Abstract
Primary sinonasal tract carcinoma ex-pleomorphic adenoma (CEPA) is very uncommon, with adenoid cystic carcinoma (ACC) CEPA exceptional. These tumors are often misclassified. This is a retrospective study. Nine cases of ACC CEPA included 7 females and 2 males, aged 39–64 years (mean, 51.1 years). Patients presented most frequently with obstructive symptoms (n = 5), epistaxis (n = 3), nerve changes or pain (n = 3), present for a mean of 25 months (men: 9.5 versus women: 29.4 months; p = 0.264). The tumors involved the nasal cavity alone (n = 5), nasopharynx (n = 2), or a combination of locations (n = 2) with a mean size of 2.9 cm (females: 3.3; males: 1.7; p = 0.064). Most patients presented at a low clinical stage (n = 7, stage I), with one patient each in stage II and IV, respectively. Histologically, the tumors showed foci of PA associated with areas of ACC. Tumors showed invasion (lymph-vascular: n = 4; perineural: n = 6; bone: n = 6). The neoplastic cells were arranged in tubules, cribriform and solid patterns, with peg-shaped cells arranged around reduplicated basement membrane and glycosaminoglycan material. Mitoses ranged from 0 to 33, with a mean of 8.7 mitoses/10 HPFs. Necrosis (n = 2) and atypical mitotic figures (n = 1) were seen infrequently. Immunohistochemical studies showed positive reactions for cytokeratin, CK5/6, p63, CK7, EMA, SMA, calponin, S100 protein and CD117, several highlighting luminal versus basal cells components. GFAP, CK20 and MSA were non-reactive. p53 and Ki-67 were reactive to a variable degree. Surgery (n = 8), accompanied by radiation therapy (n = 5) was generally employed. Five patients developed a recurrence, all of whom died with disease (mean, 8.4 years), while 4 patients are either alive (n = 2) or had died (n = 2) without evidence of disease (mean, 15.9 years). In summary, ACC CEPA probably arises from the minor mucoserous glands of the upper aerodigestive tract, usually presenting in patients in middle age with obstructive symptoms in a nasal cavity based tumor. Most patients present with low stage disease (stage I and II), although invasive growth is common. Recurrences develop in about a 55 % of patients, who experience a shorter survival (mean, 8.4 years) than patients without recurrences (mean, 15.9 years). The following parameters, when present, suggest an increased incidence of recurrence or dying with disease: bone invasion, lymph-vascular invasion, and perineural invasion.
Keywords: Sinonasal tract, Carcinoma ex-pleomorphic adenoma, Adenoid cystic carcinoma, Pleomorphic adenoma, Nasal cavity, Nasopharynx, Review, Immunohistochemistry, Prognosis
Introduction
Carcinomas arising within the sinonasal tract (nasal cavity and paranasal sinuses; SNT) are uncommonly encountered by pathologists. Adenocarcinoma is separated into salivary gland-type adenocarcinoma and non-salivary gland-type adenocarcinoma [1–4]. The latter is divided into two major categories: intestinal-type adenocarcinoma and non-intestinal type adenocarcinoma. Adenocarcinomas of sinonasal tract can originate from the respiratory epithelium or the underlying mucoserous glands, with the majority arising from the mucoserous glands (60 %) [5]. Specifically, adenoid cystic carcinoma (ACC), the most common salivary gland malignancy in the SNT, is thought to arise from the minor mucoserous glands which lie within the mucosa, below the respiratory-type epithelium of the nasal cavity and paranasal sinuses [1]. However, the presence of an antecedent or contemporaneous benign tumor is very rare. Specifically, the presence of pleomorphic adenoma and ACC as the carcinoma component of a CEPA is exceedingly uncommon (Table 1). [6, 7] The varied clinical behavior, treatment alternatives, and long term patient prognosis of ACC CEPA is not well developed or understood. There is no large comprehensive evaluation of primary sinonasal tract ACC CEPA with respect to their clinical features, imaging findings, histomorphology, immunohistochemical reactivity, treatment outcomes, and clinical behavior.
Table 1.
| Characteristics | CEPA Total: n = 6 |
|---|---|
| Gender | |
| Women | 5 |
| Men | 1 |
| Age (in years) | |
| Range | 41–76 |
| Mean | 57.3 |
| Women (mean) | 53.6 |
| Men (mean) | 76.0 |
| Symptom duration (in months) | |
| Range | 1–60 |
| Mean | 15.8 |
| Women (mean) | 18.8 |
| Men (mean) | 1.0 |
| Symptoms at presentation* | |
| Obstructive symptoms | 4 |
| Epistaxis | 3 |
| Drainage, discharge, crusting | 1 |
| Headaches | 1 |
| Location | |
| Mixed (more than one anatomic site) | 2 |
| Septum | 3 |
| Nasal cavity only | 1 |
| Laterality | |
| Right | 4 |
| Left | 2 |
| Size (mean, cm)† | 2.67 |
| Tumor type (for malignancy) | |
| Adenocarcinoma, NOS | 3 |
| Adenoid cystic carcinoma* | 2 |
| Squamous cell carcinoma* | 2 |
| Mucoepidermoid carcinoma* | 1 |
| Treatment | |
| Surgery | 4 |
| Surgery and radiation | 2 |
| Recurrence | 1 |
| Patients with follow-up (n = 5) (follow-up in years) | |
| Follow-up range | 0.08–1 |
| Alive, no evidence of disease (n = 3) | 0.4 |
| Dead, no evidence of disease (n = 1) | 0.3 |
| Dead, with disease (n = 1) | 1.0 |
* More than one parameter may have been present
† Parameter was not stated in all cases
Materials and Methods
The records of 89 cases of primary sinonasal tract adenoid cystic carcinomas were selected involving the nasal cavity, paranasal sinuses (sphenoid, maxillary, ethmoid, and frontal sinuses) and nasopharynx. The cases were retrieved from the files of the Otorhinolaryngic-Head & Neck Tumor Registry of the Armed Forces Institute of Pathology (AFIP), Washington, DC, between 1970 and 2000 and the senior author’s consultation files. Of these patients, the records of 9 patients with tumors diagnosed as ACC CEPA of the nasal cavity, paranasal sinuses (sphenoid, maxillary, ethmoid, and frontal sinuses) and nasopharynx were selected. Materials within the files were supplemented by a review of the patient demographics (gender, age); symptoms and physical findings and duration at presentation including mass, nasal obstruction, polyps, difficulty breathing, changes in breathing, epistaxis, discharge, chronic rhinosinusitis, pain, headaches, nerve paralysis, visual changes; and past medical and surgical history. In addition, we reviewed imaging, surgical pathology, and operative reports and obtained follow-up information by direct written or oral communication with the referring pathologist, patient’s physician, oncology data services and tumor registries, or the patient (patient’s family member[s]). Follow-up data, available for all patients, included information regarding tumor location, presence of recurrent disease, treatment modalities used, and the current patient status. Since most samples were submitted in a fragmented fashion, definitive margins were not assessed nor were margins identified by the surgeons. Furthermore, as the cases were consultations, margin status was unreliable if inking had not been performed. Patients who were found to have a contiguous site primary (minor mucoserous glands of the palate; orbit) were excluded from further consideration. No patients in this series were part of a syndrome associated kindred (no familial cancer syndrome). It is important to add that as a tertiary pathology review center, conducting a retrospective review of these patients, we did not treat the patients. This clinical investigation was conducted in accordance and compliance with all statutes, directives, and guidelines of an Internal Review Board authorization (#5968) performed under the direction of Southern California Permanente Medical Group.
The macroscopic pathology observations noted within this study were gathered from the individual gross descriptions of the neoplasms given by the contributing pathologists. Hematoxylin and eosin-stained slides from all cases were reviewed, with a range of 1–21 slides reviewed per case (mean, 5 slides). The following specific features were documented: exact tumor location; lateralization; tumor size (greatest dimension in centimeters); tumor encapsulation (presence or absence); tumor extension (bone[s], soft tissue); architectural patterns of growth (solid, tubular, trabeculae, cribriform, glandular); cell population; surface origin; surface ulceration; presence or absence of necrosis; perineural invasion; lymph-vascular invasion; cellular pleomorphism (mild, moderate, severe [anaplastic]); presence of nucleoli; mitotic figures (number of mitotic figures per 10 high power fields [magnification at 40× with a 10× objective lens using an Olympus BX41 microscope]); atypical mitotic figures (present or absent, and defined by abnormal chromosome spread, tripolar or quadripolar forms, circular forms, or indescribably bizarre); and the presence of other microscopic pathologic findings in the remaining tissues.
Immunophenotypic analysis was performed in cases with sufficient suitable material by a standardized Envision™ method employing 4 μm-thick, formalin-fixed, paraffin-embedded sections on a single representative block, attempting to choose one which showed the benign and malignant in the same block. Table 2 documents the pertinent, commercially available immunohistochemical antibody panel used. However, the biopsies were often small, yielding a limited amount of tissue for additional examination. Epitope retrieval was performed, as required by the manufacturer guidelines. Standard positive controls were used throughout, with serum used as the negative control. The antibody reactions were graded as absent to weak (0 to 1+), moderate (2+ to 3+) and strong (4+) staining, and the fraction of positive cells was determined by separating them into four groups: <10 % (focal), 11-50 % (patchy), 51–90 % (majority), and >90 % (diffuse); proliferation markers were separated into <2 %, 2–10 %, >10 %.
Table 2.
Immunohistochemical panel
| Antigen/antibody/clone | Type | Company | Dilution | Antigen recovery |
|---|---|---|---|---|
| Cytokeratin (AE1/AE3:M3515 and CAM5.2) | mm | Dako, Carpinteria, CA Boehringer Mannheim Biochemicals, Indianapolis, IN |
1:40 1:8 |
CC1, 30 min |
| CK5/6 (D5/16 B4) | mm | Dako | 1:25 | E2, 20 min |
| CK7 (OV-TL-12/30) | mm | Dako | 1:200 | CC1, 30 min |
| CK20 (KS20.8) | mm | Ventana Medical Systems, Tucson, AZ | Neat | CC1, 30 min |
| Epithelial membrane antigen (E29) | mm | Ventana | Neat | CC1, 30 min |
| CAM5.2 (CK8/18) | mm | Covance, Princeton, NJ | 1:8 | CC1, 30 min |
| p63 (7jul) | mm | Leica Microsystems, Buffalo Grove, IL | 1:40 | E2, 30 min |
| CEA (CLO1-cea ab-1) | mm | Lab Vision/NeoMarkers, Fremont, CA | 1:250 | CC1, 30 min |
| Calponin | mm | Abcam, Cambridge, England | Neat | CC1, 30 min |
| Muscle specific actin (HHF35) | mm | Enzo Life Sciences, Farmingdale, NY | 1:100 | CC1, 30 min |
| Smooth muscle actin (asm-1) | mm | Leica, Wetzlar, Germany | 1:200 | E2, 20 min |
| Smooth muscle myosin heavy chain (SMMS1) | mm | Dako | 1:100 | CC1, 30 min |
| S100 protein | rp | Dako | 1:2,000 | CC1, 30 min |
| CD117 (C-Kit) | rp | Dako | 1:400 | CC1, 30 min |
| GFAP (6F2) | mm | Dako | 1:200 | CC1, 30 min |
| p53 (DO-7) | mm | Dako | Neat | CC1, 30 min |
| Ki-67 (MIB-1) | mm | Dako | 1:100 | CC1, 30 min |
mm mouse monoclonal, rp rabbit polyclonal
A review of publications in English (MEDLINE 1966–2012) was performed, with all cases reported as carcinoma ex-pleomorphic adenoma, malignant mixed tumor, carcinoma ex-mixed tumor of the sinonasal tract included in the review [8–18]. However, many cases were excluded if the lesion arose primarily in the oral cavity, upper lip or oropharynx or represented a different tumor type, or if the information was too generalized and non-specific to make a meaningful interpretation of the demographics, histologic features, or patient outcome.
Statistical evaluation was performed using a standard statistics software package with categorical variables analyzed using Chi-square tests and Fisher’s Exact tests to compare observed and expected frequency distributions. Comparison of means between groups were made with one-way analysis of variance. Confidence intervals of 95 % were generated for all positive findings. The alpha level was set at p <0.05.
Results
Clinical
The patients included 7 women and 2 men (Table 3). Their ages ranged from 39 to 64 years of age, with an overall mean age at presentation of 51.1 years. The average age at presentation for women was younger than men, at 48 and 62 years, respectively, but it did not quite reach statistical significance (p = 0.053). Patients most frequently presented with obstructive symptoms (including difficulty breathing, chronic sinusitis) (n = 5). Other symptoms included epistaxis (n = 3), headache, sinusitis, teeth dehiscence, and serous otitis media (n = 1 each). By definition, none of the tumors were centered in the oral cavity or orbit. The duration of symptoms ranged from 1 to 60 months, with an average of 25.0 months. On average, women (mean, 29.4 months) experienced a longer duration of symptoms than men (mean, 9.5 months), but this finding was not significant (p = 0.264). When separated by anatomic site, the mean duration of symptoms was quite different: nasal cavity alone: 14.6 months; maxillary sinus alone: 60 months; nasopharynx alone: 31 months; combination of nasal cavity and sinuses: 30 months, although due to limited number of cases in each group, there was no statistical significance (p = 0.257). Three patients had epistaxis, a symptom which dictates immediate clinical assessment, but they still had a mean duration of symptoms of 20 months. There was no statistically significant difference in survival based on duration of symptoms (p = 0.434). Either a computed tomography or magnetic resonance imaging study was performed in most patients (Fig. 1). The findings were non-specific, although they showed a soft tissue mass, often complex with heterogeneous enhancement involving a specific anatomic structure, which could help guide the surgery.
Table 3.
Clinical characteristics of adenoid cystic carcinoma ex-pleomorphic adenoma
| Clinical characteristics | Number |
|---|---|
| Gender | |
| Females | 7 |
| Males | 2 |
| Age (in years) | |
| Range | 39–64 |
| Mean | 51.1 |
| Women (mean) | 48.0 |
| Men (mean) | 62.0 |
| Symptoms* | |
| Duration (range, in months) | 1–60 |
| Duration (mean, in months) | 25.0 |
| Women (mean, in months) | 29.4 |
| Men (mean, in months) | 9.5 |
| Obstructive symptoms | 5 |
| Epistaxis | 3 |
| Headache | 1 |
| Mass, otitis media, sinusitis, dehiscence | 4 |
| Stage (p = 0.288) | |
| I | 7 |
| II | 1 |
| III | 0 |
| IV | 1 |
* More than one parameter may have been present
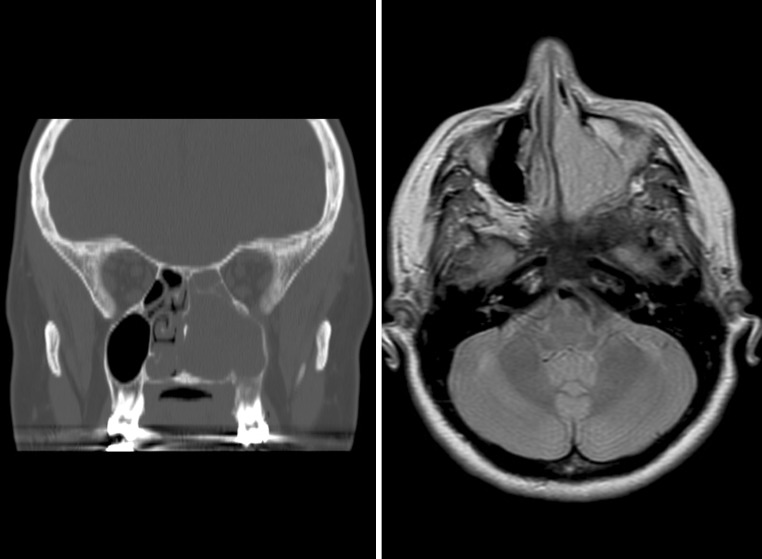
Fig. 1.
Imaging studies were performed to identify the exact site and extent of the tumor. Left: Computed tomography study demonstrating a left sided mass within the maxillary sinus and nasal cavity, expanding into adjacent sinuses. Right: An MRI shows a heterogeneously enhancing mass within the nasal cavity and expanding into the maxillary sinus, although without bone destruction
Pathologic Features
Macroscopic
The tumors ranged from 1 up to 4.5 cm in greatest single linear dimension (Table 4). The average size was 2.9 cm. Females had larger tumors than males (female, mean: 3.3 cm; male, mean: 1.7 cm), but this did not reach statistical significance (p = 0.064) Tumors were submitted in multiple fragments in most cases, precluding a definitive evaluation of margin status. The cases presented with tumors confined to the nasal cavity (n = 5), nasopharynx (n = 2), or maxillary sinus and nasal cavity (n = 2). Based on the clinical presentation, endoscopic evaluation, radiographic findings, and intraoperative observations, the cases were placed in appropriate stage, utilizing current staging criteria [19]. Seven patients were stage I; one patient: stage II; and one patient: stage IV. Due to the anatomic site of involvement, specific tumor macroscopic features were not unique. The tumors were described as pale, white, yellow to reddish-tan, showing a glistening, mucinous, myxoid appearance. Some of the fragments were gritty, no doubt related to fragments of bony tissue normally present in turbinate tissue or samples removed via curettage of the sinuses. Cartilage was described, but may have been part of the septum.
Table 4.
Pathology findings of adenoid cystic carcinoma ex-pleomorphic adenoma
| Pathology characteristic | Number of cases |
|---|---|
| Size (cm) | |
| Range | 1.0–4.5 |
| Mean | 2.9 |
| Female (mean) (p = 0.063) | 3.3 |
| Male (mean) | 1.7 |
| Anatomic site | |
| Nasal cavity alone | 5 |
| Nasopharynx only | 2 |
| Maxillary sinus and nasal cavity | 2 |
| Invasion | |
| Perineural invasion (p = 0.004) | 6 |
| Bone invasion (p = 0.004) | 6 |
| Invasion/destruction of parenchyma | 5 |
| Lymph-vascular invasion (p = 0.05) | 4 |
| Necrosis present (p = 0.479) | 2 |
| Pleomorphism | |
| Mild | 8 |
| Moderate | 1 |
| Severe | 0 |
| Nucleoli | |
| Small and focal | 3 |
| Prominent | 2 |
| Not identified | 4 |
| Nuclear to cytoplasmic ratio | |
| Intermediate | 7 |
| High | 2 |
| Mitotic figures | |
| Present | 6 |
| Mean (per 10 HPF) | 8.7 |
| Range | 0–33 |
| Atypical figures (present) (p = 0.288) | 1 |
Microscopic
None of the tumors showed surface derivation or connection. By definition, a benign pleomorphic adenoma was present in the sample (Figs. 2, 3). This ranged from approximately 5 % up to about 70 % of the tumor volume, although given the fragmentation and number of slides examined in each case, this may not be a meaningful separation. The classic features of pleomorphic adenoma were present, including a myxochondroid matrix material and both epithelial and myoepithelial cells intermingled (Figs. 2, 3). Small tubules and glandular profiles were noted (Figs. 2, 3). The pleomorphic adenoma component showed an increased sclerosis or fibrosis, sometimes associated with calcification or ossification. There was usually an abrupt transition of the pleomorphic adenoma to the carcinoma (Figs. 2, 3). In the areas of carcinoma, there was lymph-vascular invasion (n = 4), bone invasion (n = 6; Fig. 4), or perineural invasion (n = 6; Fig. 4), along with showing infiltration or destruction of minor mucoserous glands (n = 5, Fig. 5) (Table 4). A variable architectural appearance was characteristic both between tumors as well as within tumors. Two tumors were equally split between cribriform and tubular (50 % each), but a specific architectural pattern tended to predominate in each case: tubular (n = 4) and cribriform (n = 3), with a range of 0–95 % of the tumor showing a particular pattern (Fig. 6). The solid pattern was never >30 %, and so, no tumor qualified as the solid type. The characteristic “blue-goo” mucinous matrix material was seen, along with reduplicated basement membrane material (n = 8). Necrosis was present to a limited degree in two cases. Nuclear pleomorphism was usually mild to moderate, with the nuclei displaying a “carrot” or “peg” shape as they palisaded around the periphery of the tumor nests. Prominent nucleoli were seen in 2 cases, while small and focal nucleoli were seen in 3 cases. Most cells had an intermediate nuclear to cytoplasmic ratio, although a high N:C ratio could be found. Mitotic figures were seen in most cases (n = 6), with a range of 0–33, with a mean of 8.7 mitotic figures per 10 high power fields (Fig. 6). Atypical mitotic figures were only present in a single case, and did not seem to affect outcome. Squamous metaplasia was noted along with cystic change in two tumors. The following factors were analyzed for an impact on patient survival outcome:
Perineural invasion present: p = 0.004 (mean survival: 19.8 years when absent; 7.64 years when present);
Bone invasion present: p = 0.004 (mean survival 19.8 years when absent; 7.64 years when present);
Lymph-vascular invasion present: p = 0.05 (mean survival: 15.8 years when absent; 6.9 years when present);
Necrosis: p = 0.479;
Atypical mitoses: p = 0.288;
Size: p = 0.874;
Stage: p = 0.103.
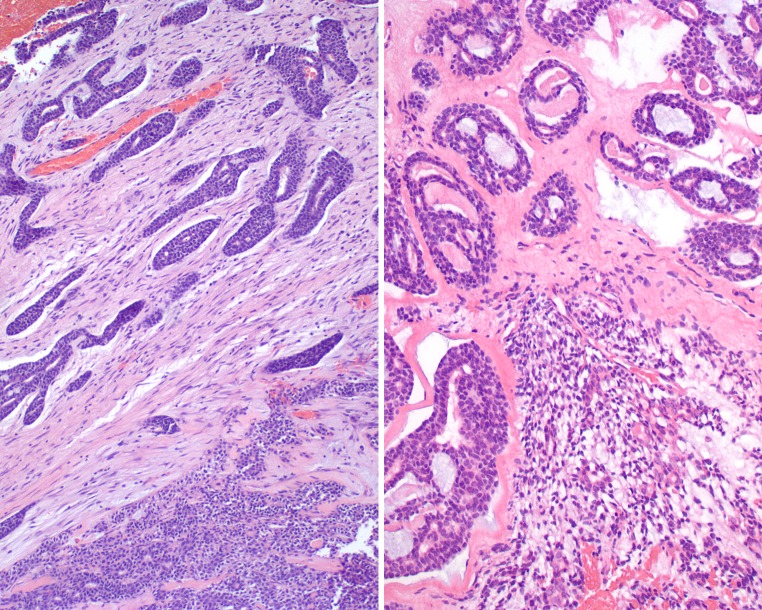
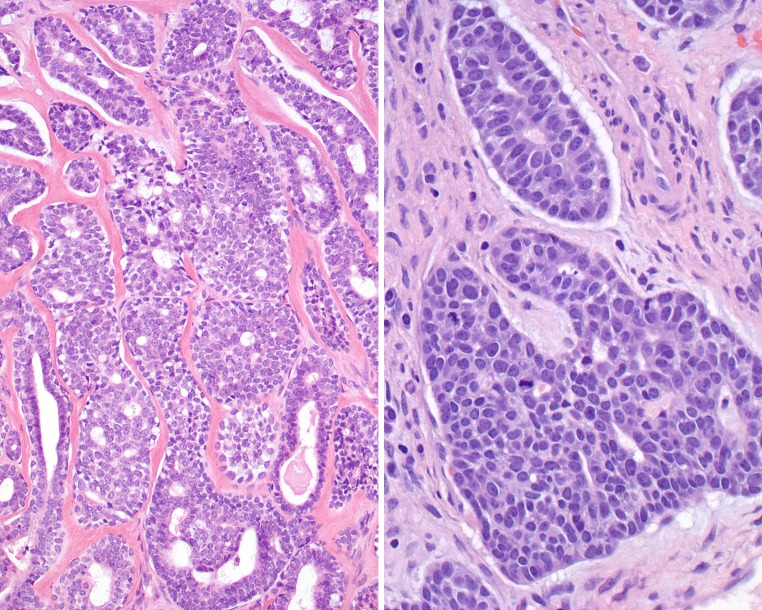
Fig. 2.
A combination of adenoid cystic carcinoma and pleomorphic adenoma can be seen. Left:Upper left side shows an abrupt transition from the lower right pleomorphic adenoma. Right: The lower central field shows characteristic pleomorphic adenoma intimately associated with the areas of adenoid cystic carcinoma
Fig. 3.
The juxtaposition of pleomorphic adenoma to adenoid cystic carcinoma was variable. Left: The classic features of pleomorphic adenoma, including the chondro-myxoid matrix and epithelial islands. Right: The left half of the field shows pleomorphic adenoma blending imperceptibly with the right half of the field which shows adenoid cystic carcinoma
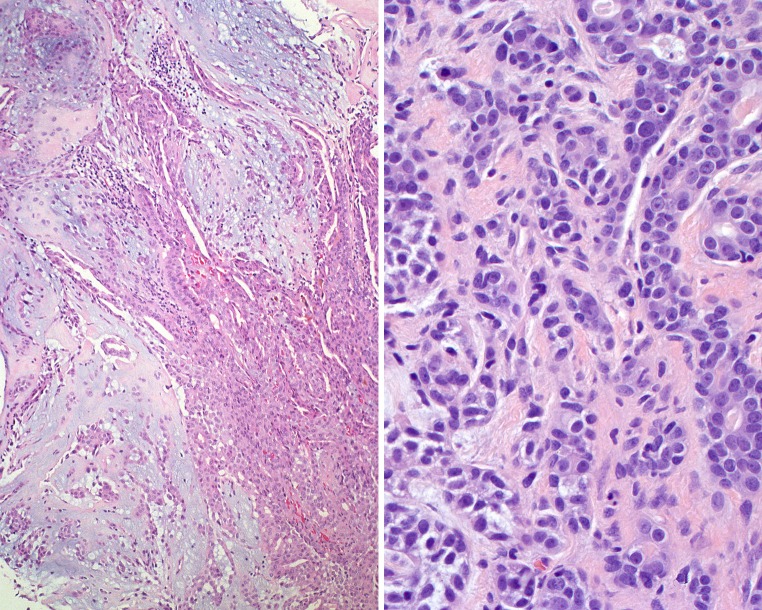
Fig. 4.
Left: Adenoid cystic carcinoma invading into and destroying bone. Right: Perineural invasion by adenoid cystic carcinoma
Fig. 5.
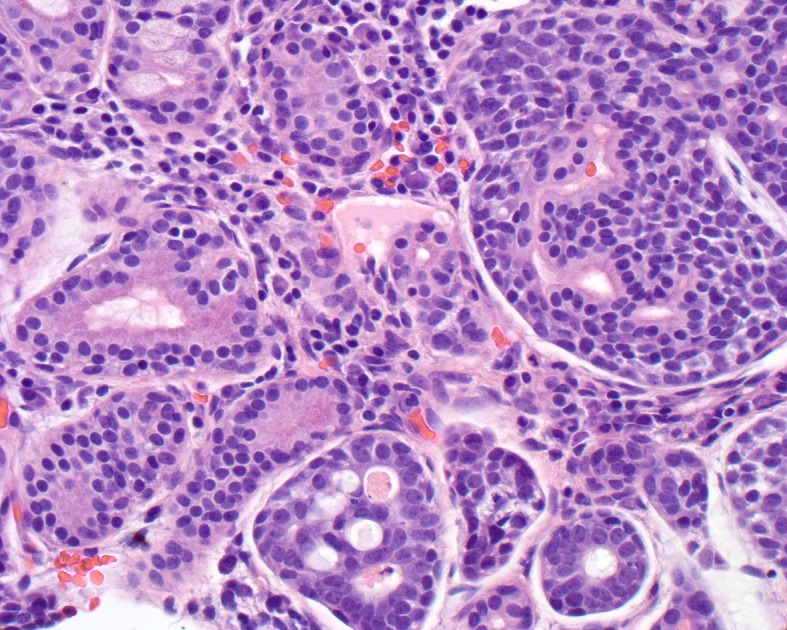
Minor mucoserous glands of the sinonasal tract (left) are invaded and partially destroyed by the adenoid cystic carcinoma cells (right). The cells show a cribriform pattern (lower mid field)
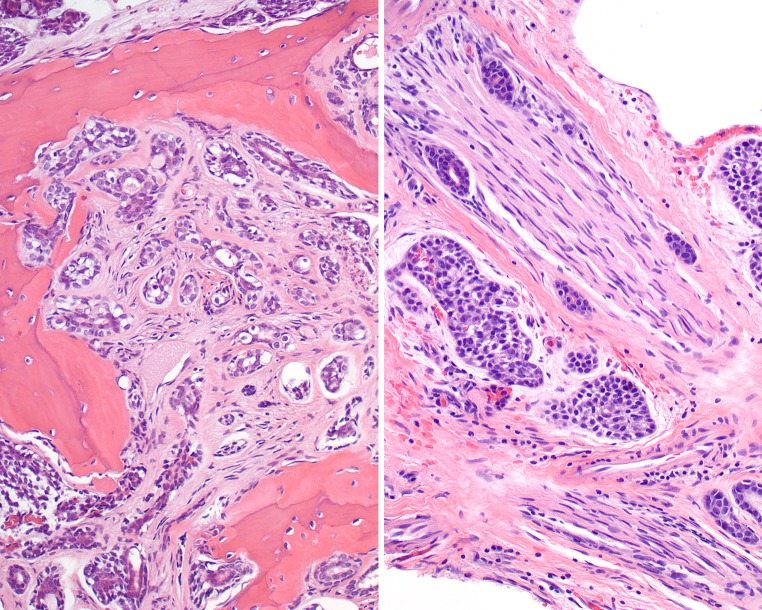
Fig. 6.
Left: Heavy stromal hyalinization separates the neoplastic groups in this focus of adenoid cystic carcinoma. There is a cribriform and tubular-glandular appearance. Right: Three mitoses are noted within a single group of cells in this adenoid cystic carcinoma
Immunohistochemistry
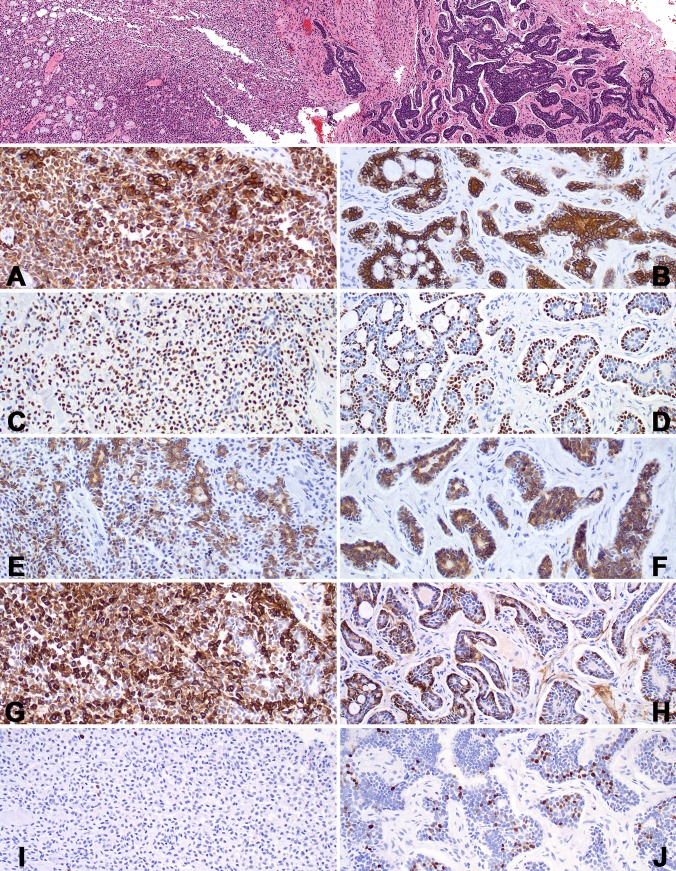
As would be expected, the pleomorphic adenoma and adenoid cystic carcinoma components showed some similarity in immunohistochemical reactivity, since they are both epithelial and myoepithelial. Both tumors showed diffuse and strong pan-cytokeratin positivity, focally showing an accentuation of the luminal and tubular foci. CK5/6 and CK7, along with EMA usually showed a strong and diffuse cytoplasmic reactivity in most cases, focally with a luminal distribution (Fig. 7). p63 (Fig. 7), calponin, smooth muscle actin, and smooth muscle myosin heavy chain (Fig. 7) showed a variably strong and diffuse basal cell positive reaction. S100 protein showed a variable reaction in both the nuclei and cytoplasm of lesional cells, accentuating the abluminal cells, but positive in all cells in some cases. This was also the case with the pleomorphic adenoma components. CD117 showed a strong and diffuse luminal and tubular positive reaction, again in both benign and malignant components (Fig. 7). m-CEA was identified focally, usually within tubular areas. There was no reaction with CK20, MSA, or GFAP in the malignant component, although GFAP was expressed in the pleomorphic adenoma regions. Over-expression of p53 was only seen in one case, where it was positive in approximately 30 % of the nuclei. The Ki-67 proliferation index ranged from 0 to approximately 5 %, although most were <1 % (Fig. 7).
Fig. 7.
A lower power field shows a pleomorphic adenoma (left) transitioning into adenoid cystic carcinoma (right). The immunohistochemistry reactions are shown for the pleomorphic adenoma (left) and the adenoid cystic carcinoma (right) in each row. A and B: CK7: Note the luminal cell accentuation in the ACC. C and D: p63: There is a basal accentuation in the ACC. E and F: CD117. G and H: SMMHC: There is variable accentuation in the ACC. I and J: Ki67. Note the number of positive nuclei in the ACC in comparison to the PA
Treatment and Follow-up
Nearly all patients were managed with surgery as the initial management modality (n = 8) (Table 5). The types of procedures varied based on the specific anatomic site of involvement. Excision, wide local excision, maxillectomy, and exenteration were employed to remove the primary tumor. Five patients were further managed with radiation a few weeks after surgery, while only a single patient was treated by radiation alone without surgery (other than biopsy). No patients were managed with chemotherapy. Five patients developed a recurrence (Table 5). The recurrences developed within 23–100 months after the initial management. There were four females and 1 male, average age, 51 years. All of the tumors were identified in only a single site. The mean size was 3.1 cm, while four patients were stage I, and one stage II. All of these patients had died of disease, either local or metastatic (mean, 8.4 years). The survival ranged from 4.6 up to 12.5 years, with a worse outcome for those with recurrence compared to those without a recurrence (p = 0.001).
Table 5.
Summary of patient outcome
| All patients | A, NED | D, NED | D, D | |
|---|---|---|---|---|
| All patients with follow-up (mean years) | 9 (11.7) | 2 (11.1) | 2 (20.8) | 5 (8.4) |
| Follow-up range (years) | 4.0–26.5 | 4.0 and 18.2 | 15.0 and 26.5 | 4.6–12.5 |
| Gender (p = 0.859) | ||||
| Females | 7 (12.0) | 2 (11.1) | 1 (26.5) | 4 (8.8) |
| Males | 2 (10.9) | n/a | 1 (15.0) | 1 (6.7) |
| Age (p = 0.859) | ||||
| <60 years | 7 (12.0) | 2 (11.1) | 1 (26.5) | 4 (8.8) |
| ≥60 years | 2 (10.9) | n/a | 1 (15.0) | 1 (6.7) |
| Size (p = 0.551) | ||||
| <4.0 cm | 7 (12.0) | 2 (11.1) | 2 (20.8) | 3 (6.7) |
| ≥4.0 cm | 2 (10.9) | n/a | n/a | 2 (10.9) |
| Anatomic site (p = 0.101) | ||||
| Nasal cavity alone | 5 (16.2) | 1 (18.2) | 2 (20.8) | 2 (10.6) |
| Nasopharynx alone | 2 (8.0) | n/a | n/a | 2 (8.0) |
| Mixed | 2 (4.3) | 1 (4.0) | n/a | 1 (4.6) |
| Stage (p = 0.101) | ||||
| I | 7 (13.8) | 1 (18.2) | 2 (20.8) | 4 (9.3) |
| II | 1 (4.6) | n/a | n/a | 1 (4.6) |
| IV | 1 (4.0) | 1 (4.0) | n/a | n/a |
| Recurrence (p = 0.001) | ||||
| Absent | 4 (15.9) | 2 (11.1) | 2 (20.8) | n/a |
| Present | 5 (8.4) | n/a | n/a | 5 (8.4) |
A, NED Alive, no evidence of disease, D, NED dead, no evidence of disease, D, D dead with disease, n/a not applicable
Overall, there was a good long term survival, with overall 11.7 years of average follow-up for the 9 patients (Table 5). Five patients died with disease (local or with distant metastases), an average of 8.4 years after diagnosis. This is in sharp contrast to those who were either alive or had died, but without evidence of disease at last follow-up (average, 15.9 years). If the patients were >60 years of age, 50 % of the patients died, but 57 % of patients died if they were <60 years of age (p = 0.859). All of the patients died of disease if the tumor was ≥4 cm, while 43 % of patients died if the tumor was <4 cm (p = 0.055). All of the patients with nasopharyngeal tumors died of their disease. Patients with high stage tumors had a much shorter outcome when compared with low stage tumors: stage I: mean, 13.8 years; Stage II–IV: 4.3 years, but this difference did not reach statistical significance (p = 0.101). Due to the limited number of cases in each category, differences between age, gender, tumor location, tumor size, and stage did not reach statistical significance.
Discussion
Carcinoma ex pleomorphic adenoma (CEPA) is defined as a carcinoma arising from a primary or recurrent benign pleomorphic adenoma (PA) [1, 20, 21]. Pleomorphic adenoma are uncommon in the sinonasal tract [22], but when present, tend to affect the nasal septum. ACC is the most common salivary gland type primary adenocarcinoma of the sinonasal tract [1, 21]. However, there are only isolated case reports of CEPA in which the carcinoma is ACC [6, 7, 18, 23]. Other carcinomas, such as mucoepidermoid carcinoma [18, 24, 25], squamous cell carcinoma [18] and adenocarcinoma, not otherwise specified (NOS), [6, 23] are also recognized but are equally infrequent. Benign salivary gland neoplasms are less common than malignant tumors, and include pleomorphic adenoma, myoepithelioma, and oncocytoma. Overall, CEPA ACC is very rare.
Clinical
Combining the 9 cases from this clinical series and the 6 cases from a review of the pertinent literature (Table 6), there is a remarkable gender difference, with 12 women and 3 men (4:1). However, women seem to be more likely than men to die from their disease: 6 of 12 females died of disease (mean: 7.2 years; 50 %); while only 1 of 3 males died (mean: 6.7 years; 33 %), although this did not reach a significant probability (one tailed = 0.21). Patients range in age from 39 to 76 years, with a mean age at presentation of 54.0 years. Men were statistically older than women, with a mean of 67 versus 50 years, respectively (p = 0.012). Patients presented with basically non-specific symptoms, with obstructive symptoms, epistaxis, and headaches the most frequently noted signs and symptoms. Symptoms were usually present for an average of 21.3 months, suggesting the non-specific nature of the presenting signs and symptoms. Interestingly, when symptoms were stratified by gender, women experienced symptoms for a longer duration (24.8 months) than men (7.3 months), although this did not reach statistical significance (p = 0.201). Tumors involved a single site (nasal cavity, nasopharynx) more often than multiple sites. In general, tumors were on average 2.5 cm, without a side predilection. As would be expected, nasal cavity tumors were smaller (mean, 2.8 cm) than mixed site tumors (mean, 3.2 cm). Likewise, patients who had a tumor ≥4 cm were more likely to die of their disease (100 %) than patients with tumors <4 cm (33.3 %).
Table 6.
Combination of current study with literature
| Characteristics | Total: n = 15 |
|---|---|
| Gender | |
| Women | 12 |
| Men | 3 |
| Age (in years) | |
| Range | 39–76 |
| Mean | 54.0 |
| Women (mean) (p = 0.012) | 50.0 |
| Men (mean) | 67.0 |
| Symptom duration (in months) | |
| Range | 1–60 |
| Mean | 21.3 |
| Women (mean) (p = 0.201) | 24.8 |
| Men (mean) | 7.3 |
| Symptoms at presentation* | |
| Obstructive symptoms | 9 |
| Epistaxis | 6 |
| Headaches | 2 |
| Other (sinusitis, blurred vision, otitis media, rhinorrhea, teeth dehiscence) | 6 |
| Location (p = 0.288) | |
| Nasal cavity only, including septum | 9 |
| Mixed (more than one anatomic site) | 4 |
| Nasopharynx only | 2 |
| Size† | |
| Range | 1–4.5 |
| Mean | 2.9 |
| Female (mean) (p = 0.018) | 3.1 |
| Male (mean) | 1.7 |
| Tumor Stage (p = 0.288) | |
| Stage 1 | 11 |
| Stage 2 | 3 |
| Stage 3 | 0 |
| Stage 4 | 1 |
| All patients with follow-up (n = 14) (mean years of survival) | 14 (7.7) |
| Follow-up range | 0.3–26.5 |
| Alive, no evidence of disease (n = 5) | 4.7 |
| Dead, no evidence of disease (n = 3) | 13.9 |
| Dead, with disease (n = 6) | 7.1 |
| Alive/dead, no evidence of disease (n = 8) | 8.1 |
| Dead, with disease (n = 6) | 7.1 |
| Patients with recurrence (n = 6) | 7.1 |
| Patients without recurrence (n = 8) | 8.2 |
* Patients may have experienced more than one symptom
† Parameter was not stated in all cases
Most patients presented with low stage disease (11 patients with T1 tumors; 4 patients with T2-T4 tumors). The higher the stage of disease, the more likely patients were to die from disease: 4 of 11 (36 %) patients with Stage I tumors died of disease (mean: 9.3 years); 2 of 3 (66.6 %) patients with Stage II-IV tumors died of disease (mean: 2.8 years). This did not reach statistical significance (p = 0.288) due to the very limited follow-up periods in patients who were still alive (the average follow-up for the single case reports in the literature was only 6 months).
If a patient developed recurrence, they were also more likely to die from disease, a finding which reached statistical significance (p = 0.001). Six patients developed local recurrence, while 8 patients did not. Of the patients with recurrence, 100 % died of disease (mean, 7.2 years); in contrast, when there was no recurrence, none of the patients died from disease (mean, 8.1 years). Overall, when combined with the literature, 57.1 % of patients were either alive or had died without evidence of disease, with a mean follow-up of 8.1 years. This is in contrast to the 42.9 % of patients who had died with disease, with a mean follow-up of 7.1 years. Overall, the raw survival rate at 5 years is 50 %, and 28.6 % at 10 years, suggesting the survival is similar to adenoid cystic carcinoma in general.
Although difficult to achieve given the anatomic confines of the region, wide excision is the treatment of choice, and was used exclusively in 7 patients. Four patients were alive or had died without evidence of disease (mean, 0.4 years); while 2 patients had died with disease (mean, 9.0 years), with one patient lost to follow-up. Again, the lack of prolonged follow-up in single case reports, makes a meaningful interpretation of this mode of therapy impossible. Clear margins are very difficult to assess in many cases, but are considered to be a reliable indicator of surgical extirpation. In this clinical series, no reliable information could be obtained on margin status, and so this postulation cannot be further evaluated. Radiation therapy was employed in 57 % (n = 8), although combined with surgery in 7 patients. Four patients were alive or had died without evidence of disease (mean, 12.2 years), while 4 had died with disease (mean, 6.2 years). Therefore, a definitive statement about the effectiveness of radiation therapy in sinonasal tract disease is equivocal.
Pathology Features
ACC CEPA in this anatomic site are invasive by definition. Tumors infiltrate into the adjacent parenchyma, soft tissues, bone, and mucoserous glands, while frequently displaying perineural and/or lymph-vascular invasion. Perineural invasion (p = 0.004), bone invasion (p = 0.004) and lymph-vascular invasion (p = 0.05) are all associated with a worse clinical outcome when present. Necrosis was identified infrequently (n = 2). In general, mitoses were seen frequently, but atypical mitoses were uncommon.
p53 is well known to be increased in malignant or pre-malignant neoplasms, showing a gradient of increased nuclear staining as the tumor becomes less well differentiated. Since there is no staining in pleomorphic adenoma, and 75 % of the cases in this series showed increased staining (between 5 and 30 %), the presence of any p53 expression may help with separating areas of benign from malignant transformation, especially on small biopsies. Correlation of p53 immunohistochemistry with p53 gene mutation is variable. However, in general, strong expression indicates aberrant stabilization of the p53 protein. The immunohistochemistry results are similar to those of major salivary gland studies. As expected, there is variable expression between the tubular and more solid areas, as well as differential expression between the epithelial and myoepithelial-basal cells within the tumors.
Differential Diagnosis
Given the anatomic site, the most important consideration is recognizing the benign PA component and separating it from the ACC. Distinction can usually be made based on invasion (into minor salivary gland, around nerves, into bone, and within lymph-vascular spaces), difference in patterns of growth, presence of reduplicated basement membrane material, and an increased number of mitoses, including atypical forms. The myxochondroid matrix material is lost in carcinoma. There is an increased expression of p53 and an increased Ki-67 index (>1 %).
Summary
ACC CEPA are exceedingly rare in the sinonasal tract. Patients are usually middle aged women who present with obstructive symptoms and epistaxis, usually present for just less than 2 years. Most patients present with low stage disease (stage I and II) with tumors that are 2.9 cm on average. Recurrences develop in about 42 % of patients, and when present strongly predict dying from disease (100 % of patients). Surgery, with or without radiation, is the treatment of choice, attempting to achieve clear margins. Overall survival is about 7.7 years, although with a 50 % 5-year and a 29 % 10-year survival, long term clinical follow-up is mandatory.
Acknowledgments
The authors thank Ms. Hannah Herrera for her research assistance. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of Southern California Permanente Medical Group.
References
- 1.Eveson JW. Salivary gland-type carcinomas. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics: head and neck tumours. Lyon: IARC Press; 2005. pp. 24–25. [Google Scholar]
- 2.Franchi A, Santucci M, Wenig BM. Adenocarcinoma. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics head and neck tumours. Lyon: IARC Press; 2005. pp. 20–23. [Google Scholar]
- 3.Kleinsasser O, Schroeder HG. Adenocarcinomas of the inner nose after exposure to wood dust. Morphological findings and relationships between histopathology and clinical behavior in 79 cases. Arch Otorhinolaryngol. 1988;245:1–15. doi: 10.1007/BF00463541. [DOI] [PubMed] [Google Scholar]
- 4.Barnes L. Intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Am J Surg Pathol. 1986;10:192–202. doi: 10.1097/00000478-198603000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Gnepp DR, Heffner DK. Mucosal origin of sinonasal tract adenomatous neoplasms. Mod Pathol. 1989;2:365–371. [PubMed] [Google Scholar]
- 6.Cimino-Mathews A, Lin BM, Chang SS, Boahene KD, Bishop JA. Carcinoma ex pleomorphic adenoma of the nasal cavity. Head Neck Pathol. 2011;5:405–409. doi: 10.1007/s12105-011-0262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman SR, Sloan P, Carpentier J. Carcinoma ex-pleomorphic adenoma of the nasal septum with adenoid cystic and squamous carcinomatous differentiation. Rhinology. 2003;41:118–121. [PubMed] [Google Scholar]
- 8.Shukla NK, Hazarika S, Deo S, Kar M, Kumar S, Samaiya A, et al. Salivary gland tumours: profile and management at a tertiary cancer centre. J Indian Med Assoc. 2011;109:381–385. [PubMed] [Google Scholar]
- 9.Nordkvist A, Roijer E, Bang G, Gustafsson H, Behrendt M, Ryd W, et al. Expression and mutation patterns of p53 in benign and malignant salivary gland tumors. Int J Oncol. 2000;16:477–483. doi: 10.3892/ijo.16.3.477. [DOI] [PubMed] [Google Scholar]
- 10.Saleh ER, Franca CM, Marques MM. Neural adhesion molecule (N-CAM) in pleomorphic adenoma and carcinoma ex-pleomorphic adenoma. J Oral Pathol Med. 2003;32:562–567. doi: 10.1034/j.1600-0714.2003.00076.x. [DOI] [PubMed] [Google Scholar]
- 11.Perez DE, Pires FR, Almeida OP, Kowalski LP. Epithelial lacrimal gland tumors: a clinicopathological study of 18 cases. Otolaryngol Head Neck Surg. 2006;134:321–325. doi: 10.1016/j.otohns.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Katori H, Nozawa A, Tsukuda M. Increased expression of cyclooxygenase-2 and Ki-67 are associated with malignant transformation of pleomorphic adenoma. Auris Nasus Larynx. 2007;34:79–84. doi: 10.1016/j.anl.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Ding CS, Yap WM, Teo CH, Giron D, Chuah KL. Tracheal carcinoma ex pleomorphic adenoma: a rare tumour with potential problems in diagnosis. Histopathology. 2007;51:868–871. doi: 10.1111/j.1365-2559.2007.02865.x. [DOI] [PubMed] [Google Scholar]
- 14.Ide F, Mishima K, Yamada H, Saito I. Adenoid cystic carcinoma ex pleomorphic adenoma of the parotid gland. Head Neck Pathol. 2009;3:159–162. doi: 10.1007/s12105-009-0108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo YL, Tu TY, Chang CF, Li WY, Chang SY, Shiao AS, et al. Extra-major salivary gland pleomorphic adenoma of the head and neck: a 10-year experience and review of the literature. Eur Arch Otorhinolaryngol. 2011;268:1035–1040. doi: 10.1007/s00405-010-1437-2. [DOI] [PubMed] [Google Scholar]
- 16.Farman AG, George DI, Jr, Clear RM. Computerized tomography of combined carcinomas arising in pleomorphic adenoma. Oral Surg Oral Med Oral Pathol. 1985;59:96–101. doi: 10.1016/0030-4220(85)90124-0. [DOI] [PubMed] [Google Scholar]
- 17.Schramm VL, Jr, Imola MJ. Management of nasopharyngeal salivary gland malignancy. Laryngoscope. 2001;111:1533–1544. doi: 10.1097/00005537-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Chimona TS, Koutsopoulos AV, Malliotakis P, Nikolidakis A, Skoulakis C, Bizakis JG. Malignant mixed tumor of the nasal cavity. Auris Nasus Larynx. 2006;33:63–66. doi: 10.1016/j.anl.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 19.AJCC Cancer Staging Manual, 7th ed. New York: Springer; 2009.
- 20.Lewis JE, Olsen KD, Sebo TJ. Carcinoma ex pleomorphic adenoma: pathologic analysis of 73 cases. Hum Pathol. 2001;32:596–604. doi: 10.1053/hupa.2001.25000. [DOI] [PubMed] [Google Scholar]
- 21.Gnepp DR. Malignant mixed tumors of the salivary glands: a review. Pathol Annu. 1993;28(Pt 1):279–328. [PubMed] [Google Scholar]
- 22.Wenig BL, Sciubba JJ, Cohen A, Abramson AL. Pleomorphic adenoma of the nasal septum. Otolaryngol Head Neck Surg. 1985;93:432–436. doi: 10.1177/019459988509300328. [DOI] [PubMed] [Google Scholar]
- 23.Cho KJ, el-Naggar AK, Mahanupab P, Luna MA, Batsakis JG. Carcinoma ex-pleomorphic adenoma of the nasal cavity: a report of two cases. J Laryngol Otol. 1995;109:677–679. doi: 10.1017/S0022215100131019. [DOI] [PubMed] [Google Scholar]
- 24.Carinci F, Curioni C, Padula E, Calearo C. Cancer of the nasal cavity and paranasal sinuses: a new staging system. Int J Oral Maxillofac Surg. 1996;25:34–39. doi: 10.1016/S0901-5027(96)80009-9. [DOI] [PubMed] [Google Scholar]
- 25.Wolfish EB, Nelson BL, Thompson LD. Sinonasal tract mucoepidermoid carcinoma: a clinicopathologic and immunophenotypic study of 19 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2012;6(2):191–207. [DOI] [PMC free article] [PubMed]