Abstract
1,3-Butadiene (BD) is a known rodent and human carcinogen that is metabolized mainly by P450 2E1 to three epoxides, 1,2-epoxy-3-butene (EB), 1,2:3,4-diepoxybutane (DEB) and 1,2-epoxy-3,4-butanediol (EB-diol). The individual epoxides vary up to 200-fold in their mutagenic potency, with DEB being the most mutagenic metabolite. It is important to understand the internal formation of the individual epoxides to assign the relative risk for each metabolite and to understand the molecular mechanisms responsible for major species differences in carcinogenicity. We have conducted extensive exposure-biomarker studies on mice, rats and humans. Using low exposures that range from current occupational levels to human exposures from tobacco smoke has provided evidence that mice are very different from humans, with mice forming ~200 times more DEB than humans at exposures of 0.1–1.5 ppm BD. While no gender differences have been noted in mice and rats for globin adducts or N-7 guanine adducts, female rats and mice had 2–3-fold higher Hprt mutations and DNA-DNA cross-links, suggesting a gender difference in DNA repair. Numerous molecular epidemiology studies have evaluated globin adducts and Hprt mutations, SCEs and chromosomal abnormalities. None of the blinded studies have shown evidence of human genotoxicity at current occupational exposures and studies of globin adducts have shown similar or lower formation of adducts in females than males. If one calculates the EB dose-equivalents for the three species, mice clearly differ from rats and humans, being ~44 and 174 times greater than rats and humans, respectively. These data provide a scientific basis for improved risk assessment of BD.
Keywords: butadiene, globin adducts, species comparisons, low exposures, metabolism, EB-equivalents
1. Introduction
1,3-Butadiene (BD)1 is an important industrial chemical widely used for synthetic rubber and resin production [1]. It is also found in gasoline, motor vehicle exhaust and cigarette smoke [2;3], including being rated the cigarette constituent with the highest cancer risk index [4]. BD is classified as a human carcinogen following inhalation exposure by the EPA [5] and IARC [6]. This classification was based on epidemiologic studies of exposed workers in the butadiene rubber industry and laboratory animal data. Human epidemiologic studies of occupationally exposed workers have presented evidence for an increased incidence of leukemia, lymphatic and hematopoietic cancer, compared with unexposed controls [6–8]. BD has been shown to be a potent carcinogen in mice, inducing neoplasms at multiple sites. When rats and mice were exposed to high concentrations, rats were less susceptible. Furthermore, mice developed cancer at exposures 160 times lower than rats. An important caveat, however, is that lower exposures to rats have not been evaluated for carcinogenicity [1]. A gender difference in susceptibility was also demonstrated in mice, with female mice developing tumors at lower exposures than males. To date, the complexity of BD carcinogenesis largely has been attributed to species-dependent differences in BD metabolism.
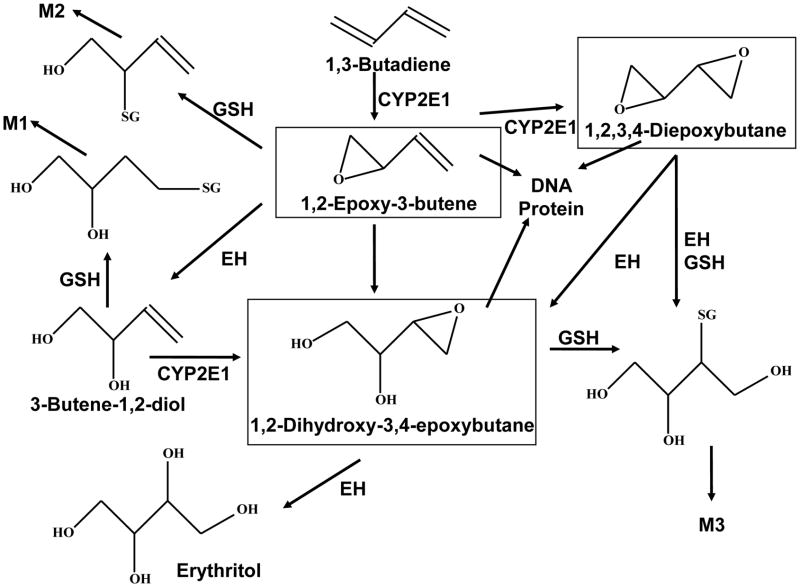
Bioactivation to yield reactive epoxides of BD in animal and human tissues is proposed to be an important determinant of the mutagenic and carcinogenic effects of BD [9]. BD is metabolized by cytochrome P450, forming reactive epoxides: 1,2-epoxybutene (EB), 1,2:3,4-diepoxybutane (DEB) and 3,4-epoxy-1,2-butanediol (EB-diol) [1] that are known to bind to DNA and proteins. A simplified metabolic scheme for BD is shown in Scheme 1. Of critical importance is the fact that BD derived epoxides differ in their mutagenic potencies by up to 200-fold, with DEB being the most potent mutagenic metabolite due to its capacity as a bifunctional electrophile [9–12].
Scheme 1. Metabolism of BD.
Showing enzymes involved in BD metabolism and the major detoxification metabolites excreted in urine (M1, M2, and M3).
Species and gender differences related to BD metabolism can be examined by measuring suitable biomarkers. Protein adducts have been widely used as surrogate biomarkers for exposure. Measurement of protein adducts provides useful information on exposure-specific internal formation of individual epoxide metabolites. In contrast to DNA adducts, protein adducts are not repaired. The three main globin adducts: N-(2-hydroxy-3-buten-1-yl)-valine (HB-Val), N,N-(2,3-dihydroxy-1,4-butadiyl)-valine (pyr-Val) and 1,2,3-trihydroxybutyl-valine (THB-Val) are produced by EB, DEB and EB-diol. The latter adduct can also be formed in small amounts from binding of DEB, followed by hydrolysis of the remaining epoxide. Furthermore, THB-Val has been demonstrated in unexposed animals and humans from endogenous sources [13]. Earlier studies utilized Edman chemistry, followed by GS/MS analysis, however, this method could not be used for pyr-Val. More recently, a proteomics approach has been established for the analysis of globin adducts in rodents and humans [14;15]. This method utilizes trypsin hydrolysis and immunoaffinity purification prior to quantitation of the N-terminal peptide adducts by LC-MS/MS.
Integration of these data can provide information relevant to understanding species differences in mutagenic and carcinogenic potency of BD and its epoxide metabolites by using an EB-dose equivalent [16]. Conversion of individual hemoglobin adduct values into EB-equivalents is calculated on the basis of the combined internal dose of the three globin adducts and the relative genotoxic potency of the respective epoxides, as inferred from their efficiency of inducing mutations at the Hprt locus. Using EB as the reference, the relative mutagenic potency was suggested to be 1: 32: 0.21 for EB, DEB, and EB-diol, respectively [16]. We will compare the biomarkers of exposure (hemoglobin adducts) and biomarkers of effect (mutations) across species using a similar approach for EB equivalents, but with slightly different numerical values.
1.1 BD Epoxides and DNA
DNA adduct formation, mutagenesis and gene expression can provide translational information on similarities and differences in animal-human carcinogenic potential. The molecular dose and tissue distribution provides insight into differences in metabolic activation, epoxide stability, mutagenic efficiency and those mechanisms of repair/loss that are involved in BD-induced DNA lesions. DEB is formed in much smaller amounts than EB-diol, but is the most mutagenic and genotoxic of all three epoxides. DEB is ~100 times more mutagenic than EB and ~200–500 times more mutagenic than EB-diol. All three butadiene epoxides (EB, DEB, EB-diol) react with DNA and globin to form covalent adducts. The DNA adducts can either be monoadducts (EB and EB-diol), or DNA-DNA, or DNA-protein cross-links (DEB).
The primary BD monoadducts include N-7-(2-hydroxy-3-buten-1-yl)guanine (HB-G I) and N-7-(1-hydroxy-3-buten-2-yl)guanine (HB-G II), and N-7-(2,3,4-trihydroxy-3-buten-2-yl)guanine (THB-G). HB-G adducts are formed by the reaction of EB with the N7 position of guanine. EB-diol reacts with N7 of guanine to form THB Gua stereoisomers [17;18]. The third epoxide, DEB, consists of three epoxide stereoisomers (R,R, S,S and meso). All of the stereoisomers can form N7-G monoadducts, which can go on to form BD DNA-DNA and DNA-protein cross-links, or be hydrolyzed to THB-guanine. The N7 DEB adducts will produce different adducts depending if they react with another guanine in the N7 position or an adenine in the N1 position. N7G-N7G-BD adducts are: racemic bis-N7G-BD (S,S and R,R) or meso-bis-N7G-BD. The N7G-N1A-BD can rearrange to N7G-N6A-BD.
Like globin adducts, BD derived DNA adducts are biomarkers of exposure that can be used to determine exposure-responses for metabolic activation resulting in the formation of adducts from EB, EBD and DEB. In contrast to globin adducts, DNA adducts undergo DNA repair and loss from chemical depurination, so there are less consistent half-lives to model exposure. The N7G monoadducts have been considered non-promutagenic due to their chemical instability and the fact that the N7 position does not participate in Watson Crick base pairing [19]. They are lost from DNA primarily by chemical depurination, with a half-life of ~4 days. This results in the formation of abasic sites, which if not repaired, can result in mutations. However, Meng et al. [10] has shown the 2R,3S isomer is 30-fold more cytotoxic and mutagenic than the other three forms of EB-diol. DNA-DNA cross-links have different half-lives, with intra-strand bis-N7G-BD cross-links being 35h, while the inter-strand bis-N7G-BD adducts are 147h. Interstrand cross-links are the most toxic because they affect both DNA strands, preventing DNA transcription, replication, and repair.
2. Materials and methods
2.1 Animals and Human subjects
The rodent exposures and globin adduct measurements were done as previously reported [15]. Tissues for DNA adducts were harvested and snap-frozen for storage at − 80°C until DNA and globin extraction [20;21]. The human data came from a previously reported study on 83 male workers from the Czech Republic [22].
2.2 Biomarkers
DNA isolation and hydrolysis and quantitation were performed as described previously with some modifications in extraction [20;21]. HPRT mutations were measured in both rodents and occupationally exposed workers as previously described [9;22–25]. Amounts of BD epoxide globin adducts were analyzed based on the immunoaffinity nano-UPLC-MS/MS or GC/HRMS methods as described previously [15;22].
The internal adduct dose (D; in nMh·ppmh−1) was calculated from the measured Hb adducts level (A; in mol·g−1). The reaction rate constant of the electrophilic agent towards the nucleophile is known as kval in [L·g−1·h−1]. In this study, the formation of adducts to N-terminal valine in Hb measured shortly after exposure was used in Equation 1.
| (Equ. 1) |
The rate constant for the reaction of the epoxy metabolites of BD were used as reported [16]. The different values of kval used in calculations for each epoxide are as follows: EB 4.0×10−5 L·g−1·h−1, DEB 5.5×10−5 L·g−1·h−1 and EB-diol 2.3×10−5 L·g−1·h−1 [16].
In a study using human TK6 cells it was shown that EB, DEB, and EB-diol were effective at inducing mutations in Hprt loci. Meng et al. [25] has reported the initial treatment concentration of individual epoxides inducing a doubling of the mutation frequency were 200 μM, 2 μM, and 1,000 μM for EB, DEB and EB-diol, respectively. We used this reference as our estimate for the relative mutagenic potencies, with EB as a reference having a value of 1, DEB having a value of 100 and EB-diol having a value of 0.2. The EB dose equivalent was then calculated by multiplying the internal hemoglobin adduct dose (D) of the respective epoxide with the corresponding mutagenic potency (Equation 2).
| (Equ. 2) |
Equation 2 is expressed as EB dose equivalents (nMh) per exposure dose of BD (ppmh).
3. Results and Discussion
3.1 DNA adducts and BD exposures
THB-G adducts are clearly the most abundant DNA BD monoadducts measured in mice and rats, followed by HBG I and II, which are 10 to 20 times lower. The total number of DNA-DNA cross-links measured in rodents is similar to that of HB-G adducts, with racemic bis-N7G-BD (both S,S and R,R stereoisomers) followed by meso-N7G-BD and lastly, N7G-N1A-BD/N7G-N6A-BD being 100 times less abundant than the previous two cross-links. The tissue forming the highest abundance of adducts is liver, with other tissues having 1/3 to 1/10 as many. Mice had the highest adduct abundance for both monoadducts and DNA-DNA cross-links. For monoadducts, both THB-G and HB-G I and II showed no statistically significant differences between males and females. On the other hand, DNA-DNA cross-links were 2–2.5 times higher for racemic bis-N7G-BD and 3 times higher meso-N7G-BD when comparing female to male adducts in liver following exposures to 625 ppm BD.
Using present methods, the lowest exposure with detectability of a DNA adduct was THB-G in mice exposed to 0.5 ppm, followed by HB-Gua and bis-N7G-BD at 6.25 ppm. The rat exhibited saturation of metabolic activation for THB-G, bis-N7G-BD and N7G-N6A-BD at 62.5 ppm [20;21]. In contrast, while these higher exposures formed lesser amounts of adducts per ppm BD than low exposures in mice, saturation of metabolic activation and DNA adduct formation has not been observed in mice (Troutman et. al., unpublished data).
3.2 Hemoglobin adducts, EB dose equivalent and BD exposure
The amounts of BD-derived epoxide globin adducts formed in rodents at low and high exposures (0.5–625 ppm) are presented in Table 1 and EB dose equivalents for mice, rats and humans are presented in Table 2. The globin adduct data provide valuable insight for cross-species comparisons at low exposures and represents the most comprehensive data set available for the three species. The amounts of all three epoxide adducts increased with exposure in the mouse, rat and human (human data not shown) [15;22]. Within each species, the amount of HB-Val and pyr-Val were similar. Based on the EB dose-equivalent, the highest dose per ppm exposure was observed at sub-part per million exposures. This demonstrates that metabolism of BD to epoxides is most effective at low exposures. EB and DEB are formed in amounts roughly similar to each other in the rat and in the mouse, however, mice make 10–30 fold greater amounts of the epoxides than rats (Table 1).
Table 1.
Hemoglobin adducts from rats and mice exposed to butadiene for 10 days
| B6C3F1 Mice (pmol/g globin) | F344 Rats (pmol/g globin) | |||||
|---|---|---|---|---|---|---|
| Dose (ppm) | HB-Val | Pyr- Val | THB-Vala | HB-Val | Pyr- Val | THB-Vala |
| 0.5 | 18.8 ± 6.4 | 23.2 ± 2.5 | 157.4 ± 23.0 | 0.9 ± 0.1 | 0.8 ± 0.1 | 2.4 ± 0.3 |
| 1 | 21.5 ± 4.0 | 66.8 ± 5.9 | 525 ± 307 | 1.6 ± 0.5 | 1.4 ± 0.2 | 29 ± 3 |
| 1.5 | 26.5 ± 9.2 | 82.3 ± 6.5 | 633 ± 108 | 2.5 ± 0.4 | 2.0 ± 0.5 | 59 ± 6 |
| 6.25 | 71.4 ± 4.5 | 272 ± 17 | 1043 ± 259 | 8.1 ± 0.7 | 6.7 ± 0.7 | 220 ± 19 |
| 62.5 | 430 ± 70 | 1632 ± 283 | 1155 ± 126 | 60.0 ± 3.0 | 46.5 ± 8.3 | 481 ± 53 |
| 200 | N/A | N/A | N/A | 148 ± 19 | 123 ± 8 | 606 ± 75 |
| 625 | N/A | N/A | N/A | 410 ± 15 | 124 ± 11 | 737 ± 74 |
N/A: Not available
Endogenous THB-Val was subtracted in all group using data from control animal; 30.0 pmol/g and 36.8 pmol/g globin in rats
Table 2.
EB equivalents in humans, rats and mice exposed to butadiene.
| Species / Duration | BD Exposure | Doses in blood nMh/ppmh | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| ppm | ppmh | EB | DEB | EBdiola | EB-equiv. | |
|
| ||||||
| 2 Month Human (Czech I), 8 hr/day | 0.29 | 92.8 | 0.06 | 0.03 | 19.8 | 10 |
| 0.81 | 259.2 | 0.11 | 0.02 | 52.4 | 13 | |
|
| ||||||
| 10 days Female Rats (F344) | 0.5 | 30 | 0.73 | 0.48 | 3.5 | 49 |
| 1 | 60 | 0.67 | 0.42 | 21.2 | 47 | |
| 1.5 | 90 | 0.64 | 0.40 | 28.3 | 46 | |
| 6.25 | 375 | 0.54 | 0.32 | 26.7 | 38 | |
|
| ||||||
| 10 days Male Rats (F344) | 0.5 | 30 | 0.67 | 0.42 | 3 | 46 |
| 1.5 | 90 | 0.63 | 0.40 | 20 | 43 | |
|
| ||||||
| 10 days Female Mice (B6C3F1) | 0.5 | 30 | 22 | 16 | 250 | 1672 |
| 1 | 60 | 12 | 24 | 416 | 2495 | |
| 1.5 | 90 | 10 | 19 | 334 | 1977 | |
| 6.25 | 375 | 7 | 15 | 132 | 1533 | |
In human studies, the average exposures were 0.29 ppm and 0.81 ppm in the monomer workers and polymer workers, respectively [22]. In addition, the period of exposure was 5.3 times longer than the rodents. The internal adducts dose have been adjusted with regard to the duration of exposure and the disappearance of adducts due to the life span of erythrocytes (ter). For species differences in this study, ter is 45 days for mouse, 63 days for rat, and 126 days for human. After adjustement, the EB dose-equivalent in humans was 4 times lower than rats: 13 nMh/ppmh in humans and 47 nMh/ppmh in rats (Table 2). Comparing the EB dose-equivalents for the three species, mice clearly differ from rats and humans, being ~50–200 times greater at similar exposures with significant differences in all three biomarkers. Furthermore, it demonstrates that DEB contributes little to the human EB-equivalent, being ~20-fold lower than the rat. Using the EB-equivalent, EB-diol presents the greatest risk for mutations in humans. This provides important information relevant to susceptibility issues and species differences for BD.
3.3 Gene and chromosomal mutations
Research has shown that BD is genotoxic in rodents. Differences in Hprt Mf were significantly higher in both species when exposed to 1250 ppm BD for 2 weeks compared to controls (3.5 fold, rats/control) [9]. The lowest exposure to BD that produced a significant increase in the Hprt Mf was 3 ppm in mice and 62.5 ppm in rats, representing an 8.5 fold lower exposure [9]. Three blinded human epidemiological studies evaluated Hprt mutations in exposed workers, but failed to find any increase in Hprt Mf [22;23;26]. It must be remembered, however, that the human exposures in these studies were lower than the exposures in the positive mouse or rat studies.
It is of interest, however, that recent in vitro studies with TK6 cells exposed to nine different stereochemical configurations of BD epoxides and using cloning assays with multiplex PCR analysis for both Hprt and TK genes concluded that (2R,3S)-EB-diol was the most mutagenic monoepoxide stereoisomer, being 30 times more mutagenic than the other 3 forms of EB-diol and 5–10-fold less mutagenic than DEB [25]. The only stereospecific studies on the DNA adducts of EB-diol were reported by Kovisto et al. [17;18].
3.4 Gender differences
Multiple BD studies have revealed gender differences in the development of neoplasia, Hprt and amounts of DNA-DNA cross-links measured. Mutagenicity studies measuring BD-induced Hprt gene mutations in mice and rats revealed gender differences in sensitivity, with rat and mouse females having 2.3–3 fold greater Hprt Mfs than their male counterparts [9;10]. Furthermore, long-term BD inhalation studies in B6C3F1 mice demonstrated male and female mice formed tumors in similar tissues, but for the most part, females developed tumors at lower exposure concentrations. For example, lung and liver tumors in female mice were observed at exposure concentrations of 6.25 and 20 ppm BD, respectively. By comparison, the respective exposure concentrations of BD that produced lung and liver tumors in male mice were 62.5 and 200 ppm, which represents a 10-fold increase in exposure concentration [8]. Female rats and mice had more bis-N7G-BD, racemic and meso DNA-DNA cross-links than males at high exposures (200 ppm and 625 ppm) [21]. However, a recent study of globin adducts showed no significant gender differences at the exposure concentrations studied ([15], data in Table 1). In addition, there were no gender differences in the amount of N-7 guanine monoadducts in mice. Pyr-Val data and HB-G and THB-G indicate that there is no difference in BD metabolism in female and male rats and mice at multiple exposure concentrations and durations. This comprehensive data set strongly suggests that a deficiency in DNA cross-link repair in females is the most plausible hypothesis for the gender differences in mutagenic and carcinogenic susceptibility. Unpublished preliminary results from our lab demonstrated that homologous recombination and nucleotide excision repair are critical pathways for DEB, while defects in mismatch repair or non-homologous end joining in DT-40 cells showed no difference from wild type.
The observations in rodents differ from findings in humans, however, in that the molecular epidemiology study comparing male and female workers exposed to BD showed similar or lower formation of adducts in females ([24] and unpublished data). Data on globin adducts (pyr-Val and THB-Val) suggest that human males metabolize BD to a greater extent than females. The blinded studies in humans have shown no significant differences in gene mutation (Hprt Mfs) or sister chromatid exchanges (SCEs) between male and female workers [22;23;26].
3.5 Application to risk assessment
The data presented suggest that species differences observed in BD-induced cancer are directly related to differences in the extent of BD metabolism to its epoxides. Examination of the EB dose equivalent for all three species revealed that humans showed the lowest amount of EB-equivalent per ppm exposure for BD metabolite formation. In contrast, mice clearly differed, being ~50 times greater than rats and 200-fold higher than humans using calculated data on the basis of internal dose and relative mutagenic potency [described in Materials and Methods]. Based on mutagenicity and DNA cross-links, DEB appears to be a primary metabolite resulting in genotoxicity. However, the new mutagenicity data of Meng et al [25] on stereoisomers of EB-diol suggest that it is important not to lose sight of the amount of THB-Val in all three species. Furthermore, it appears that the rat is a more representative animal model to study human effects following BD exposure than is the mouse. It is unfortunate that only one high exposure carcinogenicity study has been conducted in the rat at 0, 1000 or 8000 ppm BD, while numerous studies have focused on the mouse, a species that markedly differs from humans.
DEB-mediated DNA-protein cross-link (DPC) formation have been observed in human cervical carcinoma cells [27]. This observation positively identified DPC between cysteine thiols within proteins and the N-7 guanine positions within DNA. Similar studies have not been reported in rodent or human tissues, but it is likely that they will form proportionate to DEB formation. The formation of these bulky, helix-distorting lesions would have the potential to interfere with the critical cellular process such as replication, transcription, and triggering programmed cell death or inducing genotoxicity.
Nevertheless, the comprehensive data reviewed here demonstrates the strength of Mode of Action (MOA) research in clarifying the relevance of animal models of disease and improving the scientific basis of risk assessment. Current occupational exposures are much lower than 30–70 years ago. It is also lower than human exposure from cigarette smoke. The human studies reported here cover 83 individuals. Additional data from two other molecular epidemiology studies are being evaluated that will bring the total up to over 300 individuals. Relative to the human population, this represents a very small proportion. Therefore, we cannot state with confidence that more susceptible individuals do not exist. Nevertheless, major species differences are clear for the complex metabolism of BD and these differences do impact risk for the general human population.
Additional research will be necessary to identify the mechanism(s) responsible for gender differences in mutagenesis and cancer sensitivity, although differences in DNA repair are strongly suggested. Future risk assessment can benefit from accurate studies on mutagenesis at low exposures, as this MOA is likely to drive the biology leading to the induction of cancer in all species. It may well explain human effects of past high exposures. In addition, however, it may suggest minimal concern for current levels of occupational exposure if based on high quality scientific information, rather than relying on default assumptions.
Acknowledgments
This research was supported in part by grants from the NIH (1 R01 ES012689, 5 P30-ES10126), the Health Effects Institute (agreements 99-5 and 05-12) and the American Chemistry Council.
Footnotes
Abbreviations: BD, 1,3-butadiene; EB, 1,2-epoxybutene; DEB, 1,2:3,4-diepoxybutane; HB-Val, N-(2-hydroxy-3-buten-1-yl)-valine; pyr-Val, N,N-(2,3-dihydroxy-1,4-butadiyl)-valine; THB-Val, 1,2,3-trihydroxybutyl-valine; HB-G I, N-7-(2-hydroxy-3-buten-1-yl)guanine; HB-GII N-7-(1-hydroxy-3-buten-2-yl)guanine; THB-G, N-7-(2,3,4-trihydroxy-3-buten-2-yl)guanine; bis-N7G-BD, 1,4-bis-(guan-7-yl)-2,3-butanediol; N7G-N1A-BD, 1-(guan-7yl)-4-(aden-1-yl)-2,3-butanediol; N7G-N6A-BD, 1-(guan-7yl)-4-(aden-6-yl)-2,3-butanediol; M1, 1,2-dihydroxy-4-(N-acetylcysteinyl)-butane; M2, 1-hydroxy-2-(N-acetylcysteinyl)-3-butane; M3, 1,3,4-trihydroxy-2-(N-acetylcysteinyl)-butane.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Himmelstein MW, Acquavella JF, Recio L, Medinsky MA, Bond JA. Toxicology and epidemiology of 1,3-butadiene. Crit Rev Toxicol. 1997;27:1–108. doi: 10.3109/10408449709037482. [DOI] [PubMed] [Google Scholar]
- 2.Agency for Toxic Substances and Disease Registry (ATSDR) Draft toxicological profile for 1,3-butadiene. U.S. Department of Health and Human Services; Atlanta, Georgia: 2009. [Google Scholar]
- 3.Morrow NL. Significance of 1,3-butadiene to the US air toxics regulatory effort. Chem -Biol Interact. 2001;135–136:137–43. doi: 10.1016/s0009-2797(01)00174-0. [DOI] [PubMed] [Google Scholar]
- 4.Törnqvist M, Fred C, Haglund J, Helleberg H, Paulsson B, Rydberg P. Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. J Chromatog B. 2002;778:279–308. doi: 10.1016/s1570-0232(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 5.US Environmental Protection Agency. Health Assessment of 1,3-Butadiene. National Center for Environmental Assessment; Washington, DC: 2002. [Google Scholar]
- 6.IARC. 1,3-Butadiene, Ethylene Oxide and Vinyl Halides (Vinyl Fluoride, Vinyl Chloride and Vinyl Bromide) International Agency for Research on Cancer; Lyons, France: 2008. [Google Scholar]
- 7.Santos-Burgoa C, Matanoski GM, Zeger S, Schwartz L. Lymphohematopoietic cancer in styrene-butadiene polymerization workers. Am J Epidemiol. 1992;136:843–54. doi: 10.1093/aje/136.7.843. [DOI] [PubMed] [Google Scholar]
- 8.National Toxicology Program. Report on Carcinogens. U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program; Washington, D.C: 2005. [Google Scholar]
- 9.Walker VE, Walker DM, Meng Q, McDonald JD, Scott BR, Bauer MJ, et al. Genotoxicity of 1,3-butadiene and its epoxy intermediates. Health Effects Institute, Capital City Press; Montpelier, VT: 2009. [PubMed] [Google Scholar]
- 10.Meng Q, Redetzke DL, Hackfeld LC, Hodge RP, Walker DM, Walker VE. Mutagenicity of stereochemical configurations of 1,2-epoxybutene and 1,2:3,4-diepoxybutane in human lymphblastoid cells. Chem -Biol Interact. 2007;166:207–18. doi: 10.1016/j.cbi.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Cochrane JE, Skopek TR. Mutagenicity of butadiene and its epoxide metabolites: I. Mutagenic potential of 1,2-epoxybutene, 1,2,3,4-diepoxybutane and 3,4-epoxy-1,2-butanediol in cultured human lymphoblasts. Carcinogenesis. 1994;15:713–7. doi: 10.1093/carcin/15.4.713. [DOI] [PubMed] [Google Scholar]
- 12.Cochrane JE, Skopek TR. Mutagenicity of butadiene and its epoxide metabolites: II. Mutational spectra of butadiene, 1,2-epoxybutene and diepoxybutane at the hprt locus in splenic T cells from exposed B6C3F1 mice. Carcinogenesis. 1994;15:719–23. doi: 10.1093/carcin/15.4.719. [DOI] [PubMed] [Google Scholar]
- 13.Himmelstein MW, Turner MJ, Asgharian B, Bond JA. Comparison of blood concentrations of 1,3-butadiene and butadiene epoxides in mice and rats exposed to 1,3-butadiene by inhalation. Carcinogenesis. 1994;15:1479–86. doi: 10.1093/carcin/15.8.1479. [DOI] [PubMed] [Google Scholar]
- 14.Boysen G, Georgieva NI, Upton PB, Jayaraj K, Li Y, Walker VE, et al. Analysis of diepoxide-specific cyclic N-terminal globin adducts in mice and rats after inhalation exposure to 1,3-butadiene. Cancer Res. 2004;64:8517–20. doi: 10.1158/0008-5472.CAN-04-3184. [DOI] [PubMed] [Google Scholar]
- 15.Georgieva NI, Boysen G, Bordeerat N, Walker VE, Swenberg JA. Exposure-response of 1,2:3,4-diepoxybutane-specific N-terminal valine adducts in mice and rats after inhalation exposure to 1,3-butadiene. Toxicol Sci. 2010;115:322–9. doi: 10.1093/toxsci/kfq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fred C, Törnqvist M, Granath F. Evaluation of cancer tests of 1,3-butadiene using internal dose, genotoxic potency, and a multiplicative risk model. Cancer Res. 2008;68:8014–21. doi: 10.1158/0008-5472.CAN-08-0334. [DOI] [PubMed] [Google Scholar]
- 17.Koivisto P, Sorsa M, Pacchierotti F, Peltonen K. 32P-Postlabelling/HPLC assay reveals an enantioselective adduct formation in N7 guanine residues in vivo after 1,3-butadiene exposure. Carcinogenesis. 1997;18:439–43. doi: 10.1093/carcin/18.2.439. [DOI] [PubMed] [Google Scholar]
- 18.Koivisto P, Kilpelainen I, Rasanen I, Adler ID, Pacchierotti F, Peltonen K. Butadiene diolepoxide- and diepoxybutane-derived DNA adducts at N7-guanine: a high occurrence of diolepoxide-derived adducts in mouse lung after 1,3-butadiene exposure. Carcinogenesis. 1999;20:1253–9. doi: 10.1093/carcin/20.7.1253. [DOI] [PubMed] [Google Scholar]
- 19.Boysen G, Pachkowski BF, Nakamura J, Swenberg JA. The formation and biological significance of N7-guanine adducts. Mutat Res. 2009;678:76–94. doi: 10.1016/j.mrgentox.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koc H, Tretyakova NY, Walker VE, Henderson RF, Swenberg JA. Molecular dosimetry of N-7 guanine adduct formation in mice and rats exposed to 1,3-butadiene. Chem Res Toxicol. 1999;12:566–74. doi: 10.1021/tx980265f. [DOI] [PubMed] [Google Scholar]
- 21.Goggin M, Swenberg JA, Walker VE, Tretyakova N. Molecular dosimetry of 1,2,3,4-diepoxybutane-induced DNA-DNA cross-links in B6C3F1 mice and F344 rats exposed to 1,3-butadiene by inhalation. Cancer Res. 2009;69:2479–86. doi: 10.1158/0008-5472.CAN-08-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albertini RJ, Srám RJ, Vacek PM, Lynch J, Nicklas JA, Van Sittert NJ, et al. Biomarkers in Czech workers exposed to 1,3-butadiene: a transitional epidemiologic study. Health Effects Institute, Capital City Press; Montpelier, VT: 2003. [PubMed] [Google Scholar]
- 23.Albertini RJ, Sram RJ, Vacek PM, Lynch J, Rossner P, Nicklas JA, et al. Molecular epidemiological studies in 1,3-butadiene exposed Czech workers: Female-male comparisons. Chem -Biol Interact. 2007;166:63–77. doi: 10.1016/j.cbi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Vacek PM, Albertini RJ, Sram RJ, Upton P, Swenberg JA. Hemoglobin adducts in 1,3-butadiene exposed Czech workers: Female-male comparisons. Chem Biol Interact. 2010 doi: 10.1016/j.cbi.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Meng RQ, Hackfeld LC, Hodge RP, Wisse LA, Redetzke DL, Walker VE. Mutagenicity of stereochemical configurations of 1,3-butadiene epoxy metabolites in human cells. Health Effects Institute; Cambridge, MA: 2010. [PubMed] [Google Scholar]
- 26.Hayes RB, Zhang L, Yin S, Swenberg JA, Xi L, Wiencke J, et al. Genotoxic markers among butadiene polymer workers in China. Carcinogenesis. 2000;21:55–62. doi: 10.1093/carcin/21.1.55. [DOI] [PubMed] [Google Scholar]
- 27.Michaelson-Richie ED, Loeber RL, Codreanu SG, Ming X, Liebler DC, Campbell C, et al. DNA–protein cross-linking by 1,2,3,4-diepoxybutane. Journal of Proteome Research. 2010;9:4356–67. doi: 10.1021/pr1000835. [DOI] [PMC free article] [PubMed] [Google Scholar]