Abstract
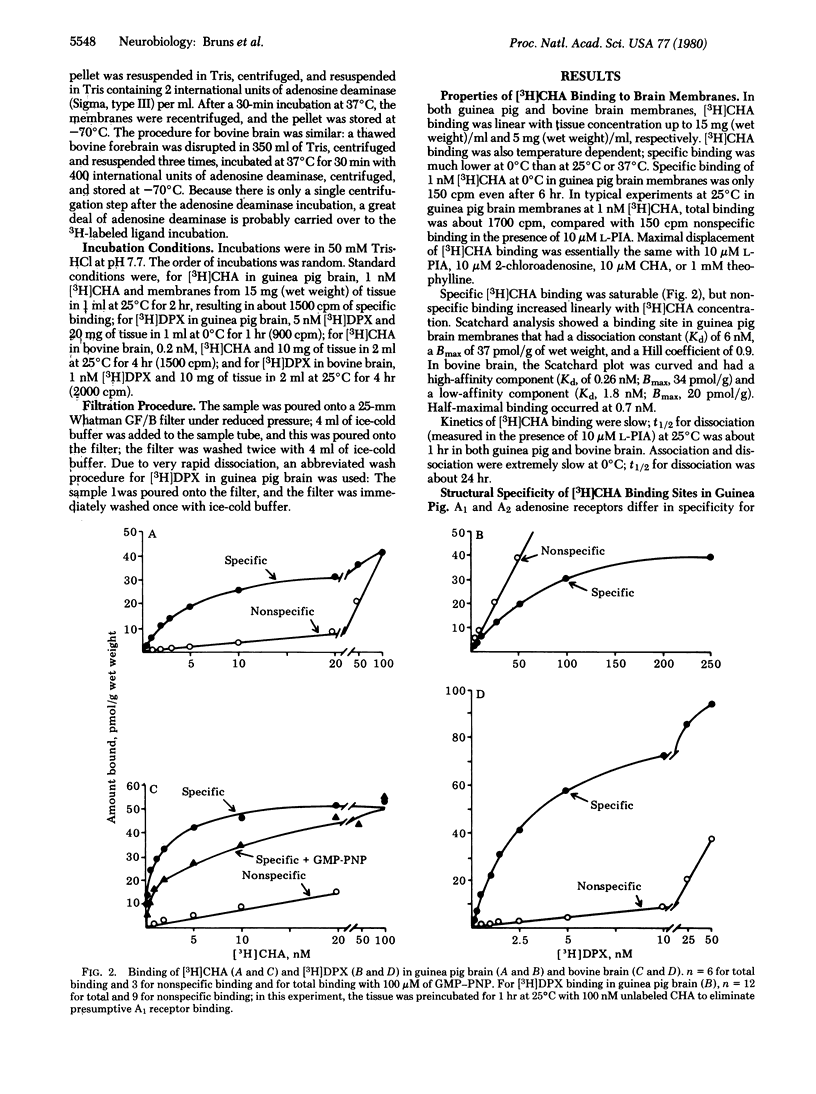
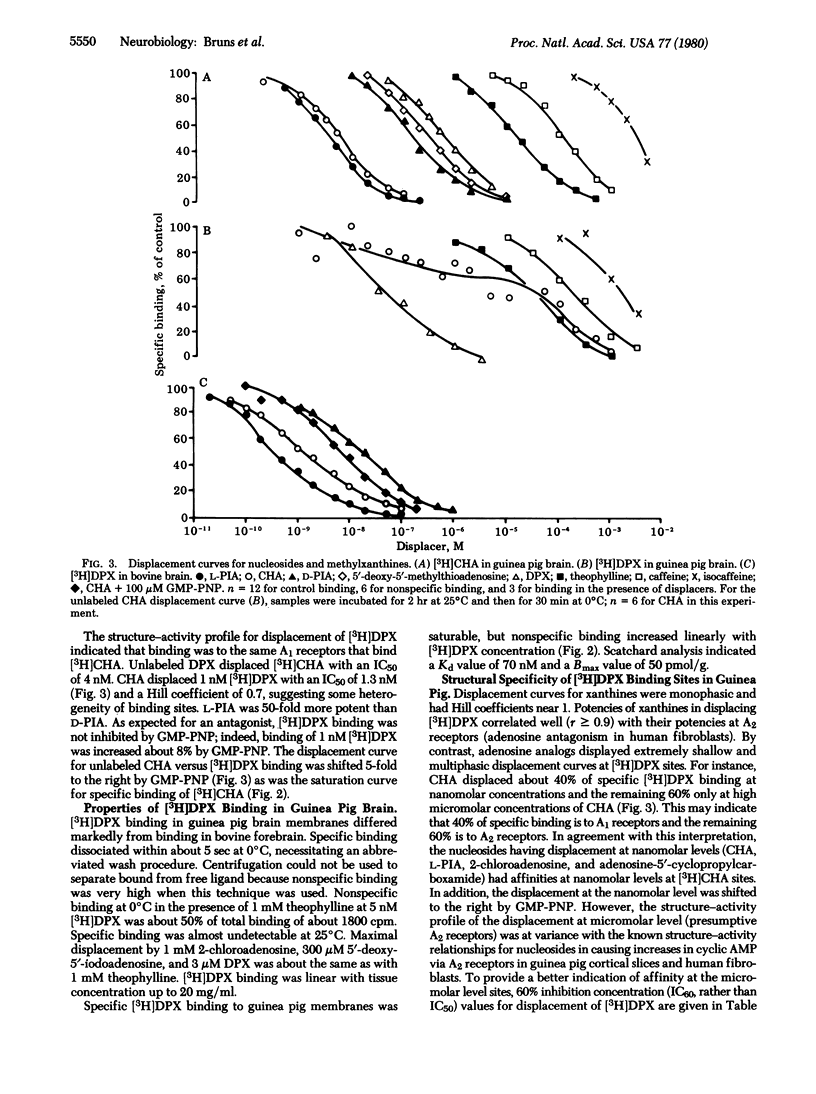
N6-Cyclohexyl[3H]adenosine ([3H]CHA) and 1,3-diethyl-8-[3H]phenylxanthine ([3H]DPX) to bind to adenosine receptors in brain membranes. The agonist [3H]CHA has high affinity in both bovine and guinea pig brain (Kd, 0.7 nM and 6 nM, respectively). [3H]CHA binding kinetics are slow (dissociation t1/2;60 min); binding is much higher at 25 degrees C than at 0 degrees C and is inhibited by guanine nucleotides. Potencies of nucleosides and xanthines in competing for [3H]CHA sites indicate that specific binding is entirely to A1 adenosine receptors. In bovine brain, the antagonist [3H]DPX exhibits high-affinity binding (Kd, 5 nM) to the same A1 receptors that bind [3H]CHA. Binding kinetics are rapid (dissociation t1/2, 1 min), and binding is moderately higher at 0 degrees C than at 25 degrees C. In guinea pig brain, [3H]DPX binding has only moderate affintiy (Kd 50 nM), and about 60% of specific binding is to sites that resemble A2 adenosine receptors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruns R. F. Adenosine receptor activation in human fibroblasts: nucleoside agonists and antagonists. Can J Physiol Pharmacol. 1980 Jun;58(6):673–691. doi: 10.1139/y80-110. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Gaudette L. A., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Specific binding of the inhibitor nitrobenzylthioinosine to nucleoside transport sites in the erythrocyte membrane. Biochim Biophys Acta. 1974 Apr 12;345(1):1–10. doi: 10.1016/0005-2736(74)90239-9. [DOI] [PubMed] [Google Scholar]
- Dutta P., Mustafa S. J. Saturable binding of adenosine to the dog heart microsomal fraction: competitive inhibition by aminophylline. J Pharmacol Exp Ther. 1979 Dec;211(3):496–501. [PubMed] [Google Scholar]
- HAGER G. P., KRANTZ J. C., Jr, HARMON J. B., BURGISON R. M. Theophylline derivatives. II. 8-Aralkyltheophyllines and related compounds. J Am Pharm Assoc Am Pharm Assoc. 1954 Mar;43(3):152–155. doi: 10.1002/jps.3030430308. [DOI] [PubMed] [Google Scholar]
- Kuroda Y. Physiological roles of adenosine derivatives which are released during neurotransmission in mammalian brain. J Physiol (Paris) 1978;74(5):463–470. [PubMed] [Google Scholar]
- Londos C., Wolff J. Two distinct adenosine-sensitive sites on adenylate cyclase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5482–5486. doi: 10.1073/pnas.74.12.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbon C. C., Hert R. C., Fain J. N. Characterization of [3H]adenosine binding to fat cell membranes. J Biol Chem. 1978 May 10;253(9):3114–3122. [PubMed] [Google Scholar]
- Paton D. M. Presynaptic inhibition of neurotransmission in rat vas deferens by 2-(p-methoxyphenyl) adenosine, ethyl adenosine-5'-carboxylate and N-cyclopropyl adenosine-5'-carboxamide. J Pharm Pharmacol. 1980 Feb;32(2):133–135. doi: 10.1111/j.2042-7158.1980.tb12869.x. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Edstrom J. P., Kostopoulos G. K., Kirkpatrick J. R. Effects of adenosine and adenine nucleotides on synaptic transmission in the cerebral cortex. Can J Physiol Pharmacol. 1979 Nov;57(11):1289–1312. doi: 10.1139/y79-194. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Kiffe H., Puchstein C., Trost T. Specific binding of 3H-adenosine to rat brain membranes. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(1):59–67. doi: 10.1007/BF00499875. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Klein-Szanto A. J., Fischer S. M., Weeks C. E., Nelson K., Major S. Studies on mechanism of action of anti-tumor-promoting agents: their specificity in two-stage promotion. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2251–2254. doi: 10.1073/pnas.77.4.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smellie F. W., Daly J. W., Dunwiddie T. V., Hoffer B. J. The dextro and levorotatory isomers of N-phenylisopropyladenosine: stereospecific effects on cyclic AMP-formation and evoked synaptic responses in brain slices. Life Sci. 1979 Nov 12;25(20):1739–1748. doi: 10.1016/0024-3205(79)90477-6. [DOI] [PubMed] [Google Scholar]
- Smellie F. W., Davis C. W., Daly J. W., Wells J. N. Alkylxanthines: inhibition of adenosine-elicited accumulation of cyclic AMP in brain slices and of brain phosphodiesterase activity. Life Sci. 1979 Jun 25;24(26):2475–2482. doi: 10.1016/0024-3205(79)90458-2. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]



