the hormonal activation of cAMP production in pancreatic β-cells is accompanied by an increase of cytosolic [cAMP] that can be measured in real time through the use of a cAMP sensor (Epac1-camps) that exhibits a decrease of fluorescence resonance energy transfer (FRET) when it binds to cAMP (9, 15, 18). When this cAMP sensor is paired with the fluorescent Ca2+ indicator fura-2, it is possible to perform simultaneous measurements of cytosolic [cAMP] and [Ca2+] in single living β-cells (9). Studies performed in this manner have revealed oscillations in the levels of both second messengers, a phenomenon that can be imaged to evaluate the spatial distribution and temporal dynamics of cAMP and Ca2+ (15). Fridlyand et al. (6) now report a new mathematical model that seeks to explain how oscillations of cAMP and Ca2+ are generated. A primary focus of the model is glucagon-like peptide-1 (GLP-1), a blood glucose-lowering hormone that stimulates β-cell cAMP production and that also potentiates glucose-stimulated insulin secretion (4, 7, 12–14). An additional focus of the model concerns d-glucose, a metabolizable sugar that increases the cytosolic [Ca2+] of β-cells and that is generally considered to be the primary physiological stimulus regulating β-cell insulin secretion (11). The computational approach devised by Fridlyand et al. is of interest because it may help resolve a long-standing debate within the field of endocrinology: specifically, how do GLP-1 and glucose metabolism interact to stimulate insulin secretion from β-cells?
A prior study (24) has demonstrated a powerful synergistic interaction of GLP-1 and glucose to stimulate insulin secretion. Moreover, a wealth of data exists indicating that the insulin secretagogue actions of GLP-1 and glucose are secondary to their ability to stimulate an increase of cytosolic [cAMP] and [Ca2+] (5, 7, 12–14). GLP-1 stimulates cAMP production as a consequence of its binding to the β-cell GLP-1 receptor (22), a class II G protein-coupled receptor that activates Gs proteins and that stimulates transmembrane adenylyl cyclase (tmAC) (Fig. 1). In contrast, glucose must be metabolized by the β-cell for it to stimulate an increase of [Ca2+] (11) (Fig. 1). Glucose metabolism increases the cytosolic [ATP]-to-[ADP] ratio, which promotes the closure of ATP-sensitive K+ (KATP) channels and initiates bursts of Ca2+-dependent action potentials, causing oscillations of [Ca2+] (11, 19). The ensuing increase of cytosolic [Ca2+] triggers the fusion of insulin-containing secretory granules with the plasma membrane. Because cAMP facilitates the action of Ca2+ to stimulate secretory granule exocytosis in β-cells (20), GLP-1 potentiates glucose-stimulated insulin secretion. In view of the fact that pancreatic insulin secretion is stimulated after the ingestion of a meal when levels of GLP-1 and glucose increase in the systemic circulation (14), the model proposed by Fridlyand et al. predicts that the synergistic interaction of GLP-1 and glucose to stimulate insulin secretion results from synchronous and in-phase oscillations of β-cell [cAMP] and [Ca2+] (6).
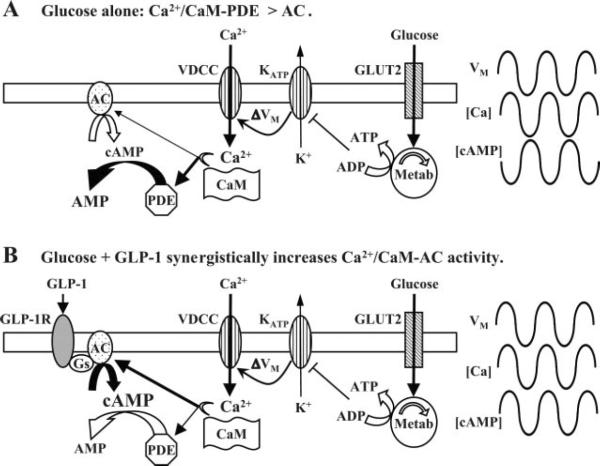
Fig. 1.
Changes in the synchrony of [Ca2+] and [cAMP] induced by treatment of β-cells with glucose or glucose plus glucagon-like peptide-1 (GLP-1). A: in the presence of glucose alone, metabolism (Metab) of the sugar induces ATP-sensitive K+ (KATP) channel closure, membrane depolarization [change in membrane voltage (ΔVM)], electrical bursting activity, and periodic influx of Ca2+. This Ca2+ influx activates calmodulin (CaM), which weakly stimulates transmembrane adenylyl cyclase (AC) 1/8 (tmAC1/8) but strongly stimulates phosphodiesterase 1C (PDE1C). Since PDE1C activity exceeds that of tmAC1/8, cAMP levels fall when [Ca2+] increases, and these oscillations are antiphasic. Because glucose alone has little effect on basal [cAMP], [cAMP] oscillates at levels close to the basal concentration. VDCC, voltage-dependent Ca2+ channel; GLUT2, glucose transporter 2. B: exposure of β-cells to GLP-1 in the presence of glucose results in the synergistic activation of tmAC1/8 by Gαs and Ca2+/CaM, whereas PDE1C is also activated by Ca2+/CaM. When [Ca2+] increases, the rate of cAMP production exceeds the rate of cAMP hydrolysis so that [cAMP] oscillates in phase with the increase of [Ca2+]. Since GLP-1 is a strong stimulus for cAMP production under conditions of elevated cytosolic [Ca2+], [cAMP] oscillates at levels greatly in excess of basal [cAMP]. GLP-1R, GLP-1 receptor. Note that these two models of cAMP and Ca2+ dynamics predict that oscillations of [cAMP] will either have little effect (A) or a strong stimulatory effect (B) on glucose-stimulated insulin secretion.
The model of Fridlyand et al. proposes that the amplitude, periodicity, and phase characteristics of [cAMP] oscillations will be determined by two principal factors: 1) Ca2+/calmodulin-dependent activation of cyclic nucleotide phosphodiesterase (PDE) and 2) Ca2+/calmodulin-dependent activation of tmAC (Fig. 1). The computational approach of Fridlyand et al. provides an ingenious prediction: that when β-cells are exposed to elevated concentrations of glucose, the binding of GLP-1 to the GLP-1 receptor converts anti-phasic oscillations of [cAMP] and [Ca2+] into in-phase oscillatory activity and in so doing allows the concentrations of both cAMP and Ca2+ to rise simultaneously (Fig. 1). Once this in-phase oscillatory activity is established, the frequency and amplitude of each oscillation dictate the efficacy with which GLP-1 potentiates glucose-stimulated insulin secretion.
This model developed by Fridlyand et al. is an outgrowth of the earlier study of Landa and co-workers (15) in which synchronous but anti-phasic oscillations of [cAMP] and [Ca2+] were detected in an insulin-secreting cell line (MIN6) not treated with GLP-1. In that study, anti-phasic oscillations of cAMP and Ca2+ were observed after exposure of MIN6 cells to buffered saline containing glucose and a K+ channel blocker (TEA). Under such conditions, the peak of [cAMP] coincided with the nadir of [Ca2+] for each oscillation, a finding that prompted Landa and co-workers to conclude that in the absence of GLP-1, anti-phasic oscillations of [cAMP] are induced by the periodic activation of Ca2+-dependent PDEs (Fig. 1A) (15). Consistent with this concept, evidence exists for the expression of a Ca2+/calmodulin-regulated PDE designated as PDE1C in β-cells (8, 15).
It is of interest to note that in the study of Landa et al., oscillations of [Ca2+] were induced by exposure of MIN6 cells to a solution that contained both glucose and TEA. However, in the absence of TEA, glucose failed to stimulate oscillations of [Ca2+] (15). This finding is understandable if TEA reduces a K+ conductance that normally suppresses oscillations of membrane potential in this cell type. Thus, MIN6 cells differ substantially from primary β-cells, as evidenced by the fact that treatment of whole islets of Langerhans with glucose induces periodic membrane depolarization, bursting electrical activity, and oscillations of β-cell [Ca2+] (19). In an innovative manner, the model of Fridyland et al. takes these unique features of β-cell physiology into account by simulating the glucose-dependent oscillations of membrane potential that generate anti-phasic oscillations of [Ca2+] and [cAMP] (Fig. 1A) and that are predicted to occur in the absence of GLP-1.
Fridlyand et al. also take advantage of findings generated in prior studies of R15, a neuron in the sea slug Aplysia. Although R15 is not glucose responsive, it exhibits oscillations of membrane potential that are sensitive to both cAMP and Ca2+. In a computational study (25) of R15 electrical activity, a model was developed in which the activity of tmAC was considered to be stimulated or inhibited by the binding of Ca2+ to calmodulin. The model of Fridlyand et al. takes these predictions concerning Ca2+/calmodulin-dependent regulation of tmAC into account when considering how Ca2+ influences β-cell cAMP production. Attention is focused on tmAC1 and tmAC8, isoforms of tmAC that are expressed in β-cells and that are known to act as molecular coincidence detectors (3, 15, 23). Available evidence indicates that tmAC1 and tmAC8 are synergistically activated by 1) Gαs proteins and 2) Ca2+/calmodulin (2). In the model of Fridlyand et al., binding of GLP-1 to the GLP-1 receptor activates Gαs proteins, whereas glucose metabolism promotes the increase of [Ca2+] that allows for the association of Ca2+ with calmodulin. Under conditions in which β-cells are exposed to both GLP-1 and glucose, tmAC1 and tmAC8 become synergistically activated, and cAMP production exceeds cAMP degradation. For this reason, [cAMP] and [Ca2+] oscillate in phase despite the fact that Ca2+ influx also generates periodic activation of PDE (Fig. 1B).
In summary, Fridlyand et al.'s model, supported by published data, demonstrates that cAMP oscillations are contin gent upon, and entrained by, oscillations of [Ca2+]. Exactly how such oscillations of [Ca2+] are generated during the exposure of β-cells to glucose is a topic of substantial debate. In simulations of R15 electrical activity, oscillations of [Ca2+] can be generated as a consequence of Ca2+ and cAMP-dependent mechanisms controlling the activity of ion channels (25). Thus, there is reason to believe that oscillations of [Ca2+] in β-cells might have a similar ionic basis (19). However, β-cells differ from R15 in that the initiation of Ca2+ oscillations is contingent on the glucose metabolism-dependent reduction of plasma membrane KATP channel conductance. Furthermore, glucose stimulates both fast and slow oscillations of [Ca2+] in the β-cell, and it is the slow oscillations of [Ca2+] that Fridlyand et al. have incorporated into their model of cAMP and Ca2+ dynamics. With these points in mind, it seems reasonable to speculate that oscillations of [Ca2+] in β-cells might have a metabolic basis in addition to an ionic basis. Indeed, a dual oscillator model for β-cell Ca2+ handling has been described, one in which fast oscillations of [Ca2+] result from an ionic mechanism, whereas slow oscillations result from oscillations of metabolism (1). In the dual oscillator model, glucose metabolism generates glycolytic oscillations that are dependent on the activity of the allosterically regulated enzyme phosphofructokinase (21). Pulsatile delivery of pyruvate to the tricarboxylic acid (TCA) cycle leads to oscillations in the cytosolic [ATP]-to-[ADP] ratio, thereby generating oscillations of KATP channel activity, membrane potential, and [Ca2+]. It is important to note, however, that there is also evidence for an intramitochondrial source of oscillatory activity in the β-cell. Exposure of isolated β-cell mitochondria to pyruvate generates oscillations in the levels of TCA cycle intermediates (16, 17), whereas exposure of β-cells to the TCA cycle intermediates α-ketoisocaproate or methyl-pyruvate (a membrane permeable analog of pyruvate) induces oscillations of [Ca2+] in the absence of glucose (10). Thus, it may be that oscillations of [Ca2+] and [cAMP] find their genesis in a complex interplay between ionic, glycolytic, and mitochondrial mechanisms of oscillatory control in β-cells.
Acknowledgments
GRANTS G. G. Holz acknowledges the support of National Institutes of Health (NIH) Grants R01-DK-045817 and R01-DK-069575. E. Heart acknowledges the support of NIH Grant P41-RR-001395 (to P. J. S. Smith). C. A. Leech acknowledges the support of an American Diabetes Association Research Grant Award.
REFERENCES
- 1.Bertram R, Sherman A, Satin LS. Metabolic and electrical oscillations: partners in controlling pulsatile insulin secretion. Am J Physiol Endocrinol Metab. 2007;293:E890–E900. doi: 10.1152/ajpendo.00359.2007. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem J. 2003;375:517–529. doi: 10.1042/BJ20031061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delmeire D, Flamez D, Hinke SA, Cali JJ, Pipeleers D, Schuit F. Type VIII adenylyl cyclase in rat beta cells: coincidence signal detector/generator for glucose and GLP-1. Diabetologia. 2003;46:1383–1393. doi: 10.1007/s00125-003-1203-8. [DOI] [PubMed] [Google Scholar]
- 4.Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide-1 in the pancreas. Pharmacol Ther. 2007;113:546–593. doi: 10.1016/j.pharmthera.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyachok O, Isakov Y, Sagetorp J, Tengholm A. Oscillations of cyclic AMP in hormone-stimulated insulin-secreting beta-cells. Nature. 2006;439:349–352. doi: 10.1038/nature04410. [DOI] [PubMed] [Google Scholar]
- 6.Fridlyand LE, Harbeck MC, Roe MW, Philipson LH. Regulation of cAMP dynamics by Ca2+ and G protein-coupled receptors in the pancreatic β-cell: a computational approach. Am J Physiol Cell Physiol. 2007 Oct 10; doi: 10.1152/ajpcell.00555.2006. doi: 10.1152/ajpcell.00555.2006. [DOI] [PubMed] [Google Scholar]
- 7.Gromada J, Holst JJ, Rorsman P. Cellular regulation of islet hormone secretion by the incretin hormone glucagon-like peptide 1. Pflügers Arch. 1998;435:583–594. doi: 10.1007/s004240050558. [DOI] [PubMed] [Google Scholar]
- 8.Han P, Werber J, Surana M, Fleischer N, Michaeli T. The calcium/ calmodulin-dependent phosphodiesterase PDE1C down-regulates glucose-induced insulin secretion. J Biol Chem. 1999;274:22337–22344. doi: 10.1074/jbc.274.32.22337. [DOI] [PubMed] [Google Scholar]
- 9.Harbeck MC, Chepurny O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. Simultaneous optical measurements of cytosolic Ca2+ and cAMP in single cells. Sci STKE. 2006;16 doi: 10.1126/stke.3532006pl6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heart E, Smith PJ. Rhythm of the β-cell oscillator is not governed by a single regulator: multiple systems contribute to oscillatory behavior. Am J Physiol Endocrinol Metab. 2007;292:E1295–E1300. doi: 10.1152/ajpendo.00648.2006. [DOI] [PubMed] [Google Scholar]
- 11.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 12.Holz GG. New insights concerning the glucose-dependent insulin secretagogue action of glucagon-like peptide-1 in pancreatic beta-cells. Horm Metab Res. 2004;36:787–794. doi: 10.1055/s-2004-826165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holz GG, Habener JF. Signal transduction crosstalk in the endocrine system: pancreatic beta-cells and the glucose competence concept. Trends Biochem Sci. 1992;17:388–393. doi: 10.1016/0968-0004(92)90006-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 15.Landa LR, Jr, Harbeck M, Kaihara K, Chepurny O, Kitiphongspattana K, Graf O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 beta-cell line. J Biol Chem. 2005;280:31294–31302. doi: 10.1074/jbc.M505657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacDonald MJ, Al-Masri H, Jumelle-Laclau M, Cruz MO. Oscillations in activities of enzymes in pancreatic islet subcellular fractions induced by physiological concentrations of effectors. Diabetes. 1997;46:1996–2001. doi: 10.2337/diab.46.12.1996. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald MJ, Fahien LA, Buss JD, Hasan NM, Fallon MJ, Kendrick MA. Citrate oscillates in liver and pancreatic beta cell mitochondria and in INS-1 insulinoma cells. J Biol Chem. 2003;278:51894–51900. doi: 10.1074/jbc.M309038200. [DOI] [PubMed] [Google Scholar]
- 18.Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem. 2004;279:37215–37218. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- 19.Nunemaker CS, Bertram R, Sherman A, Tsaneva-Atanasova K, Daniel CR, Satin LS. Glucose modulates [Ca2+]i oscillations in pancreatic islets via ionic and glycolytic mechanisms. Biophys J. 2006;91:2082–2096. doi: 10.1529/biophysj.106.087296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renstrom E, Eliasson L, Rorsman P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J Physiol. 1997;502:105–118. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richard AM, Webb DL, Goodman JM, Schultz V, Flanagan JN, Getty-Kaushik L, Deeney JT, Yaney GC, Dunaway GA, Berggren PO, Tornheim K. Tissue-dependent loss of phosphofructokinase-M in mice with interrupted activity of the distal promoter: impairment in insulin secretion. Am J Physiol Endocrinol Metab. 2007;293:E794–E801. doi: 10.1152/ajpendo.00168.2007. [DOI] [PubMed] [Google Scholar]
- 22.Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci USA. 1992;89:8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wayman GA, Impey S, Wu Z, Kindsvogel W, Prichard L, Storm DR. Synergistic activation of the type I adenylyl cyclase by Ca2+ and Gs-coupled receptors in vivo. J Biol Chem. 1994;269:25400–25405. [PubMed] [Google Scholar]
- 24.Weir GC, Mojsov S, Hendrick GK, Habener JF. Glucagon-like peptide-1 (7–37) actions on endocrine pancreas. Diabetes. 1989;38:338–342. doi: 10.2337/diab.38.3.338. [DOI] [PubMed] [Google Scholar]
- 25.Yu X, Byrne JH, Baxter DA. Modeling interactions between electrical activity and second-messenger cascades in Aplysia neuron R15. J Neurophysiol. 2004;91:2297–2311. doi: 10.1152/jn.00787.2003. [DOI] [PubMed] [Google Scholar]