Abstract
Adenomatoid odontogenic tumor (AOT) is an uncommon benign epithelial lesion of odontogenic origin and, thus far, only few studies regarding the frequency of its many histopathologic features have been published in the literature. Thus, the aim of this study was to perform a retrospective analysis in a case series of AOT, with emphasis on the histopathological features. Fifteen cases of AOT were studied considering their clinical, radiographic and histopathologic aspects. Twelve cases affected females and the mean age was 16.2 years. The anterior maxilla was the most common site (66.6 %) and radiographically most cases showed a unilocular radiolucency with well-defined borders (57.1 %). Histologically, most cases exhibited predominantly a solid growth pattern (46.7 %) or a similar proportion of solid and cribriform patterns (46.7 %). Eosinophilic amorphous material (“tumor droplets”) was found in all cases (100 %). Most tumors showed duct-like spaces (93.3 %) and convoluted structures (60.0 %) whereas a minor proportion of cases presented calcifying epithelial odontogenic tumor (CEOT)-like areas (26.7 %). Variable amounts of calcified material were found in most AOTs (80.0 %) whereas osteodentin and perivascular hyalinization were seen only rarely (6.7 % each one). Five (33.3 %) cases had areas mimicking a dentigerous cyst and most of these were diagnosed in females (80.0 %). Regarding the histopathologic features, our results suggest that AOTs usually show predominance of solid pattern or a similar proportion of solid and cribriform patterns while osteodentin and perivascular hyalinization are rarely seen in these tumors. In addition, areas mimicking a dentigerous cyst and CEOT-like areas are relatively infrequent findings in AOTs.
Keywords: Adenomatoid odontogenic tumor, Odontogenic tumor, Clinical, Histopathology
Introduction
Adenomatoid odontogenic tumor (AOT) is an uncommon benign epithelial lesion of odontogenic origin, representing approximately 3.0 % of all odontogenic tumors [1, 2]. Credit has been giving to Harbiz [3] for reporting the earliest case demonstrating irrefutable proof of an AOT, in 1915. On the other hand, Stafne, in 1948, was the first author who considered AOT as an entity although he did not propose a specific name for it [4]. In 1969, Philipsen and Birn [5] introduced the term ‘adenomatoid odontogenic tumor’, to be adopted by the World Health Organization (WHO) classification of odontogenic tumors in 1971 [6], and it is now the generally accepted nomenclature.
The AOT can occur in three forms: a follicular type, an extrafollicular type and a peripheral type. The follicular type occurs intraosseously and presents as a well-defined, unilocular radiolucency associated with the crown and often part of the root of an unerupted tooth thus mimicking a dentigerous cyst [2]. The extrafollicular type also occurs within the bone but is not associated with an unerupted tooth [7]. This variant presents as a well-defined, unilocular radiolucency found between, above or superimposed upon the roots of erupted, permanent teeth. The peripheral type occurs extraosseously and often appears as a gingival fibroma or epulis attached to the labial gingival [2, 7]. This variant may show erosion of the alveolar bone crest but rarely produces radiographically detectable changes [2]. Clinically, all variants of AOT are characterized by slow but progressive growth, with few or no subjective symptoms. In intraosseous variants, cortical expansion is a common finding, while perforation of the cortical plate is unusual [8, 9].
Microscopically, AOT is usually surrounded by a well-developed fibrous capsule and the tumor is composed of solid nodules of spindle-shaped, polygonal or cuboidal epithelial cells forming nests, rosette-like structures, duct-like spaces and strands of epithelium with a trabecular or cribriform configuration [2]. Between the epithelial cells and in the center of the rosette-like structures, eosinophilic amorphous material (often described as ‘tumor droplets’) is present. Additionally, variably amounts of calcified material are present in most lesions [10].
Despite the relatively large number of studies about demographic, clinical and radiologic features of AOT [4, 7, 9, 11, 12], information regarding the frequency of the many histopathologic aspects of this lesion is scarce [8, 13, 14]. Thus, the aim of this study was to perform a retrospective analysis in a case series of AOT, with emphasis on the histopathological features, in order to provide useful additional data to the literature.
Materials and Methods
After approval by the Ethics Committee of the Federal University of Rio Grande do Norte (protocol number 337/2010), all cases of AOT kept in the archives of an Oral Pathology Service, and diagnosed between January 1970 and December 2009 were reviewed. Data regarding patient age and gender, site of occurrence, location, radiographic aspects, size, time of disease and symptomatology of all cases were compiled from the clinical data sent together with the biopsy records. With respect to the site of occurrence, the jaw was divided in two areas: anterior region (from canine to canine) and posterior region (posterior to the canine). According to a topographical division, location of the tumors was considered as incisor, canine, premolar and molar areas, and when the lesion extended to more than one area, the location of the central part of the lesion was considered as the affected site [8].
For histopathologic analysis, all slides containing hematoxylin/eosin-stained 5-μm-thick sections were reassessed. Under light microscopy (Olympus CX31; Olympus Japan Co., Tokyo, JPN), AOTs were histologically reviewed by two observers. The cases were classified according to the predominant histological pattern (solid or cribriform), as well as according to the presence or absence of the following features: duct-like spaces, convoluted structures (cuboidal to columnar cells forming convoluted cords in complicated patterns that often exhibit invaginations), eosinophilic amorphous material (“tumor droplets”), calcified material, melanin pigmentation, calcifying epithelial odontogenic tumor (CEOT)-like areas, calcifying cystic odontogenic tumor-like areas [15], areas mimicking dentigerous cysts [16] and perivascular hyalinization [17].
The data were tabulated and analyzed by descriptive statistics using the Statistical Package for the Social Sciences, version 17.0 (SPSS, Inc., Chicago, IL).
Results
Clinical and Radiographic Findings
Of the 273 cases of odontogenic tumors diagnosed at our Oral Pathology Service during the study period (1970–2009), fifteen (5.4 %) AOTs were identified. Twelve (80 %) cases affected females and 3 (20.0 %) occurred in males, with a female/male proportion of 4:1. Patients’ age ranged from 12 to 26 years, with a mean of 16.2 years. Ten (66.7 %) cases affected the maxilla and 5 (33.3 %) the mandible. With respect to the site of occurrence, it was observed a remarkable predilection for the anterior region (93.3 %) of the jaws, with only 1 (6.7 %) case in the posterior region, which affected the mandible. The information regarding anatomic location was available in 10 cases and revealed seven (70.0 %) lesions in the canine area (5 in maxilla and 2 in mandible), 2 (20.0 %) in the maxillary incisive area, and 1 (10.0 %) in the mandibular premolar area.
The size of the lesion was known in 10 cases and ranged from 0.4 to 4.7 cm, with a mean of 2.4 cm. Reported length of disease varied from 2 to 84 months, with a mean of 21.9 months. Asymptomatic swelling was the main complaint in 9 (75.0 %) cases, whereas 3 (25.0 %) patients reported mild pain. Radiographically, of the 14 cases presenting information, all showed unilocular radiolucency with well-defined borders, and in 6 (42.9 %) of these, discrete radiopaque foci were also seen (Fig. 1). Thirteen (86.7 %) cases were follicular (8 in maxilla and 5 in mandible), 1 (6.7 %) was extrafollicular and 1 (6.7 %) was peripheral, these two last cases affecting the maxilla.
Fig. 1.
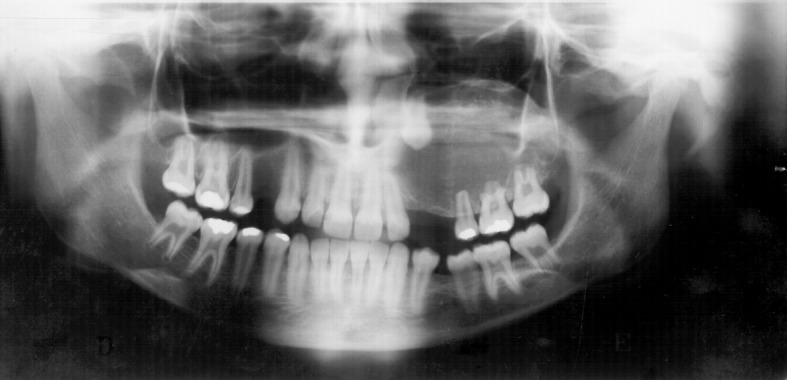
Panoramic radiograph showing unilocular radiolucency with discrete radiopaque foci associated with impacted left maxillary canine
Histopathological Findings
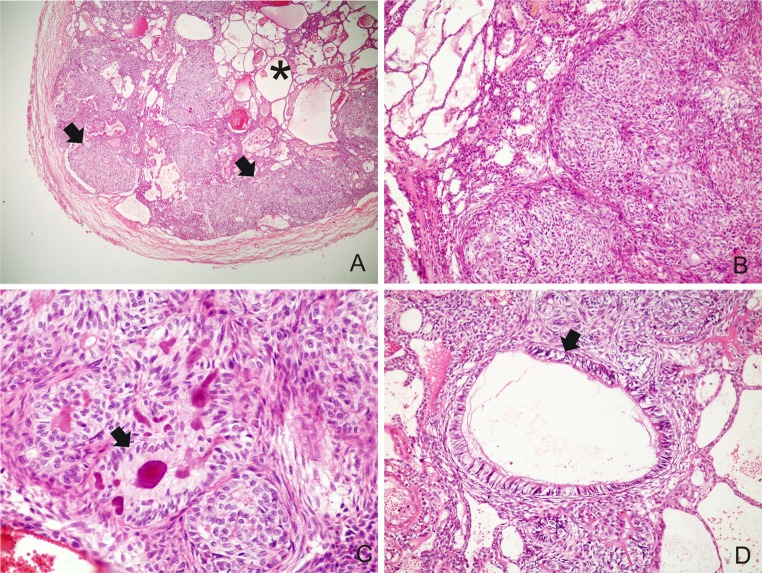
The histopathological findings of AOT are summarized in Table 1. All cases revealed the typical findings of AOT at light microscopy, with varying sized solid nodules of columnar or cuboidal epithelial cells forming nests or rosette-like structures. Interlacing strands of epithelium composed of oval, angular, and elongated cells, forming a cribriform pattern were seen at the periphery of the more solid areas. All tumors presented minimal mature connective tissue stroma, which was generally loosely structured and contained thin-walled congested vessels (Fig. 2a, b).
Table 1.
Frequency of the histopathological aspects of AOT from the present study and others studies
| Histopathological aspects | Present study | Leon et al. [8] | Jivan et al. [14] |
|---|---|---|---|
| Predominant pattern | |||
| Solid | 7 (46.7 %) | NS | NS |
| Cribriform | 1 (6.6 %) | ||
| Solid/cribriform | 7 (46.7 %) | ||
| Duct-like spaces | |||
| Present | 14 (93.3 %) | 39 (100.0 %) | NS |
| Absent | 1 (6.7 %) | 0 (0.0 %) | |
| Convoluted structures | |||
| Present | 9 (60.0 %) | NS | NS |
| Absent | 6 (40.0 %) | ||
| Tumor droplets | |||
| Present | 15 (100.0 %) | 39 (100.0 %) | 51 (100.0 %) |
| Absent | 0 (0.0 %) | 0 (0.0 %) | 0 (0.0 %) |
| Calcified material | |||
| Present | 12 (80 %) | NS | 15 (29.4 %) |
| Absent | 3 (20 %) | 36 (70.6 %) | |
| Melanin pigmentation | |||
| Present | 0 (0.0 %) | NS | NS |
| Absent | 15 (100.0 %) | ||
| CEOT-like areas | |||
| Present | 4 (26.7 %) | 36 (92.3 %) | NS |
| Absent | 11 (73.3 %) | 3 (7.7 %) | |
| CCOT-like areas | |||
| Present | 0 (0.0 %) | NS | NS |
| Absent | 15 (100.0 %) | ||
| Perivascular hyalinization | |||
| Present | 1 (6.7 %) | 7 (17.9 %) | NS |
| Absent | 14 (93.3 %) | 32 (82.1 %) | |
NS not stated
Fig. 2.
a A well-encapsulated lesion showing cribriform (asterisk) and solid patterns (arrow) (H&E ×40); bSolid nodules of columnar or cuboidal epithelial cells and interlacing strands of epithelium composed of oval, angular and elongated cells (H&E ×200); c Rosette-like structures with eosinophilic amorphous material (“tumor droplets”) (arrow) (H&E ×400); d Duct-like spaces lined by a single row of columnar epithelial cells (arrow) (H&E ×200)
With respect to the predominant histological pattern, 7 (46.7 %) cases showed predominance of solid pattern, 1 (6.6 %) revealed predominance of cribriform pattern, and 7 (46.7 %) had both in similar proportions (Fig. 2a). Between the epithelial cells of nodules and in the center of the rosette-like configurations, eosinophilic amorphous material (“tumor droplets”) was present in all cases (100.0 %) (Fig. 2c). The duct-like spaces were lined by a single row of columnar epithelial cells, with the nuclei polarized away from luminal surface and were found in 14 (93.3 %) cases (Fig. 2d). Convoluted structures of tall columnar epithelial cells were found in 9 (60.0 %) cases (Fig. 3a).
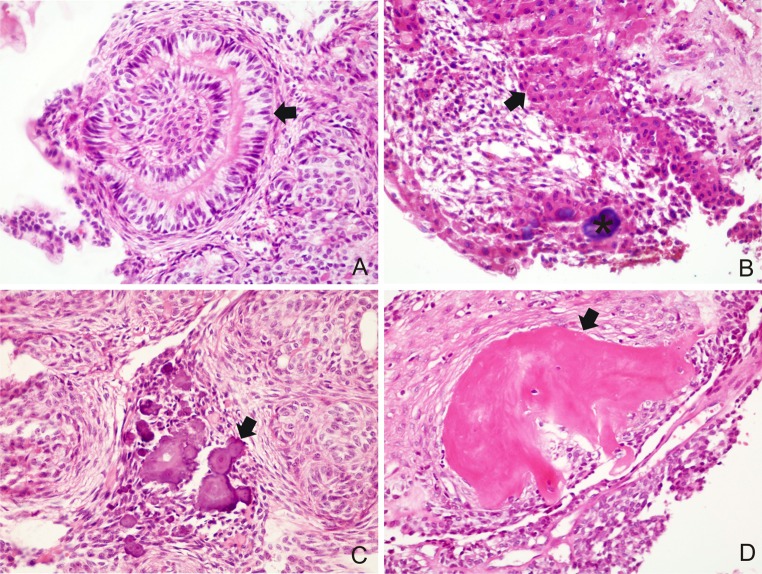
Fig. 3.
a Convoluted structures of tall columnar epithelial cells (arrow) (H&E ×400); b CEOT-like areas consisting of polyhedral, eosinophilic epithelial cells of squamous appearance with distinct cell boundaries and intercellular bridges (arrow) with calcified material (asterisk) (H&E ×400); c calcified material between the solid nests (arrow) (H&E ×400); d osteodentin juxtaposed to tumor cells (arrow) (H&E ×400)
CEOT-like areas, characterized by nodules consisting of polyhedral, eosinophilic epithelial cells of squamous appearance with distinct cell boundaries and intercellular bridges, were observed in 4 (26.7 %) cases (Fig. 3b). Variable amounts of calcified material were found in most lesions (80.0 %) (Fig. 3c) and they were commonly seen in the solid pattern and in CEOT-like areas (Fig. 3b). Calcified material in the cribriform pattern was an unusual finding. One (6.7 %) case exhibited osteodentin in focal area (Fig. 3d).
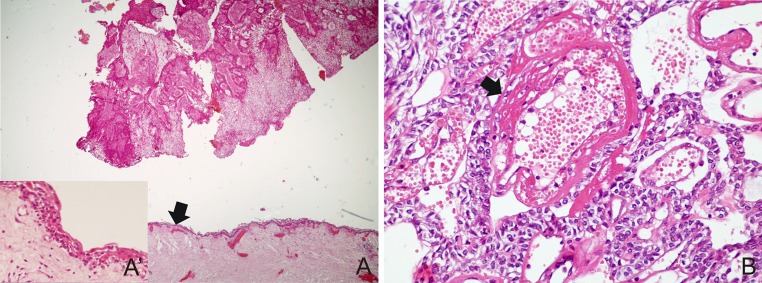
Five (33.3 %) cases had areas mimicking dentigerous cyst (Fig. 4a) and most of them were diagnosed in females (80.0 %). Perivascular hyalinization was observed in 1 (6.7 %) case, which demonstrated a concentric disposition of hyaline layers in the perivascular connective tissue and modified the endothelial layer (Fig. 4b). None of the cases showed melanin pigmentation or calcifying cystic odontogenic tumor-like areas.
Fig. 4.
a AOT with area exhibiting thin non-keratinized stratified squamous lining epithelium mimicking a dentigerous cyst (arrow) (H&E ×40) and in detail (A′) (H&E ×400); b blood vessels of tumor stroma showing degenerative changes of endothelial lining and vessel wall (arrow), surrounded by cells with ovoid basophilic nuclei (H&E ×400)
Discussion
The AOT is a benign odontogenic lesion that has been regarded either as a non-aggressive, non-invasive neoplasm or as a developmental hamartomatous growth [4, 18]. It has been generally reported that AOT corresponds to 2.2–8.7 % of all odontogenic tumors [2, 10, 19]. However, in a worldwide collaborative retrospective study, the relative frequency of AOT ranged from 0.6 to 38.5 % [4]. Interestingly, the highest percentages were found in studies from Nigeria, which might suggest geographic differences in the frequency of these tumors [4]. Nonetheless, it must be emphasized that the recent inclusion of odontogenic keratocysts in the group of odontogenic tumors [20] may make in some instances a marked impact on the reported relative frequency of AOT [4]. Thus, according to Philipsen et al. [4], it is likely that it will take some years to accumulate reliable data on the relative frequency of AOT.
Clinically, AOTs are characterized by slow but progressive growth with few or no subjective symptoms [7, 8]. The size of intraosseous lesions ranges from 1.0 to 3.0 cm [10]. Similar findings were observed in the present case series. With respect to age distribution, it has been reported that more than two-thirds of AOTs are diagnosed in the second decade of life and 90 % are found before the age of 30 [2, 4, 12]. Our results are in agreement with these reports.
Similar to the present study, retrospective studies with large case series reveal a female predominance for AOT, with a global female: male ratio of 1.9:1 [2, 4]. However, variations in gender distribution have been reported depending on the geographic region. In some Asian and African countries the female: male ratio may reach 3.2:1 [21] and 5.6:1 [12], respectively.
The follicular type is the most common variant of AOT, accounting for 70.8 % of the cases, followed by the extrafollicular type which accounts for 26.9 % of the cases [4]. The peripheral type is rare and accounts for only 2.3 % [4] of all AOTs. The central variants are more commonly found in the anterior maxilla [9, 12], with a maxilla: mandible ratio ranging from 1.5:1 to 2.1:1 [2, 4, 8, 9]. Permanent maxillary canines account for almost 40.0 % of the cases of unerupted teeth associated with the follicular variant of AOT [2]. The peripheral variant is found almost exclusively in the anterior maxillary gingiva with this location accounting for 88.0 % [2]. The results of the present study regarding the frequency and anatomic distribution of AOT variants agree with those commonly reported in the literature.
Radiographically, it has been reported that two-thirds of intraosseous AOTs appear as well-demarcated unilocular radiolucency containing small radiopaque foci [2, 22]. In contrast, Arotiba et al. [12] and Leon et al. [8] observed a higher frequency of central AOTs presenting as unilocular radiolucencies without radiopaque foci, similar to our findings.
The histogenesis of AOT is still debatable. Philipsen et al. [23] suggested that AOT may be derived from the complex system of dental lamina or its remnants. However, results from ultrastructural and immunohistochemical studies might support the origin of these tumors from the reduced enamel epithelium [21, 24, 25].
In the present study, histopathologic analysis revealed the typical findings of AOT in all cases. Most cases exhibited predominance of solid pattern or a similar proportion of solid and cribriform patterns. Only one tumor revealed predominance of cribriform pattern, which is in accordance with the scarcity of such reports in the literature [19].
The frequency of duct-like spaces, a conspicuous finding in AOT [2], was similar to that reported by Leon et al. [8]. The duct-like spaces vary in diameter and may not be present in all AOTs [2, 15]. However, due to the overall distinctive histomorphology of AOT, the correct diagnosis can usually be reached without the presence of duct-like structures [2]. Results from an immunohistochemical investigation suggest that cells of duct-like structures are differentiated once to ameloblasts but fail to mature further due to increased production of basement membrane-associated extracellular matrix molecules and due to their retention in the lumina [26]. In addition to forming duct-like spaces, cuboidal to columnar cells of AOT can form convoluted structures [2, 15]. To the best of our knowledge, there are no studies about the frequency of convoluted structures in AOTs. The results of the present investigation suggest that these structures are relatively frequent in AOTs.
Eosinophilic amorphous material (“tumor droplets”) was observed in all AOTs evaluated in this study. Similar findings have been reported by Leon et al. [8] and Jivan et al. [14]. It has been shown that tumor droplets in AOTs are immunopositive for enamel proteins, such as enamelin, amelogenin and sheathlin, supporting a probable enamel nature for this material [27, 28]. Variable amounts of calcified material have been reported in most cases of AOT [2, 8, 22] and the present results corroborate these observations. In contrast, Jivan et al. [14] found calcified material in only 29.4 % of AOTs evaluated in their study.
Hyaline, dysplastic material or calcified osteodentin, rarely with concomitant abortive enamel, has been infrequently reported in AOTs [29, 30]. In the present study, only one case exhibited osteodentin in a focal area, which corroborates the low frequency of this microscopic feature. According to Philipsen and Nikai [15], these materials are likely the result of a metaplastic process, as odontogenic ectomesenchyme is not present in AOTs, and therefore should not be interpreted as an induction phenomenon.
The AOT may present areas mimicking CEOT [8], calcifying cystic odontogenic tumor [31], developing odontomas [32], and other odontogenic tumors or hamartomas [2]. In the present sample, we noted fewer cases of AOT exhibited CEOT-like areas compared to the multicentric retrospective study [8]. Mosqueda-Taylor et al. [33] stress that CEOT-like areas in AOTs do not present as solid, infiltrative nests, as it appears in true CEOT. In addition, the CEOT-like areas in AOTs lack the typical pleomorphism that is found in the epithelial component of CEOT [33] and their presence do not influence the biologic behavior and growth potential of AOTs [2, 34]. Thus, CEOT-like areas should be considered normal features within the histomorphologic spectrum of AOT [33, 34].
The presence of cystic areas in AOTs mimicking odontogenic cysts, such as dentigerous cyst, has been reported in the literature [8, 16]. In the study performed by Leon et al. [8], 56.4 % of AOTs exhibited cystic epithelium. On the other hand, in the present series, 5 (33.3 %) tumors showed dentigerous cyst-like areas. In a recent systematic review of the literature, Gadewar and Srikant [16] found a higher frequency of AOTs exhibited cystic epithelium in males. In contrast, in the present sample, most cases of AOT associated with dentigerous cyst were diagnosed in females. These conflicting results may be due to the relatively small number of cases in both series, which does not allow obtaining a definite tendency of gender distribution of AOTs with odontogenic cyst-like areas.
El-Labban and Lee [17] performed an ultrastructural analysis in 3 cases of AOT and verified that an estimated 70.0–90.0 % of the blood vessels found in the stroma show degenerative changes affecting both the endothelial lining and the perivascular connective tissue. According to these authors, these perivascular alterations may be derived from the multiplication of the basal lamina, associated with degradation of the layered collagen surrounding these vessels. Philipsen et al. [10] emphasize that these degenerative changes may be so pronounced that they can be easily detected in the light microscopy. In spite of these aspects, Leon et al. [8] observed perivascular hyalinization in few cases, and in the present study, only one case, suggesting that this feature may not be so commonly detectable in these tumors by light microscopy.
The neural crest interacts in the development of teeth [35] and it is thus not surprising that melanocytes can occur in odontogenic tissues and their related lesions. However, there are only few reports of AOTs exhibiting melanin pigmentation, particularly in Black [36, 37] and Asian people [38]. In a retrospective study of 31 Nigerian cases of AOT, Effiom and Odukoya [39] verified occurrence of melanin pigment in only 1 (3.2 %) tumor. According to these authors, melanin pigmentation is rarely demonstrated in AOT even in a predominantly Black population. In the present study, none of the cases showed melanin pigmentation, corroborating the rarity of this microscopic feature in AOTs. In view of the limited number of cases of AOT showing melanin pigmentation, a hypothesis of a racial factor in the development of these lesions cannot be drawn.
In conclusion, the clinic-radiographic profile of AOTs observed in this study agrees with that commonly reported in the literature. Regarding the histopathologic features, our results suggest that AOTs usually show predominance of solid pattern or a similar proportion of solid and cribriform patterns, with presence of duct-like spaces, convoluted structures, tumor droplets and calcified material while perivascular hyalinization are rarely seen in these tumors. In addition, areas mimicking a dentigerous cyst and CEOT-like areas may be relatively infrequent findings in AOTs.
Conflict of interest
The authors deny any conflicts of interest.
Contributor Information
Lélia Batista de Souza, Email: leliabsouza@gmail.com.
Roseana de Almeida Freitas, Phone: +55-84-32154138, FAX: +55-84-32154138, Email: phelipematos@yahoo.com.br, Email: roseanafreitas@hotmail.com.
References
- 1.Vera Sempere FJ, Artes Martinez MJ, Vera Sirera B, Bonet Marco J. Follicular adenomatoid odontogenic tumor: immunohistochemical study. Med Oral Patol Oral Cir Bucal. 2006;11(4):E305-8. [PubMed]
- 2.Philipsen HP, Reichart PA. Adenomatoid odontogenic tumour: facts and figures. Oral Oncol. 1999;35(2):125–131. doi: 10.1016/S1368-8375(98)00111-0. [DOI] [PubMed] [Google Scholar]
- 3.Harbitz F. On cystic tumors of the maxilla, and especially on adamantine cystadenomas (adamantomas) Dent Cosmos. 1915;57:1081–1093. [Google Scholar]
- 4.Philipsen HP, Reichart PA, Siar CH, Ng KH, Lau SH, Zhang X, et al. An updated clinical and epidemiological profile of the adenomatoid odontogenic tumour: a collaborative retrospective study. J Oral Pathol Med. 2007;36(7):383–393. doi: 10.1111/j.1600-0714.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 5.Philipsen HP, Birn H. The adenomatoid odontogenic tumour. Ameloblastic adenomatoid tumour or adeno-ameloblastoma. Acta Pathol Microbiol Scand. 1969;75(3):375–398. [PubMed] [Google Scholar]
- 6.Pindborg JJ, Kramer JR, Torloni H. Histological typing of odontogenic tumours, jaw cysts and allied lesions. Geneva: WHO; 1971. [Google Scholar]
- 7.Mohamed A, Singh AS, Raubenheimer EJ, Bouckaert MM. Adenomatoid odontogenic tumour: review of the literature and an analysis of 33 cases from South Africa. Int J Oral Maxillofac Surg. 2010;39(9):843–846. doi: 10.1016/j.ijom.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Leon JE, Mata GM, Fregnani ER, Carlos-Bregni R, Almeida OP, Mosqueda-Taylor A, et al. Clinicopathological and immunohistochemical study of 39 cases of Adenomatoid Odontogenic Tumour: a multicentric study. Oral Oncol. 2005;41(8):835–842. doi: 10.1016/j.oraloncology.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Swasdison S, Dhanuthai K, Jainkittivong A, Philipsen HP. Adenomatoid odontogenic tumors: an analysis of 67 cases in a Thai population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(2):210–215. doi: 10.1016/j.tripleo.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Philipsen HP, Reichart PA, Zhang KH, Nikai H, Yu QX. Adenomatoid odontogenic tumor: biologic profile based on 499 cases. J Oral Pathol Med. 1991;20(4):149–158. doi: 10.1111/j.1600-0714.1991.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 11.Mendis BR, MacDonald DG. Adenomatoid odontogenic tumour. A survey of 21 cases from Sri Lanka. Int J Oral Maxillofac Surg. 1990;19(3):141–143. doi: 10.1016/S0901-5027(05)80129-8. [DOI] [PubMed] [Google Scholar]
- 12.Arotiba GT, Arotiba JT, Olaitan AA, Ajayi OF. The adenomatoid odontogenic tumor: an analysis of 57 cases in a black African population. J Oral Maxillofac Surg 1997;55(2):146–8; discussion 9-50. [DOI] [PubMed]
- 13.Takahashi H, Fujita S, Shibata Y, Yamaguchi A. Adenomatoid odontogenic tumour: immunohistochemical demonstration of transferrin, ferritin and alpha-one-antitrypsin. J Oral Pathol Med. 2001;30(4):237–244. doi: 10.1034/j.1600-0714.2001.300408.x. [DOI] [PubMed] [Google Scholar]
- 14.Jivan V, Altini M, Meer S. Secretory cells in adenomatoid odontogenic tumour: tissue induction or metaplastic mineralisation? Oral Dis. 2008;14(5):445–449. doi: 10.1111/j.1601-0825.2007.01399.x. [DOI] [PubMed] [Google Scholar]
- 15.Philipsen HP, Nikai H. Chapter 6: odontogenic tumours, adenomatoid odontogenic tumour. In: Pathology and genetics of head and neck tumours. Lyon, France: IARC Press; 2005. p. 304–5.
- 16.Gadewar DR, Srikant N. Adenomatoid odontogenic tumour: tumour or a cyst, a histopathological support for the controversy. Int J Pediatr Otorhinolaryngol. 2010;74(4):333–337. doi: 10.1016/j.ijporl.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 17.el-Labban NG, Lee KW. Vascular degeneration in adenomatoid odontogenic tumour: an ultrastructural study. J Oral Pathol 1988;17(6):298–305. [DOI] [PubMed]
- 18.Rick GM. Adenomatoid odontogenic tumor. Oral Maxillofac Surg Clin North Am. 2004;16(3):333–354. doi: 10.1016/j.coms.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Santos JN, Lima FO, Romerio P, Souza VF. Adenomatoid odontogenic tumor: an unusual case exhibiting cribriform aspect. Quintessence Int. 2008;39(9):777–781. [PubMed] [Google Scholar]
- 20.Barnes L, Eveson JW, Reichart P, Sidransky D. World health organization classification of tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 21.Yamamoto H, Kozawa Y, Hirai G, Hagiwara T, Nakamura T. Adenomatoid odontogenic tumor: light and electron microscopic study. Int J Oral Surg. 1981;10(4):272–278. doi: 10.1016/S0300-9785(81)80071-3. [DOI] [PubMed] [Google Scholar]
- 22.Philipsen HP, Reichart PA, Nikai H. The adenomatoid odontogenic tumour (AOT): an update. Oral Med Pathol. 1997;2:55–60. doi: 10.3353/omp.2.55. [DOI] [Google Scholar]
- 23.Philipsen HP, Samman N, Ormiston IW, Wu PC, Reichart PA. Variants of the adenomatoid odontogenic tumor with a note on tumor origin. J Oral Pathol Med. 1992;21(8):348–352. doi: 10.1111/j.1600-0714.1992.tb01363.x. [DOI] [PubMed] [Google Scholar]
- 24.Poulson TC, Greer RO., Jr Adenomatoid odontogenic tumor: clinicopathologic and ultrastructural concepts. J Oral Maxillofac Surg. 1983;41(12):818–824. doi: 10.1016/S0278-2391(83)80050-0. [DOI] [PubMed] [Google Scholar]
- 25.Crivelini MM, Araujo VC, Sousa SO, Araujo NS. Cytokeratins in epithelia of odontogenic neoplasms. Oral Dis. 2003;9(1):1–6. doi: 10.1034/j.1601-0825.2003.00861.x. [DOI] [PubMed] [Google Scholar]
- 26.Murata M, Cheng J, Horino K, Hara K, Shimokawa H, Saku T. Enamel proteins and extracellular matrix molecules are co-localized in the pseudocystic stromal space of adenomatoid odontogenic tumor. J Oral Pathol Med. 2000;29(10):483–490. doi: 10.1034/j.1600-0714.2000.291002.x. [DOI] [PubMed] [Google Scholar]
- 27.Saku T, Okabe H, Shimokawa H. Immunohistochemical demonstration of enamel proteins in odontogenic tumors. J Oral Pathol Med. 1992;21(3):113–119. doi: 10.1111/j.1600-0714.1992.tb00993.x. [DOI] [PubMed] [Google Scholar]
- 28.Takata T, Zhao M, Uchida T, Kudo Y, Sato S, Nikai H. Immunohistochemical demonstration of an enamel sheath protein, sheathlin, in odontogenic tumors. Virchows Arch. 2000;436(4):324–329. doi: 10.1007/s004280050454. [DOI] [PubMed] [Google Scholar]
- 29.Tajima Y, Sakamoto E, Yamamoto Y. Odontogenic cyst giving rise to an adenomatoid odontogenic tumor: report of a case with peculiar features. J Oral Maxillofac Surg. 1992;50(2):190–193. doi: 10.1016/0278-2391(92)90370-F. [DOI] [PubMed] [Google Scholar]
- 30.Takeda Y. Induction of osteodentin and abortive enamel in adenomatoid odontogenic tumor. Ann Dent. 1995;54(1–2):61–63. [PubMed] [Google Scholar]
- 31.Zeitoun IM, Dhanrajani PJ, Mosadomi HA. Adenomatoid odontogenic tumor arising in a calcifying odontogenic cyst. J Oral Maxillofac Surg. 1996;54(5):634–637. doi: 10.1016/S0278-2391(96)90650-3. [DOI] [PubMed] [Google Scholar]
- 32.Dunlap CL, Fritzlen TJ. Cystic odontoma with concomitant adenoameloblastoma (adenoameloblastic odontoma) Oral Surg Oral Med Oral Pathol. 1972;34(3):450–456. doi: 10.1016/0030-4220(72)90324-6. [DOI] [PubMed] [Google Scholar]
- 33.Mosqueda-Taylor A, Carlos-Bregni R, Ledesma-Montes C, Fillipi RZ, Almeida OP, Vargas PA. Calcifying epithelial odontogenic tumor-like areas are common findings in adenomatoid odontogenic tumors and not a specific entity. Oral Oncol. 2005;41(2):214–215. doi: 10.1016/j.oraloncology.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Ledesma-Montes C, Mosqueda-Taylor A, Romero de León E, de la Piedra Garza M, Goldberg Juakin P, Portilla Robertson J. Adenomatoid odontogenic tumor with features of calcifying epithelial odontogenic tumor. (The so-called combined epithelial odontogenic tumor) Clinico-pathological report of 12 cases. Eur J Cancer B Oral Oncol. 1993;29B:221–4. [DOI] [PubMed]
- 35.Lawson W, Abaci IF, Zak FG. Studies on melanocytes. V. The presence of melanocytes in the human dental primordium: an explanation for pigmented lesions of the jaws. Oral Surg Oral Med Oral Pathol. 1976;42(3):375–380. doi: 10.1016/0030-4220(76)90171-7. [DOI] [PubMed] [Google Scholar]
- 36.Aldred MJ, Gray AR. A pigmented adenomatoid odontogenic tumor. Oral Surg Oral Med Oral Pathol. 1990;70(1):86–89. doi: 10.1016/0030-4220(90)90184-T. [DOI] [PubMed] [Google Scholar]
- 37.Warter A, George-Diolombi G, Chazal M, Ango A. Melanin in a dentigerous cyst and associated adenomatoid odontogenic tumor. Cancer. 1990;66(4):786–788. doi: 10.1002/1097-0142(19900815)66:4<786::AID-CNCR2820660431>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Takeda Y. Pigmented adenomatoid odontogenic tumour. Report of an undescribed case and review of the literature of pigmented intraosseous odontogenic lesions. Virchows Arch A Pathol Anat Histopathol. 1989;415(6):571–575. doi: 10.1007/BF00718652. [DOI] [PubMed] [Google Scholar]
- 39.Effiom OA, Odukoya O. Adenomatoid odontogenic tumour: a clinico-pathological analysis and melanin pigmentation study of 31 Nigerian cases. Niger Postgrad Med J. 2005;12(2):131–135. [PubMed] [Google Scholar]