Abstract
Immobilized Candida antarctica (Novozyme 435) catalyzed synthesis of N-acylethanolamines is described. Treatment of methyl esters with lipase and amines yielded the desired amides within 2–24 hrs with yields ranging from 41–98%.
Keywords: N-Acylethanolamines, Candida antarctica, Endogenous ligand, Amidation, Biocatalysis
N-Acylethanolamines (NAEs), ethanolamides of various long-chain fatty acids, constitute a class of bioactive lipid molecules formed from glycerophospholipids through the phosphodiesterase-transacylation pathway consisting of Ca2+-dependent N-acyltransferase and N-acylphosphatidylethanolamine-hydrolyzing phospholipase D.1,2 Among the NAEs, N-arachidonoylethanolamine, known as anandamide (figure 1), is a physiologically important lipid signaling molecule acting as a receptor ligand in the endocannabinoid system and has been studied extensively.2 Recently, other NAEs such as palmitoylethanolamine, and N-oleoylethanolamine (figure 1) also gained much attention due to their anti-inflammatory and analgesic activities, and anorexic activity, respectively.3
Figure 1.
NAEs including anandamide are not stored in the cell but rather produced on demand, and their endogenous levels are regulated directly by enzymes responsible for their formation and degradation. Anandamide has a relatively rapid onset of action, but is rapidly hydrolyzed by fatty acid amide hydrolase (FAAH) which accounts for its short duration of action. Early studies on structure-activity relationships (SAR) focused on the preparation of various amides of arachidonic acid and established that amides from chloroethylamine, cylopropylamine and R-(2)-aminopropanol showed excellent improvement in their respective affinities to the cannabinoid CB1 receptor while exhibiting enhanced metabolic stability towards FAAH.4–8 Recently, SAR studies on the modification of the hydrophobic chain have gained more attention and various analogs with fully saturated fatty acid chains or alternatively encompassed alkyne moieties were synthesized. Furthermore, our laboratory designed and synthesized high affinity covalent anandamide probes for the CB1 receptor by introducing either electrophilic isothiocyanato or a photoactivatable azido groups at the terminal carbon of the arachidonic acid moiety.9 We have also studied the effect of aryl substitutions with variable methylene linker at the distal end of arachidonic acid.10
All the synthetic schemes use the esters of the substituted fatty acids as a starting point and convert them to the needed amides using base mediated conventional hydrolysis of an ester to carboxylic acid followed by activation of carboxylic acid with either EDCI or CDI and treatment with various amines to provide the respective amides. In few cases, a protected form of ethanolamine is also used which, however, requires an additional deprotection step.
Several methods have been reported for the direct conversion of esters to amides including Mg(OCH3)2 and CaCl2,11 sodium cyanide,12 metal catalysts,13–15 and Alcalase.16 However, most of these suffer from incomplete conversion, longer reaction times, and possible functional group instability of the final products under the conditions used. Here, we report a highly selective lipase mediated mild conversion of esters to biologically important amides.
Lipases have found wide use as biocatalysts for many chemical transformations. Many lipases have been studied for their use in amide formation,17–18 such as, amidation of benzyl esters,19 synthesis of acetamides in the presence of ionic liquids,20 and acylation of amines with acids.21 Most of these methods utilize either carboxylic acids or vinylesters of carboxylic acids as reactants and the reactions require relatively high temperatures. In the kinetic resolution of amines, Nechab et al. reported that the reaction conditions required 80 °C and 3–10 h to acylate chiral amines with Candida antarctica (CAL) and ethyl acetate.22 The aminolysis of linoleyl ethyl ester with ethanolamine, catalyzed by CAL, in a solvent free system produced the linoleylethanolamide only in 24% yield in 20 h including the presence of the unwanted o-acylation product.23 While these examples show the use of lipases for the amidation of esters, there is limited reported work on the use of lipases as a direct method for the synthesis of biologically active NAEs with regard to functional group sensitivity common in the synthesis of modified fatty acid moieties. We have thus focused our efforts on the synthesis of biologically active NAEs with immobilized CAL from methyl esters and various amines. Development in this area will ameliorate the synthesis of multistep tail-modified N-acylethanolamine as well as other biologically important fatty acid amide analogs.
To optimize reaction conditions, we chose methyl arachidonate and cyclopropyl amine as reactants and hexane as a solvent. When carried out at room temperature in the presence of immobilized CAL, the reaction proceeded smoothly, but very slow as it required 24 h for completion. When heated to 45 °C, reaction completion was observed in a much improved 3 h. For amines not sufficiently soluble in n-hexane, the reaction proceeded equally well in a 1:1 hexanes-diisopropyl ether mixture (Scheme 1). The results are outlined in Table 1.
Scheme 1.
Table 1.
Amidation of esters with immobilized Candida antarctica in 1:1 hexane-diisopropylethera
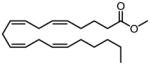
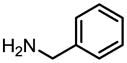
| Entry | Ester | Amine | Amide
|
|
|---|---|---|---|---|
| Isolated yield | Time | |||
| 1 |

|
|
85% | 3h |
| 2 |
|

|
98% | 24h |
| 3 |

|
|
89% | 2h |
| 4 |

|
|
85% | 24h |
| 5 |

|
|
60% | 24h |
| 6 |
|

|
41% | 24h |
| 7 |
|
|
85% | 3h |
| 8 |

|
|
84% | 3h |
| 9 |

|
|
95% | 24h |
| 10 |

|
|
90% | 24h |
| 11 |

|

|
91% | 24h |
Candida antarctica (Novozym 435, 100 mg) and amine (0.24 mmol, 1.2 eq) were added to a stirred solution of ester (0.20 mmol, 1 eq) in a 1:1 mixture of hexanes and isopropyl ether (1 mL). The reaction was heated to 45 °C and stirred until completion (TLC monitoring). The reaction was diluted with diethyl ether, filtered, and concentrated. The resulting residue was chromatographed on silica gel to yield the amide.
Esters and amines were chosen based on their biological importance. Thus methyl arachidonate was treated with cyclopropyl amine, ethanolamine and (R)-2-aminopropanol to provide arachidonoylcyclopropylamide (ACPA), anandamide and R-methanandamide respectively in excellent yields. Unprotected ethanolamine was directly used in the preparation of various ethanolamides (2, 3, 4 and 10). When performed with a substituted fatty acid carrying a terminal hydroxyl group (4) the reaction proceeded smoothly to provide the desired amide. There was no observable transesterification product in any reactions where hydroxyl groups were present either in the amine or the fatty acid moieties. To investigate general applicability of the method, we chose various esters and amines and showed that reactions proceeded within 24 h in good yield. Variation in yield was mainly dependent on the amine used. Primary amines, including benzylic amines, underwent amidation smoothly and in excellent yields after 24 h. Conversely, cyclohexylamine exhibited slower reactivity with decreased yield under the present conditions. Longer reaction times and increased temperature did not improve the yield significantly. Esters of non-fatty acids (9, 10 and 11) underwent amidation with amines in excellent yields and in all the cases the reaction time appeared to be more dependent on the amine used.
In summary, we have demonstrated that CAL can be useful for achieving direct formation of amides from various amines and esters containing skipped polyenes, allyl alcohol, allyl azide, alkyne, and aryl moieties. The method described in this report, is a simple, efficient and environmentally friendly and does not require any protection of other susceptible functional groups. This transacylation reaction provides excellent yields and is selective. It may find general utility in the synthesis of amides from the corresponding esters without requiring prior hydrolysis of the esters, as it can be difficult to synthesize amides directly from esters under mild conditions. The method should prove to be useful in the synthesis of drug intermediates and biologically important natural products.
Acknowledgments
One of the authors (S.K.V.) acknowledges the nancial support for this research from NIDA (R03 DA029184–02)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coulon D, Faure L, Salmon M, Wattelet V, Bessoule JJ. Plant Sci. 2012;184:129–140. doi: 10.1016/j.plantsci.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Ezzili C, Otrubova K, Boger DL. Bioorg Med Chem Lett. 2010;20:5959–5968. doi: 10.1016/j.bmcl.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ueda N, Tsuboi K, Uyama T. BBA - Mol Cell Biol L. 2010;1801:1274–1285. doi: 10.1016/j.bbalip.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, Makriyannis A. Journal of Medicinal Chemistry. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- 5.Goutopoulos A, Fan P, Khanolkar AD, Xie XQ, Lin S, Makriyannis A. Biorg Med Chem. 2001;9:1673–1684. doi: 10.1016/s0968-0896(01)00088-8. [DOI] [PubMed] [Google Scholar]
- 6.Bezuglov V, Bobrov M, Gretskaya N, Gonchar A, Zinchenko G, Melck D, Bisogno T, Di Marzo V, Kuklev D, Rossi JC, Vidal JP, Durand T. Bioorg Med Chem Lett. 2001;11:447–449. doi: 10.1016/s0960-894x(00)00689-2. [DOI] [PubMed] [Google Scholar]
- 7.El Fangour S, Balas L, Rossi J-C, Fedenyuk A, Gretskaya N, Bobrov M, Bezuglov V, Hillard CJ, Durand T. Bioorg Med Chem Lett. 2003;13:1977–1980. doi: 10.1016/s0960-894x(03)00348-2. [DOI] [PubMed] [Google Scholar]
- 8.Urbani P, Cavallo P, Cascio MG, Buonerba M, De Martino G, Di Marzo V, Saturnino C. Bioorg Med Chem Lett. 2006;16:138–141. doi: 10.1016/j.bmcl.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Xu W, Vadivel SK, Fan P, Makriyannis A. J Med Chem. 2005;48:6423–9. doi: 10.1021/jm050272i. [DOI] [PubMed] [Google Scholar]
- 10.Yao F, Li C, Vadivel SK, Bowman AL, Makriyannis A. Bioorg Med Chem Lett. 2008;18:5912–5915. doi: 10.1016/j.bmcl.2008.07.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bundesmann MW, Coffey SB, Wright SW. Tetrahedron Lett. 2010;51:3879–3882. [Google Scholar]
- 12.Hoegberg T, Stroem P, Ebner M, Raemsby S. J Org Chem. 1987;52:2033–2036. [Google Scholar]
- 13.Gnanaprakasam B, Milstein D. J Am Chem Soc. 2011;133:1682–1685. doi: 10.1021/ja109944n. [DOI] [PubMed] [Google Scholar]
- 14.Han C, Lee JP, Lobkovsky E, Porco JA. J Am Chem Soc. 2005;127:10039–10044. doi: 10.1021/ja0527976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishihara K, Kuroki Y, Hanaki N, Ohara S, Yamamoto H. J Am Chem Soc. 1996;118:1569–1570. [Google Scholar]
- 16.Nuijens T, Cusan C, Kruijtzer JAW, Rijkers DTS, Liskamp RMJ, Quaedflieg PJLM. J Org Chem. 2009;74:5145–5150. doi: 10.1021/jo900634g. [DOI] [PubMed] [Google Scholar]
- 17.Gotor V. Biorg Med Chem. 1999;7:2189–2197. doi: 10.1016/s0968-0896(99)00150-9. [DOI] [PubMed] [Google Scholar]
- 18.Bistline R, Bilyk A, Feairheller S. J Am Oil Chem Soc. 1991;68:95–98. [Google Scholar]
- 19.Adamczyk M, Grote J. Tetrahedron Lett. 1996;37:7913–7916. [Google Scholar]
- 20.Dhake KP, Qureshi ZS, Singhal RS, Bhanage BM. Tetrahedron Lett. 2009;50:2811–2814. [Google Scholar]
- 21.Tufvesson P, Annerling A, Hatti-Kaul R, Adlercreutz D. Biotechnol Bioeng. 2007;97:447–53. doi: 10.1002/bit.21258. [DOI] [PubMed] [Google Scholar]
- 22.Nechab M, Azzi N, Vanthuyne N, Bertrand M, Gastaldi S, Gil G. J Org Chem. 2007;72:6918–23. doi: 10.1021/jo071069t. [DOI] [PubMed] [Google Scholar]
- 23.Couturier L, Taupin D, Yvergnaux F. J Mol Catal B: Enzym. 2009;56:29–33. [Google Scholar]




