Abstract
Arsenic (As) is a well documented human carcinogen. However, its mechanisms of toxic action and carcinogenic potential in animals have not been conclusive. In this research, we investigated the biochemical and genotoxic effects of As and studied its distribution in selected tissues of Sprague-Dawley rats. Four groups of six male rats, each weighing approximately 60 ± 2 g, were injected intraperitoneally, once a day for 5 days with doses of 5, 10, 15, 20 mg/kg bw of arsenic trioxide. A control group was also made of 6 animals injected with distilled water. Following anaesthetization, blood was collected and enzyme analysis was performed by spectrophotometry following standard protocols. At the end of experimentation, the animals were sacrificed, and the lung, liver, brain and kidney were collected 24 h after the fifth day treatment. Chromosome and micronuclei preparation was obtained from bone marrow cells. Arsenic exposure significantly increased (p<0.05) the activities of plasma alanine aminotransferase-glutamate pyruvate transaminase (ALT/GPT), and aspartate aminotransferase-glutamate oxaloacetate transaminase (AST/GOT), as well as the number of structural chromosomal aberrations (SCA) and frequency of micronuclei (MN) in the bone marrow cells. In contrast, the mitotic index in these cells was significantly reduced (p<0.05). These findings indicate that aminotransferases are candidate biomarkers for arsenic-induced hepatotoxicity. Our results also demonstrate that As has a strong genotoxic potential, as measured by the bone marrow SCA and MN tests in Sprague-Dawley rats. Total arsenic concentrations in tissues were measured by inductively coupled plasma mass spectrometry (ICP-MS). A dynamic reaction cell (DRC) with hydrogen gas was used to eliminate the ArCl interference at mass 75, in the measurement of total As. Total As doses in tissues tended to correlate with specific exposure levels.
Keywords: arsenic exposure, tissue distribution, genotoxicity, hepatotoxicity, rats
1 Introduction
Arsenic is a ubiquitous element present in food, soil, water and air, and it is released into the environment from both natural and man-made sources [1, 2]. The major inorganic forms of arsenic include the trivalent meta arsenite As+3 and the pentavalent arsenate As+5. Trivalent arsenic form has a higher affinity for thiol groups [3] and is more cytotoxic and genotoxic than As+5 [4]. Individuals who accumulate the trivalent intermediates are thought to be greater risk of arsenic-induced diseases [4]. Some of the organic forms include the methylated metabolites – monomethylarsonic acid (MMA), dimethylarsenic acid (DMA) and trimethylarsine oxide (TMAO) as well as arsenobetaine (AsB), arsenocholine and arsenosugars. More than 80% of commercially utilized arsenic compounds are used to manufacture products with agricultural applications such as insecticides, herbicides, fungicides, algicides, sheep dips, wood preservatives, dye-stuffs, and medicines for the eradication of tapeworms in sheep and cattle. Arsenic compounds have been used for at least a century in the treatment of syphilis, yaws, amoebic dysentery, and trypanosomiasis [5]. Despite the well known toxicity of arsenic, arsenic trioxide has long been of biomedical interest, dating to traditional Chinese medicine, where it is known as Pi Shuang and is still used to treat cancer and other conditions [6], and to homeopathy, where it is called arsenicum album. Some discredited patent medicines, e.g., Fowler’s solution, contained derivatives of arsenic oxide. Arsenic trioxide under the trade name Trisenox (manufacturer: Cephalon) is a chemotheraputic agent of idiopathic function used to treat leukemia that is unresponsive to “first line” agents. It is suspected that arsenic trioxide induces cancer cells to undergo apoptosis. Due to the toxic nature of arsenic, this drug carries significant risks. Use as a cytostatic in the treatment of refractory promyelocytic (M3) subtype of acute myeloid leukemia [7, 8]. The combination therapy of arsenic trioxide and all-trans retinoic acid (ATRA) has been approved by the U.S. Food and Drug Administration (FDA) for treatment of certain leukemias [9] and its therapeutic action has been attributed to the induction of programmed cell death (apoptosis) in leukemia cells [10].
Occupational sources of arsenic to human workers include vineyards, ceramics, glass making, smelting and refining of metallic ores, during production and use of arsenic containing agricultural products like pesticides and herbicides. Exposure to arsenic occurs via the oral route (ingestion), inhalation, dermal contact, and the parenteral route to some extent. Humans can be exposed to arsenic through the intake of air, food and water [11]. Epidemiological and clinical studies indicate that arsenic is a paradoxical human carcinogen that does not easily induce cancer in animal models [12].
The toxicity of arsenic depends on its chemical state. Inorganic arsenic in its trivalent form is more toxic than pentavalent arsenic. The toxicity of arsenic also depends on the exposure dose, frequency and duration, the biological species, age, and gender, as well as on individual susceptibilities, genetic and nutritional factors [13, 14]. By binding to thiol or sulfhydryl groups on proteins, As (III) can inactivate over 200 enzymes. This is the likely mechanism responsible for arsenic’s widespread effects on different organ system. As (V) can replace phosphate, which is involved in many biochemical pathways [15–17]. The major metabolic pathway for inorganic arsenic in humans is methylation. Arsenic trioxide is methylated to two major metabolites via a non-enzymatic process to MMA, which is further methylated enzymatically to DMA before excretion in the urine [18–20]. Hepatic cancer and other hepatic disorders are considered to be the major causes of arsenic-related mortality. Hepatic function, liver diseases and drug-induced liver injury can be assessed by various routinely ordered liver function tests, i.e., clinical investigations that measure the levels of various biomarkers (proteins or enzymes) in the blood. These proteins/enzymes reflect different aspects of a normal functioning liver. For example, ALT and AST indicate hepatocellular integrity [21, 22].
Tests for genotoxicity have indicated that arsenic compounds inhibit DNA repair, and induce chromosomal aberrations, sister-chromatid exchanges, and micronuclei formation in both human and rodent cells in culture [18, 23–26] and in cells of exposed humans [14]. Reversion assays with Salmonella typhimurium fails to detect mutations that are induced by arsenic compounds. Although arsenic compounds are generally perceived as weak mutagens in bacterial and animal cells, they exhibit clastogenic properties in many cell types in vivo and in vitro [18, 23, 25–27]. In the absence of animal models, in vitro cell transformation studies become a useful means of obtaining information on the carcinogenic mechanisms of arsenic toxicity. Arsenic and arsenical compounds are toxic to and induce morphological transformations of Syrian hamster embryo (SHE) cells as well as mouse C3H10T1/2 cells and BALB/3T3 cells [28–30]. Based on the comet assay, it has been reported that arsenic trioxide induces DNA damage in human lymphophytes [31], colon cancer cells [32] and also in mice leukocytes [33]. Arsenic compounds have also been shown to induce gene amplification, arrest cells in mitosis, inhibit DNA repair, and induce expression of the c-fos gene and the oxidative stress protein heme oxygenase in mammalian cells [34, 35]. They have been implicated as promoters and comutagens for a variety of toxic agents [36]. Recent studies in our laboratory have demonstrated that arsenic trioxide is cytotoxic and able to transcriptionally induce a significant number of stress genes and related proteins in human liver carcinoma cells [37], also demonstrated induction of cytotoxicity and genotoxicity in HL-60 cells [38].
Analyzing the toxic effects of arsenic is complicated because it exists in many different inorganic and organic compounds, and its toxicity varies according to its oxidation state, its solubility and many other factors including the exposure dose, frequency and duration, the biological species, age and gender, as well as individual susceptibilities, genetic and nutritional factors [39–41]. Most cases of human toxicity from arsenic have been associated with exposure to inorganic arsenic. Inorganic trivalent arsenite (As+3) is 2–10 times more toxic than pentavalent arsenate (As+5) [42]. Interest in the toxicity of arsenic has been heightened by recent reports of large populations in West Bengal, Bangladesh, Thailand, Inner Mongolia, Taiwan, China, Mexico, Argentina, Chile, Finland and Hungary that have been exposed to high concentrations of arsenic in their drinking water and are displaying various clinico-pathological conditions, the major effects being skin alterations and skin cancer. General health effects that are associated with arsenic exposure include cardiovascular and peripheral vascular disease, developmental anomalies, neurologic and neurobehavioural disorders, diabetes, hearing loss, portal fibrosis, hematologic disorders (anemia, leukopenia and eosinophilia) and multiple cancers: significantly higher standardized mortality rates and cumulative mortality rates for cancers of the skin, lung, liver, urinary bladder, kidney, and colon in many areas of arsenic pollution [13, 43, 44].
Although arsenic and arsenic containing compounds has been the subject of important toxicology research, there exists a lack of appropriate animal model for carcinogenicity assessment, as well as a scarcity of scientific data describing the tissue distribution of arsenic in relation to the biomarkers of arsenic-induced hepatotoxicity and genotoxicity in in vivo systems. Therefore, the present work was undertaken to study the distribution of arsenic in tissues, as well as the hepatotoxic and cytogenetic effects in Sprague-Dawley rats. Serum aminotransferases (ALT, AST), structural chromosomal aberrations (SCA), micronuclei (MN) formation and mitotic index (MI) in bone marrow cells were used as biomarkers of toxic effects. Cytogenetic biomarkers (SCA, MN) play an important role in toxicological hazard evaluation as the first step towards quantification of cancers. Biomarkers serve as internal indicators of environmental or occupational exposures and have the potential for prevention of effects of carcinogen exposure by early detection. The possible use of biomarkers representing intermediate steps in the exposure-to-disease continuum to estimate health risk in human populations has gained increasing attention.
2. Material and Methods
2.1 Chemicals
Arsenic trioxide (As2O3) with an active ingredient of 100% arsenic in 10% nitric acid, methanol, glacial acetic acid, and superfrost microscope slides were purchased from Fischer-Scientific Houston, TX, USA. Potassium chloride solution (0.075M) and Giemsa stain stock solution (0.4%) was obtained from Sigma-Aldrich (St. louis, MO, USA). Hanks Balanced Salt Solution was purchased from GIBCO (Grand Island, NY, USA). Fetal Bovine Serum (FBS) was obtained from Hyclone (Logan, UT).
2.2 Animal maintenance
Healthy adult male Sprague-Dawley rats (8–10 weeks of age, with average body weight (BW) of 60 ± 2 g were used in this study. They were obtained from Harlan-Sprague-Dawley Breeding laboratories in Indianapolis, Indiana, USA. The animals were randomly selected and housed in polycarbonate cages (three rats per cage) with steel wire tops and corn-cob bedding. They were maintained in a controlled atmosphere with a 12h:12h dark/light cycle, a temperature of 22 ± 2°C and 50–70% humidity with free access to pelleted feed and fresh tap water. The animals were supplied with dry food pellets commercially available from PMI Feeds Inc. (St. Louis, Missouri). They were allowed to acclimate for 10 days before treatment. The local Ethics committee for animal experiments [Institutional Animal Care and Use Committee] at Jackson State University, Jackson MS, (USA) approved this study. Procedures involving the animals and their care conformed to the institutional guidelines, in compliance with national and international laws and guidelines for the use of animals in biomedical research [45].
2.3 Chemical administration
Groups of six rats each were administered intraperitoneally with four different arsenic trioxide dose levels, 5, 10, 15 and 20 mg/kg BW, respectively. Arsenic trioxide was diluted with distilled water (as required) and intraperitoneally administered to animals at the doses of 0, 5, 10, 15 and 20 mg/kg BW, one dose per 24h given for 5 days. We have selected intraperitoneal injection because it is the most commonly used method that is simple and also for many agents such as arsenic compounds, it will tend to maximize chemical exposure to the bone marrow. Treatment by multiple injections was done for two reasons, firstly from pharmacological evidence it indicates the necessity for multiple injections to obtain required doses to the bone marrow [46] and secondly in order to induce tumors in rodents very high doses of arsenic compounds are required [18]. Each rat received a total of five doses at 24h intervals. The cumulative doses of arsenic trioxide given to rats were thus 25, 50, 75 and 100 mg/kg BW. Distilled water was administered to the 6 animals of control group in the same manner as in the treatment groups. The same dose regimes were used in both chromosome aberration (CA) and micronuclei (MN) assays.
2.4 Chromosome aberration assay
The rats were sacrificed by cervical dislocation 24h after administration of the last dose for chromosome aberration assay. Cytogenetic analysis was performed on bone marrow cells according to the recommendations of Preston et al [46], with slight modifications. Experimental animals were injected (i.p.) with colchicine (4mg/kg) 1.5 h prior to sacrifice. Both femora were dissected out and cleaned of any adhering muscle. Bone-marrow cells were collected from both femora by flushing in KCL (0.075 M, at 37° C) and incubated at 37° C for 25 min. Collected cells were centrifuged at 2000 x g for 10 min, and fixed in aceto-methanol (acetic acid:methanol, 1:3, v/v). Centrifugation and fixation were repeated five times at an interval of 20 min. The cells were resuspended in a small volume of fixative, dropped onto chilled slides, flame-dried and stained the following day with freshly prepared 2% Giemsa stain for 3–5 min, and washed in distilled water to remove excess stain.
2.5 Mitotic index determination
The mitotic index was used to determine the rate of cell division. The slides prepared for the assessment of chromosomal aberrations were also used for calculating the mitotic index. Randomly selected views on the slides were monitored to determine the number of dividing cells (metaphase stage) and the total number of cells. At least 1000 cells were examined in each preparation.
2.6 Micronucleus test
Rats were sacrificed by cervical dislocation 30h after the last treatment. The frequency of micronucleated cells in femoral bone marrow was evaluated according to the procedure of Schmid [47], with slight modifications as reported by Agarwal and Chauhan [48]. The bone marrow was flushed out from both femora using 2ml of Fetal Calf Serum and Hanks Balanced Salt Solution (3:1) and centrifuged at 2000xg for 10 min. The supernatant was discarded. Evenly spread bone marrow smears were stained using the May-Grunwald and Giemsa protocol.
2.7 Scoring of slides
Bone marrow preparations for the analysis of chromosome aberrations in metaphase cells were obtained using the technique by Preston et al [46]. The slides were stained with Giemsa. Well-spread metaphases presenting 42 ± 1 chromosomes were analyzed. One hundred metaphases per animal were screened to a total of 500 metaphases for each treatment and control to obtain the total number of chromosomal aberrations. The mitotic indices were obtained by counting the number of mitotic cells in 1000 cells per animal to a total of 5000 cells per treatment and control. The mitotic index was calculated as the ratio of the number of dividing cells to the total number of cells, multiplied by 100. A total of 3000 cells/treatment were scored, on coded slides to evaluate the frequency of micronucleated cells in bone marrow under an Olympus microscope.
2.8 Serum Biochemical Analysis
Following anesthetization, blood specimens were immediately collected using heparinized syringes, and transferred into polypropylene tubes. Each sample was allowed to clot for a minimum of 30 min (maximum 60 min). After clotting, the sample was centrifuged at 6000 RPM for 10 min. The serum or plasma then was pipetted from the cellular elements (erythrocytes, platelets, leucocytes) and transferred to an acid-washed polypropylene tube, properly labeled, and stored at 4° C until ready for analysis. The activities of certain liver enzymes such as alanine (GPT) and aspartate (GOT) aminotransferases in the serum samples were determined using colorimetric assay kits from Sigma (St. Louis MO, USA).
To determine the activities of alanine aminotransferase-glutamate pyruvate transaminase (GPT), and aspartate aminotransferase-glutamate oxaloacetate transaminase (GOT) in serum a method by Reitman and Frankel [49] was followed. Human serum contains many different transaminases. The two most commonly determined are AST and ALT. These enzymes catalyze transfer of alpha amino groups from specific amino acids to alpha-ketoglutaric acid [AKG] to yield glutamic acid and oxacetic acid or pyruvic acid. The keto acids are then determined colorimetrically after their reaction 2,4-dinitrophenyl hydrazine [DNP]. The absorbance of the resulting color is then measured at wavelength of approximately 505 nm to take advantage in the absorption that exists between the hydrazones of AKG and the hydrazones of oxalacetic acid or pyruvic acid.
The reaction for GOT is as follows:
The reaction for GPT is as follows:
2.9 Total Arsenic analysis
Brain, kidneys, liver and lungs were surgically excised from Sprague-Dawley rats under diethyl ether anesthesia. The organs were washed with ice-cold normal saline (0.9%NaCl) and 20 mM EDTA to remove blood, cut into small pieces, and fixed immediately in 10% phosphate-buffered formalin for 48 hrs prior to the histopathological analysis. Other set of tissues were also cut and placed in plastic tubes without fixation solution and immediately frozen at −70°C, and stored until ready to be processed for analysis.
For total arsenic analysis 50mg of frozen non-fixed tissue specimen were digested in 2 mL nitric acid and 1mL hydrogen peroxide using a microwave digestion system (MARS 5, CEM, Mathews, NC). The digestion included 20 min ramp to a constant pressure of 8.5 barr followed by holding the pressure at 8.5 barr for additional 60 min. The temperature corresponding to the constant pressure digestion was ~180°C. After the digestion was complete, distilled dionized water was added to a total volume of 10 mL. For quality control purposes with each digestion 2 blank and 2 NIST CRM 1577b were processed in the same manner as the unknown samples. The samples were analyzed for As levels using a PerkinElmer ELAN 6100 DRC (PerkinElmer, Waltham, MA). The method of standard additions was used for total arsenic quantification with additions of 0.5 ng/mL and 5 ng/mL in the sample aspirated to the ICP-MS. Whenever the As concentration in the samples was higher than the second addition (5 ng/mL), the samples were diluted to 5 ng/mL or lower level. The detection limit was calculated based on three times the standard deviation obtained from 7 digestion blank measurements. To minimize the presence of ArCl+ at m/z 75, we used hydrogen gas (5% H2/95%Ar). The instrumental parameters are shown Table 1.
Table 1.
Operating Conditions of the ICP-MS for total arsenic (As) analysis in rat tissues
| ICP Operating Parameters | Setting |
|---|---|
| Plasma Power | 1350 W |
| Auxiliary gas flow | 1.2 L min−1 |
| Plasma gas flow | 15 L min−1 |
| Nebulizer Gas Flow | 0.98 L min−1 |
| Sampler and skimmer cones | Pt |
| DRC gas flow | 0.3 L min−1 (5% H2/95Ar) |
| RPq | 0.6 |
| Mass Spectrometer Acquisition setting
| |
| Monitored signal | m/z: 74.9216 |
| Dwell Time | 500 ms |
| Scan Mode | Peak Hopping |
| Sweeps/Reading | 25 |
| Readings/Replicate | 1 |
| Replicates | 5 |
2.10 Statistical Analysis
Data were compared by ANOVA (two-way analysis of variance, one-way analyses for the As levels in the tissues). Statistical analysis was performed using SAS for Windows 98 package program. Using the Dunnett test, multiple comparisons were performed. All values were reported as Means ± SDs. For all the experiments, the significance level was set at p ≤ 0.05.
3. Results
3.1 Biomarkers of hepatotoxicity
3.1.1 Alanine aminotransferase
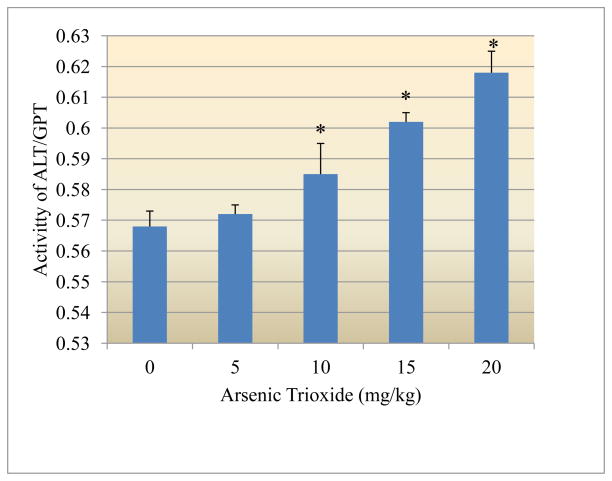
Figure 1 presents the experimental data obtained from the analysis of alanine aminotransferase (GPT). The results yielded the optical density readings of 0.568±0.005, 0.572±0.003, 0.585±0.010, 0.602±0.003, and 0.618±0.007 for 0, 5, 10, 15 and 20mg arsenic trioxide/kg bodyweight, respectively. As shown in this figure there was a dose-response relationship with respect to arsenic-induced release of GTP from the liver cells.
Figure 1.
Effect of arsenic trioxide on the activity of alanine aminotransferase (GPT)
3.1.2 Aspartate aminotransferase
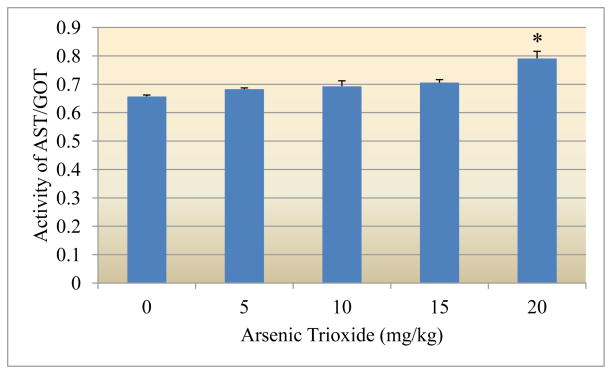
Figure 2 presents the experimental data obtained from the analysis of aspartate aminotransferase (GOT). Arsenic exposure resulted in a significant increase (p<0.05) of the activity of GOT. Optical density readings of 0.657±0.005, 0.683±0.004, 0.693±0.019, 0.706±0.010 and 0.791±0.025 were recorded for 0, 5, 10, 15, and 20mg arsenic trioxide/kg bodyweight, respectively.
Figure 2.
Effect of arsenic trioxide on the activity of aspartate aminotransferase (GOT)
3.2 Genotoxicity biomarkers
3.2.1 Chromosome Aberrations
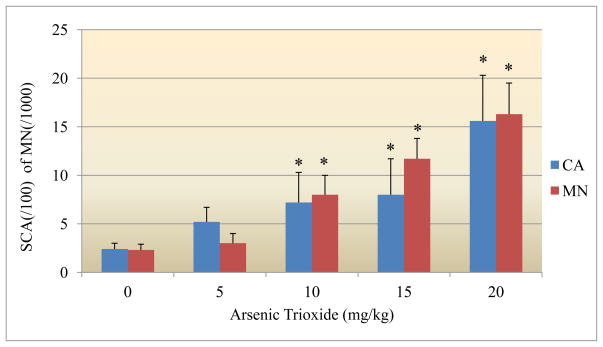
Data obtained from the chromosomal aberration assay with bone marrow cells are summarized in Figure 3. The frequency of chromosomal aberrations (CA) also increased with increasing doses of As2O3, and statistically significant differences from the control were observed (p<0.05). The mean percentages of the induced CAs were 2.4 ± 0.6 %, 5.2 ± 1.5%, 7.2 ± 3.1%, 8.0 ± 3.7%, and 15.6 ± 4.7% at As2O3 doses of 0, 5, 10, 15 and 20 mg/kg BW, respectively. The metaphase analysis of bone-marrow cells revealed various types of chromosomal aberrations, which consisted of chromatid and isochromatid types of gaps, breaks, unions, and fragments. Chromatid gaps and breaks were noted to be more frequent than others. Relatively higher frequencies of gaps were observed for all the doses tested. A quantitative assessment of the distribution of breaks and gaps revealed that the distal regions of the chromosomes were more vulnerable to the effects of As2O3 [50].
Figure 3.
Effect of arsenic trioxide on structural chromosome aberrations and micronuclei induction
3.2.2 Micronuclei induction
The micronuclei frequencies in bone marrow cells of Sprague-Dawley rats pre-exposed to arsenic trioxide are summarized in Figure 3. Arsenic trioxide induced a dose-related increase in micronuclei frequency. Significant differences (p<0.05) were observed between controls and cells obtained from arsenic trioxide-treated animals. The mean numbers of micronucleated cells were 2.33 ± 1.15, 3.0 ± 1.0, 8.0 ± 2.0, 11.66 ± 2.08, and 16.33 ± 3.21 per 1000 cells for arsenic trioxide doses of 0, 5, 10, 15 and 20 mg/kg BW, respectively.
3.2.3 Mitotic index
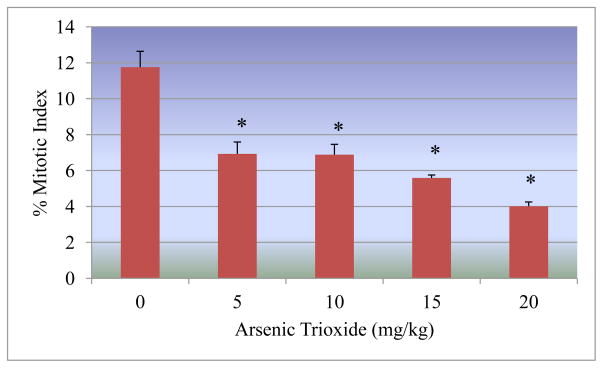
The mitotic index was used to determine the rate of cell division in bone marrow cells of Sprague-Dawley rats pre-exposed to arsenic trioxide. It was found that the mitotic index significantly decreased as the arsenic trioxide doses increased. Mitotic indices of 11.76 ± 0.88%, 6.93 ± 0.66%, 6.89 ± 0.57%, 5.59 ± 0.16%, and 4.01 ± 0.24% were recorded for As2O3 doses of 0, 5, 10, 15, 20 mg/kg BW, respectively (Figure 4).
Figure 4.
Effect of arsenic trioxide on the mitotic index.
3.3 Arsenic distribution in tissues
The detection limit for the As quantification in the tissue samples was 0.023 g/g based on 50 mg sample size. The accuracy was checked using NIST liver CRM 1577b (0.05 g/g information value) with resulting recoveries of 87–105%. Table 2 shows the distribution of arsenic levels in rat liver, kidney, lung and brain tissues as a function of increasing dose. The data represents results obtained for non-exposed Sprague-Dawley control rats and animals exposed to 5 mg/kg, 10 mg/kg, 15 mg/kg and 20 mg/kg. Each data point is an average of two to five preparations from different rats exposed to the same quantity of As. The As levels measured in all four tissues were linear with respect to dose for exposure levels upto 15 mg/kg, and were statistically different from the controls (p<0.005) The highest arsenic levels were found in the lung for exposures upto 15 mg/kg followed by the kidney, liver and brain. After 15 mg/kg exposure, the kidney As level in the lung leveled off, whereas the levels in the kidney, liver and brain continued to at a higher rate than observed for exposures upto 15 mg/kg.
Table 2.
Arsenic levels in liver, kidney, brain and lung rat tissues based on exposure levels
| All units in mg As/kg wet tissue
| |||||
|---|---|---|---|---|---|
| Control | 5mg/kg | 10mg/kg | 15mg/kg | 20mg/kg | |
| Liver | 0.122 ± 0.075 | 3.08 ± 0.95 | 2.63 ± 1.84 | 6.85 ± 2.37 | 17.3 ± 1.7 |
| Kidney | 0.433 ± 0.778 | 2.50 ± 0.42 | 3.49 ± 0.61 | 8.74 ± 1.60 | 19.2 ± 10.8 |
| Brain | 0.065 ± 0.114 | 0.400 ± 0.064 | 0.415 ± 0.032 | 1.09 ± 0.32 | 1.73 ± 0.97 |
| Lung | 0.216 ± 0.049 | 4.18 ± 0.14 | 8.17 ± 2.18 | 19.3 ± 4.6 | 14.4 ± 5.5 |
4 Discussion
4.1 Hepatotoxicity biomarkers
The data obtained from this study clearly show that arsenic trioxide significantly increased the activities of both serum alanine (ALT/GPT) and aspartate (AST/GOT) aminotransferases in a dose-dependent manner. Alanine and aspartate aminotransferases are released from liver when hepatocytes are damaged or destroyed. Serum activities of these enzymes have been reported to increase in virtually all cases of viral hepatitis and in cases of hepatocellular damage due to toxic substances. Previous experiments conducted in our laboratory and those of other investigators have also reported elevated levels of serum aminotransferases following arsenic toxicity [21, 22, 51–53]. It has been reported that the initial biochemical evidence of toxicity is damage to hepatocyte membrane, probably due oxidation stress subsequent to the reduction of glutathione level and antioxidant enzymes [52, 53]. It has also been suggested that hepatocyte injury following metal exposure may be due to binding in the inner membrane and accumulation in the mitochondria, leading to the collapse of the mitochondrial membrane, followed by plasma membrane depolarization and cell death [54]. Hepatic disorders appear to be one of the major causes of arsenic-related mortality. Our findings therefore indicate that aminotransferases may be useful as biomarkers for arsenic-induced hepatotoxicity.
4.2 Genotoxicity biomarkers
Data generated from this study clearly indicate a significant increase of cytogenetic damage in the bone marrow cells, due to arsenic trioxide exposure. The percentages of aberrant cells in bone marrow of exposed groups showed statistically significant increase (p<0.05) as compared to the controls. Out of all types of aberrations, chromatid breaks and gaps were the predominant forms of CA observed. Chromosome type aberrations such as dicentrics were also observed. Also, a significant increase (p<0.05) in micronuclei was observed in arsenic-treated cells compared to the controls. The types of cytogenetic damage observed in this study have been attributed to several modes of arsenic-induced toxicity including the inhibition of various enzymes involved in DNA repair and expression [55], the induction of reactive oxygen species capable of inflicting DNA damage [56] or the induction of gene expression of a number of stress response proteins leading to alteration in DNA repair mechanism causing DNA damage [57].
Cytogenetic effects of arsenic compounds have also been studied in different rodents using CA and MN assays. These genotoxic effects have been observed in in-vivo experiments with mouse fetal chromosome [58] as well as with mouse fibroblasts [59]. Arsenic-induce SCAs has also been demonstrated in in in-vitro studies with CHO cells [60–62], V79 cells [63], and SHE cells [23, 64]. The induction of micronuclei in rat bone marrow exposed to As2O3 in our study was found to be dose-dependent which is in accordance with other previously published studies [65, 66]. Different in vitro studies have also demonstrated positive induction of MN after exposure to arsenic compounds, confirming its sensitivity as a biomarker of genotoxicity [12, 34, 67, 68].
Both CAs and MNs are among the genotoxicity biomarkers most commonly considered for risk assessment purposes [69–75]. Although their presence does not necessarily lead to adverse health outcomes, high levels indicate that cells have been exposed to mutagens/carcinogens. A clear connection between cytogenetic changes and cancer has not been clearly elucidated. However, such biochemical alterations modulate the expression of growth control genes which are important in carcinogenesis or apoptosis [76].
4.3 Total As levels in tissues from exposed Sprague-Dawley rats
Our data indicates that As levels increase correspondingly with exposure dose levels. Liver, kidney levels show a slower increase for exposures to 15 mg/kg, whereas at 20 mg/kg exposure, tissue total As levels increase rapidly. Linear trends of exposure to the levels measured in all tissues were observed for all four tissues analyzed. This is most probably due to the inability of the rats to metabolize and excrete As at high exposure levels, such as 20 mg/kg. Although brain levels were much lower, they showed the same trend as liver and kidney accumulation. The lung tissue demonstrated higher arsenic accumulation, particularly at the 10 and 15 mg/kg dose increments, leveling off at the 20 mg/kg dose. Kenyon et al, studied the As tissues distribution after oral exposure to arsenate and observed that tissue arsenic accumulation was greatest in kidney > lung > urinary bladder ⋙ skin > blood > liver [77]. The difference in tissue distribution is most likely based on the route of exposure, oral vs. intraperitoneal, arsenic oxidation state, and chronic vs. near-acute exposure for our study at 20 mg/kg dose.
HIGHLIGHTS.
Evaluated arsenic-induced biochemical and genotoxic effects in a rat model.
Evaluated distribution of arsenic in selected tissues as a function of arsenic dose.
Described a robust method for the determination of arsenic in tissues by ICP-MS.
Acknowledgments
This research was financially supported by National Institutes of Health-RCMI Grant No. 1G12RR13459 awarded to PBT.
Footnotes
Disclaimer: The views, opinions and assertions expressed herein are those of the authors and do not reflect the official policy or position of The Joint Pathology Center, the Department of the Army, the Department of Defense, United States Geological Survey or the US Government. Citation of commercial organizations or trade names in this report do not constitute an official Joint Pathology Center, Department of the Army or US Government endorsement or approval of the products or services of these organizations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.IARC (International Agency for Research on Cancer) Monographs on evaluation of carcinogenic risk to humans. Some Drinking Water Disinfectants and Contaminants, including Arsenic. 2004;84:269–477. [PMC free article] [PubMed] [Google Scholar]
- 2.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Arsenic TP-92/09. Center for Disease Control; Atlanta, Georgia: 2000. [Google Scholar]
- 3.Vahter M, Concha G. Role of metabolism in arsenic toxicity. Pharmacol Toxico. 2001;89:1–5. doi: 10.1034/j.1600-0773.2001.d01-128.x. [DOI] [PubMed] [Google Scholar]
- 4.Rossman TG, Uddin AN, Burns FJ. Evidence that arsenic acts as a cocarcinogen in skin cancer. Toxicol Appl Pharmacol. 2004;198:394–404. doi: 10.1016/j.taap.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Tchounwou PB, Centeno JA, Patlolla AK. Arsenic toxicity, mutagenesis, and carcinogenesis- A health risk assessment and management approach. Mol Cell Biochem. 2004;255:47–55. doi: 10.1023/b:mcbi.0000007260.32981.b9. [DOI] [PubMed] [Google Scholar]
- 6.Gielen M, Tiekink Edward RT. Metallotherapeutic Drugs and Metal-Based Diagnostic Agents. Wiley; 2005. p. 298. [Google Scholar]
- 7.Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, Stone RM, Kalaycio M, Scheinberg DA, Steinherz P, Sievers EL, Coutré S, Dahlberg S, Ellison R, Warrell RP., Jr United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19(18):3852–3860. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- 8.Antman HK. Introduction: the history of arsenic trioxide in cancer therapy. Oncologist. 2001;6(Suppl 2):1–2. doi: 10.1634/theoncologist.6-suppl_2-1. [DOI] [PubMed] [Google Scholar]
- 9.Zhu J, Chen Z, Lallemand-Breitenbach V, de Thé H. How acute promyelocytic leukaemia revived arsenic. Nat Rev Cancer. 2002;2(9):705–13. doi: 10.1038/nrc887. [DOI] [PubMed] [Google Scholar]
- 10.Rousselot P, Laboume S, Marolleau JP, Larghero T, Noguera ML, Brouet JC, Fermand JP. Arsenic trioxide and melarsoprol induce apoptosis in plasma cell lines and in plasma cells from myeloma patients. Cancer Res. 1999;59:1041–1048. [PubMed] [Google Scholar]
- 11.Tchounwou PB, Wilson BA, Ishaque A. Important considerations in the development of public health advisories for arsenic and arsenic containing compounds in drinking water. Rev Environ Hlth. 1999;14:1–19. doi: 10.1515/reveh.1999.14.4.211. [DOI] [PubMed] [Google Scholar]
- 12.Basu A, Mahata J, Gupta S, Giri AK. Genetic Toxicology of a paradoxical human carcinogen, arsenic: a review. Mutat Res. 2001;488:171–194. doi: 10.1016/s1383-5742(01)00056-4. [DOI] [PubMed] [Google Scholar]
- 13.Tchounwou PB, Patlolla AK, Centeno JA. Carcinogenic and Systemic Health effects associated with arsenic exposure-A critical review. Toxicol Pathol. 2003;31(6):575–588. doi: 10.1080/01926230390242007. [DOI] [PubMed] [Google Scholar]
- 14.Tchounwou PB, Wilson BA, Ishaque A, Schneider J. Atrazine potentiation of arsenic trioxide induced cytotoxicity and gene expression in human liver carcinoma cells. Mol Cell Biochem. 2001;222:49–59. [PubMed] [Google Scholar]
- 15.Abernathy CO, Liu YP, Longfellow D, Aposhian HV, Beck B, Fowler B, Goyer R, Menzer R, Rossman T, Thompson C, Waalkes M. Arsenic: Health effects, mechanisms of actions and research issues. Environ Health Perspect. 1999;107:593–597. doi: 10.1289/ehp.99107593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol Lett. 2002;133:1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- 17.Goyer RA. Toxic effects of metals. In: Klaassen CD, editor. Cassarett & Doull’s toxicology – The Basic Science of Poisons. McGraw Hill; New York: 1996. pp. 691–736. [Google Scholar]
- 18.Wang Z, Rossman TG. In: The Toxicology of Metals. Cheng LW, editor. Vol. 1. CRC Press; Boca Raton, FL: 1996. pp. 221–243. [Google Scholar]
- 19.Wang A, Wolf DC, Sen B, Knapp GW, Holladay SD, Huckle WR, Caceci T, Robertson JL. Dimethylarsinic acid in drinking water changed the morphology of urinary bladder but not the expression of DNA repair genes of bladder transitional epithelium in F344 rats. Toxicol Pathol. 2009;37(4):425–437. doi: 10.1177/0192623309334147. [DOI] [PubMed] [Google Scholar]
- 20.Naranmandura H, Carew MW, Xu S, Lee J, Leslie EM, Weinfeld M, Le XC. Comparative toxicity of arsenic metabolites in human bladder cancer EJ-1 cells. Chem Res Toxicol. 2011;24(9):1586–1596. doi: 10.1021/tx200291p. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt E, Schmidt FW. Enzyme diagnosis of liver diseases. Clin Biochem. 1993;26:241–251. doi: 10.1016/0009-9120(93)90123-n. [DOI] [PubMed] [Google Scholar]
- 22.Islam K, Haque A, Karim R, Fajol A, Hossain E, Salam KA, Ali N, Saud ZA, Rahman M, Rahman M, Karim R, Sultana P, Hossain M, Akhand AA, Mandal A, Miyataka H, Himeno S, Hossain K. Dose-response relationship between arsenic exposure and the serum enzymes for liver function tests in the individuals exposed to arsenic: a cross sectional study in Bangladesh. Environ Health. 2011;10:64–74. doi: 10.1186/1476-069X-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrett JC, Lamb JW, Wang TC, Lee TC. Mechanisms of arsenic-induced cell transformation. Biol Trace Elem Res. 1989;21:421–429. doi: 10.1007/BF02917284. [DOI] [PubMed] [Google Scholar]
- 24.Hartmann A, Speit G. Comparative investigations of the genotoxic effects of metals in the single cell gel (SCG) assay and the sister chromatid exchange test. Environ Mol Mutagen. 1994;23:299–305. doi: 10.1002/em.2850230407. [DOI] [PubMed] [Google Scholar]
- 25.Jha AN, Noditi M, Nilsson R, Natarajan AT. Genotoxic effects of sodium arsenite on human cells. Mutat Res. 1992;284:215–221. doi: 10.1016/0027-5107(92)90005-m. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh P, Basu A, Singh KK, Giri AK. Evaluation of cell types for assessment of cytogenetic damage in arsenic exposed population. Mol Cancer. 2008;7:45–51. doi: 10.1186/1476-4598-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobson Kram D, Montalbano D. The reproductive effects assessment groups report on the mutagenicity of inorganic arsenic. Environ Mutagen. 1985;7:789–804. doi: 10.1002/em.2860070515. [DOI] [PubMed] [Google Scholar]
- 28.Landolph JR. Molecular and cellular mechanisms of transformation of C3H/10T1/2C18 and diploid human fibroblasts by unique carcinogenic, non- mutagenic metal compounds. A review. Biol Trace Elem Res. 1989;21:459–467. doi: 10.1007/BF02917289. [DOI] [PubMed] [Google Scholar]
- 29.Lee TC, Oshimura M, Barrett JC. Comparison of arsenic-induced cell transformation, cytotoxicity, mutation and cytogenetic effects in Syrian hamster embryo cells in culture. Carcinogenesis. 1985;6(10):1421–1426. doi: 10.1093/carcin/6.10.1421. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi M, Barrett JC, Tsutsui T. Transforamtion by inorganic arsenic compounds of normal Syrian hamster embryo cells into a neoplastic state in which they become anchorage-independent and cause tumors in newborn hamsters. Int J Cancer. 2002;99:629–634. doi: 10.1002/ijc.10407. [DOI] [PubMed] [Google Scholar]
- 31.Schaumloffel N, Gebel T. Heterogeneity of the DNA damage provoked by antimony and arsenic. Mutagenesis. 1998;13:281–286. doi: 10.1093/mutage/13.3.281. [DOI] [PubMed] [Google Scholar]
- 32.Stevens JJ, Graham B, Walker AM, Tchounwou PB, Rogers C. The effects of arsenic trioxide on DNA synthesis and genotoxicity in human colon cancer cells. Int J Environ Res Public Health. 2010;7(5):2018–2032. doi: 10.3390/ijerph7052018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saleha B, Danadevi K, Jamil K, Ahuja YR, Visweswara Rao K, Ishaq M. In vivo genotoxic effect of arsenic trioxide in mice using comet assay. Toxicol. 2001;162:171–177. doi: 10.1016/s0300-483x(01)00359-6. [DOI] [PubMed] [Google Scholar]
- 34.Gonsebatt ME, Vega L, Salazar AM, Montero R, Guzman P, Blas J, Del Razo LM, Garcia-Vargas G, Albores A, Cebrian ME, Kelsh M, Ostrosky-Wegman P. Cytogenetic effects in human exposure to arsenic. Mutat Res. 1997;386(3):219–228. doi: 10.1016/s1383-5742(97)00009-4. [DOI] [PubMed] [Google Scholar]
- 35.Ramirez P, Eastmond DA, Laclette JP, Ostrosky-Wegman P. Disruption of microtubule assembly and spindle formation for the induction of aneuploid cells by sodium arsenite and vanadium pentoxide. Mutat Res. 1997;386:291–298. doi: 10.1016/s1383-5742(97)00018-5. [DOI] [PubMed] [Google Scholar]
- 36.Huang SC, Lee TC. Arsenite inhibits mitotic division and perturbs spindle dynamics in HeLa S3 cells. Carcinogenesis. 1998;19:889–896. doi: 10.1093/carcin/19.5.889. [DOI] [PubMed] [Google Scholar]
- 37.Tchounwou PB, Yedjou CG, Dorsey WC. Arsenic trioxide- induced transcriptional activation of stress genes and expression of related proteins in human liver carcinoma cells [HepG2] Cell & Mol Biol. 2003;49(7):1071–1079. [PubMed] [Google Scholar]
- 38.Yedjou CG, Tchounwou PB. In-vitro cytotoxic and genotoxic effects of arsenic trioxide on human leukemia (HL-60) cells using the MTT and alkaline single cell gel electrophoresis (Comet) assays. Mol Cell Biochem. 2007;301(1–2):123–30. doi: 10.1007/s11010-006-9403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venugopal B, Lucky TD. Metal Toxicity in Mammals. Plenum Press; New York: 1978. [Google Scholar]
- 40.Marafante E, Vahter M. Solubility, retention, and metabolism of intratracheally and orally administered inorganic arsenic compounds in the Hamster. Environ Res. 1987;42:72–82. doi: 10.1016/s0013-9351(87)80008-7. [DOI] [PubMed] [Google Scholar]
- 41.Chen CJ, Lin LJ. Human carcinogenicity and atherogenicity induced by chronic exposure to inorganic arsenic. In: Nriagu JO, editor. Arsenic in the Environment; Part II:Human Health and Ecosystem Effects. John Wiley & Sons, Inc; New York, NY: 1994. pp. 109–131. [Google Scholar]
- 42.Kosnett MJ. Arsenic. In: Olson KK, editor. Poisoning and Drug Overdose. Appleton & Lange; Norwalk, CT: 1994. pp. 87–89. [Google Scholar]
- 43.Tseng CH, Tai TY, Chong CK, Tseng CP, Lai MS, Lin BJ, Chiou HY, Hsueh YM, HSU KH, Chen CJ. Long-term arsenic exposure and incidence of non-insulin-dependent diabetes milletus: A cohort study in arsenicosis hyperendemic villages in Taiwan. Environ Health Perspect. 2000;108:847–851. doi: 10.1289/ehp.00108847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tseng CH, Chang CK, Tseng CP, Hsueh YM, Chiou HY, Tseng CC, et al. Long-term arsenic exposure and ischemic heart disease in arseniasis-hyperendemic villages in Taiwan. Toxicol Lett. 2003;137:15–21. doi: 10.1016/s0378-4274(02)00377-6. [DOI] [PubMed] [Google Scholar]
- 45.Giles AR. Guidelines for the use of animals in biomedical research. Thromb Haemost. 1987;58(4):1078–84. [PubMed] [Google Scholar]
- 46.Preston RJ, Dean BJ, Galloway S, Holden H, McFee AF, Shelby M. Mammalian in vivo cytogenetic assays: Analysis of chromosome aberrations in bone marrow cells. Mutat Res. 1987;189:157–165. doi: 10.1016/0165-1218(87)90021-8. [DOI] [PubMed] [Google Scholar]
- 47.Schmid W. The micronucleus test for cytogenetic analysis. In: Hollander A, editor. Chemical mutagens, Principles and Methods for their detection. Vol. 4. Plenum Press; New York: 1976. pp. 31–53. [Google Scholar]
- 48.Agarwal DK, Chauhan LKS. An improved chemical substitute for fetal calf serum for the micronucleus test. Biotechnol Histochem. 1993;68:187–188. doi: 10.3109/10520299309104695. [DOI] [PubMed] [Google Scholar]
- 49.Reitman S, Frankel SA. Colorimeteric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 50.Patlolla AK, Tchounwou PB. Cytogenetic evaluation of arsenic trioxide toxicity in Sprague-Dawley rats. Mutation Research. 2005;687:126–133. doi: 10.1016/j.mrgentox.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Tchounwou PB, Patlolla AK, Centeno JA. Serum animotransferates as biomarkers of arsenic-induced hepatotoxicity. Metal Ions in Biology and Medicine. 2004;8:284–288. [Google Scholar]
- 52.Santra A, Maiti A, Das S, Lahiri S, Charkaborty SK, Mazumder DN. Hepatic damage caused by chronic arsenic toxicity in experimental animals. J Toxicol Clin Toxicol. 2000;38(4):395–405. doi: 10.1081/clt-100100949. [DOI] [PubMed] [Google Scholar]
- 53.Hernandez-Zavala A, Del Razo LM, Aguilar C, Garcia-Vargas GG, Borja VH, Cebrian ME. Alteration in bilirubin excretion in individuals chronically exposed to arsenic in Mexico. Toxicol Lett. 1998;99(2):79–84. doi: 10.1016/s0378-4274(98)00115-5. [DOI] [PubMed] [Google Scholar]
- 54.Chang LW, Suzuki T. Toxicology of Metals. CRC Press; Boca Raton. FL: 1996. pp. 885–899. [Google Scholar]
- 55.Gebel TW. Genotoxicity of arsenical compounds. Int J Hyg Environ Health. 2001;203:249–262. doi: 10.1078/S1438-4639(04)70036-X. [DOI] [PubMed] [Google Scholar]
- 56.Hei TK, Liu SX, Waldren C. Mutagenicity of arsenic in mammalian cells: role of reactive oxygen species. Proc Natl Acad Sci. 1998;95:8103–8107. doi: 10.1073/pnas.95.14.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitchin KT. Recent advances in arsenic carcinogenesis: modes of action, animal model systems, and methylated arsenic metabolites. Toxicol appl Pharmacol. 2001;172:249–261. doi: 10.1006/taap.2001.9157. [DOI] [PubMed] [Google Scholar]
- 58.Nagymajtenyi K, Selypes A, Berencsi G. Chromosomal aberrations and fetotoxic effects of atmospheric arsenic exposure in mice. J Appl Toxicol. 1985;5:61–63. doi: 10.1002/jat.2550050204. [DOI] [PubMed] [Google Scholar]
- 59.King H, Lunford RJ. The relation between the constitution of arsenicals and their action on cell division. J Chem Soc. 1950;8:2086–2088. [Google Scholar]
- 60.Lee TC, Wang-Wu S, Huang RY, Lee KC, Jan KY. Differential effects of pre- and post – treatment of sodium arsenite on the genotoxicity of methylmethane sulphonate in Chinese hamster ovary cells. Cancer Res. 1986;46:1854–1857. [PubMed] [Google Scholar]
- 61.Jan KY, Yang JL, Tzeng YJ, Huang RY, Lee TC. Sodium arsenite mediated synergistical increase in chromosome aberrations induced by alkylating agents in mammalian cells. In: Dillon HK, Ho MH, editors. Biological Monitoring of Exposure to Chemicals: Metals. Wiley; New York: 1991. pp. 111–118. [Google Scholar]
- 62.Huang H, Huang CF, Huang JS, Wang TC, Jan KY. The transition from late G1 to early S phase is most vulnerable to the coclastogenic effect of ultraviolet radiation plus arsenite. Int J Radiat Biol. 1992;61:57–62. doi: 10.1080/09553009214550621. [DOI] [PubMed] [Google Scholar]
- 63.Endo G, Kuroda K, Okamoto A, Horiguchi S. Dimethylarsenic acid induces tetraploids in CHO cells. Bull Environ Contam Toxicol. 1992;48:131–137. doi: 10.1007/BF00197495. [DOI] [PubMed] [Google Scholar]
- 64.Mass MJ. Human carcinogenesis by arsenic. Environ Geochem Health. 1992;14:49–54. doi: 10.1007/BF01783628. [DOI] [PubMed] [Google Scholar]
- 65.Pashin IV, Kozachenko VI, Toroptsev SN. Arsenic trioxide inhibition of the thiophophamide induction of mutations in mouse germ and somatic cells. Genetika. 1984;20:365–366. [PubMed] [Google Scholar]
- 66.Deknudt G, Leonard A, Arany J, Jenar-Du-Buission G, Delavinette E. In vivo studies in male mice on the mutagenic effects of inorganic arsenic. Mutagenesis. 1986;1:33–34. doi: 10.1093/mutage/1.1.33. [DOI] [PubMed] [Google Scholar]
- 67.Gurr JR, Liu F, Lynn S, Jan KY. Calcium-dependent nitric oxide production is involved in arsenite-induced micronuclei. Mutat Res. 1998;416:137–148. doi: 10.1016/s1383-5718(98)00076-x. [DOI] [PubMed] [Google Scholar]
- 68.Gebel TW, Leister M, Schumann W, Hirsch-Ernst K. Low-level self tolerance to arsenite in human HepG2 cells is associated with a depressed induction of micronuclei. Mutat Res. 2002;514:245–255. doi: 10.1016/s1383-5718(01)00343-6. [DOI] [PubMed] [Google Scholar]
- 69.Miller WH, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide – review. Cancer Res. 2002;62:3893–3903. [PubMed] [Google Scholar]
- 70.Bonassi S, Hagmar L, Stromberg U, Montagud AH, Tinnerberg H, Forni A, Heikkila P, Wanders S, Wilhardt P, Hansteen IL, Knudsen LE, Norppa H. Chromosomal aberrations in lymphocytes predict human cancer independently of exposure to carcinogens. Cancer Res. 2000;60:1619–1625. [PubMed] [Google Scholar]
- 71.Liou SH, Lung JC, Chen YH, Yang T, Hsieh LL, Chen CJ, Wu TN. Increased chromosome-type chromosome aberration frequencies as biomarkers of cancer risk in a blackfoot endemic area. Cancer Res. 1999;59:1481–1484. [PubMed] [Google Scholar]
- 72.Martinez V, Creus A, Venegas W, Arroyo A, Beck JP, Gebel TW, Surralles J, Marcos R. Micronuclei assessment in buccal cells of people environmentally exposed to arsenic in northern Chile. Toxicol Lett. 2005;155(2):319–327. doi: 10.1016/j.toxlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 73.Chakraborty TDeM. Clastogenic effects of inorganic arsenic salts on human chromosomes in vitro. Drug Chem Toxicol. 2009;32(2):169–73. doi: 10.1080/01480540802594509. [DOI] [PubMed] [Google Scholar]
- 74.Salazar AM, Sordo M, Ostrosky-Wegman P. Relationship between micronuclei formation and p53 induction. Mutat Res. 2009;672(2):124–8. doi: 10.1016/j.mrgentox.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 75.Martinez V, Creus A, Venegas W, Arroyo A, Beck JP, Gebel TW, Surralles J, Marcos R. Evaluation of micronucleus induction in a Chilean population environmentally exposed to arsenic. Mutat Res. 2004;564(1):65–74. doi: 10.1016/j.mrgentox.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 76.Tchounwou PB, Centeno JA. Toxicologic Pathology. In: Gad SC, editor. Preclinical Development Handbook. John Wiley & Sons; New York, NY: 2008. pp. 551–580. [Google Scholar]
- 77.Kenyon EM, Hughes MF, Adair BM, Highfill JH, Crecelius EA, Clewell HJ, Yager JW. Tissue distribution and urinary excretion of inorganic arsenic and its methylated metabolites in C57BL6 mice following subchronic exposure to arsenate in drinking water. Toxicol Appl Pharmacol. 2008;232(3):448–455. doi: 10.1016/j.taap.2008.07.018. [DOI] [PubMed] [Google Scholar]