mimic other epithelial or mesenchymal malignancies because of advanced acantholysis and dyskeratosis [5, 11, 12]. Because of its rarity, most of the previous studies have been case reposts and information concerning the immunohistochemical features of ASCC is limited.
We herein report on four additional cases of head and neck mucosa ASCC, including one from the nasal cavity, to widen the clinicopathological and immunohistochemical spectrum of this rare variant of HNSCC. Clinically, these tumors were associated with pain, ulcer, mass effects and locally aggressive growth and/or metastasis. The immunohistochemical profile was different from conventional HNSCC. All tumors showed significant reductions of cytokeratin19 (CK19) and E-cadherin expression and concomitant up-regulation of vimentin expression. These findings suggest an epithelial-mesenchymal transition, a biologic process that is associated with a high proclivity for tumor invasion and dissemination, occurs during ASCC tumor development and progression. In contrast to other solid malignant neoplasms, an epithelial mesenchymal transition phenotype change in ASCC is not limited to the invasive front of the peripheral tumor but, rather, diffusely involves the entire lesion. Loss of CK19 in ASCC needs to be remembered when this tumor is included in the differential diagnosis especially, when significant number of intercellular spaces and intratumoral lumina are present. In addition, CK19 would be an insensitive marker for following up or detecting disseminated tumor cells in patients with the acantholytic variant SCC of the upper aerodigestive tract.
Materials and Methods
Case Selection
Four cases of ASCC were collected from the 1995 to 2010 surgical pathology archives at our institute. Clinical study of one case (case number 1) has been reported previously. Detailed morphological review was performed. Acantholytic squamous cell carcinoma was defined as a malignant neoplasm with squamous differentiation that had significant acantholysis in tumor nests creating intercellular spaces, pseudo-glandular and/or pseudo-luminal appearances. In cases where the tumor cells had no obvious squamous features, pan-keratin and p63 immunostains were performed to confirm the squamous epithelial origin. The Clinical information and follow up data were obtained from patients’ chart review.
Histology Methods
Resection tissue specimens for diagnosis were fixed in 4 % buffered formalin and embedded in paraffin. The slides were stained with H & E or special histochemical stains for light microscopy. For immunohistochemical stains, four-micron sections were subjected to deparaffinization, hydration, endogenous peroxidase blocking and antigen retrieval in a Ventana automatic stainer system. Pre-diluted antibodies against to AE1/AE3, p63, CK19, E-cadherin, vimentin, desmin and smooth muscle actin were obtained from Ventana. Detection of the staining reaction was achieved by an enzyme conjugated polymer complex adapted for an automatic stainer from Ventana (Ventana Medical Systems, Tucson, AZ, USA). Positive and negative controls were included for quality control purpose. For each case, estimated staining intensity (0 = no stain, 1+ = unequivocal but weak, 2+ = moderate, 3+ = strong), and staining extent (focal: estimated percentage of stained cells was less than 10 %; intermediate: percentage of stained cells was between 10 and 50 %; and diffuse: percentage of stained cells was more than 50 %) was recorded.
Results
Clinicopathological Characteristics
The clinical data are summarized in the Table 1. The range of patients’ age was from 38 to 72 years. The male to female ratio was 3:1. Except for one patient (lost in follow up), the follow up time ranged from 6 to 48 months. Tumor arising from oral mucosa was seen in three cases, and tumor arising from nasal mucosa was seen in one case. Clinically, all patients complained of pain with or without local swelling and mass effects. In two cases, the tumors were described as rapidly growing masses in clinical examination. One patient showed lymph node metastasis at the time of initial diagnosis. There were local recurrences in two cases, both of them occurred within 12 months of the initial excisions.
Table 1.
Summary of clinical information
| Case no | Age/Sex | Clinical symptoms | Site | Rec/Met/DOD | Follow-up months |
|---|---|---|---|---|---|
| 1 | 38/F | Pain, mass with ulcer | Buccal mucosa | Met and DOD | 7 |
| 2 | 61/M | Pain and mass | Buccal mucosa | N/A | N/A |
| 3 | 72/M | Pain and mass | Nasal mucosa | Recurred | 6 |
| 4 | 70/M | Pain and ulcer | Palate mucosa | Recurred | 48 |
Rec/Met/DOD recurrent/metastasis/dead of disease, NA not applicable
Histopathology and Immunohistochemical Results
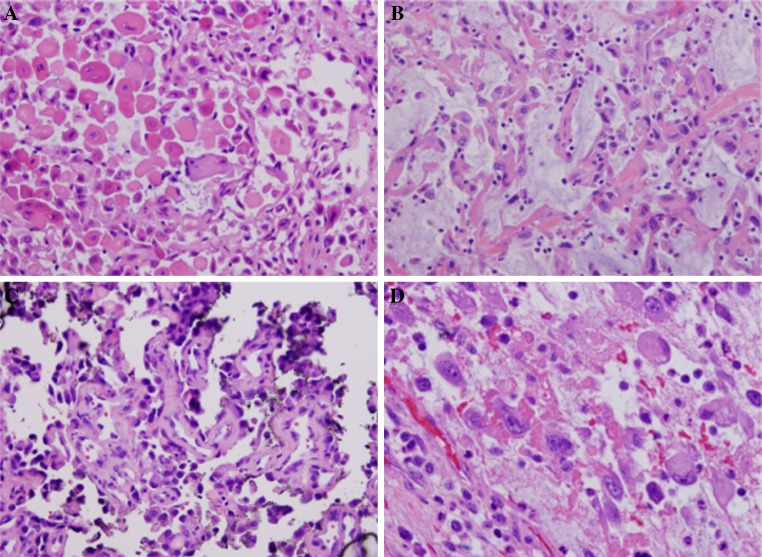
Histologically, all tumors revealed significant acantholysis with separation of tumor cells (Fig. 1a). Intratumoral spaces and lumina were either diffusely or focally present. Extensive necrosis and features of conventional SCC were observed in focal areas in two cases. Areas with the accumulation of myxoid or mucinous material in the stroma mimicking adenocarcinoma were also noted (Fig. 1b). There were areas with irregular rudimentary lumina and highly pleomorphic tumor cells emulating angiosarcoma (Fig. 1c).
Fig. 1.
ASCC shows prominent dyskeratosis with separation of tumor cells (a). Accumulation of myxoid or mucin-like materials in the stroma or empty spaces may be present (b). There are areas with irregular rudimentary lumina and highly pleomorphic tumor cells emulating angiosarcoma (c). Large undifferentiated tumor cells with dense eosinophilic cytoplasm mimicking high-grade malignant mesenchymal neoplasm (d)
The tumor cells showed high nuclear pleomorphism. The nuclei varied from hyperchromatic and slightly enlarged (Fig. 1c) to large and/or multinucleated nuclei with open and fine chromatin. Mitoses were not uncommon (Fig. 1d). The tumor cells also revealed significant variations in cytoplasm. Some acantholytic tumor cells showed prominent dyskeratosis with dense eosinophilic cytoplasm (Fig. 1a). In some tumors, there were scattered large cells containing large amounts of deeply eosinophilic material and peripherally located vesicular nuclei mimicking high-grade sarcoma. Some discohesive tumor cells were also relatively small with little cytoplasm as shown in Fig. 1c.
The results of immunohistochemical and special chemical studies were presented in Table 2 and Fig. 2. In the immunohistologic studies, all tumors revealed moderate to strong reactions for cytokeratin AE1/AE3 and p63 (Fig. 2a, b). For CK19, as shown in Fig. 2c, the immunoreactive reactions were either totally negative (in 3 cases) or showed only a focal weak reaction (1 case). Reaction for E-cadherin was significantly reduced in all tumors and in most tumor cells (0 to 1+). The reaction for E-cadherin was preserved only in areas that showed features close to conventional SCC (2+ to 3+). Interestingly, three of four cases revealed diffuse and strong reactions for vimentin (Fig. 2d). In all tumors, the tumor cells were negative for desmin and smooth muscle actin; in mucicarmine stains, no intracytoplasmic reactions were identified in any of the tumors.
Table 2.
Results of immunohistochemical study
| Case no | Pan keratin (AE1/AE3) | CK 19 | p63 | E-cadherin | Vimentin |
|---|---|---|---|---|---|
| 1 | 3+ | 0 | 2+ | 0 | 2+ |
| 2 | 2+ | Focal 1+ | 3+ | Focal 1+ | 2+ |
| 3 | 2+ | 0 | 2+ | 1+ | 3+ |
| 4 | 3+ | Focal 1+ | 2+ | 1 + to focal 2+ | 2+ |
Fig. 2.
Results of immunohistochemical study. a Tumor cells are positive for cytokeratin AE1/AE3. b Tumor cells reveal positive nuclear stain for p63. c All tumor cells are negative for CK19. d Majority tumor cells are either negative or minimal reaction for E-cadherin. Rare tumor cells show a moderate peripheral cytoplasmic reaction for E-cadherin. e Tumor cells show strong reaction for vimentin
Discussion
ASCC is an uncommon variant of SCC characterized by marked acantholysis, loss of cohesion and dyskeratosis [2, 3]. Morphologically, ASCC shows intratumoral spaces or lumina giving the tumor a pseudo-glandular or pseudo-vascular appearance. ASCC was first described by Lever in 1947 as a cutaneous tumor and was initially described as an adenoacanthoma of eccrine tissue [13]. Later studies proved that ASCC also arises from noneccrine tissue. The most common site of ASCC is sun-exposed skin [9]. Mucosal ASCC is rare but has been reported in multiple locations in the aerodigestive tract particularly the oral cavity including the buccal mucosa, tongue, gingiva, floor of mouth and larynx [6, 14, 15]. Rare cases of ASCC in the cecal mucosa [16] and breast parenchyma [8, 17] have also been reported. Although mucosal ASCC is well defined and well recognized, most studies were based on sporadic case reports and information concerning the immunohistochemical profile is limited. In this report, we have analyzed clinicopathological and immunohistochemical findings from four ASCCs in the upper aerodigestive tract. This report is also, to the best of our knowledge, the first time documented occurrence of ASCC in the nasal cavity, broadening the anatomical locations that can be affected by this variant of SCC in the upper aerodigestive tract.
Clinical studies of aerodigestive ASCC showed mixed results in terms of tumor aggressiveness and disease outcome. In cases of lip ASCC, a good prognosis was noted. Perhaps similar to skin ASCC, lip ASCC is related to sun-exposure injury. The nature of location and superficial position of this tumor may lead to early medical attention that might result in a better ultimate prognosis. The prognosis of mucosal ASCC in other locations has been controversial [18]. Some cases of ASCC have been aggressive with a greater risk of local recurrence and metastases than conventional SCCA. However, others report cases with a relatively good control after complete surgical excision.
In our cases, the head and neck mucosal ASCC showed relatively aggressive clinical behavior. Two cases recurred within 12 months after treatment. One patient showed a fast growing mass that grew to 6 cm in a short time. There was regional lymph node metastasis and the patient eventually died of disease. Pain was also a constant clinical feature, irrespective of mucosal ulceration.
Histologically, as reported in previous studies, our cases showed typical features of acantholysis (Fig. 1a–d). The tumor cells lost intercellular junctions and cellular polarity. Intercellular spaces and intratumoral lumina were present, and created a pseudo-vascular or pseudo-glandular appearance. Background myxoid and mucinous changes were present in some tumors. The tumor cells showed significant pleomorphism, as typically seen in high-grade malignant neoplasms, such as hyperchromatic nuclei, irregular nuclear membranes and prominent nucleoli. Mitotic figures were present and could be brisk in focal areas. The amount of cytoplasm was variable, and in some tumor cells the cytoplasm was dense and eosinophilic.
In immunohistochemical studies, as reported in literature, our cases showed strong cytoplasmic immunoreaction for high molecular weight cytokeratins AE1/AE3 and nuclear staining for p63 supporting a squamous epithelial origin (Fig. 2a, b and Table 2). Since ASCC may lose morphologic features of squamous differentiation, application of ancillary studies are often necessary. P40 a relatively new and more specific marker for epithelial cells of squamous origin might also be applied in this situation. However, in contrast to conventional aerodigestive SCC, reactions for CK19 were significantly reduced (Fig. 2c). Interestingly, the tumor cells also showed significant down regulation of E-cadherin (Fig. 2d) and up regulation for the mesenchymal marker vimentin (Fig. 2e). Reactions for other more specific mesenchymal markers, such as desmin and smooth muscle actin were all negative (data not shown).
CK19 is an intermediate filament with a molecular weight of 44 kDa belonging to the acidic type of cytokeratins. CK19 normally presents in aerodigestive squamous mucosa and is restricted in the cells located in the basal and supra basal layers. In the process of aerodigestive SCC development, a series of transitional events occur. In high-grade squamous dysplasia, the expression of CK19 is increased and extends to all dysplastic cells in all layers of the squamous epithelium [19]. Previous studies of the conventional type of invasive SCC revealed that CK19 mRNA level and protein expression are elevated in the tumor cells [20]. In those studies, the tumor cells revealed strong reactions in immunohistochemical studies and elevated protein expression using western blot analyses. Expression of CK19 is also elevated in some glandular epithelial malignancies such as breast carcinoma, pancreatobiliary carcinoma and thyroid carcinoma [21].
E-cadherin, an important cell adhesion molecule, is present in almost all normally developed epithelial cells and many solid malignant tumors of epithelial origin [22]. In certain malignancies, such as lobular carcinoma of the breast, E-cadherin expression is typically decreased. In addition to cell–cell adhesion, E-cadherin is also a morphogenetic regulator and plays an important role in the modulation of tissue maturation and tumor differentiation [23]. Aberrant E-cadherin expression is associated with invasiveness of tumor cells and poor clinical prognoses [24]. During tumor growth and progression, the local microenvironment and growth factors may induce epithelial tumor cells to dedifferentiate and acquire an epithelial mesenchymal transition like phenotype characterized by loss of cell to cell adhesion, down regulation of E-cadherin and a shift of intermediate filaments to vimentin [25].
Suppression of CK19 expression in carcinomas during tumor differentiation has been reported in both human tissues, such as breast cancers, and cultured malignant cell lines [26, 27]. Down-regulation of CK19 expression and overexpression of vimentin were found in highly aggressive breast cancer cells with strong migratory and invasive abilities [28]. In most malignant epithelial neoplasms, the tumor cells express distinctive cytokeratins and adhesion molecules. Studies have implied that, in tumor differentiation and progression, different cytokeratins are associated with different function in carcinogenesis [29] and that may result in the phenotypic changes as seen in our cases as well as in previous studies.
Many studies have proved that CK19 expression is significantly elevated in conventional SCC of the upper aerodigestive tract. In general, there is no need to apply CK19 for the diagnosis of HNSCC, although several recently published studies have used CK19 or CK19 mRNA as a potential marker for monitoring hematologically disseminated tumor cells in patients with head and neck SCC. In the pathologic diagnosis, when there are significant numbers of intercellular spaces and intratumoral lumina and ASCC is in the differential diagnosis, this loss of CK19 staining needs to be remembered. In patients with ASCC, CK19 is likely to be insensitive as a tumor marker for morphologic diagnosis or for the detection of disseminated tumor cells in lymph nodes and peripheral blood.
In our cases, the results of morphologic and immunohistochemical studies indicated that ASCC tumor cells acquire an epithelial mesenchymal transition like phenotype. As reported in many cancer studies, characteristic features of an epithelial mesenchymal transition like phenotype have been mainly identified in the individual tumor cells distributed at the invasive front of the peripheral tumor, and were assumed to be related to tumor invasion and metastasis [30, 31]. Studies also suggested that an epithelial mesenchymal transition like phenotype in these individual cells could be transient, and these invasive cells would regain E-cadherin expression and their epithelial cohesive characteristics after forming invasive tumor foci. In contrast to conventional SCC, the acquisition of an epithelial mesenchymal transition like phenotype in our cases was not only limited to individual peripheral cells, but appeared rather homogenously in a majority of the tumor cells, suggesting that molecular and cellular events related to epithelial mesenchymal transition in ASCC has been widely spread.
Epithelial mesenchymal transition is associated with aggressive tumor behavior and poor response to chemoradiation therapy. Morphologic and immunohistochemical features of ASCC suggest that epithelial mesenchymal transition related genes, such as Snail and Twist, have been activated [32, 33]. As indicated in previous studies, overexpression of these genes could also be linked to tumor aggressiveness as we noted in ASCC.
In conclusion, upper aerodigestive ASCC is a rare variant of SCC that often shows aggressive biological behavior. Expression of CK19 protein and E-cadherin in ASCC are significantly down regulated with concurrent up-regulation of vimentin, suggests activation of epithelial mesenchymal transition related pathways in carcinogenesis and tumor progression. Different from other solid tumors, expression of epithelial mesenchymal transition in ASCC is diffuse and homogenous.
Acknowledgments
This study is accepted for presentation at the International Pathology Conference 2012 (OMICS), August 27–29, Philadelphia, PA, USA.
References
- 1.Landis SH, Murray T, Bolden S, et al. Cancer statistics, 1998. CA Cancer J Clin. 1998;48:6–29. doi: 10.3322/canjclin.48.1.6. [DOI] [PubMed] [Google Scholar]
- 2.Thomas LDR. Squamous cell carcinoma variants of the head and neck. Curr Diagnostic Pathol. 2003;9:384–396. doi: 10.1016/S0968-6053(03)00069-3. [DOI] [Google Scholar]
- 3.Cardesa A, Zidar N, Alos L. Acantholytic squamous cell carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. p. 129. [Google Scholar]
- 4.Zidar N, Gale N, Zupevc A, et al. Pseudovascular adenoid squamous-cell carcinoma of the oral cavity—A report of two cases. J Clin Pathol. 2006;59:1206–1208. doi: 10.1136/jcp.2005.029553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driemel O, Műller-Richter UD, Hakim SG, et al. Oral acantholytic squamous cell carcinoma shares clinical and histological features with angiosarcoma. Head Face Med. 2008;4:17. doi: 10.1186/1746-160X-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeoh MS, Kim DD, Ghali GE. Acantholytic sqaumous cell carcinoma of the buccal mucosa: Report of a case. J Oral Maxillofac Surg. 2011; Oct 17, E-public ahead of print. [DOI] [PubMed]
- 7.Nappi O, Wick MR, Pettinato G, et al. Psuedovascular adenoid squamous cell carcinoma of the skin. A neoplasm that may be mistaken for angiosarcoma. Am J Surg Pathol. 1992;16:429–438. doi: 10.1097/00000478-199205000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Eusebi V, Lamovec J, Cattani MC, et al. Acantholytic variant of squamous-cell carcinoma of the breast. Am J Surg Pathol. 1986;10:855–861. doi: 10.1097/00000478-198612000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Johnson WC, Helwig EB. Adenoid squamous cell carcinoma (adenoacanthoma): a clinicopathologic study of 155 patients. Cancer. 1966;19:1639–1650. doi: 10.1002/1097-0142(196611)19:11<1639::AID-CNCR2820191131>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 10.Papadopoulou E, Tosios KI, Nikitakis N, et al. Acantholytic squamous cell carcinoma of the gingiva: report of a case and review of the literature. Oral Sur Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e67–e71. doi: 10.1016/j.tripleo.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee SS, Eyden BP, Wells S, et al. Pseudoangiosarcomatous carcinoma: a clinicopathological study of seven cases. Histopathology. 1992;21(1):13–23. doi: 10.1111/j.1365-2559.1992.tb00338.x. [DOI] [PubMed] [Google Scholar]
- 12.Chute DJ, Stelow EB. Cytology of head and neck squamous cell carcinoma variants. Diagn Cytopathol. 2010;38:65–68. doi: 10.1002/dc.21134. [DOI] [PubMed] [Google Scholar]
- 13.Lever WF. Adenoacanthoma of sweat-gland—Carcinoma of sweat glands with glandular and epidermal elements—Report of four cases. Arch Dermatol. 1947;56:157. doi: 10.1001/archderm.1947.01520080017002. [DOI] [PubMed] [Google Scholar]
- 14.González-Vela MC, Val-Bernal JF, Mayorga M, et al. Adenoid squamous cell carcinoma of the larynx: an uncommon histological variant of squamous cell carcinoma. APMIS Acta Pathol Moicrobio Immunol Scand. 2006;114:470–473. doi: 10.1111/j.1600-0463.2006.apm_376.x. [DOI] [PubMed] [Google Scholar]
- 15.Jones AC, Freedman PD, Kerpel SM. Oral adenoid squamous cell carcinoma: a report of three cases and review of the literature. J Oral Maxillofac Surg. 1993;51:676–681. doi: 10.1016/S0278-2391(10)80269-1. [DOI] [PubMed] [Google Scholar]
- 16.Jukić Z, Ledinsky I, Ulamec M, et al. Primary acantholytic squamous cell carcinoma of the cecum: a case report. Diagn Pathol. 2011;6:5. doi: 10.1186/1746-1596-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aulmann S, Schnabel PA, Helmchen B, et al. Immunohistochemical and cytogenetic characterization of acantholytic sqaumous cell carcinoma of the breast. Vichows Arch. 2005;446:305–309. doi: 10.1007/s00428-004-1163-5. [DOI] [PubMed] [Google Scholar]
- 18.Kerawala CJ. Acantholytic squamous cell carcinoma of the oral cavity: a more aggressive entity? Br J Oral Maxillofac Surg. 2009;47:123–125. doi: 10.1016/j.bjoms.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Zhong LP, Zhao SF, Chen GF, et al. Increased levels of CK19 mRNA in oral squamous cell carcinoma tissue detected by relative quantification with real-time polymerase chain reaction. Arch Oral Biol. 2006;51:112–119. doi: 10.1016/j.archoralbio.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Xu XC, Lee JS, Lippman SM, et al. Increased expression of cytokeratins CK8 and CK19 is associated with head and neck carcinogenesis. Cancer Epidemiol Biomarkers Prev. 1995;4:871–876. [PubMed] [Google Scholar]
- 21.Jain R, Fischer S, Serra S, et al. The use of cytokeratin 19 (CK19) immunohistochemistry in lesions of the pancreas, gastrointestinal tract, and liver. Appl Immunohistochem Mol Morphol. 2010;18:9–15. doi: 10.1097/PAI.0b013e3181ad36ea. [DOI] [PubMed] [Google Scholar]
- 22.Eidelman S, Damsky CH, Wheelock MJ, et al. Expression of the cell–cell adhesion glycoprotein cell-CAM 120/80 in normal human tissues and tumors. Am J Pathol. 1989;135:101–110. [PMC free article] [PubMed] [Google Scholar]
- 23.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;22:251(5000):1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Z, Ge J, Sun Y, et al. Is E-cadherin immunoexpression a prognostic factor for head and neck squamous cell carcinoma (HSCC)? A systematic review and meta-analysis. Oral Oncol. 2012; March 26, Epub ahead of print. [DOI] [PubMed]
- 25.Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010 Oct; 21 Suppl 7:vii89–vii92. [DOI] [PMC free article] [PubMed]
- 26.Willipinski-Stape B, Riethdorf S, Assmann V, et al. Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res. 2005;11:8006–8014. doi: 10.1158/1078-0432.CCR-05-0632. [DOI] [PubMed] [Google Scholar]
- 27.Jeong H, Ryu YJ, An J, et al. Epithelial-mesenchymal transition in breast cancer correlates with high histological grade and triple-negative phenotype. Histopathology. 2102; Mar 22, Epub ahead of print. [DOI] [PubMed]
- 28.Hendrix MJ, Seftor A, Seftor RE, et al. Experimental coexpression of vimentin and keratin intermediate filaments in human breast cancer cells results in phenotypic interconversion and increased invasive behavior. Am J Pathol. 1997;150:483–495. [PMC free article] [PubMed] [Google Scholar]
- 29.Willipinski-Stapelfeldt B, Riethdorf S, Assmann V, et al. Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res. 2005;15:8006–8014. doi: 10.1158/1078-0432.CCR-05-0632. [DOI] [PubMed] [Google Scholar]
- 30.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 31.Wever O, Pauwels P, Craene B, et al. Molecular and pathological signatures of epithelial-mesenchymal transitions at the cancer invasion front. Histochem Cell Biol. 2008;130:481–494. doi: 10.1007/s00418-008-0464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thiery JP, Acloque H, Huang RYJ, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Bolos V, Peinado H, Perez-Moreno MA, et al. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116:499–551. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]