Abstracts
Objectives
To evaluate the correlation of AgNOR count and colposcopy in cases of suspicious cervix.
Methods
A hundred women presenting in OPD with complaints of postcoital bleeding, discharge per vaginum, pain in the lower abdomen, or intermenstrual bleeding were subjected to colposcopy. After colposcopy, a cervical biopsy was done. Routine paraffin sectioning was done for these biopsy specimens. Histopathologic diagnosis was first established on these sections using the routine (H & E) stain. Then, further sections were cut from prepared blocks and were subjected to AgNOR staining technique. AgNOR count was taken as the mean number of black dots per 100 cells observed under a 100× oil immersion objective.
Results
In our study, 60 % cases were colposcopy positive and 40 % were colposcopy negative. The sensitivity of colposcopy in diagnosing all grades of dysplasias was 95.4 % and the specificity was 67.86 % in our study. The positive predictive value was 70 % and the negative predictive value was 95 %. The mean AgNOR score in our study was 1.21 ± 0.51 in chronic cervicitis, 2.44 ± 0.56 in mild dysplasia, 3.48 ± 0.35 in moderate dysplasia, and 4.58 ± 0.43 and 5.91 ± 0.51 in squamous cell carcinoma, showing a progressive increase in the score. AgNOR count showed an increase from CIN to SCC in our study. The correlation coefficient between the colposcopy and AgNOR count found in our study is 0.892 and p value is <0.0001 which is highly significant.
Conclusion
The results of the colposcopy and AgNOR when used together can provide strength to the clinician and histopathologist in diagnosing early carcinoma in cases of suspicious cervix.
Keywords: Silver-stained nucleolar organizing regions, Hematoxylene and eosin, Colposcopy
Introduction
Noncommunicable diseases are emerging as important health problems with changes in the lifestyles and demographic profiles of developing countries, which demand an appropriate control program before they assume epidemic proportions. One of these is the problem of cancer. In India, cervical cancer is one of the leading causes of cancer deaths in women and is the fourth most common cancer among women all over the world [1]. In India, it is most common in Bangalore and Chennai and the second most common in Mumbai and Thiruvananthapuram, followed by Dibrugarh in the 3rd place [2]. It is a significant problem in terms of incidence, mortality, and morbidity. Cervical cancer is a disease that can be prevented through both primary prevention and early detection using screening techniques due to its well-defined natural history and a long detectable preclinical phase. Several screening modalities like PAP smear, VIA, Cervicography, and Colposcopy are now available for early detection of cervical cancer and its precursor lesions. They all differ with regard to their test characteristics, feasibility, and economic considerations. For a definitive diagnosis, a histopathologic examination is done.
Sometimes, cytology fails to identify high risk low grade and high grade SIL which would progress to invasive cancers; then, molecular tumor markers can be used. One such marker is AgNOR which stands for silver-stained nucleolar organizing regions [3]. Nucleolar organizing regions are loops of DNA encoded for ribosomal RNA production which are located in the short arm of five acrocentric chromosomes, no. 13, 14, 15, 21, 22. NORs are argyrophilic and can be demonstrated easily in routinely processed histologic sections by silver impregnation techniques as black or brown dots within the nucleus [4]. So, the technique is of potential value in diagnostic histopathology. The number of AgNORs in a cell nucleus reliably reflects the cell kinetics and proliferative activity. The silver staining technique identifies the acidic proteins (nonhistone proteins) also called AgNOR proteins associated with the site of rRNA transcription. These proteins are designated B23, C23 AgNOR protein and RNA polymerase [5].
The normal (typical) transformation zone on the cervix represents the area of physiologic (normal) metaplastic epithelium that has replaced the columnar epithelium. This site is of the greatest interest to the colposcopists as it has the potential for developing neoplasia of the cervix. The introduction of carcinogens at this point results in an atypical transformation zone [6].
In the present study, an attempt has been made to study the correlation of colposcopy and AgNOR count in the case of suspicious cervix.
Material and Methods
The present study was carried out in 100 patients attending Obstetrics and Gynecology OPD of Rajindra Hospital, Patiala who presented with complaints of discharge per vaginum, low backache, pain in the lower abdomen, and irregular or postcoital bleeding. These patients were subjected to colposcopy followed by cervical biopsy. Colposcopy is an optical method for visualizing the lower female genital tract under bright illumination using stereoscopic vision varying from 4 to 40 folds. A colposcope is used to visualize any abnormality in the transformation zone. Colposcopic findings are recorded on the basis of Reid’s combined colposcopic index. This was given by [7]. They defined three categories based on four colposcopic signs. Each category is given a score of 0–2 and the grade of the lesion is predicted according to the combined score obtained on adding all the scores. Then, silver nitrate staining was done for the sections prepared from paraffin-embedded tissue blocks of biopsy specimens. The mean number of AgNOR dots per cell was calculated and expressed as the AgNOR count.
Results
In the present study, 100 cases of cervical lesions were studied. The maximum number of patients presented with a complaint of discharge per vaginum. The next common complaint was low backache and pain in the lower abdomen, followed by irregular and postcoital bleeding. The women in the study belonged to the reproductive age group of 18–60 years. The mean age was 30.25 years. A majority (52 %) of women screened were para 3 or 4. The mean parity was 1.6. About 54 % of females were from a rural background and 46 % were from the urban area. This is because the rural population is largely illiterate and most of the early symptoms like discharge per vaginum and pain in the lower abdomen are ignored. Moreover, the access to health care services and screening programs is also limited. 70 % of the women screened had first coitus between 21 and 24 years of age and the mean age was 21.2. Forty percent of women screened were illiterate and the prevalence was low of women receiving higher education. Approximately 72 % women belonged to the low income group. Cervical cancer is known to be the disease of poverty, which results from an early age of marriage, unhygienic conditions, and lack of access to family planning and health care services.
Out of 100 patients, 40 were having a normal colposcopy (Reid’s index of 0–2). 53 were having abnormal colposcopic findings and 7 patients were having an unsatisfactory colposcopy in which the endocervix was then exposed with an endocervical speculum and colposcopic findings were noted. Those with a Reid’s index of 6–8 were all positive on biopsy. Out of those with an index of 3–5, 43.75 % were positive on biopsy. In 40 cases with a Reid’s index of 0–2, only 2 were positive on biopsy. This shows that the sensitivity of colposcopy is 95.4 %, the specificity is 67.8 %. The positive predictive value is 70 % and the negative predictive value of the colposcopy is 95 %. Table 1 shows a comparison of sensitivity and specificity of colposcopy in reference to biopsy as studied by different authors.
Table 1.
Sensitivity and specificity of colposcopy with reference to biopsy
Table 2 shows colposcopic versus histopathologic findings. Out of 40 patients with a Reid’s index of 0–2 (i.e., colposcopy negative), 38 were having chronic cervicitis and 2 had mild dysplasia on HPE. In colposcopy positive cases with a Reid’s index of 3–5 (32 cases), 18 had chronic cervicitis and 14 had mild dysplasia. In 28 cases with a Reid’s index of 6–8, 12 were having moderate dysplasia, 8 had severe dysplasia, and another 8 cases showed invasive carcinoma on histopathology.
Table 2.
Colposcopic versus histopathologic findings
| Reid’s index | Ch cervicitis | Mild dysplasia | Mod dysplasia | Severe dysplasia | Invasive Ca |
|---|---|---|---|---|---|
| 0–2 | 38 | 2 | 0 | 0 | 0 |
| 3–5 | 18 | 14 | 0 | 0 | 0 |
| 6–8 | 0 | 0 | 12 | 8 | 8 |
Table 3 shows a variation of AgNOR count when compared with the histopathologic findings. It shows that the mean AgNOR count increased progressively from chronic cervicitis to carcinoma.
Table 3.
Mean AgNOR count in different lesions of the cervix
| AgNOR | ||||
|---|---|---|---|---|
| Range | Mean | SD | ||
| Ch cervicitis | 56 | 0.7–2.0 | 1.216 | 0.514 |
| Mild dysplasia | 16 | 1.8–3.0 | 2.44 | 0.561 |
| Mod dysplasia | 12 | 3–4.2 | 3.482 | 0.352 |
| Sev dysplasia | 8 | 3.8–5 | 4.586 | 0.434 |
| Ca | 8 | 5–6.4 | 5.911 | 0.521 |
As shown in Table 4, out of 60 cases having an AgNOR count of 0–2, the majority (56) had chronic cervicitis on HPE. 12 cases with a count between 2.1 and 3 had mild dysplasia on HPE. In another 12 cases with an AgNOR count of 3.1–4, 11 were having moderate dysplasia and 1 had severe dysplasia. In 8 patients with a count of 4.1–5, 7 were with severe dysplasia and in 8 patients with invasive carcinoma, AgNOR was >5.
Table 4.
Correlation of biopsy findings with AgNOR count
| AgNOR | Ch cervicitis | Mild dysplasia | Moderate dysplasia | Severe dysplasia | Invasive Ca |
|---|---|---|---|---|---|
| 0–2 | 56 | 4 | 0 | 0 | 0 |
| 2.1–3 | 0 | 12 | 0 | 0 | 0 |
| 3.1–4 | 0 | 0 | 11 | 1 | 0 |
| 4.1–5 | 0 | 0 | 1 | 7 | 0 |
| >5 | 0 | 0 | 0 | 0 | 8 |
Table 5 shows that 40 cases having a Reid’s index of 0–2, i.e., colposcopy negative, were having a mean AgNOR count of 1.137 with a range of 0.7–2.5. Cases with an AgNOR count between 3 and 5 were having a mean AgNOR count of 1.859 with range of 0.7–3, while out of 28 cases with a 6–8 Reid’s index, the mean AgNOR count was 4.571 with a range between 3.2 and 6.4 (Table 6).
Table 5.
Correlation of colposcopy findings with AgNOR count and Reid Index
| AgNOR | ||||
|---|---|---|---|---|
| Range | Mean | SD | ||
| 0–2 | 40 | 0.7–2.5 | 1.1375 | 0.4796 |
| 3–5 | 32 | 0.7–3 | 1.859 | 0.634 |
| 6–8 | 28 | 3.2–6.4 | 4.571 | 1.096 |
Table 6.
Comparisons of AgNOR per cell in various studies done on cervical biopsies
Discussion
Cervical cancer is both preventable and curable if detected early in preinvasive or at an early invasive stage by screening procedures as it has a well-defined natural history and a long detectable preclinical phase. If detected and treated early in the preinvasive and early invasive stages, the disease has virtually a 100 % cure rate. However, in advanced cancers, the 5-year survival rate drops to less than 35 %. In countries where cervical programs have been established, the incidence of cervical cancer has markedly decreased [8].
Colposcopy used for screening the suspicious cervix can be highly accurate, but skill levels vary. Cervical biopsy and AgNOR count add to the diagnostic accuracy of colposcopy. The number and size of NOR dots in the malignant cells are significantly different from those in normal and benign cells and reflect the current phase of transcription of the cells.
The mean AgNOR score in our study was 1.21 ± 0.51 in chronic cervicitis, 2.44 ± 0.56 in mild dysplasia, 3.48 ± 0.35 in moderate dysplasia, and 4.58 ± 0.43 and 5.91 ± 0.51 in squamous cell carcinoma, showing a progressive increase in the score. The differences in the AgNOR count between CIN-I and CIN-II and between CIN-II and CIN-III and between CIN-III and CIN-I were statistically significant. Egan et al. [9] observed that the mean AgNOR count increased steadily, whereas the mean size of the AgNOR dots decreased from CIN-I to CIN-III.
An Indian study done by Pratibha and Kuruvilla [10] on the role of AgNOR in diagnosing premalignant and malignant lesions of the cervix showed that the mean AgNOR count progressively increased from normal to CIN-I, CIN-II, CIN-III, and invasive carcinoma. The mean AgNOR per nucleus was 1.2, 1.8, 3.0, and 4.3 in the Normal cervix, CIN-I, II then CIN-III, and squamous cell carcinoma, respectively, in their study. The difference between counts in CIN-I and CIN-II and in the normal cervix and between counts in CIN-III and in invasive cancer was statistically significant.
However, Rowlands [11], in a study on NORs in CIN, did not find any significant difference in the AgNOR count in squamous epithelium of the normal cervix, CIN-I, and CIN-II, but there was a small, but significant, increase in CIN-III group. The mean AgNOR count per cell also increased from 2.07 ± 0.22 to 2.73 ± 0.66 to 4.49 ± 1.97 to 5.99 ± 1.71 to 7.35 ± 2.73 in normal, CIN-I, CIN-II, CIN-III, and squamous cell carcinoma, respectively, in a study conducted by Dey et al. [12]. Kaushik et al. [13] determined AgNOR per cell as 1.36 in normal, 2.56 in CIN-I, 4.28 in CIN-II, 5.16 in CIN-III, and 6.40 in squamous cell carcinoma.
Our figures matched with the AgNOR per cell quoted by Kaushik et al. [13] and Pratibha and Kuruvilla [10] for CIN-I, CIN-II, and III. But, for the AgNOR count in the normal cervix and in squamous cell carcinoma, our figures matched with Kaushik et al. The AgNOR count reported by Pratibha and Kuruvilla was higher (7.35) as compared to our figure, i.e., (5.91). The AgNOR count showed an increase from CIN to SCC in our study.
AgNOR is an effective tool reflecting the proliferation rate of the tumor and has a prognostic value in tumor pathology [14]. But, there are some problems during AgNOR counting; first, the number of dots visible in each cell is fewer with paraffin wax sections than with a cell smear or chromosome preparation [15]. Second, the number of dots revealed in interphase nuclei by AgNOR staining does not necessarily correspond to the actual number of NORs in the karyotype because AgNOR are often small, coalescent, and overlapping, and their apparent number is invariably less than the actual number present [16].
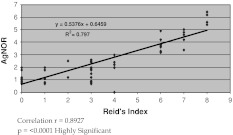
The correlation coefficient between colposcopy and the AgNOR count found in our study is 0.892 and p value is <0.0001 which is highly significant, as shown in Graph 1. Another study done by Stewart et al. [17] showed that an association between colposcopic impression and biopsy histology was significant (p value <0.001), but the strength of correlation was poor (0.20). Reid and colleagues [18] reported that the scoring index was highly correlated with biopsy histology with the coefficient as 0.86.
Graph 1.
The correlation between colposcopic findings and AgNOR count is highly significant. Correlation r = 0.8927; p = < 0.0001 highly significant
Conclusion
Colposcopy is a good method of screening cases of suspicious cervix and its diagnostic efficacy can be improved with the help of the AgNOR count. This simple silver staining technique can be used as an adjunct to routine colposcopy and histopathologic examination especially for grading dysplasias, thus rendering earlier diagnosis and treatment. AgNOR count can be used to assess the cellular proliferation rate as there is an increase in the AgNOR count from chronic cervicitis to dysplasias and malignancy. To conclude, the results of colposcopy and AgNOR when used together can provide strength to the clinician and histopathologist in diagnosing early carcinoma in cases of suspicious cervix.
References
- 1.Parkin DM, Bray F, Ferlay J. Estimating the world cancer burden. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.NRCP consolidated report of hospital based cancer registry programme (2001–2003), 2007.
- 3.Singh U, Singh R, Srivastava An, et al. AgNOR count and its diagnostic significance in cervical intraepithelial neoplasia. Obstet Gynecol India. 2006;56(3):244–6.
- 4.Pratibha D, Kuruvilla S. Value of AgNORs in premalignant and malignant lesions of cervix. Indian J Pathol Microbiol. 1995;38(1):11–16. [PubMed] [Google Scholar]
- 5.Buys CHCM, Osinga J. Abundance of protein bound sulphydral and disulphide groups at chromosomal NORs. Chromosoma. 1980;77:1–11. doi: 10.1007/BF00292037. [DOI] [PubMed] [Google Scholar]
- 6.Baliga BS. Principles and practice of colposcopy, Vol. I;
- 7.Reid R, Scalzi P. Genital warts and cancer. An improved colposcopic index for differentiating benign papilloma viral infection from high- grade cervical intraepithelial neoplasia. Am J Obstet Gynaecol. 1985;153:611–618. doi: 10.1016/s0002-9378(85)80244-1. [DOI] [PubMed] [Google Scholar]
- 8.Christine B, Johney A, Sylvie A. Cervical smear: histories of 585 women with biopsy proven carcinoma in situ. Acta Cytol. 1997;41:1676–1680. doi: 10.1159/000333167. [DOI] [PubMed] [Google Scholar]
- 9.Egan MJ, Freeth M, Croker J. Relationship between intraepithelial neoplasia of the cervix and the size and number of the nucleolar organizer regions. Gynaecol Oncol. 1990;36:147–151. doi: 10.1016/0090-8258(90)90104-S. [DOI] [PubMed] [Google Scholar]
- 10.Pratibha D, Kuruvilla S. Value of AgNORs in premalignant and malignant lesions of cervix. Indian J Pathol Microbiol. 1995;38(1):11–16. [PubMed] [Google Scholar]
- 11.Rowlands DC. Nucleolar organizer regions in cervical intraepithelial neoplasia. J Clin Pathol. 1988;41:1200–1202. doi: 10.1136/jcp.41.11.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dey P, Das R. Sabudin. Correlation between apoptotic and proliferative indices in cervical intraepithelial neoplasia and carcinoma. Indian. J Pathol. 2000;43(3):271–275. [PubMed] [Google Scholar]
- 13.Kaushik R, Sharma V, Gulati A, et al. AgNOR counts in cervical lesions. Indian J Pathol Microbiol. 2003;46(2):201–203. [PubMed] [Google Scholar]
- 14.Akhtar K, Mehdi G, Maheshwari V, et al. Diagnostic and prognostic significance of AgNOR counts in radiotherapy treated squamous cell carcinoma of the cervix. J Obstet Gynaecol India. 2005;55(2):163–166. [Google Scholar]
- 15.Platon D, Menanger M, Jeannesson P, et al. Improvement of staining in visualization of agyrophillic proteins of the nucleolar organizing region at optical level. Histochem J. 1986;18:5–14. doi: 10.1007/BF01676192. [DOI] [PubMed] [Google Scholar]
- 16.Underwood JCE, Giri DD. Nucleolar organizing regions as diagnostic deterimants for malignancy (editorial) J Pathol. 1988;155:95–96. doi: 10.1002/path.1711550203. [DOI] [PubMed] [Google Scholar]
- 17.Stewart L, Collins YC. Strength of correlations between colposcopic impression and biopsy histology. Gynecol Oncol. 2003;89:424–428. doi: 10.1016/S0090-8258(03)00082-9. [DOI] [PubMed] [Google Scholar]
- 18.Reid R, Stanhope R. Genital warts and cervical cancer : IV. A colposcopic index for differentiating subclinical papillomaviral infection from CIN. Am. J Obstet Gynaecol. 1984;149:815–823. doi: 10.1016/0002-9378(84)90597-0. [DOI] [PubMed] [Google Scholar]
- 19.Lozowski MS, Mishriki Y, Talebian F, Solitare G. The combined use of cytology and colposcopy in enhancing diagnostic accuracy in preclinical lesions of the uterine cervix. Acta Cytol. 1982;26:285–291. [PubMed] [Google Scholar]