Abstract
The use of totally implantable venous access devices developed as a medical device allowing mid- and long-term, frequent, repeated, or continuous injection of therapeutic products, by vascular, cavitary, or perineural access. The effective flushing of these devices is a central element to assure long-lasting use. Our experimental work demonstrates that directing the Huber point needle opening in the diametrically opposite direction of the implantable port exit channel increases the flushing efficiency. These results are consolidated by numerical computations, which support recommendations not only for their maintenance, but also for their use.
Keywords: implantable ports, totally implantable venous access devices (TIVADs), flushing, obstruction, prevention
Introduction
Totally implantable venous access devices (TIVADs) have been in use for more than two decades. They allow repeated vascular (venous, arterial), body cavity (pleural, peritoneal, and intradural), and perineural accesses for drug administration ( anticancer, antibiotics, analgesics, parenteral nutrition, blood transfusion) as well as blood collection.
These devices have proved to be safe and effective in overcoming problems of repeated venous access when patients need regular intravenous therapy. Not only do they respect the venous capital, but they also bring comfort, autonomy, and safety to the patients, and ease and reliability of use for the health care workers. Their characteristics, risks, and benefits are well described.1–7 Their setting, use, and maintenance are detailed in various protocols and in at least one national recommendation.8–12 If these documents mention that an appropriate flushing is an absolute necessity, none have described the technique to be used.
Previous works on central and peripheral intravenous access devices13,14 showed that flushing efficacy was technique-dependent (pulsed flushing versus continuous flushing) and, therefore, flushing efficacy could be optimized with a potential increase of the lifespan of devices and a decrease of obstructive and infectious risks.
The flushing efficacy of a cavity, catheter, or an implantable port depends on two groups of factors: on one hand, the physicochemical process of molecule adhesion and deadhesion and, on the other hand, a hydrodynamic process linked to the shear stress forces of the flush on the internal surface. In the case of implantable ports, this force is based on the intensity of the flow and on the orientation of the Huber point needle (HPN) bevel.
The objective of this experimental work was to quantify the effect of the HPN bevel orientation with respect to the exit channel on the flushing efficacy of an implantable port without catheter. The experimental results were completed by a numerical computation describing the dynamics of the outflow.
Material and methods
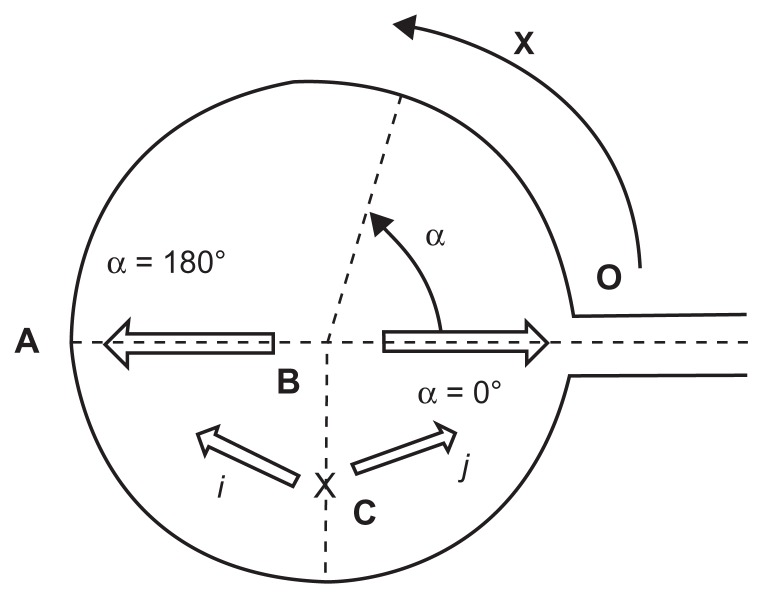
The port geometry is shown in Figure 1. The reservoir is cylindrical and there is an exit channel at the port base which connects to the central venous catheter. Its top is made of a compressed silicone membrane (septum) which can be punctured by a specific needle (HPN).
Figure 1.
Bidimensional schematic representation of a port: (A) The diametrically opposite point of the exit channel (bottom); (B) Septum center; (α) the angle between the needle bevel opening and the exit channel; (i) and (j) indicate the directions of the needle bevel opening when the needle insertion point is C; (x) the curvilinear coordinate from O to A.
The port characteristics (Polysite®, séries 2000, 3000, and 4000; Pérouse Médical, Ivry le Temple, France) are summarized in Table 1.
Table 1.
Port characteristics
| Type: Polysite®; Perouse Médical | 2000 | 3000 | 4000 |
|---|---|---|---|
| Port diameter (mm) | 8 | 11 | 13 |
| Port internal volume (mL) | 0.15 | 0.3 | 0.4 |
| Septum diameter (mm) | 7.5 | 10.5 | 12.1 |
Each port was filled with an aqueous solution of 0.1% dodecyl sulfate sodium (DSS) by a syringe and an HPN inserted in the septum center (point B). After an incubation of 48 hours at 37°C, the ports were emptied and flushed with 30 mL of pure water with a sloped outflow channel, to completely washout the DSS.
Once cleansed, each port was filled with an aqueous solution of fibronectin (5 μg/mL) and kept for 2 hours at 37°C. The nonadherent fibronectin was eliminated by a slow injection of air through the HPN. A saline solution of bovine serum albumin (10 mg/mL) was then injected and the ports incubated 24 hours at 37°C. Again, each port was flushed by a slow injection of air through the HPN to eliminate the nonadherent albumin. The recovered amount was titrated. Fibronectin and albumin were selected because both are physiological blood proteins. Furthermore, fibronectin easily and strongly adheres to biomaterials and blood products, and also promotes the adhesion of albumin and other proteins.15–19 The ports were flushed with 10 mL of NaCl 0.9% by an autopulsed syringe (55–1144; Harvard Apparatus, Les Ulis, France) locked to the 19G and 22G HPN.8 Two series of tests were performed with the HPN inserted in the septum center (point “B” in Figure 1), one series with the bevel opening directed toward the exit channel (α = 0°), and another with the bevel opening toward the bottom of the port (α = 180°).
As good clinical practices recommend changing the needle insertion point, a site (point “C” in Figure 1) located at half the radius length of the port side, on an axis perpendicular to the central site (A-O) was selected. Two other series of tests were performed with the HPN inserted there, one with the bevel opening directed toward the exit channel (j), and another with the bevel opening toward the bottom of the port (i). The flush solution was collected to obtain the proteins extracted by the flushing.
At insertion point B, six tests were performed for each of the 36 combinations: three port sizes, two HPN gauges (19G and 22G), two orientations of the bevel (α = 0° and α = 180°), and three flow rates (0.25–0.5−1 mL/second).
At insertion point C, six tests were performed with one port size (13 mm diameter), a 22G HPN, two orientations of the bevel (i directed to A and j directed to O; see Figure 1), and three flow rates (0.25–0.5−1 mL/second).
Albumin was titrated using an ultraviolet spectrophotometer at 280 nm (Gilson 112 UV/VIS detector; Gilson, Middleton, WI). Results are reported in percent of extracted albumin and are calculated by the amount recovered after flushing with NaCl divided by the amount filled minus the amount recovered after flushing with air.
Statistical analysis
The Mann–Whitney U test was used on means. Statistical significance thresholds are shown in Figures 2–5 using the classical correspondence: *0.01 < P < 0.05; **0.001 < P < 0.01; ***P < 0.
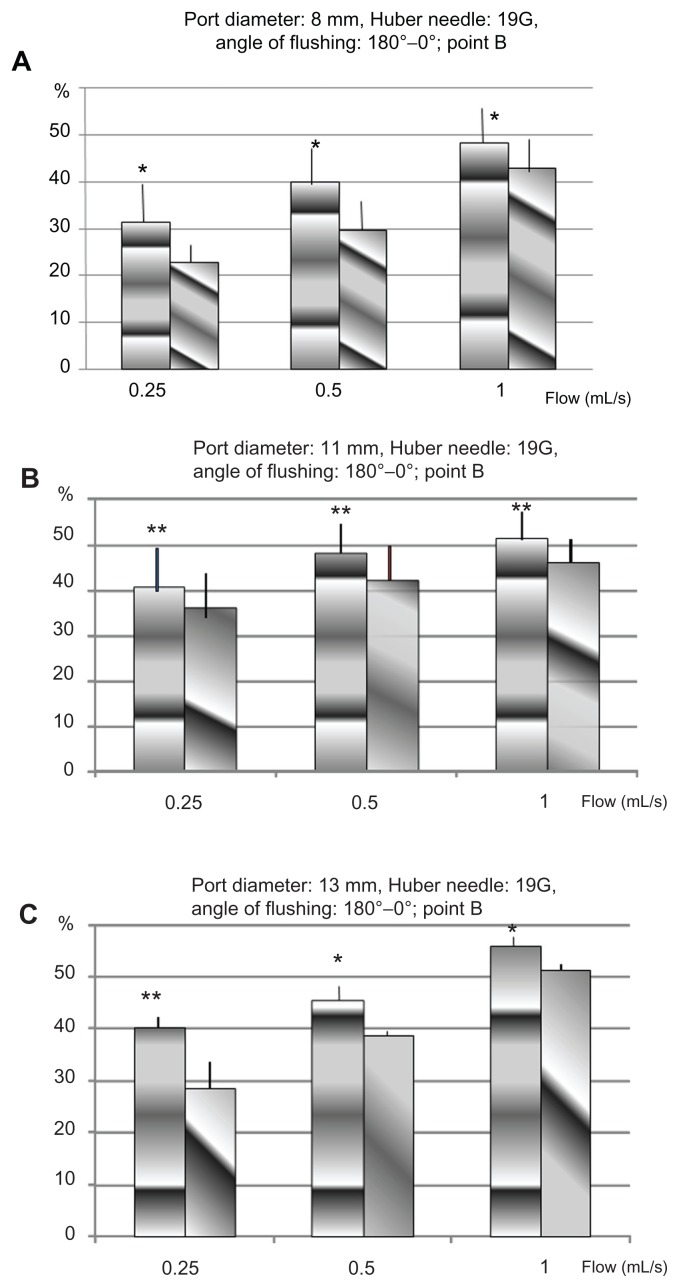
Figure 2.
Percentage of of proteins removed in TIVADs of diameters 8 mm (A), 11 mm (B), and 13 mm (C), with a Huber point needle of 19G, for two orientations of the flow, from the point B: 0° towards the exit channel, and 180° towards the bottom of the cavity.
Abbreviations: G, gauge; TIVAD, totally implantable venous access device.
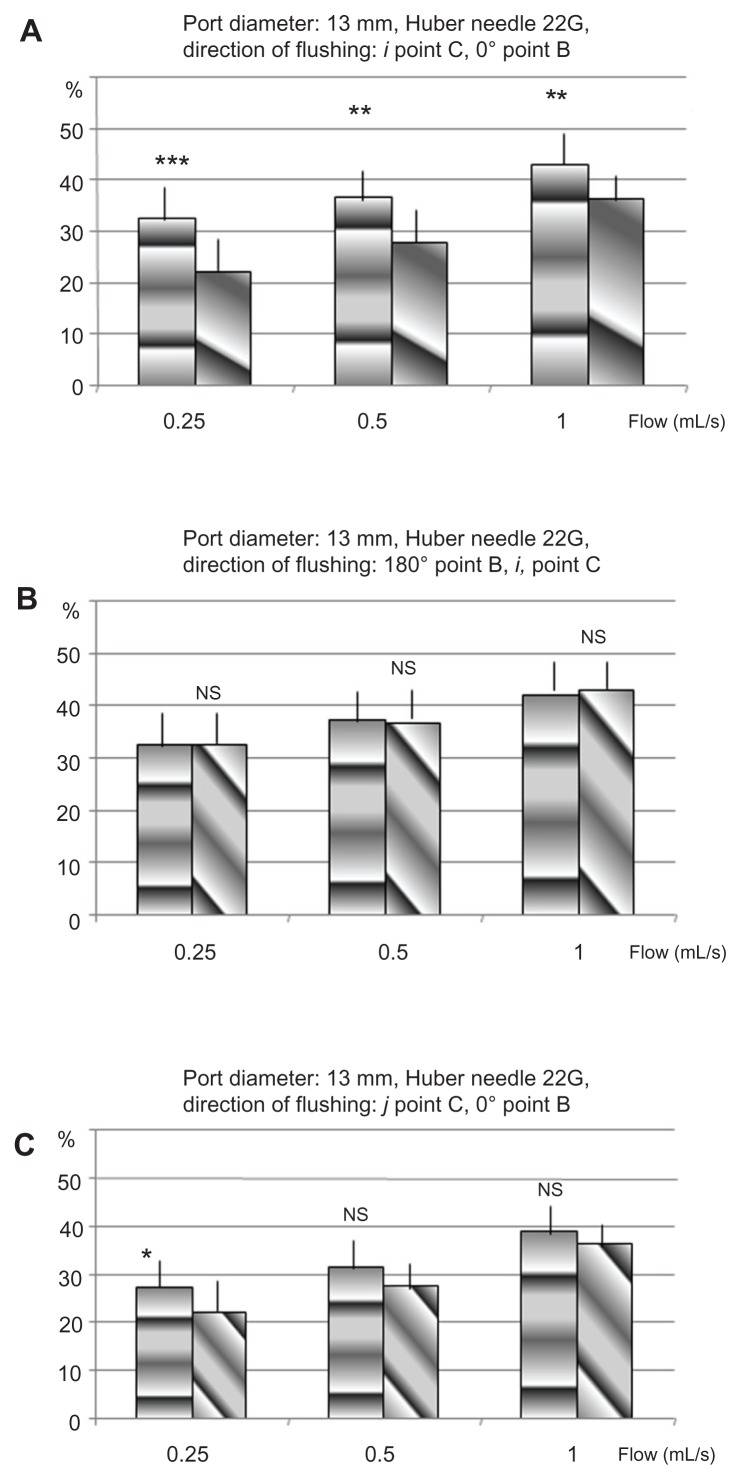
Figure 5.
Crossed comparison of the percentage of removed proteins for different orientations of the flow from the points B and C in TIVAD of 13 mm, with a HPN of 22G.
Abbreviations: G, gauge; HPN, Huber point needle; NS, nonsignificant; TIVAD, totally implantable venous access devices.
Experimental results
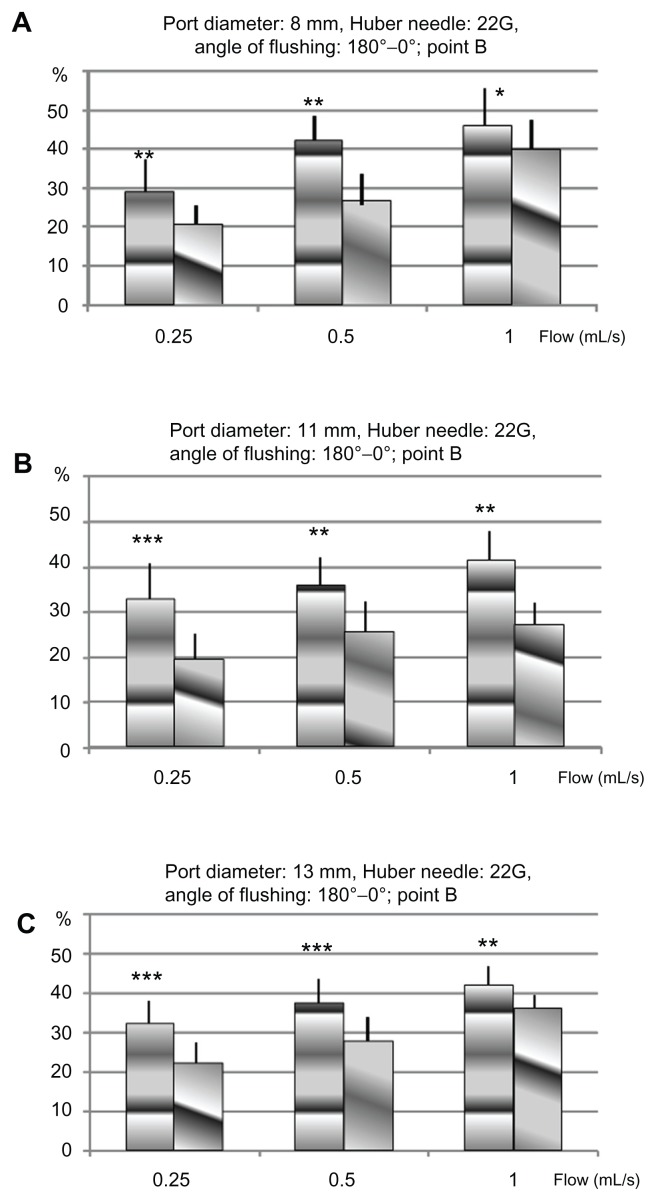
The results from insertion point B are summarized in Figures 2 and 3. Each column represents the mean percentage of extracted proteins according to the flushing flow for each type of implantable port size, the needle gauge, and the bevel-opening orientation. The mean percentage of extracted proteins ranged between 21% and 55%. The lowest amount was recovered with a 22G HPN, including the slowest flow rate (0.25 mL/second) with the bevel opening directed toward the outflow channel (α = 0°). The port size did not influence the amount received. The highest amount was recovered with a 19G HPN, including the fastest flow rate (1 mL/second) with the bevel opening toward the bottom of the port (α = 180°) at the largest port size (13 mm diameter) (see Figure 2B). The flushing efficacy increased with the flow rate, whatever the mix of needle gauge, port diameter, or orientation of the needle bevel opening. Furthermore, the efficiency increased in every configuration of flow rate, needle gauge, and port diameter when the needle bevel opening was directed toward the bottom of the port (α = 180°).
Figure 3.
Percentage of proteins removed in TIVADs of diameters 8 mm (A), 11 mm (B), and 13 mm (C), with a Huber point needle of 22G, for two orientations of the flow, from the point B: 0° towards the exit channel, and 180° towards the bottom of the cavity.
Abbreviations: G, gauge; TIVAD, totally implantable venous access device.
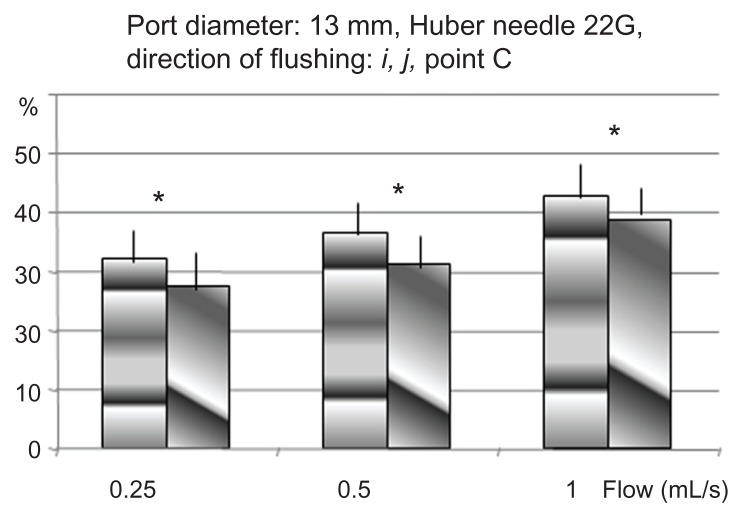
The results of insertion point C expressed in average percentage of extracted proteins are shown in Figures 4 and 5. These results are in agreement with the results from insertion point B: the highest efficiency is obtained when the needle bevel opening is directed toward the bottom of the port (i) as shown in Table 2.
Figure 4.
Percentage of proteins removed in TIVAD of 13 mm (c), with an HPN of 22G, for two orientations of the flow from the point C: i towards the bottom of the cavity, j towards the exit channel.
Abbreviations: G, gauge; HPN, Huber point needle; TIVAD, totally implantable venous access devices.
Table 2.
Percentage of extracted proteins
| Flow rate | i | j | P value |
|---|---|---|---|
| 0.25 mL/second | 32.4% | 27.4% | 0.01 < P < 0.05 |
| 0.50 mL/second | 36.7% | 31.3% | 0.01 < P < 0.05 |
| 1 mL/second | 42.9% | 38.9% | 0.01 < P < 0.05 |
Note: Bevel opening toward the outflow channel versus bottom of the port with an off-centered needle insertion point.
Cross comparisons show that the needle insertion point has no or little effect on flushing efficiency (see Figure 5A and B; Tables 3 and 4).
Table 3.
Percentage of extracted proteins
| Flow rate | j | α = 0° | P value |
|---|---|---|---|
| 0.25 mL/second | 27.1% | 22.1% | Nonsignificant |
| 0.50 mL/second | 31.3% | 27.7% | Nonsignificant |
| 1 mL/second | 38.9% | 36.2% | Nonsignificant |
Note: Bevel opening toward the outflow channel with a centered versus off-centered needle insertion point.
Table 4.
Percentage of extracted proteins.
| Flow rate | α = 180° | i | P value |
|---|---|---|---|
| 0.25 mL/second | 32.4% | 32.4% | Nonsignificant |
| 0.50 mL/second | 37.4% | 36.7% | Nonsignificant |
| 1 mL/second | 42% | 42.9% | Nonsignificant |
Note: Bevel opening toward the port bottom (α = 180°) with a centered versus off-centered needle insertion point.
Numerical computation
The experimental results are consolidated by numerical computations that show the flow structure (laminar flow) in a cavity filled with a liquid (as ports in use are). The cavity bidimensional geometry is shown in Figure 1. The circular cavity has a diameter of 8 mm. The dynamic viscosity of the flush solution (normal saline) used is η = 10−3 Pa-s, and its volume mass was ρ = 103 kgm−3. The needle was inserted in the port center (point B). The angle of injection α = 0° corresponds to the axis between the point B and the exit channel O.
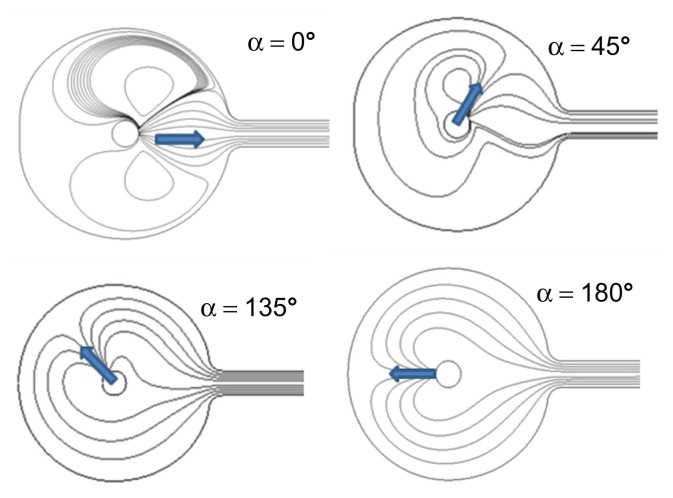
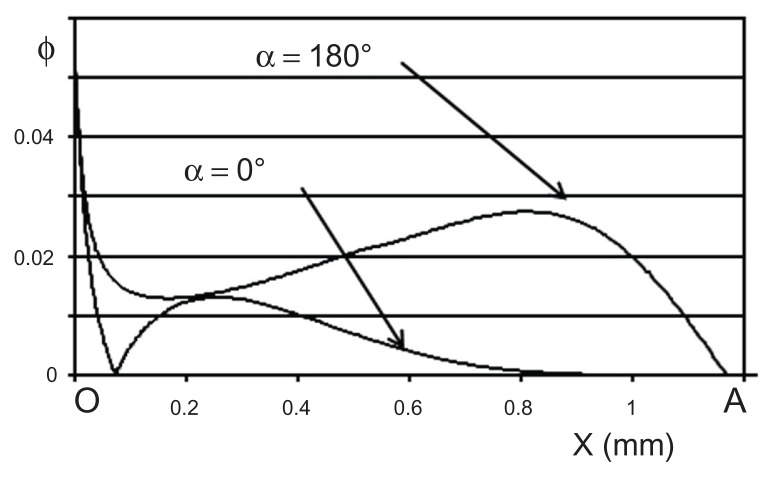
Figure 6 shows the lines of a continuous flow for four injection angles α = 0°, 45°, 135°, and 180°. For α = 180°, the lines fold up on the cavity bottom and follow the outline of the reservoir wall. The flow is drawn toward the exit channel. On the other hand, when α = 0°, the flow divides. One part recirculates, in the rheological sense, and remains inside the cavity. One can quantify the flushing efficiency by calculating the shear stress force at each point along the cavity inside wall. The value, quoted as Φ, is proportional to the friction force by surface unit of the cavity and therefore, represents the local density of flow of proteins extracted by friction. Figure 7 shows that Φ is always higher when α = 180° than when α = 0°. This is obviously in agreement with the tests results. One also notices that Φ is always close to 0 in an area more or less important around point A at the bottom of the cavity. This is due to a stop point of the flow for both α = 180° and α = 0°.
Figure 6.
Lines of flow in a circular cavity for four injection angles.
Figure 7.
Φ numerical values along the wall of the cavity according to the curvilinear abscissa (X) between points O and A for the two directions of the flow α = 180° and α = 0°.
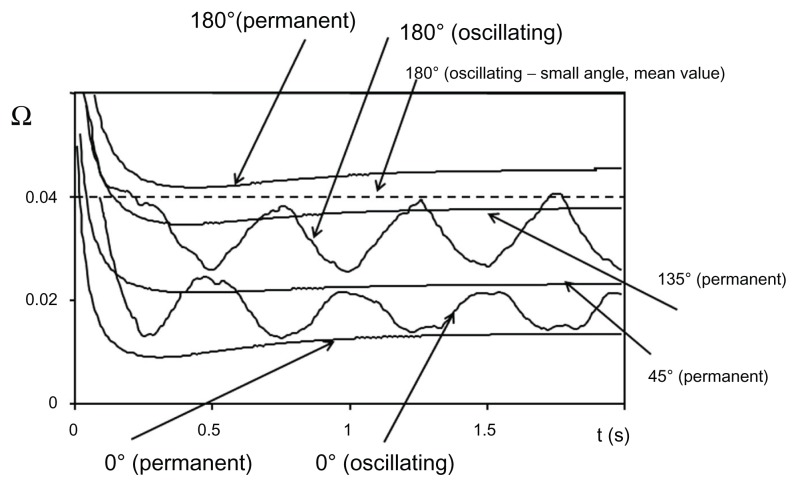
The global efficiency of the flushing can be estimated by integrating Φ. The computation leads then to a value, quoted as Ω, which is proportional to the mass flow collected by flushing. Results are reported in Figure 8 which shows the Ω variations according to time from the initial moment before beginning to flush the cavity and according to the flushing technique used, needle bevel-opening orientation, and with or without a movement of oscillation around its vertical axis.
Figure 8.
Distribution of numerical values as a function of time for different situations of flushing in permanent or impermanent conditions.
The results without oscillations are presented on Figure 8 for four bevel needle-opening orientations (α = 0°, 45°, 135°, and 180°). The numerical computation reproduces the experimental test data. The most efficient flushing is obtained when α = 180° and the less efficient when α = 0°. Keeping in mind that an unflushed area remains in the neighborhood of the point A, a movement of oscillation of the needle around its axis could be performed (as recommended by numerous clinicians). The results of that technique, with an oscillation of ±90° for both α = 0° and 180° are shown in Figure 8. As expected, the efficiency lies between that of α = 180° and α = 0° without needle oscillations. A compromise could be to impose a movement of oscillation of very small amplitude ≈ ±10° around α = 180°. The result is show as the dotted line in Figure 8. We obtained a global efficiency close to (but always below) the maximum while avoiding the absence of flushing in the neighborhood of point A.
Discussion
The purpose of this work was to test, under controlled conditions, the effect of the needle bevel orientation on the efficiency of flushing implantable ports with a continuous infusion of normal saline. Maintaining the vacuity of vascular access, specifically venous access, is of upmost importance for the medical, psychological, and financial costs. To avoid obstruction is the first and foremost reasonable use to limit deposits in TIVADs by conducting effective flushing. Flushing with the correct solution and technique is then fundamental to maintain patency of the catheter. The use of heparinized solution (10 U/mL) to ensure catheter patency is controversial and efficacy is not proven. Flushing with Heparin solution is no more beneficial than flushing with NaCl 0.9% alone.9,11 Because an intraluminal fibrin sheet or blood clot might serve as a nidus for microbial colonization after the perfusion of intravascular catheters by medication or parenteral nutrition, it is very important to realize effective flushing and to withdraw any biological or chemical component.
We specified the effective pulsed flushing technique,13 but no publication specifies the influence of the direction of the opening of HPN in the port. The experimental results of the study pollution from flushing of ports, consolidated by the results of numerical computation, show that the shear stress forces are always more important when the injected flow is directed towards the bottom of the port, even if the HPN is not inserted in the center of the septum port. When the flow is directed towards the bottom of the port (point A, α = 180°), the flushing flow redistributes on the totality of the walls of the port and the intensity of the shear stress force of cutting is major. If the flow is directed towards the exit channel, only a small part of the flow is redirected towards the walls of the port. The intensity of the shear stress force is strongly decreased and the recirculating flow favors the parietal deposit of all or any of the perfusate (proteins, lipids, contrast medium) or of figurative elements of the blood. This experimental work expresses and confirms fundamental basic principles to establish a protocol of TIVAD use: flushing, perfusing, and blood collection. These principles are physically valid whatever the position of the insertion point of the HPN in the septum. Optimal flushing is obtained with a small-gauge HPN with its bevel opening opposite to the exit channel. This corresponds to the recommendations of use of this type of needle. It may be interesting, during the flushing, to perform an oscillating movement of very low amplitude (for example at point B, α = 180° ± ≈10°) to try to eliminate the deposits in the stop point A at the bottom of the port (see Figure 6). Oscillations of big amplitude around the needle insertion points (B or C, see Figure 1) are only of average efficiency, and much less than in the fixed injection. The rotations of HPN on its vertical axis, as recommended by some protocols, are also less effective and must not be performed. Furthermore this movement sometimes causes pain in patients. It seems desirable to direct the flow of a drip to the bottom of the port, although the flow is mostly lower than the flow of the flushing. Indeed, the hydrodynamic effect which accounts for flushing occurs in the same way during the infusion to prevent or limit the parietal deposition. If the flow is directed towards the output channel, the redistribution of the flow leads to a recirculation, which promotes the wall deposition of all or part of the perfusate, which is then trapped in the port.
Conclusion
The prevention of the occlusion of TIVADs is imperative. The definition of protocols of use of the TIVADs based on the hemodynamic principles mentioned above is a simple, effective and economic therapeutic for use by nursing teams to avoid or reduce the obstruction of the TIVADs. The knowledge of the physical principles which govern the flows in these devices allows for the reduction of mechanical manipulations, which are sources of medical complications10–12 in particular infections. Nevertheless, no article or recommendations appear in the literature concerning the orientation of the opening of HPN used with TIVADs.
We have shown that the direction of the jet of flushing and perfusion is an important factor for the prevention of the accumulation of biological and/or chemical deposit (contrast agent for example, due to an increasing use of TIVADs) and to assure the patency of the device. We hope that this study will contribute to the definition of good practices in an area of increasing importance.
Aknowledgments
The authors thank Perouse Medical for supplying them with the TIVADs necessary for the study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Adlera A, Yanivb I, Steinbergc R, et al. Infectious complications of implantable ports and Hickman catheters in paediatric haematology–oncology patients. J Hosp Infect. 2006;62(3):358–365. doi: 10.1016/j.jhin.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Ahmadi J, Izadyar M, Ashjaei B, et al. Study of advantages and disadvantages of totally implanted venous access device. Acta Med Iran. 2006;44(3):199–202. [Google Scholar]
- 3.Hall P, Cedermark B, Swedenborg J. Implantable catheter system for long-term intravenous chemotherapy. J Surg Oncol. 1989;41(1):39–41. doi: 10.1002/jso.2930410112. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann KA, Waggershauser T, Sittek H, Reiser MF. Liver intraarterial chemotherapy: use of the femoral artery for percutaneous implantation of catheter-port systems. Radiology. 2000;215(1):294–299. doi: 10.1148/radiology.215.1.r00ap14294. [DOI] [PubMed] [Google Scholar]
- 5.Hirota T, Yamagami T, Tanaka O, et al. Brain infarction after percutaneous implantation of port-catheter system via the left subclavian artery. Br J Radiol. 2002;75(898):799–804. doi: 10.1259/bjr.75.898.750799. [DOI] [PubMed] [Google Scholar]
- 6.Yamagami T, Kato T, Iida S, Hirota T, Nishimura T. Management of end hole in placement of port-catheter system for continuous hepatic arterial infusion chemotherapy using the fixed catheter tip method. Am J Roentgenol. 2005;184(4):1332–1339. doi: 10.2214/ajr.184.4.01841332. [DOI] [PubMed] [Google Scholar]
- 7.Yamagami T, Terayama K, Yoshimatsu R, Matsumoto T, Miura H, Nishimura T. Use of N-butyl cyanoacrylate in implantation of a portcatheter system for hepatic arterial infusion chemotherapy with the fixed-catheter-tip method: is it necessary? Am J Roentgenol. 2008;191(5):1523–1529. doi: 10.2214/AJR.07.3717. [DOI] [PubMed] [Google Scholar]
- 8.MALER G. ANAES Lettre Circulaire. DGS/EM 1. Octobre. 1996. Sécurité des dispositifs médicaux et utilisation des chambres à cathéters implantables et des aiguilles. N° 96-6225. [Google Scholar]
- 9.MALER G. ANAES Lettre Circulaire. DGS/DH N° 98-249. Avril. 1998. Evaluation de la qualité de l’utilisation et de la surveillance des chambres à cathéters implantables. [Google Scholar]
- 10.Baskin JL, Pui CH, Reiss U, et al. Management of occlusion and thrombosis associated with long term indwelling central venous catheters. Lancet. 2009;374(9684):159–169. doi: 10.1016/S0140-6736(09)60220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pittiruti M, Hamilton H, Biffi R, MacFie J, Pertkiewicz M. ESPEN Guidelines on Parenteral Nutrition: central venous catheters (access, care, diagnosis and therapy of complications) Clin Nutr. 2009;28(4):365–377. doi: 10.1016/j.clnu.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Ragni MV, Journeycake JM, Brambilla DJ. Tissue plasminogen activator to prevent central venous access device infections: a systematic review of central venous access catheter thrombosis, infection, and thromboprophylaxis. Haemophilia. 2008;14(1):30–38. doi: 10.1111/j.1365-2516.2007.01599.x. [DOI] [PubMed] [Google Scholar]
- 13.Guiffant G, Durussel JJ, Merckx J, Flaud P, Vigier J-P, Mousset P. Flushing of intravascular access devices (IVADs) Efficacy of pulsed and continuous infusions. J Vasc Access. 2011 Jul 11; doi: 10.5301/JVA.2011.8487. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 14.Vigier JP, Merckx J, Coquin JY, Flaud P, Guiffant G. The use of a hydrodynamic bench for experimental simulation of flushing venous catheters: impact of the technique. ITBM-RBM Innovation et Technologie en Biologie Medicine-Revue de Tech Biomediale. 2005;26(2):147–149. [Google Scholar]
- 15.Chevallier P, Haïdopoulos M, Mantovani D. Biomatériaux: implantation d’une prothèse artérielle synthétique. Le médecin du Québec. 2003;38(6):115–123. [Google Scholar]
- 16.Drumheller PD. Materials and methods for the immobilization of bioactive species onto polymeric substrates. United States Patent 5897955. 1999 Apr 27;
- 17.Horbett TA. The role of adsorbed proteins in animal cell adhesion. Colloids Surf B Biointerfaces. 1994;2(1–3):225–240. [Google Scholar]
- 18.Pellenc D, Berry H, Gallet O. Adsorption-induced fibronectin aggregation and fibrillogenesis. J Colloid Interface Sci. 2006;298(1):132–144. doi: 10.1016/j.jcis.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 19.Potts JR, Campbell ID. Fibronectin structure and assembly. Curr Opin Cell Biol. 1994;6(5):648–655. doi: 10.1016/0955-0674(94)90090-6. [DOI] [PubMed] [Google Scholar]