Abstract
The regional and cellular distribution of guanosine 3',5'-cyclic monophosphate (cGMP)-dependent protein kinase (ATP:protein phosphotransferase,EC 2.7.1.37) in mammalian brain was examined by use of the photoaffinity label 8-azidoinosine 3',5'-cyclic monophosphate. Of the regions examined, cerebellum had by far the highest concentration of this enzyme. The cellular localization of cGMP-dependent protein kinase within the cerebellum was determined by examination of mutant mice missing specific types of cerebellar neurons. Mutant mice lacking Purkinje cells had greatly reduced amounts of cGMP-dependent protein kinase, whereas the loss of another cell type, granule cells, did not reduce cGMP-dependent protein kinase levels. By using the same strains of mutant mice, a 23,000-dalton soluble cerebellar substrate for cGMP-dependent protein kinase was also shown to be enriched in Purkinje cells. In contrast, the concentration of type I 3',5'-cyclic AMP-dependent protein kinase in the cerebellum was unaffected by the absence of Purkinje cells and only slightly reduced by the absence of granule cells. The enrichment in Purkinje cells of the cGMP-dependent protein kinase and its substrate suggests an important role for cGMP and cGMP-dependent protein phosphorylation in the function of this type of neuronal cell.
Full text
PDF
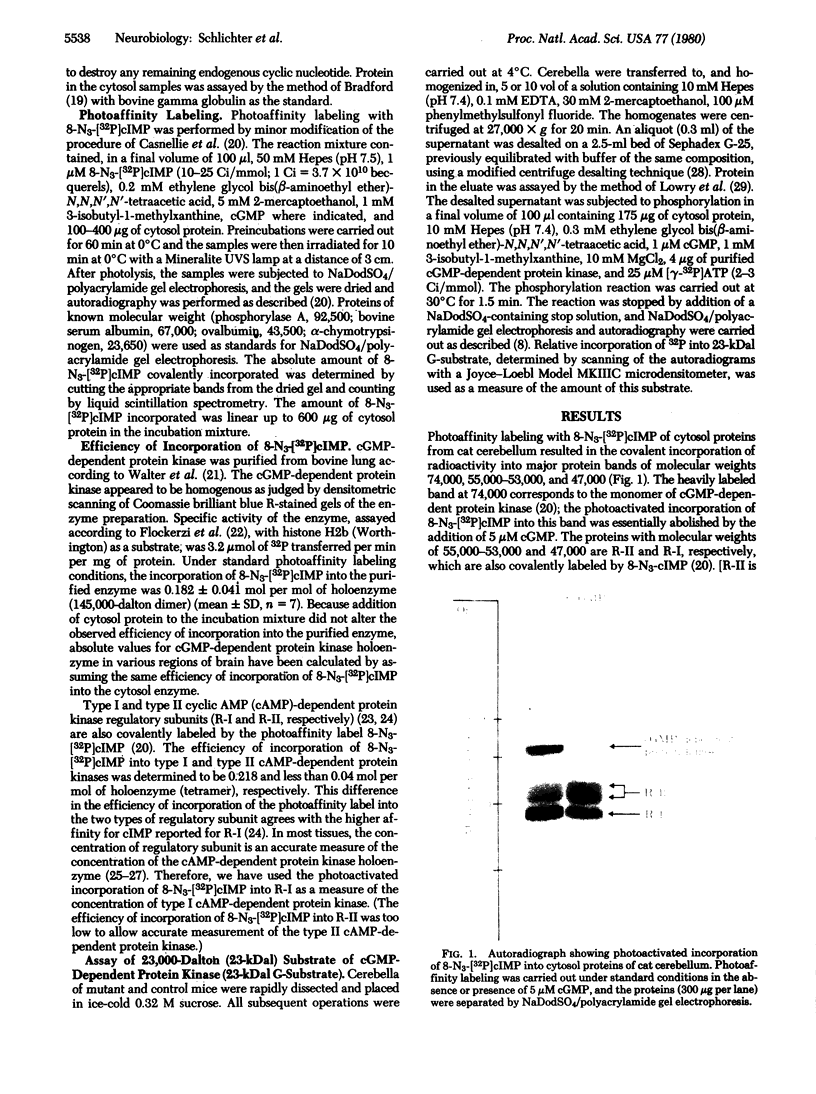
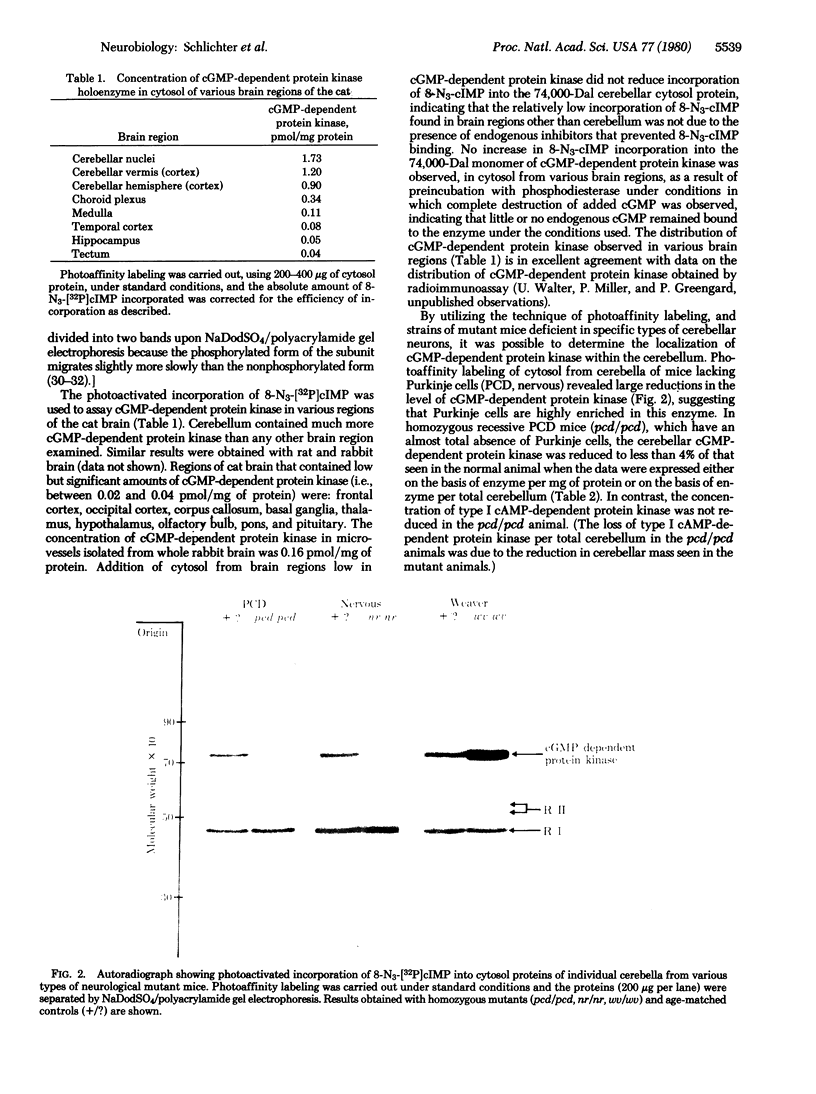
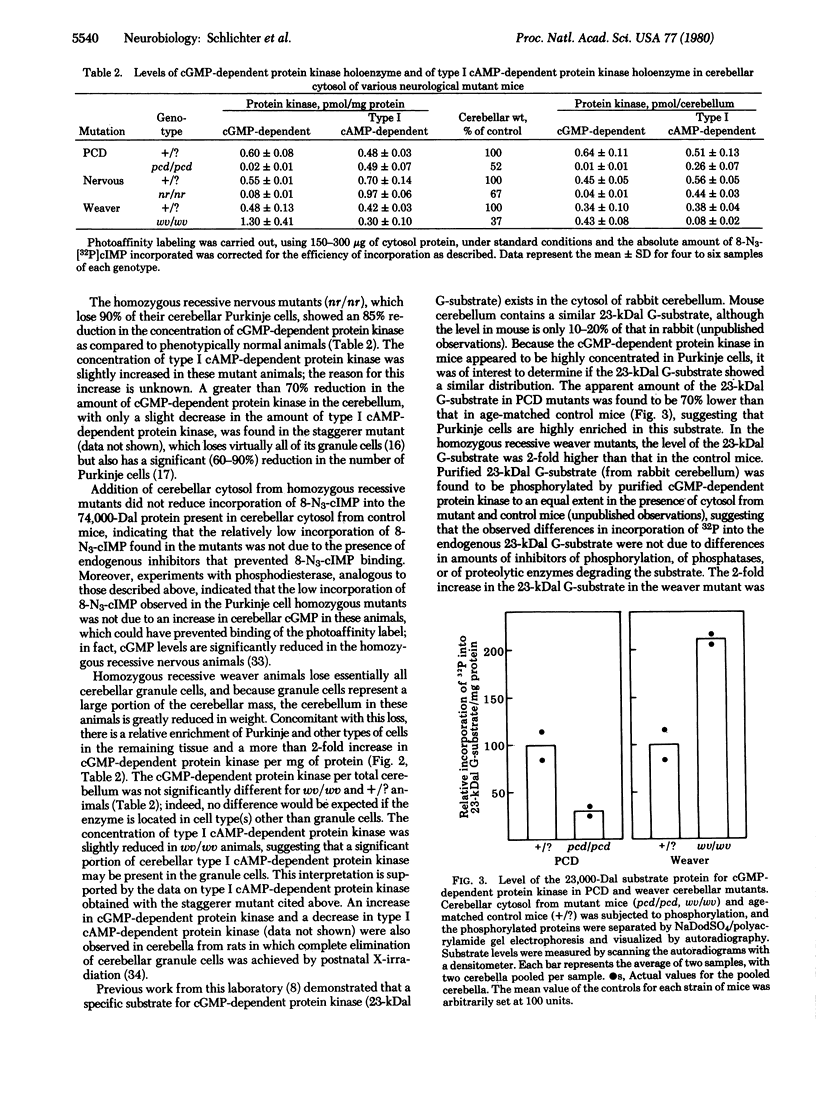

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J., Anderson W. J. Experimental reorganization of the cerebellar cortex. I. Morphological effects of elimination of all microneurons with prolonged x-irradiation started at birth. J Comp Neurol. 1972 Nov;146(3):355–406. doi: 10.1002/cne.901460305. [DOI] [PubMed] [Google Scholar]
- Bandle E., Guidotti A. Studies on the cell location of cyclic 3',5'-guanosine monophosphate-dependent protein kinase in cerebellum. Brain Res. 1978 Nov 10;156(2):412–416. doi: 10.1016/0006-8993(78)90530-9. [DOI] [PubMed] [Google Scholar]
- Bloom F. E. The role of cyclic nucleotides in central synaptic function. Rev Physiol Biochem Pharmacol. 1975;74:1–103. doi: 10.1007/3-540-07483-x_19. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Casnellie J. E., Ives H. E., Jamieson J. D., Greengard P. Cyclic GMP-dependent protein phosphorylation in intact medial tissue and isolated cells from vascular smooth muscle. J Biol Chem. 1980 Apr 25;255(8):3770–3776. [PubMed] [Google Scholar]
- Casnellie J. E., Schlichter D. J., Walter U., Greengard P. Photoaffinity labeling of a guanosine 3':5'-monophosphate-dependent protein kinase from vascular smooth muscle. J Biol Chem. 1978 Jul 10;253(13):4771–4776. [PubMed] [Google Scholar]
- Corbin J. D., Sugden P. H., Lincoln T. M., Keely S. L. Compartmentalization of adenosine 3':5'-monophosphate and adenosine 3':5'-monophosphate-dependent protein kinase in heart tissue. J Biol Chem. 1977 Jun 10;252(11):3854–3861. [PubMed] [Google Scholar]
- Flockerzi V., Speichermann N., Hofmann F. A guanosine 3':5'-monophosphate-dependent protein kinase from bovine heart muscle. Purification and phosphorylation of histone I and IIb. J Biol Chem. 1978 May 25;253(10):3395–3399. [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Greengard P. Possible role for cyclic nucleotides and phosphorylated membrane proteins in postsynaptic actions of neurotransmitters. Nature. 1976 Mar 11;260(5547):101–108. doi: 10.1038/260101a0. [DOI] [PubMed] [Google Scholar]
- Helmerhorst E., Stokes G. B. Microcentrifuge desalting: a rapid, quantitative method for desalting small amounts of protein. Anal Biochem. 1980 May 1;104(1):130–135. doi: 10.1016/0003-2697(80)90287-0. [DOI] [PubMed] [Google Scholar]
- Herrup K., Mullen R. J. Regional variation and absence of large neurons in the cerebellum of the staggerer mouse. Brain Res. 1979 Aug 17;172(1):1–12. doi: 10.1016/0006-8993(79)90891-6. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Beavo J. A., Bechtel P. J., Krebs E. G. Comparison of adenosine 3':5'-monophosphate-dependent protein kinases from rabbit skeletal and bovine heart muscle. J Biol Chem. 1975 Oct 10;250(19):7795–7801. [PubMed] [Google Scholar]
- Hofmann F., Bechtel P. J., Krebs E. G. Concentrations of cyclic AMP-dependent protein kinase subunits in various tissues. J Biol Chem. 1977 Feb 25;252(4):1441–1447. [PubMed] [Google Scholar]
- Hofmann F., Sold G. A protein kinase activity from rat cerebellum stimulated by guanosine-3':5'-monophosphate. Biochem Biophys Res Commun. 1972 Nov 15;49(4):1100–1107. doi: 10.1016/0006-291x(72)90326-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landis D. M., Sidman R. L. Electron microscopic analysis of postnatal histogenesis in the cerebellar cortex of staggerer mutant mice. J Comp Neurol. 1978 Jun 15;179(4):831–863. doi: 10.1002/cne.901790408. [DOI] [PubMed] [Google Scholar]
- Landis S. C., Mullen R. J. The development and degeneration of Purkinje cells in pcd mutant mice. J Comp Neurol. 1978 Jan 1;177(1):125–143. doi: 10.1002/cne.901770109. [DOI] [PubMed] [Google Scholar]
- Landis S. C. Ultrastructural changes in the mitochondria of cerebellar Purkinje cells of nervous mutant mice. J Cell Biol. 1973 Jun;57(3):782–797. doi: 10.1083/jcb.57.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann S. M., Walter U., Greengard P. Protein kinases in developing rat brain. J Cyclic Nucleotide Res. 1978 Dec;4(6):445–452. [PubMed] [Google Scholar]
- Mao C. C., Guidotti A., Landis S. Cyclic GMP: reduction of cerebellar concentrations in "nervous" mutant mice. Brain Res. 1975 Jun 13;90(2):335–339. doi: 10.1016/0006-8993(75)90316-9. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A. Cyclic nucleotides and nervous system function. Physiol Rev. 1977 Apr;57(2):157–256. doi: 10.1152/physrev.1977.57.2.157. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A., Glaser G. H. Identification of beta-adrenergic-sensitive adenylate cyclase in intracranial blood vessels. Nature. 1979 Apr 5;278(5704):567–569. doi: 10.1038/278567a0. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Moskowitz M. A., Reinhard J. F., Jr, Snyder S. H. Neurotransmitter receptor binding in bovine cerebral microvessels. Science. 1980 May 9;208(4444):610–612. doi: 10.1126/science.6102801. [DOI] [PubMed] [Google Scholar]
- Rakic P., Sidman R. L. Organization of cerebellar cortex secondary to deficit of granule cells in weaver mutant mice. J Comp Neurol. 1973 Nov 15;152(2):133–161. doi: 10.1002/cne.901520203. [DOI] [PubMed] [Google Scholar]
- Rakic P., Sidman R. L. Sequence of developmental abnormalities leading to granule cell deficit in cerebellar cortex of weaver mutant mice. J Comp Neurol. 1973 Nov 15;152(2):103–132. doi: 10.1002/cne.901520202. [DOI] [PubMed] [Google Scholar]
- Rangel-Aldao R., Kupiec J. W., Rosen O. M. Resolution of the phosphorylated and dephosphorylated cAMP-binding proteins of bovine cardiac muscle by affinity labeling and two-dimensional electrophoresis. J Biol Chem. 1979 Apr 10;254(7):2499–2508. [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Roffler-Tarlov S., Sidman R. L. Concentrations of glutamic acid in cerebellar cortex and deep nuclei of normal mice and Weaver, Staggerer and nervous mutants. Brain Res. 1978 Feb 24;142(2):269–283. doi: 10.1016/0006-8993(78)90635-2. [DOI] [PubMed] [Google Scholar]
- Schlichter D. J., Casnellie J. E., Greengard P. An endogenous substrate for cGMP-dependent protein kinase in mammalian cerebellum. Nature. 1978 May 4;273(5657):61–62. doi: 10.1038/273061a0. [DOI] [PubMed] [Google Scholar]
- Takai Y., Nishiyama K., Yamamura H., Nishizuka Y. Guanosine 3':5'-monophosphate-dependent protein kinase from bovine cerebellum. Purification and characterization. J Biol Chem. 1975 Jun 25;250(12):4690–4695. [PubMed] [Google Scholar]
- Walter U., Greengard P. Quantitative labeling of the regulatory subunit of type II cAMP-dependent protein kinase from bovine heart by a photoaffinity analog. J Cyclic Nucleotide Res. 1978 Dec;4(6):437–444. [PubMed] [Google Scholar]
- Walter U., Lohmann S. M., Sieghart W., Greengard P. Identification of the cyclic AMP-dependent protein kinase responsible for endogenous phosphorylation of substrate proteins in synaptic membrane fraction from rat brain. J Biol Chem. 1979 Dec 10;254(23):12235–12239. [PubMed] [Google Scholar]
- Walter U., Miller P., Wilson F., Menkes D., Greengard P. Immunological distinction between guanosine 3':5'-monophosphate-dependent and adenosine 3':5'-monophosphate-dependent protein kinases. J Biol Chem. 1980 Apr 25;255(8):3757–3762. [PubMed] [Google Scholar]