Abstract
Background
Long-acting somatostatin receptor ligands (SRL) with product-specific formulation and means of administration are injected periodically in patients with acromegaly and neuroendocrine tumors. A simple decision-tree model aimed at comparing cost savings with ready-to-use Somatuline Autogel® (lanreotide) and Sandostatin LAR® (octreotide) for the UK, France, and Germany. The drivers of cost savings studied were the reduction of time to administer as well as a reduced baseline risk of clogging during product administration reported for Somatuline Autogel®.
Methods
The decision-tree model assumed two settings for SRL administration, ie, by either hospital-based or community-based nurses. In the case of clogging, the first dose was assumed to be lost and a second injection performed. Successful injection depended on the probability of clogging. Direct medical costs were included. A set of scenarios were run, varying the cost drivers, such as the baseline risk of clogging, SRL administration time, and percentage of patients injected during a hospital stay.
Results
Costs per successful injection were less for Somatuline Autogel®/Depot, ranging from Euros (EUR) 13-45, EUR 52-108, and EUR 127-151, respectively, for France, Germany, and the UK. The prices for both long-acting SRL were the same in France, and cost savings came to 100% from differences other than drug prices. For Germany and the UK, the proportion of savings due to less clogging and shorter administration time was estimated to be around 32% and 20%, respectively. Based on low and high country-specific patient cohort size estimations of individuals eligible for SRL treatment among the patient population with acromegaly and neuroendocrine tumors, annual savings were estimated to be up to EUR 2,000,000 for France, EUR 6,000,000 for Germany, and EUR 7,000,000 for the UK.
Conclusion
This model suggests that increasing usage of the Somatuline device for injection of SRL might lead to substantial savings for health care providers across Europe.
Keywords: clogging, injection, acromegaly and neuroendocrine tumors, cost savings
Introduction
Somatotropin release inhibiting factor receptor ligands, also known as somatostatin receptor ligands (SRL), have constituted a major therapeutic advance in the treatment of acromegaly and gastroenteropancreatic neuroendocrine (carcinoid) tumors (GEP-NETs).1–4 Currently available active peptides (octreotide and lanreotide) are available as long acting-preparations with product-specific formulations and means of administration.5
In acromegaly, SRL are currently used as first-line pharmacotherapy after failure of a surgical procedure. They can also be prescribed as the first therapeutic intervention in some cases, eg, where surgery is contraindicated, pharmacotherapy is appropriate prior to surgery, or invasive tumors.5,6
SRL also remain the main symptomatic therapeutic modality for the management of patients with GEP-NET who have particular biochemical profiles.4 Their effects are primarily disease stabilization and symptom control, particularly in the carcinoid syndrome.
The first SRL marketed was a short-acting preparation requiring daily injection. Long-acting formulations of SRL represented a breakthrough in management of the disease by extending the injection time interval. Sustained-release lanreotide (Somatuline® LP) was the first of these long-acting preparations to reach the market. It is delivered by intramuscular injection every 10–14 days. Slow-release octreotide (Sandostatin LAR® 10, 20 or 30 mg) was subsequently launched, and is administered by intramuscular injection once a month.
Somatuline Autogel® is the most recently available long-acting SRL formulation, and has demonstrated noninferiority compared with Sandostatin LAR®.5 Somatuline Autogel® is available at various doses (60, 90, 120 mg) in a small-volume, prefilled ready-to-use syringe. It is injected subcutaneously once a month.
A multicenter quantitative study has investigated the time needed for preparation and administration of long-acting Somatuline Autogel® and Sandostatin LAR® as well as nurse practitioner perceptions of the success rate of these products in France, Germany, the UK, and the US.7 Somatuline Autogel® was found to have a shorter administration time (66 seconds) compared with Sandostatin LAR® (329 seconds). Somatuline Autogel® was not associated with any clogging events over the course of 79 injections, whereas two (2.5%) clogging events occurred with Sandostatin LAR®, leading to product wastage. Similar technical problems during Sandostatin LAR® administration have been reported in another clinical study.8
A cost-consequence study was conducted to estimate the health economic outcomes in three European countries (France, Germany, and the UK) based on the aforementioned quantitative study. A decision-tree model was developed to assess the extent to which Somatuline Autogel®, via its ready-to-use prefilled syringe, could result in cost savings in several patient management care settings in Europe.
Materials and methods
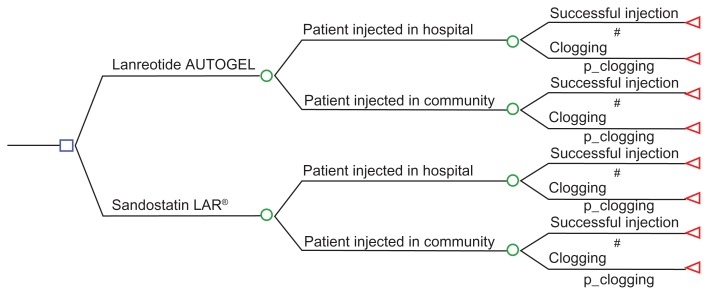
An Excel-based decision-tree model simulated two treatment arms, ie, Somatuline Autogel® and Sandostatin LAR® (Figure 1). It was assumed that patients received their injections from either hospital-based or community-based nurses. The success of injection was estimated on the basis of the number of clogging events. In the event of clogging, the first dose was assumed to be wasted and a second injection was prepared and administered. Mean cost per patient per year for each long-acting SRL was calculated. The incremental cost savings associated with Somatuline Autogel® was extrapolated to a cohort of patients based on country-specific epidemiology estimates of individuals eligible for SRL treatment among the patient population with acromegaly and neuroendocrine tumors.9–11
Figure 1.
Decision-tree structure.
Notes: P clogging represents the risk of clogging during one injection trial; #complementary probability equal to (1–p clogging).
Analyses were performed separately for the three countries. Three scenarios, ie, A, B, and C, were run by country. Scenario A was the base case, whereas B and C were based on more extreme values to assess uncertainty. A savings breakdown was developed to show the proportion of savings due to either drug rebate or reduction in consumption of medical resource units. One-way sensitivity analyses were also used to assess the key drivers within the various input parameters.
All scenarios were performed from a health care payer perspective, including only direct medical costs, such as drug consumption and administration costs. Prices from 2010 were used to estimate drug and administration costs. Somatuline Autogel® 90 mg and Sandostatin LAR® 20 mg were chosen as reference drug doses to reflect the most common posology in patients with acromegaly and GEP-NET. Retail prices for drugs were retrieved from either proprietary market databases or official list prices.12–14 UK prices were converted into 2010 Euros (EUR) based on 2010 annual average currency exchange rate (1.16 EUR = 1 GBP) to allow cross-country comparisons15 (Table 1).
Table 1.
Medical cost estimates (drug retail prices and hourly administration costs, expressed in EUR 2010)
| France | Germany | UK | |
|---|---|---|---|
| Somatuline Autogel® 90 mg | 1305 | 2322 | 875a |
| Sandostatin LAR® 20 mg | 1305 | 2351 | 991a |
| Hourly administration costs for hospital-based injection | 11–17b | 11–19.5 | 12.5–19.5 |
| Hourly administration costs for community-based injection | 15.5–21c | 17–23 | 15–21 |
Notes:
2010 annual currency exchange rate applied, 1.16 EUR = 1 GBP;15
French public hospital-based hourly nursing wage;
standard community nurse visit fees in France.
Administration unit costs were calculated by multiplying the average hourly nurse wages by the time required for injection. We derived British and German nurses’ wages by applying a relative index derived from cross-country study.16 French nurses’ wages in public hospitals were estimated based on the 2010 official salary scale17,18 for registered nurses. Concerning community-based nurses, the cost of a nurse visit for SRL injection was estimated to be EUR 15 for France, and corresponded to the official fee for a standard nurse visit to administer an injection. Rates were listed according to the Nursing Act payment nomenclature enforced by law in 201019 (Table 1).
Base case analysis
Input values used for the three scenarios A, B and C are reported in Table 2. These three scenarios used different assumptions for risk of clogging at first injection trial and time required for drug administration. Scenario A was based on values derived directly from the quantitative study. Administration times were thus assumed to be 1.1 minutes and 5.5 minutes for Somatuline Autogel® and Sandostatin LAR®, respectively. Risk of clogging at first injection trial was set to 0% and 2.6% for Somatuline Autogel® and Sandostatin LAR®, respectively. Two additional scenarios (B and C) were conducted to model worst and best cases. Scenario B combined a lower (about 25%) risk of clogging for Sandostatin LAR® (1.9%), with a smaller (about −50%) reduction of administration time and fewer clogging events at first injection trial relative to Somatuline Autogel®. Scenario C combined a higher (about +25%) risk of clogging at first injection trial for Sandostatin LAR® (3.3%) with higher (about +50%) reduction of administration time and clogging events relative to Somatuline Autogel® (100% and 80%, respectively).
Table 2.
Input parameters values of scenarios
| Parameters | Scenario A (base case) | Scenario B (low estimate) | Scenario C (high estimate) | Sensitivity analysis |
|---|---|---|---|---|
| Patients injected within hospitals (versus community, %) | 50 | 100 | 0 | 50–100 |
| Risk of clogging at first injection trial with Sandostatin LAR® | 0.026 | 0.019 | 0.033 | 0.019–0.033 |
| Risk of clogging at first injection trial with Somatuline Autogel® | 0 | 0.01 | 0 | |
| Time to prepare and administer Sandostatin LAR® (minutes) | 5.5 | 3 | 8 | 3–8 |
| Time to prepare and administer Somatuline Autogel® (minutes) | 1.1 | 1.5 | 1.6 |
One-way sensitivity analysis
Sensitivity analyses were performed for relative reduction of clogging events and proportion of patients injected within hospital versus community settings. The aforementioned relative indices for nurses’ wages had been collected prior to 2007. Taking into account the fact that they may have changed between 2007 and 2010 due to changes in annual rates of salary and price index across countries, a sensitivity analysis was performed for hospital-based and community-based nurses’ wages to assess the uncertainty surrounding these figures.
Results
Main findings
In the base case (scenario A), mean cost savings per successful injection due to Somatuline Autogel® were EUR 35, EUR 91, and EUR 143 for France, Germany, and the UK, respectively (Table 3). When considering scenarios B and C, savings per successful injection were in the range of EUR 13–45, EUR 52–108, and EUR 127–151, respectively, for France, Germany, and the UK.
Table 3.
Economic results for base case (scenario A), low estimate (scenario B), and high estimate (scenario C), expressed in EUR 2010
| France | Germany | UK | |
|---|---|---|---|
| Cost per successful injection | |||
| Somatuline Autogel® (low-high) |
1305.20 (1317.10–1 305.40) |
2322.30 (2344.30–2322.40) |
875.30 (883.60–875.50) |
| Sandostatin LAR® (low-high) |
1340.10 (1329.80–1350.20) |
2413.40 (2396.20–2430.40) |
1018.20 (1010.40–1026.10) |
| Cost savings associated with Somatuline Autogel® | |||
| CS per successful injection (Somatuline Autogel®) (low-high) |
34.90 (12.70–44.80) |
91.10 (51.90–108.00) |
142.90 (126.80–150.50) |
| CS per patient per year (Somatuline Autogel®) (low-high) |
356.40 (129–457) |
929.50 (529–1104) |
1457.50 (1294–1536) |
| Acromegaly (low-high) |
948,236 (336,377–1,187,260) |
3,176,618 (641,203–6,212,136) |
3,645,213 (1,830,826–3,929,641) |
| GEP-NETs (low-high) |
951,164 (250,953–1,555,655) |
2,559,338 (1,025,183–3,944,434) |
3,431,426 (2,172,408–4,653,502) |
Abbreviations: GEP-NETs, gastroenteropancreatic neuroendocrine tumors; CS, extrapolated cost savings associated with Somatuline Autogel® for the total patient population.
Given that the prices for both long-acting SRL were the same in France, cost savings were entirely due to differences in administration time and clogging risk. For Germany and the UK, savings were also realized due to lower drug prices for Somatuline Autogel® versus Sandostatin LAR®. The percentage savings associated with use of Somatuline Autogel® versus Sandostatin LAR® due to lower clogging incidence and administration time were around 32% and 20% for Germany and the UK, respectively.
Based on low and high country-specific patient cohort size estimations for acromegaly and neuroendocrine tumors as well as compliance rates typical for long-acting SRL (set to 85%), these cost savings led to overall annual savings in the range of EUR 1,900,000 for France, EUR 5,735,000 for Germany, and EUR 7,070,000 for the UK.
Sensitivity analyses
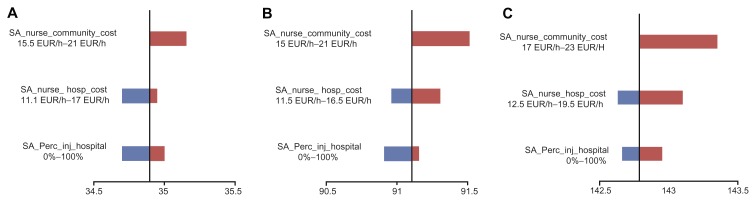
Varying hospital-based and community-based nurse wages (Table 3) had a relatively small impact on outcomes. Indeed, total administration time did not represent a significant part of the nursing workload. Similarly, the type of setting for administration (hospital or community) did not have a large impact on the results (Figure 2). These two findings are explained by the relative values of administration costs versus drug costs (about 3%).
Figure 2.
One-way sensitivity analysis on cost saving per successful injection (expressed in EUR) due to Somatuline Autogel® in France (A), Germany (B), and the UK (C).
Notes: For cost sensitivity analysis in community-based nurses (SA), 100% of patients were assumed to be injected in the community. Similarly, for hospital-based nurses (SA), 100% of patients were assumed to be injected in hospital.
Abbreviations: SA_nurse_community cost, average hourly wage of community-based nurse; SA_nurse_hosp_cost, average hourly wage of hospital-based nurse; SA_Perc_inj_hospital, proportion of patients injected within hospital (the remainder are assumed to be injected in community).
The biggest driver of cost savings was the relative reduction of clogging events associated with use of Somatuline Autogel® instead of Sandostatin LAR®. By varying this parameter alone from 50% to 100%, the mean cost savings were EUR 15–35 for France, EUR 60–91 for Germany, and EUR 140–143 for the UK.
Discussion
Fewer clogging events and the shorter administration time associated with Somatuline Autogel® in comparison with Sandostatin LAR® resulted in additional cost savings on top of direct savings due to lower drug prices. Considering the acquisition costs of long-acting SRL, drug volumes saved due to a more reliable and effective administration device resulted in direct medical cost savings for individual patients over one year of treatment. Extrapolation to the overall patient population could lead to substantial savings to payers, of up to EUR 2,000,000 for France, EUR 6,000,000 for Germany, and EUR 7,000,000 for the UK.
Very limited economic data have been published concerning the costs of medical care or health-related quality of life in patients with acromegaly. Only two economic studies were retrieved in a very recently published review addressing a comparison of disease burden between controlled and uncontrolled patient groups.20 These two studies were performed in the Italian and Spanish settings,21,22 and assessed direct costs for patients with controlled versus uncontrolled disease. In the Italian study, which enrolled a higher number of patients (134 in the Italian study versus 11 in the Spanish study), total annual costs were reported to be higher in patients with uncontrolled disease (EUR 12,533) than in those with controlled disease (EUR 7968). Results from the Spanish study were reversed because annual global treatment costs were reportedly higher in six patients with controlled disease (EUR 9874) versus five patients with uncontrolled disease (EUR 7072). This result might be explained by the difference in pharmacological treatment patterns, ie, three of the six noncontrolled patients were treated with bromocriptine, which is a less expensive drug than SRL. We did not introduce the concept of controlled versus uncontrolled patient groups into our analysis because our primary aim was to demonstrate that all administration cost components must be carefully taken into account. Therefore, the study goal was not to deliver a full assessment of the economic burden of acromegaly even though this topic is of high interest, as highlighted in the aforementioned review.20 These two economic studies reported SRL costs as major price drivers21,22 alongside surgery. Therefore, a more reliable and effective administration device has the opportunity to reduce the total economic burden of acromegaly significantly.
Our economic extrapolations must be done with caution and have to be considered as exploratory only. Indeed, clogging events and injection time have been based on drug administration by nurses into an injection pad and not on patients during everyday practice.7 These figures can be considered as proxies for real practice data, and this experimental test allowed the unambiguous attribution of these differences to the injection devices and not to patient variability (ie, patients being more or less difficult to inject). Schweinsberg et al recently presented real-life results23 that corroborate the data used in our study. In their study, mean injection times were longer than those observed in our current study, ie, 7.21 ± 0.35 minutes for Sandostatin LAR® versus 1.38 ± 0.27 minutes for Somatuline Autogel®, and a baseline risk of clogging of 3.3%.23 The relative differences in administration times were higher than in the current study and were also found to be statistically significant. Therefore, our base case assumptions can be deemed to be conservative.
Beyond economic considerations, the availability of devices that reduce workload confers several benefits. Time saved could be potentially reallocated to more thorough patient evaluation and general health monitoring. Facilitating drug administration reduces the technical workload and promotes a calm environment for the patient. As shown by the quantitative study, the Somatuline Autogel® device increased the nurse’s confidence that a full dose had been administered.7 This also helps to create a stress-free working environment.
According to our study, Somatuline Autogel® was associated with substantial cost savings (up to EUR 15,000,000 across three European countries) due to a reduced risk of clogging and shorter administration time, on top of lower retail drug prices. This therapeutic option might represent a true health economic benefit for health care payers in the context of increasing budget constraints and preferences for more cost-effective alternatives.
Conclusion
Administration costs as a whole might be important to take into account when comparing costly drugs which must be administered by injection. Simulations of reduction in risk of clogging and shorter administration times for Somatuline Autogel® (lanreotide) versus Sandostatin LAR®, on top of lower retail drug prices, predict substantial cost savings in the countries studied, ie, France, Germany, and the UK.
Acknowledgment
This study was supported by an unrestricted grant from IPSEN. IPSEN provided market sizing data and average posology for SRL administration to patients, according to internal research.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Melmed S. N Engl J Med. Vol. 335. Medical: progress; Acromegaly: 2006. pp. 2258–2273. [DOI] [PubMed] [Google Scholar]
- 2.Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest. 2009;119:3189–3202. doi: 10.1172/JCI39375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherlock M, Woods C, Sheppard MC. Medical therapy in acromegaly. Nat Rev Endocrinol. 2011;7:291–300. doi: 10.1038/nrendo.2011.42. [DOI] [PubMed] [Google Scholar]
- 4.Modlin IM, Pavel M, Kidd M, et al. Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumors. Aliment Pharmacol Ther. 2010;3:169–188. doi: 10.1111/j.1365-2036.2009.04174.x. [DOI] [PubMed] [Google Scholar]
- 5.Murray R, Melmed S. A critical analysis of clinically available somatostatin analog formulations for therapy of acromegaly. J Clin Endocrinol Metab. 2008;93:2957–2868. doi: 10.1210/jc.2008-0027. [DOI] [PubMed] [Google Scholar]
- 6.Melmed S, Casanueva F, Cavagnini F, et al. Consensus statement: medical management of acromegaly. Eur J Endocrinol. 2005;153:737–740. doi: 10.1530/eje.1.02036. [DOI] [PubMed] [Google Scholar]
- 7.Adelman DT, Burgess A, Davies PR. Nurse evaluation of long-acting somatostatin analog injection devices: a quantitative study. Presented at the 8th Annual ENETS Conference; Lisbon, Portugal. 9–11 March 2011. [Google Scholar]
- 8.Alexopoulou O, Abrams P, Verhelst J, et al. Efficacy and tolerability of lanreotide autogel therapy in acromegalic patients previously treated with octreotide LAR. Eur J Endocrinol. 2004;151:317–324. doi: 10.1530/eje.0.1510317. [DOI] [PubMed] [Google Scholar]
- 9.Ramage JK, Davies AHG, Ardill J, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut. 2005;54(Suppl IV):iv1–iv16. doi: 10.1136/gut.2004.053314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reincke M, Petersenn S, Buchfelder M, et al. The German acromegaly registry: description of the database and initial results. Exp Clin Endocrinol Diabetes. 2006;114:498–505. doi: 10.1055/s-2006-948313. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins P, Fairclough P. Screening guidelines for colorectal cancer and polyps in patients with acromegaly. Gut. 2002;51(Suppl 5):v13–v14. doi: 10.1136/gut.51.suppl_5.v13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IHS Global Insight Pricing & Reimbursement Database. [accessed April 21, 2011]. [Google Scholar]
- 13.Lauer-Taxe 2010 Pharmaceutical Database. [accessed April 21, 2011]. [Google Scholar]
- 14.MIMS Prescription Drug Database. [accessed April 21, 2011]. [Google Scholar]
- 15.European Central Bank. Statistics, Euro foreign exchange reference rates. [Accessed April 21, 2011]. Available from: http://www.ecb.int/stats/exchange/eurofxref/html/eurofxref-graph-gbp.en.html.
- 16.Observatoire des Hommes et des Organisations, Lab’Ho. Health Professionals in Europe. Staffing, training, recognition of occupations, mobility, shortage, remuneration. [Accessed April 20, 2011]. Available from: http://www.labho.fr/ French.
- 17.French National Nurses Coordination. Schedule of fees. [Accessed April 15, 2011]. Available from: http://www.coordination-nationale-infirmiere.org/index.php/Grille-des-salaires.
- 18.Decree No. 201-2761 of 7 July 2010 on increased pay for military and civilian personnel of the State, local government staff and public hospital [Décret n° 2010–2761 du 7 juillet 2010 portant majoration de la rémunération des personnels civils et militaires de l’Etat, des personnels des collectivités territoriales et des établissements publics d’hospitalisation]. Published on JORF n°0156 NOR: MTSX1017587D, July 8, 2010. French.
- 19.General nomenclature of nurses’ professional services, Order of October 17, 2008 approving the amendment No. 1 to the National Convention of Liberal Nurses [Nomenclature générale des actes professionnels infirmiers, Arrêté du 17 octobre 2008 portant approbation de l’avenant n°1 à la convention nationale des infirmières et infirmiers libéraux]; Published on JORF n°0244 NOR: SJSS0821851A, October 18t 2008, applicable since April 18, 2009 and still enforced in 2010. French.
- 20.Ben-Shlomo A, Sheppard MC, Stephens JM, Pulgar S, Melmed S. Clinical, quality of life, and economic value of acromegaly disease control. Pituitary. 2011;14:284–294. doi: 10.1007/s11102-011-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Didoni G, Grottol S, Gasco V, et al. Cost-of-illness study in acromegalic patients in Italy. J Endocrinol Invest. 2004;27:1034–1039. doi: 10.1007/BF03345306. [DOI] [PubMed] [Google Scholar]
- 22.Luque-Ramirez M, Paramo C, da Varela CC, Garcia-Mayor RV. Cost of management of invasive growth hormone-secreting macroadenoma. J Endocrinol Invest. 2007;30:541–545. doi: 10.1007/BF03346346. [DOI] [PubMed] [Google Scholar]
- 23.Schweinsberg K, Smith S, Kirshner LS. Ease of administration of somatostatin analogs, octreotide LAR versus lanreotide. Presented at the 93rd annual meeting of the Endocrine Society; June 4–7, 2011; Boston, MA. [Google Scholar]