Abstract
Pursuit of a closed-loop artificial pancreas that automatically controls the blood glucose of individuals with type 1 diabetes has intensified during the past six years. Here we discuss the recent progress and challenges in the major steps towards a closed-loop system. Continuous insulin infusion pumps have been widely available for over two decades, but “smart pump” technology has made the devices easier to use and more powerful. Continuous glucose monitoring (CGM) technology has improved and the devices are more widely available. A number of approaches are currently under study for fully closed-loop systems; most manipulate only insulin, while others manipulate insulin and glucagon. Algorithms include on-off (for prevention of overnight hypoglycemia), proportional-integral-derivative (PID), model predictive control (MPC) and fuzzy logic based learning control. Meals cause a major “disturbance” to blood glucose, and we discuss techniques that our group has developed to predict when a meal is likely to be consumed and its effect. We further examine both physiology and device-related challenges, including insulin infusion set failure and sensor signal attenuation. Finally, we discuss the next steps required to make a closed-loop artificial pancreas a commercial reality.
I. Background
The alpha and beta cells of the pancreas of a healthy individual regulate the blood glucose concentration to around 80 mg/dL. When the concentration is high, insulin is secreted by the beta cells and when the concentration is low, glucagon is secreted by the alpha cells. Individuals with Type 1 diabetes mellitus (T1DM) no longer produce insulin and, therefore, these individuals must inject insulin to regulate their blood glucose concentration. Although diabetes mellitus has been diagnosed for over 3000 years (Zajac et al., 2010) no medical treatment was possible until the discovery of insulin by Banting and Best in 1921, and the first injection of insulin into a human patient in 1922 (see Hirsch, 2004, for a concise history of insulin).
The importance of tight blood glucose control was not fully appreciated until the results of the Diabetes Control and Complications Trial (DCCT) were published in 1993. The DCCT involved a comparison of conventional therapy (one or two daily insulin injections, and daily monitoring of blood glucose or urine) with intensive insulin therapy (multiple daily injections or an insulin pump, and blood glucose measured at least 4 times per day, with daily adjustments to the insulin), and concluded that intensive therapy resulted in lower mean blood glucose values and significantly reduced complications (retinopathy, nephropathy, and macrovascular disease). The risk of complications is directly related to glycated hemoglobin, known as A1c; further, the A1c is related to the mean blood glucose values during the previous 2–3 months.
Efforts to develop a closed-loop artificial pancreas (AP) have been on-going more than 60 years. Kadish (1964) developed a system to sample blood to measure glucose concentration and deliver insulin and glucose intravenously, using an on-off controller to maintain glucose between 50 and 150 mg/dl. Bequette (2005) reviews other early AP algorithms, including the nonlinear strategy used by the Biostator (Clemens, 1979), a bedside device that was produced by Miles Laboratories.
In this paper the emphasis is on subcutaneous delivery of rapid-acting insulin using external continuous insulin infusion pumps, and continuous glucose monitors (sensors) that output a signal that is related to the interstitial glucose (just beneath the skin) and therefore an indicator of the capillary blood glucose concentration. It should be noted that research continues on the use of implantable sensors and pumps and alternative delivery routes such as the intraperitoneal cavity (Renard et al., 2010).
A large number of simulation-based studies, proposing many different algorithms, for a closed-loop AP have been published. The focus of this review is on articles and approaches that we expect to be applied in clinical studies in a relatively short term. In particular, we concentrate on projects related to the Juvenile Diabetes Research Foundation (JDRF) Artificial Pancreas Program (Kowalski, 2009) and the European AP@Home project (Heinemann et al., 2011). While many of the papers are based on simulations, we emphasize publications that involve a medical collaborator, again with a realistic plan of clinical implementation. Other recent AP reviews include Bequette (2005), Doyle et al. (2007), Hovorka (2008), Kumareswaran et al. (2009), El-Youssef et al. (2009), Cobelli et al. (2009), Harvey et al. (2010), Hovorka (2011) and Cobelli et al. (2011). Doyle (2011) presents the artificial pancreas as one of the grand challenges for control. Sherr et al. (2009) provide a broad overview of treatment options for T1DM, including disease prevention and immune suppressants. Moser et al. (2012) review insulin analogs and oral medications, in addition to devices such as insulin pens, pumps and CGMs.
Kowalski (2009) provides a roadmap to a closed-loop artificial pancreas, that includes six stages of automation: (i) pump shut-off to avoid hypoglycemia, (ii) a predictive hypoglycemia minimizing system, (iii) a system that controls glucose between low and high glucose limits (often called “control-to-range”), (iv) overnight control to a desired glucose setpoint, (v) fully closed-loop control using insulin, and, (vi) fully closed-loop control using both insulin and glucagon. Kowalski also reviews some challenges to be addressed, and suggests an ambitious timeframe for the staged implementation of various closed-loop systems. In the United States, the devices proposed by Kowalski are regulated by the Food and Drug Administration (FDA). Clinical studies require an investigational device exemption (IDE), and commercial applications require a pre-market approval (PMA). Pinkos et al. (2007) note that one of the critical path initiatives of the FDA is development and availability of a closed-loop artificial pancreas.
II. Overview of the technology and challenges
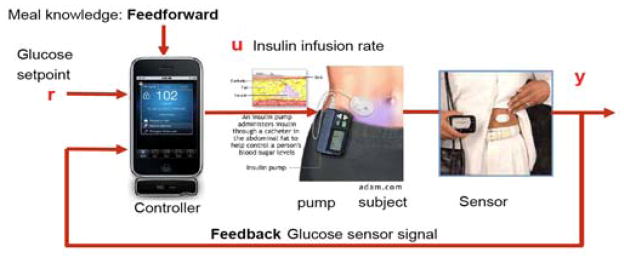
A block diagram for a closed-loop artificial pancreas is shown in Fig. 1; the actuators and sensors are available commercially. There are four continuous glucose monitors (CGM), numerous pumps, and very many self-monitoring (fingerstick) blood glucose (SMBG) meters available in the United States. While most insulin pumps have an infusion set composed of a length of tubing and a catheter inserted underneath the skin, new patch pumps are placed directly on the skin without additional tubing. Overviews of patch pump technology are provided by Anhalt and Bohannon (2010) and Schaepelynck et al. (2011). Diabetes Forecast (see Jan. 2012, for example) provides a comprehensive list of diabetes care products in its annual Consumer Guide.
Fig. 1.
Block diagram of a closed-loop artificial pancreas (adapted from Bequette, 2005). The use of meal feedforward control is often called “meal announcement.”
II.1 Current State of Care
Note that in the current standard of care, an individual serves as the feedback controller, with measurements and control decisions that are made relatively infrequently. Roughly 300,000 people, or around 20–25% of individuals in the US with T1DM, use continuous insulin pumps that continuously deliver microboluses of rapid-acting insulin (Skyler et al., 2007; Scheiner et al., 2008). A much smaller number of individuals use CGM. Individuals on intensive insulin therapy use SMBG meters to measure their blood several times a day, often before each meal and at bedtime. The insulin delivery is composed of two components: basal and bolus. Basal insulin covers the steady-state insulin needs and is either administered continuously with an infusion pump, using rapid-acting insulin, or as one or two injections of long-acting insulin each day. Meal-related insulin needs are satisfied by bolus therapy, where rapid-acting insulin is delivered at meal time, either by pump or injection.
Individuals undergoing intensive therapy often use a correction factor (CF) to help decide on the amount of insulin needed for a desired drop in glucose concentration. Similarly, an insulin to carb (I:C) ratio is used to estimate meal insulin bolus needs. Rapid-acting insulin is used for correction or meal boluses, regardless of whether the individual uses a pump or syringes. Klonoff (2012) reviews the current state of bolus calculators, which are available on most new models of insulin pumps.
While a major objective of a closed-loop artificial pancreas is to enable patients to have a more normal lifestyle without needing to constantly manage their disease, there can be a substantial economic benefit to the healthcare system. O’Grady et al. (2011) find that tighter blood glucose levels achievable with a closed-loop artificial pancreas have the potential for Medicare savings of $1.9 billion over 25 years.
II.2 Input Challenges
Manipulated inputs
There is a significant lag on the insulin effect on glucose uptake, even when rapid-acting insulin is delivered subcutaneously. Since insulin delivered in the recent past continues to have an effect, it is important to consider the remaining “insulin on board” (IOB) when deciding on a current insulin delivery rate. A further challenge is that there can be significant variability in the pharmacodynamic action of insulin.
Disturbance inputs
While meals cause a faster response in glucose concentration than insulin delivered, the time scale can be significant and highly variable; in addition, while it would be preferable to use knowledge about meal size to provide feedforward control (“meal announcement”) by injecting insulin (a “meal bolus”), it can be difficult to estimate the amount of carbohydrates in a meal. Exercise and stress levels can also have a substantial effect on glucose levels; indeed, intense (anaerobic) and moderate (aerobic) exercise can even have different short-term effects, with glucose concentration increasing under intense exercise but decreasing under moderate exercise.
II.3 Output Challenges
Currently available continuous glucose sensors suffer from a time lag between the capillary blood and the interstitial fluid, where the sensor is placed; also, there are often periods when the sensor results are biased due to the calibration procedure. Continuous glucose sensors do not eliminate the need for capillary blood glucose measurements (fingersticks); most CGMs require a calibration after a 2-hour “warm-up” period, and calibrating blood glucose measurements every 12 hours. These sensors are currently approved by the FDA for “adjunctive” use only, that is, any decision to change treatment (such as changing the insulin delivery rate) must be based on a confirmation fingerstick measurement.
II.4 Related Behavior
An individual’s insulin sensitivity and insulin delivery needs vary throughout the day, depending on meals, exercise, stress and normal diurnal variations. An individual using a pump can set different basal rates for different times during the day. The “dawn phenomena” at roughly 4:00 am, results in reduced insulin sensitivity, causing the blood glucose concentration to rise; an individual can compensate for this by programming the pump to provide a higher basal insulin delivery rate during that time period. Similarly, during periods of exercise, an individual may need to reduce their basal insulin delivery to near 0. During long periods of intense training, say for a marathon, an individual’s daily insulin demand may decrease by 50%; resistance training in adolescents was shown to increase insulin sensitivity by 23% (Landt et al., 1985).
III. Dynamics of manipulated & Disturbance inputs
A healthy pancreas has a rapid biphasic response to increases in blood glucose, with an initial spike in insulin concentration within 3 minutes of a glucose challenge (see Steil et al., 2004, for example response curves). Closed-loop artificial pancreas response times are much slower for a number of reasons, including the pharmacodynamics of subcutaneously delivered insulin.
III.1 Dynamics of Subcutaneously Delivered Insulin
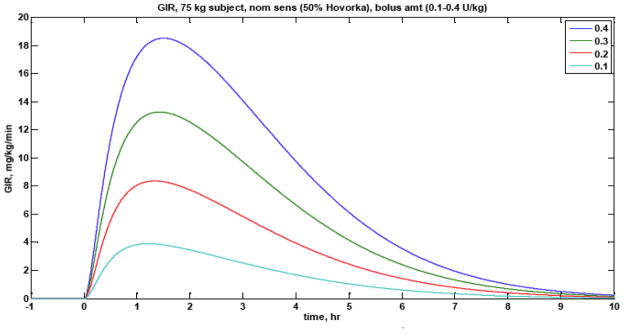
A major challenge in regulating blood glucose levels by manipulating the delivery of subcutaneous insulin, whether manually or automatically, is the long time-scale pharmacodynamic action of even rapid-acting insulin. After a bolus of insulin is delivered, the time before the maximum rate of change in blood glucose uptake (peak action) is roughly 90 minutes, and the insulin continues to have an effect on glucose for 6–8 hours; see Fig. 2 for typical pharmacodynamics profiles for rapid-acting insulin. Bequette (2009) reviews the glucose clamp procedures that are implemented clinically to estimate the profiles. The long-time scale for the effect of insulin is one reason that Cobry et al. (2010) found that delivering a bolus of rapid acting insulin 20 minutes before a meal yielded significantly better glucose control than boluses at meal-time or 20 minutes after meal initiation. There can be considerable insulin pharmacodynamic variability, as shown by El-Khatib et al. (2010), who find that five subjects (out of 11) had substantially slower insulin pharmacokinetics (plasma insulin concentration) than the other subjects.
Fig. 2.
Pharmacodynamic profiles for 4 different bolus magnitudes (0.1–0.4 U/kg) of rapid-acting insulin.
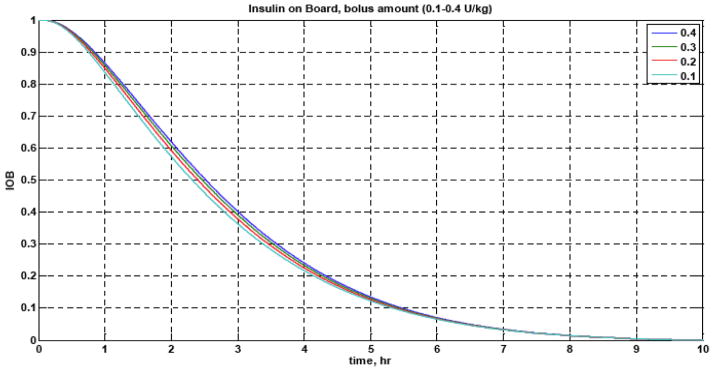
Nearly all commercially available insulin pumps provide an estimate of the “insulin on board” (IOB), which is an indicator of previously delivered insulin that will continue to have an effect on the glucose concentrations in the future (see Fig. 3). Zisser et al. (2008) review the approaches used by four different insulin pumps to estimate the IOB.
Fig. 3.
Fraction of insulin action remaining for different insulin bolus magnitudes (0.1–0.4 U/kg). After 2.5 hr, 50% of the insulin has yet to act.
III.2 Meal Dynamics
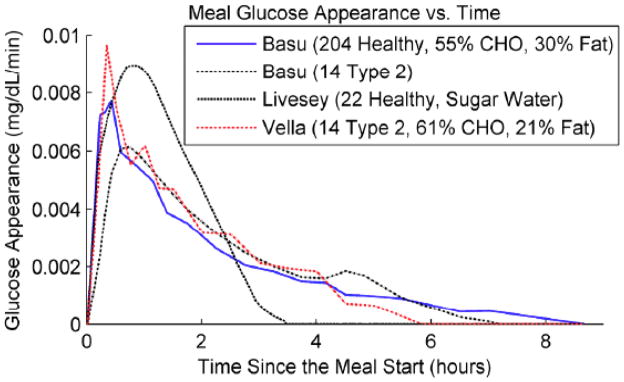
The dynamic effect of a meal on blood glucose can vary depending on a number of factors, including the fat content of the meal. The rate of glucose appearance in the blood is shown in Fig. 4 for several different studies; note that a meal effect can continue for 3–8 hours, but usually with a faster “peak” than the insulin pharmacodynamic behavior.
Fig 4.
Meal Dynamics (from Cameron et al., 2011b).
IV. Continuous Glucose Monitoring (CGM)
It is intuitive that CGM would enable an individual to better regulate their blood glucose values. The JDRF CGM Study Group (2008) showed that the use of CGM improves glycated hemoglobin (A1c) levels in individuals over the age of 25 years, whether they use continuous insulin infusion (pump) therapy, or multiple daily injections. Bergenstal et al. (2010) further showed that CGM combined with pump therapy resulted in better performance (greater reduction in A1c) than CGM combined with multiple daily injections. A review of this “sensor-augmented” insulin pump therapy is provided by Cengiz et al. (2011).
Continuous glucose monitors currently require frequent calibration by the use of a reference glucose based on a fingerstick. If there is an error in the reference glucose value, due to meter uncertainty, a user mistake in taking the blood sample, or sampling during transient conditions (particularly because of the lag between blood and interstitial fluid glucose), there is often a bias in the CGM signal until the next reference glucose sample is taken. An overview of CGM calibration and sensor signal filtering algorithms is provided by Bequette (2010).
IV.1 Hypoglycemia Detection/Prediction/Prevention
A major concern of any parent of a child with diabetes, is that the child may go hypoglycemic overnight; during the day the symptoms would be more likely to be noticed within a relatively brief period of time, but without a CGM there would be no way to detect overnight hypoglycemia. Palerm and Bequette (2007) perform a retrospective analysis of clinical CGM data to show the effect of different tuning parameters in a Kalman Filter based hypoglycemic alarm system. They show how tuning parameters could be adjusted by an individual based on their own tolerance to false alarms. Dassau et al. (2010) report clinical results for a voting-based strategy that involves several different algorithms, including a Kalman filter and a statistical prediction method (Cameron et al., 2008) to predict hypoglycemia. Harvey et al. (2012) propose metrics to evaluate the performance of hypoglycemia prediction algorithms for different applications, such as pump shut-off (see section IV.2) or rescue carbohydrate treatment.
IV.2 Pump Shut-off (Low Glucose Suspend)
Buckingham et al. (2005), in a study of the GlucoWatch G2 Biographer CGM (no longer commercially available) note that children only awoke to 29% of alarms, and their parents, when sleeping in the same room, only responded to 37% of alarms. It is desirable to take people out of the loop and simply shut-off the pump rather than sound an alarm; this approach is often called “low glucose suspend (LGS).” Two basic LGS approaches can be used: (i) threshold, where the pump is shutoff when glucose goes below a threshold value, and (ii) prediction, where the pump is shutoff when the glucose is predicted to be below a specified value within a future prediction horizon. Buckingham et al. (2009) report clinical pump shut-off results; a linear prediction algorithm with a 45-min horizon prevented hypoglycemia 80% of the time. Buckingham et al. (2010) present a voting-based strategy that prevents 84% of possible hypoglycemic events. In order to generalize the pump shut-off algorithm to handle variable sample time and sensor dropouts, a Kalman filter based approach was used by Cameron et al. (2012c) in clinical studies, preventing hypoglycemia in 73% of subjects that had datasets suitable for analysis. To-date, performance metrics have involved blood glucose samples, which are available because all studies have been performed in a clinic. Beck et al. (2011) argue that the outcome measures for outpatient studies of must be based solely on the CGM data. To-date, we have conducted 375 nights of out-patient studies, with 1/3 of the nights as “control” studies (with the pump shut-off algorithm not activated; i.e. standard care), and 2/3 of the nights as “intervention” nights (with the pump shut-off algorithm activated). Over the course of these studies, a total of three sets of tuning parameters have been used (prediction horizons of 70, 50 and 30 minutes). Buckingham et al. (2012) present out-patient results for the first 160 nights using the Cameron et al. (2012c) algorithm.
Choudhary et al. (2011), Agrawal et al. (2011), and Danne et al. (2011) report outpatient results based on the Medtronic Paradigm Veo, which can suspend the basal insulin delivery for up to 2 hours when hypoglycemia is detected by a CGM; the shutoff threshold can be set between 40 and 70mg/dl. Garg et al. (2012) study exercise-induced hypoglycemia in daytime clinical studies, with a threshold of 70 mg/dl, and find that the LGS results in reduced time in hypoglycemia compared to standard basal delivery. The Medtronic Veo low glucose suspend system is currently available in Europe but not the US, although there is a strong desire by many US clinicians for its availability (Tamborlane, 2012; Hirsch, 2012). It should be noted that insulin pump suspensions may occur naturally, as part of a fully closed-loop system, particularly during periods of a rapid decrease in blood glucose. Cengiz et al. (2009) report on pump suspensions that occurred during clinical trials of a PID algorithm, while Elleri et al. (2010) show pump suspension results during trials of a MPC algorithm.
The pump shut-off algorithms discussed above require minimal information, the measurement of blood glucose (and its rate-of-change). Hughes et al. (2010) add infusion pump data to account for the anticipated effect of insulin that has already infused, and propose a red/yellow/green light approach to hypoglycemia prevention. Hughes-Karvetski et al. (2012) propose a method to increase the aggressiveness of this system based on a patient-specific, time-varying model, using historical performance information to assess glycemic risk.
V. Other Control-related considerations
V.1 Risk Measures
Blood glucose control in diabetes is a balancing act between the long-term complications of hyperglycemia and the short-term danger of hypoglycemia. In addition, the hypoglycemic and hyperglycemic risks are asymmetric. A patient at 100 mg/dL faces a much greater risk if the glucose decreases by 50 mg/dL than if glucose increases by 50 mg/dL. An asymmetric risk measure was developed by Kovatchev et al. (1997), adapted by Cameron et al. (2011a), and used in a closed-loop MPC strategy detailed in section VI.5. Desborough et al. (2011) have noted that only the log-square and Cameron et al. (2011a) measures effectively balance the risks of both hypoglycemia and hyperglycemia.
V.2 Meal Detection/Prediction
Since meal dynamics (disturbance input) can have a significant time-scale and certainly the insulin (manipulated input) pharmacodynamics time scale is long, it is desirable that feedforward control be used to compensate for a meal; this is often called meal announcement. It is known, however, that people often forget to provide a meal-related insulin bolus (Burdick et al., 2004) resulting in higher A1c values and long-term complications. It is desirable, therefore to have a scheme that detects or predicts a meal and at least partially provides feedforward action. Dassau et al. (2008) present clinical results on a voting algorithm based procedure that detects a meal, on average, within 30 minutes after the onset of the meal. Lee and Bequette (2009) and Lee et al. (2009) present alternative procedures that also estimate the meal size. Cameron et al. (2009) present a probabilistic approach to meal detection and estimation of the rate of glucose appearance.
The meal detection methods discussed above are not “anticipatory” in nature, that is, they are based on changes in blood glucose due to meals that have already occurred (although “unannounced”). Better closed-loop performance can be obtained if meals are anticipated, perhaps through knowledge of common mealtimes. Particularly with model predictive control, if there is a high probability of a meal occurring during the “prediction horizon,” then this can be considered by the control action. Hughes et al. (2011) present a stochastic MPC algorithm that is based on a probabilistic description of the individual’s daily meal habits. Cameron et al. (2011b; 2012d) take a slightly different approach, by assigning probabilities of future meals based on the time that has lapsed since the last meal.
V.3 Exercise and Stress
Exercise and stress are known to have major impacts on insulin sensitivity and blood glucose levels. Chassin et al. (2007) review the effect of exercise on glucose levels in individuals with type 1 diabetes, noting that intense vs. moderate exercise can have qualitatively different effects. Riddell and Perkins (2009) review the effect of exercise on individuals with diabetes (both type 1 and type 2), with a focus on the possible role of CGM. Roy and Parker (2007) extend the Bergman minimal model to include the effect of exercise (based on the rate of oxygen consumption) on plasma glucose and insulin levels; model parameters were fit to data presented by Wolfe et al. (1986). Breton (2008) developed a similar model using heart rate as the exercise input; model parameters were fit based on 21 T1DM subjects undergoing a hyperinsulemic clamp. van Bon et al. (2011) find that moderate exercise increased heart rate (HR) and body acceleration counts (AC) and decreased glucose concentrations in 11 T1DM subjects, but they could not demonstrate a relationship between glucose changes and HR and AC changes. Kapitza et al. (2010) studied 16 male patients with type 1 diabetes under moderate (8) and intense (8) exercise, using CGM; the primary conclusion was that there can be a wide variability in glucose profiles before, during and after physical exercise.
The effects of carbohydrate intake, insulin boluses and exercise (based on heartrate) on blood glucose were studied by Schmidt et al. (2012), in clinical studies involving 12 subjects with T1DM. Maahs et al. (2012) performed similar outpatient studies on 30 adolescents with T1DM over five days, including more detailed meal information; physical activity was measured based on accelerometer data.
To mimic the effect of stress on insulin resistance, Bevier et al. (2008) give a 3-day course of prednisone to 10 subjects with T1DM; they find that insulin may need to be increased by 70% or more during prednisone treatment, to achieve normal blood glucose levels. Finan et al. (2010) use principle component analysis (PCA) of CGM, insulin infusion, and recorded meal data to detect “stress days” (when prednisone was given), with a 89% classification accuracy. Ward et al. (2011) mimic the effect of stress by administering hydrocortisone to individuals with type 1 diabetes, under closed-loop control; further details are presented by El-Youssef et al. (2011), as discussed in section VI.3.
V.4 Modeling for Control
While it would be ideal to use existing glucose sensor and insulin infusion data, with meal information, to develop models, this is difficult in practice. For one, the recording of meal times and carbohydrate amounts is notoriously bad and the sensor and insulin pump times are not necessarily synched with any written recordings. Also, since an insulin bolus is typically given at the same time as a meal, it is difficult for identification algorithms to distinguish the difference in the two effects. Finan et al. (2007), and Lee and Bequette (2009) show that even the sign of the gain between insulin and glucose concentration can be wrong; it should be negative, but identification techniques may yield a positive gain, due to the simultaneous effect of the meal.
Lee and Bequette (2009) propose a “human-friendly” identification-based approach to improve the development of control-relevant models; the methodology is analogous to “plant-friendly” techniques that have been developed for the process industries. Finan et al. (2009) analyzed data from nine subjects with type 1 diabetes in ambulatory conditions and found that identified ARX models yielded marginal improvements in glucose predictions, compared to simply assuming that the current glucose concentration remains constant into the future; this is another indicator of the difficulty of using normal “free living” data, rather than data generated specifically for control-relevant model development. An alternative approach is to develop model parameters based on clinically relevant information, such as total daily insulin, I:C ratio and CF, as proposed by Percival et al. (2010), who apply this approach to a first-order+deadtime model. van Heusden et al. (2012) develop personalized control-relevant models based on extensive simulations using the UVa-Padova simulator discussed in section V.5. The second-order dynamics are constant from subject-to-subject, but the gains are a function of their CF and a safety factor.
V.5 Simulation-based Testing
A realistic simulation environment with a wide variety of simulated subjects enables the development of control strategies that are robust and reliable. Indeed, the FDA accepted the UVa-Padova simulator (Kovatchev et al., 2009) for use in simulated clinical trials, enabling investigators to skip the animal trial stage; this simulator is based on a model presented by Dalla Man et al. (2007). Patek et al. (2009) discuss this approach for simulated closed-loop clinical trials. The UVa-Padova simulator contains 300 subjects (100 each of children, adolescents and adults), and includes sensor errors representative of two CGMs and the discrete resolution from two insulin pumps. Wilinska et al. (2009, 2010) discuss the use of simulation (based on the model presented in Hovorka et al., 2004a) studies for evaluating model predictive control strategies in simulated clinical trials; their simulation studies involve 18 different subject parameter sets.
V.6 Control System Platforms for Clinical Studies
While there are many commercially available subcutaneous insulin infusion pumps, and four currently available continuous glucose monitors that are approved by the US FDA, there are currently no standards to connect them with a device that contains a control algorithm. Many of the initial clinical studies have used manual entry of the CGM reading into a laptop computer, followed by manual implementation of the calculated control action into the insulin pump; this is perhaps one reason that many of the initial studies are based on a sample time of 15 minutes.
For the fully automated clinical studies that have been performed, a great deal of effort has gone into the development of hardware and software to form the closed-loop. For example, the artificial pancreas system (APS) platform developed at the University of California at Santa Barbara has been used by a number of groups involved in the JDRF AP consortium (Dassau et al., 2008b, 2009); currently two CGMs and three insulin pumps are supported by the APS.
Patek et al. (2012) summarize a modular approach, which includes the APS as the interface module, a continuous safety module, and a real-time control module. The performance of the system is demonstrated in simulated preclinical trials using the FDA accepted simulator discussed in section V.5. Keith-Hynes et al. (2012) present a cell-phone-based platform for supporting clinical trials. This platform, combined with a portable version of the APS, was used in initial out-patient clinical trials reported by Cobelli et al. (2012).
VI. Control Algorithms
VI.1 On-Off
On-off control is used by the low glucose suspend (or pump shut-off) types of systems. The decision to shut-off the pump can be based on a threshold (basically a hypoglycemic detection), or a projected violation of a threshold (a prediction that a low glucose will occur within a prediction horizon). In in-patient studies (Cameron et al., 2012c), and in on-going out-patient studies (Buckingham et al., 2012), we are using a Kalman filter based prediction for the pump shut-off algorithm; this provides more flexibility for handling sensors with different sample times, and naturally handles brief sensor drop-outs.
VI.2 Control-to-Range
A low glucose suspend controller seeks to maintain blood glucose above some minimum value. Control-to-range represents the next level of control, where the objective is to regulate blood glucose between upper and lower bounds. Kovatchev et al. (2009) present a basic structure (modular architecture) for this approach, while Grosman et al. (2010) show simulation results for a zone model predictive control strategy. Breton et al. (2012), using the platform developed by Patek et al. (2012), present clinical results for 38 subjects using two different control-to-range approaches: (i) standard, and (ii) enhanced. Both algorithms increased time spent in normoglycemia and reduced time in hypoglycemia compared to standard open-loop operation; the enhanced algorithm also showed a significant improvement in mean glucose levels.
VI.3 Proportional-Integral-Derivative (PID)
The Medtronic external physiological insulin delivery (ePID) system includes a PID controller that has been used in animal and human studies. Weinzimer et al. (2008) applied ePID to 17 adolescents and found that a small insulin priming bolus (feedforward or “meal announcement”), provided 15 minutes before meals, reduced the postprandial glucose peaks. The recent approach used by Medtronic involves model-based feedback of insulin concentration, creating a cascade type of strategy (Palerm, 2011; Steil et al., 2011), called ePID-IFB. Loutseiko et al. (2011) report studies performed in seven diabetic dogs, while Ruiz et al. (2012) study four human subjects. Marchetti et al. (2008a; 2008b) use a PID switching control strategy in simulation studies using the Hovorka et al. (2004a) simulation model.
Gopakumaran et al. (2005) developed a fading memory proportional derivative (FMPD) controller that is roughly equivalent to PID. Ward et al. (2008) manipulate both insulin and glucagon in studies conducted on rats. This approach has been used in initial human patient studies by Castle et al. (2010a), and has been extended to an adaptive strategy by El-Youssef et al. (2011); the system adapts to changing insulin sensitivity that is induced by hydrocortisone administration.
van Bon et al. (2010) use a PD controller in a clinical study of six human subjects; a glucagon pump was used to reduce the chance of hypoglycemia. van Bon et al. (2012) studied 10 patients using an adaptive PD control strategy that manipulates both insulin and glucagon. In addition to handling meals, the controller was able to respond to 30 minutes of moderate activity exercise.
VI.4 Fuzzy-Logic
A fuzzy logic-based approach that uses a combination of control-to-range and control to setpoint strategies is incorporated into the MD-Logic Artificial Pancreas System (Atlas et al., 2010), with a 5-min sample time; this has been tested in a trial on seven adults, without the use of meal announcement. Miller et al. (2011) describe the learning algorithm that extends the MD-Logic strategy to better handle interpatient variability, which is tested in simulation studies; overnight studies in seven patients are presented by Nimri et al. (2012).
Mauseth et al. (2010) describe a fuzzy logic based controller, with a 15-min sample time, that uses BG, its rate-of-change and its acceleration as inputs, and is tuned based on a personalization factor. A preliminary version was tested (without a personalization factor) on four subjects, before enhancements were made and performance demonstrated in simulation studies.
VI.5 Model Predictive Control (MPC)
MPC is a basic framework or strategy that can involve many different types of models and objective functions. Hovorka et al. (2004a) presented an approach based on a nonlinear model and Bayesian techniques to estimate parameters in simulation studies. Clinical studies were performed under fasting conditions by Hovorka et al. (2004b), based on i.v. measurements that were delayed by 30 min to mimic the time lag associated with a s.c. sensor. Hovorka et al. (2010) performed overnight studies using an MPC strategy and manually entering CGM data in the algorithm and transferring results to a pump at 15-min intervals; the major outcome was a reduction in nocturnal hypoglycemia compared to standard pump treatment. Hovorka et al. (2011) and Elleri et al. (2011) presented overnight studies based on a fully-automated MPC strategy that was initiated immediately after either dinner or a late night snack; again with a 15-min sample time.
Magni et al. (2007) present an unconstrained MPC strategy, where the model is a linearization of a nonlinear model, obtained at an average value of the population parameters. In simulation studies with a sample time of 30 minutes, they show that a single parameter, the weighting on the output predictions in the objective function, can be tuned for each individual for better performance. This approach is used in Bruttomesso et al. (2009) in a trial with six subjects; parameters included a sample time of 15 minutes and a prediction horizon of 240 minutes. Kovatchev and colleagues use a one-min sensor sample time and a 15-min actuator sample time. Kovatchev et al. (2010) report that clinical studies (each 22 hr, with 14.5 hr in closed-loop) involving 20 adults reduced nocturnal hypoglycemic events from 23 to 5, and increased the amount of time within the target range from 64% to 78% compared to standard open-loop treatment. Simulation-based studies were used to design the controllers before implementation in the clinical studies. Clarke et al. (2009) revise this approach in an overnight study of eight subjects, using individualized models based on weight, total daily insulin dose and a BG CF measured during admission. Soru et al. (2012) discuss techniques for meal compensation and individualization for better performance, in simulation studies involving four different scenarios and 100 subjects. They first use a single adjustable parameter based on clinical parameters (MPC1). They then develop low-order models to produce more a more realistic model as a basis for MPC; this model is further revised based on patient specific information (MPC2). The MPC1 algorithm in Soru et al. (2012) and the Hovorka et al. (2010) MPC algorithm were studied in trials involving 47 patients in six centers, as reported by Devries et al. (2012); while the closed-loop algorithms each had a higher mean glucose than open-loop control, both resulted in less time in hypoglycemia than open-loop control.
Ellingsen et al. (2009) use MPC based on ARX models and a 5-min sample time; IOB constraints based on I:C and CF were implemented in a simulation study. Percival et al. (2011) use low-order models based on clinical parameters (discussed in section V.4) to design a multi-parametric model predictive control strategy; multi-parametric programming enables the constrained optimization problem to be solved using a lookup table, greatly reducing the computational requirements. Wang et al. (2010) use iterative learning control (ILC) to improve the closed-loop performance of a MPC strategy in simulation studies over many days; they find that less than 10 days are required to bring the individual within the desired range of glucose values.
An adaptive generalized predictive control (GPC) approach (based on recursive identification of ARX models) is taken by El-Khatib et al. (2007) in their studies involved diabetic swine; in addition to insulin, their strategy adjusts glucagon to improve control at lower blood glucose levels. Also, El-Khatib et al. (2007) include a prediction of the insulin concentration and include it in an objective function to avoid problems associated with IOB. In the human clinical studies reported in El-Khatib et al. (2010), a PD controller that is active under certain glucose concentrations is used for glucagon. Insulin is administered based on an adaptive, discrete-time, second-order model. A sensor with a five-minute sample time was used, with a prediction horizon of one, resulting in, effectively, an adaptive PID controller. Constraints on insulin are imposed by clamping and are not part of the control algorithm. Eren-Oruklu et al. (2009) develop an adaptive GPC strategy to regulate the estimated blood glucose levels based on CGM measurements and compare their controller with Linear Quadratic Control (LQC) in simulation studies based on the Hovorka (2004a) and Glucosim (Agar et al., 2005) models.
Lee et al. (2009) and Lee and Bequette (2009) use subspace identification techniques to develop discrete state space models, and incorporate IOB constraints in MPC; additional features include a pump shut-off algorithm to avoid hypoglycemia, and meal detection and meal size estimation algorithms to handle un-announced meals. More recently, we (Cameron et al., 2011a) have developed a multiple model probabilistic predictive control (MMPPC) strategy that minimizes an asymmetric risk function subject to satisfying hypoglycemic constraints; the controller is forced to be more conservative when uncertainties are high. We are using a similar approach in on-going clinical trials.
VII. Safety and Fault Detection
There are many problems that can arise in a closed-loop artificial pancreas. Examples include sensor signals that drift or dropout, or are poorly calibrated, insulin infusion sets can fail, a planned meal that is not consumed, etc.
VII.1 Sensor Dropouts and Attenuation
There are often brief periods when CGM signals are either lost, or attenuated. Bequette (2010) discusses how a Kalman filter based approach can be used for model predictions without the measurement updates; when the state covariances indicate that there is too much uncertainty, an alarm could be activated. If this occurs overnight, the closed-loop system could be placed in some default basal delivery mode until morning.
Sensor attenuation is a difficult problem to detect, but similar approaches to the infusion set failure detection problem below (section VII.2) could be used. An obvious solution is to use multiple sensors, as proposed by Castle and Ward (2010b). Nocturnal sensor attenuation (NSA), due to individuals laying on top of their sensor is a common problem; these signals tend to attenuate for roughly 15–30 minutes, as a first-order type of decrease, before returning to near “pre-attenuation” values. An example of the effect of NSA is illustrated in Fig. 5, where the sensor attenuations caused frequent pump shut-offs to occur during overnight low glucose suspend studies (Cameron et al., 2012c). Cameron et al. (2012b) propose a Kalman filter based approach to detect invalid readings based on large negative second-derivatives of estimated glucose combined with exceeding a threshold on the glucose rate-of-change; results are reported based on 39 nights of in-patient data contain reference glucose measurements. Baysal (2012) present results for over 100 nights of data in an out-patient setting; since reference glucose measurements are not available during the night, the false positives are evaluated by experts visually interpreting the data.
Fig 5.
Illustration of nocturnal sensor attenuations causing inappropriate pump suspensions in a pump shut-off study (Cameron et al., 2012c).
A detailed analysis of biomechanical aspects of the sensor-tissue interface, including the effects of motion and pressure are presented by Helton et al. (2011a, 2011b); the authors also summarize ten other literature sources that mention the effect of motion or pressure on sensor results.
VII.2 Infusion Set Failure
A common problem encountered by diabetic patients on continuous insulin therapy is insulin infusion set failure, IISF, when Teflon catheters or steel needle infusion sets are worn for long periods of time. Common causes of IISF include blocked or dislodged sets, inflammation, or insulin leakage back to the skin surface. Clarke and Renard (2012) note that the weakest part of the infusion system remains the catheter, while Heinemann and Krinelke (2012) refer to insulin infusion sets as the “Achilles Heel” of continuous subcutaneous insulin infusion. Rojas et al. (2011a,b; 2012) use bivariate classification, principal component analysis and a combined approach to detect simulated faults in 10 subjects. Cameron et al. (2012a) use an interactive multiple model (IMM) approach to detect 27 set failures in 120 weeks of outpatient data; the infusion sets, on average, failed after 5.3 days. Cameron et al. (2012e) use a threshold-based approach (using an alarm silencing period) to detect 80% of set failures, with a false positive rate of 0.3/day. Herrero et al. (2012) use an interval analysis based technique to detect faults in simulation studies involving 10 scenarios on 10 subjects.
VII.3 Announced Meals That Are Not Consumed
While better closed-loop performance can be achieved when meal announcement is used to provide an insulin bolus, there is some risk that the meal will not be consumed, providing an argument for a using a smaller “priming bolus.” An alternative is to extend the probabilistic strategies of Cameron et al. (2011b) and Hughes et al. (2011). Even when a meal is announced, it is not necessary to assign a prior probability of 100% to the meal algorithm; a lower value would enable a risk-based controller to provide a smaller meal bolus for a safety margin over the prediction horizon.
VIII. Next Steps Toward an Artificial Pancreas
The focus of this review has been on the specific challenges to closed-loop control, with much of the emphasis on algorithms. Clearly, a successful closed-loop system has a number of components, including sensors, actuators and algorithms. There is much effort into further developing CGM and insulin pump technology, particularly since the current state of technology requires a very dedicated user. It is unlikely that a successful closed-loop artificial pancreas will be composed of the separate components of a sensor/transmitter, pump/infusion set and controller interface, with associated tubing/wiring, etc. It is more likely that components will be combined, by using patch pumps that incorporate a sensor and transmitter/receiver within the same unit.
Conclusion
Diabetes technology has advanced considerably during the past five years. The path to a fully closed-loop artificial pancreas is proceeding in stages, with hypoglycemic alarms naturally leading to pump shut-off (low glucose suspend) systems, which in turn leads to control-to-range strategies. It is expected that there will be a similar pathway for different types of devices to be approved and appear in the marketplace.
Simulation studies have provided important results that enable fewer clinical trials, particularly for full closed-loop systems, to accomplish given performance goals. Recent clinical results are very promising and a substantial number of out-patient trials are proceeding. The future for a closed-loop artificial pancreas is indeed very bright.
Acknowledgments
This work was supported in part by the Juvenile Diabetes Research Foundation (JDRF), grants 22-2011-647, 22-2009-795 and 22-2007-1801, and NIH 5R01DK085591-03. This paper has benefited from many discussions with Bruce A. Buckingham, Darrell M. Wilson and Fraser Cameron during the course of our on-going collaboration.
Footnotes
Extended version of a paper presented at the 2012 American Control Conference (Bequette, 2012).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Agar BU, Eren M, Cinar A. GLUCOSIM: educational software for virtual experiments with patients with type 1 diabetes. Proc. IEEE Eng. Med. Biolo. Soc; Shanghai, China. 2005. pp. 845–848. [DOI] [PubMed] [Google Scholar]
- Agrawal P, Welsh JB, Kannard B, Askari S, Yang Q, Kaufman FR. Usage and effectiveness of the low glucose suspend feature of the Medtronic Paradigm Veo insulin pump. J Diabetes Sci Technol. 2011;5(5):1137–1141. doi: 10.1177/193229681100500514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anhalt H, Bohannon NJV. Insulin patch pumps: their development and future in closed-loop systems. Diabetes Tech Ther. 2010;12(Suppl 1):S51–S58. doi: 10.1089/dia.2010.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas E, Nimri R, Miller S, Grunberg EA, Phillip M. MD-Logic Artificial Pancreas Systems. Diabetes Care. 2010;33(5):1072–1076. doi: 10.2337/dc09-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysal N, Cameron F, Buckingham BA, Wilson DM, Chase HP, Bequette BW. Nocturnal CGM Signal Attenuation: An Outpatient Study. Presented at the Diabetes Technology Meeting; Bethesda, MD. November, 2012. [Google Scholar]
- Beck RW, Kollman C, Xing D, Buckingham BA, Chase HP. Outcome measures for outpatient hypoglycemia prevention studies. J Diabetes Sci Technol. 2011;5:999–1004. doi: 10.1177/193229681100500423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bequette BW. A Critical Assessment of Algorithms and Challenges in the Development of an Artificial Pancreas. Diabetes Technology and Therapeutics. 2005;7(1):28–47. doi: 10.1089/dia.2005.7.28. [DOI] [PubMed] [Google Scholar]
- Bequette BW. Glucose Clamp Algorithms and Insulin Time-Action Profiles. J Diabetes Sci Technol. 2009;3:1005–1013. doi: 10.1177/193229680900300503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bequette BW. Continuous Glucose Monitoring: Real-Time Algorithms for Calibration, Filtering and Alarms. J Diabetes Sci Technol. 2010;4(2):404–418. doi: 10.1177/193229681000400222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bequette BW. Challenges and Progress in the development of a closed-loop artificial pancreas. Proceedings of the 2012 American Control Conference; Montreal: 2012. pp. 4065–4071. [Google Scholar]
- Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311–20. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- Bevier WC, Zisser HC, Jovanovic L, Finan DA, Palerm CC, Seborg DE, Doyle FJ., III Use of continuous glucose monitoring to estimate insulin requirements in patients with type 1 diabetes mellitus during a short course of prednisone. J Diabetes Sci Technol. 2008;2(4):578–583. doi: 10.1177/193229680800200408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton MD. Physical activity – the major unaccounted impediment to closed loop control. J Diabetes Sci Technol. 2008;2(1):169–174. doi: 10.1177/193229680800200127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton M, Farret A, Bruttomesso D, Anderson S, Magni L, Patek S, Dalla Man C, Place J, DeMartini S, Del Favero S, Toffanin C, Hughes C, Dassau E, Zisser H, Doyle FJ, III, de Nicolao G, Avogaro A, Cobelli C, Renard E Kovatchev on behalf of the International Artificial Pancreas (IAP) Study Group. Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes. 2012;61(9):2230–2237. doi: 10.2337/db11-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruttomesso D, Farret A, Costa S, Marescotti MC, Vettore M, Avogaro A, Tiengo A, Dalla Man C, Place J, Facchinetti A, Guerra S, Magni L, De Nicolao G, Cobelli C, Renard E, Maran A. Closed-Loop Artificial Pancreas Using Subcutaneous Glucose Sensing and Insulin Delivery and a Model Predictive Control Algorithm: Preliminary Studies in Padova and Montpellier. J Diabetes Sci Technol. 2009;3:1014–1021. doi: 10.1177/193229680900300504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham BA, Cameron F, Lum J, Maahs D, Clinton P, Harris B, Realsen J, Bequette BW, Wilson DM, Beck RW, Chase HP. Randomized outpatient in-home pilot trial of predictive nocturnal pump shut-off. Presented at the 72nd Scientific Sessions of the American Diabetes Association; Philadephia, PA. 10 June 2012. [Google Scholar]
- Buckingham BA, Block J, Burdick J, Kalajian A, Kollman C, Choy M, Wilson DM, Chase HP. Response to nocturnal alarms using a real-time glucose sensor. Diabetes Technol Ther. 2005;7:440–447. doi: 10.1089/dia.2005.7.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham B, Cobry E, Clinton P, Gage V, Caswell K, Kunselman E, Cameron F, Chase HP. Preventing hypoglycemia using predictive alarm algorithms and insulin pump suspension. Diabetes Technol Ther. 2009;11(2):93–7. doi: 10.1089/dia.2008.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham B, Chase HP, Dassau E, Cobry E, Clinton P, Gage V, Caswell K, Wilkinson J, Cameron F, Lee H, Bequette BW, Doyle FJ., III Prevention of Nocturnal Hypoglycemia Using Predictive Alarm Algorithms and Insulin Pump Suspension. Diabetes Care. 2010;33(5):1013–1018. doi: 10.2337/dc09-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick J, Chase HP, Slover R, Knievel K, Scrimgeour L, Maniatis AK, Klingensmith G. Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics. 2004;113(3 Pt 1):e221–4. doi: 10.1542/peds.113.3.e221. [DOI] [PubMed] [Google Scholar]
- Cameron F, Bequette BW, Wilson DW, Buckingham BA. Detecting insulin infusion set failure. Presented at Advanced Technologies & Treatments for Diabetes (ATTD) 2012; Barcelona. 2012a. [Google Scholar]
- Cameron F, Baysal N, Buckingham BA, Wilson DW, Bequette BW. Realtime and retrospective detection of nocturnal CGM signal attenuation. Diabetes; Poster 862 Presented at the 72nd Scientific Sessions of the American Diabetes Assocation (ADA); Philadephia, PA. 10 June 2012b; p. A219. [Google Scholar]
- Cameron F, Wilson DM, Buckingham BA, Arzumanyan H, Benzsi K, Chase HP, Lum J, Maahs DM, Calhoun PM, Bequette BW. In-patient studies of a Kalman filter based predictive pump shut-off algorithm. J Diabetes Sci Technol. 2012c;6(5):1142–1147. doi: 10.1177/193229681200600519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron F, Niemeyer G, Bequette BW. Extended multiple model prediction with application to blood glucose regulation. J Process Control. 2012d;22(8):1422–1432. [Google Scholar]
- Cameron F, Buckingham BA, Wilson DM, Bequette BW. Extending threshold based detection of infusion set failures. Presented at the 2012 Diabetes Technology Meeting; Bethesda, MD. 2012e. [Google Scholar]
- Cameron F, Bequette BW, Wilson DM, Buckingham BA, Lee H, Niemeyer GA. Closed-Loop Artificial Pancreas Based on Risk Management. J Diabetes Sci Technol. 2011a;5(2):368–379. doi: 10.1177/193229681100500226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron F, Bequette BW, Buckingham BA, Wilson DM, Lee H, Niemeyer G. Anticipatory Behavior in Blood Glucose Control: Using Prior Probabilities to Prepare for Future Meal Disturbance. preprints of the 18th IFAC World Congress; Milano: 2011b. pp. 3771–3776. [Google Scholar]
- Cameron F, Niemeyer G, Buckingham B. Probabilistic Evolving Meal Detection and Estimation of Meal Total Glucose Appearance. J Diabetes Sci Technol. 2009;3:1022–1030. doi: 10.1177/193229680900300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron F, Niemayer G, Gundy-Burlet K, Buckingham B. Statistical hypoglycemia prediction. J Diabetes Sci Technol. 2008;2(4):612–21. doi: 10.1177/193229680800200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle JR, Engle JM, El-Yousssef J, Massoud RG, Kagan R, Ward WK. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care. 2010a;33:1281–1287. doi: 10.2337/dc09-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle JR, Ward WK. Amperometric Glucose Sensors: Sources of Error and Potential Benefit of Redundancy. J Diab Sci Technol. 2010b;4:221–225. doi: 10.1177/193229681000400127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cengiz E, Swan KL, Tamborlane WV, Steil GM, Steffen AT, Weinzimer SA. Is an automatic pump suspension feature safe for children with type 1 diabetes An exploratory analysis with a closed-loop system. Diabetes Technology & Therapeutics. 2009;11(4):207–210. doi: 10.1089/dia.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cengiz E, Sherr JL, Weinzimer SA, Tamborlane WV. New-generation diabetes management: glucose sensor-augmented insulin pump therapy. Expert Rev Med Devices. 2011;8(4):449–58. doi: 10.1586/erd.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin LJ, Wilinska ME, Hovorka R. Intense exercise in type 1 diabetes: exploring the role of continuous glucose monitoring. J Diabetes Sci Technol. 2007;1(4):570–573. doi: 10.1177/193229680700100415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary P, Shin J, Wang Y, Evans ML, Hammond PJ, Kerr D, Shaw JAM, Pickup JC, Amiel SA. Insulin pump therapy with automated insulin suspension in response to hypoglycemia. Reduction in nocturnal hypoglycemia in those at greatest risk. Diabetes Care. 2011;34:2023–2025. doi: 10.2337/dc10-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke WL, Renard D. Clinical requirements for closed-loop control systems. J Diabetes Sci Technol. 2012;6(2):444–452. doi: 10.1177/193229681200600233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke WL, Anderson S, Breton M, Patek S, Kashmer L, Kovatchev B. Closed-Loop Artificial Pancreas Using Subcutaneous Glucose Sensing and Insulin Delivery and a Model-Predictive Control Algorithm: The Virginia Experience. J Diabetes Sci Technol. 2009;3:1031–1038. doi: 10.1177/193229680900300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens AH. Feedback control dynamics for glucose controlled insulin infusion systems. Med Prog Technol. 1979;6:91–98. [PubMed] [Google Scholar]
- Cobelli C, Dalla Man C, Sparacino G, Magni L, De Nicolao G, Kovatchev B. Diabetes: models, signals and control. IEEE Rev Biomed Eng. 2010;2:54–96. doi: 10.1109/RBME.2009.2036073. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobelli C, Renard E, Kovatchev B. Artificial Pancreas: Past, Present and Future. Diabetes. 2011;60(11):2672–2682. doi: 10.2337/db11-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobelli C, Renard E, Kovatchev BP, Keith-Hynes P, Brahim NB, Place J, del Favero S, Breton M, Farret A, Bruttomesso D, Dassau E, Zisser H, Doyle FJ, III, Patek SD, Avogaro A. Pilot Studies of Wearable Outpatient Artificial Pancreas in Type 1 Diabetes. Diabetes Care. 2012;35(9):e65–e67. doi: 10.2337/dc12-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobry E, McFann K, Messer L, Gage V, VanderWel B, Horton L, Chase HP. Timing of meal insulin boluses to achieve optimal postprandial glycemic control in patients with Type 1 diabetes. Diabetes Technol Ther. 2010;12(3):173–177. doi: 10.1089/dia.2009.0112. [DOI] [PubMed] [Google Scholar]
- Dalla Man C, Raimondo DM, Rizza RA, Cobelli C. GIM, simulation software of meal glucose–insulin model. Diabetes Sci Technol. 2007;1(3):323–330. doi: 10.1177/193229680700100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danne T, Kordonouri O, Holder M, Haberland H, Golembowski S, Remus K, Blasig S, Wadien T, Zierow S, Hartmann R, Thomas A. Prevention of hypoglycemia by using low glucose suspend function in sensor-augmented pump therapy. Diabetes Technol Therapeutics. 2011;13(11):1129–1134. doi: 10.1089/dia.2011.0084. [DOI] [PubMed] [Google Scholar]
- Dassau E, Cameron F, Lee H, Bequette BW, Zisser H, Jovanovic L, Chase HP, Wilson DM, Buckingham BA, Doyle FJ. Real-Time Hypoglycemia Prediction Suite Using Continuous Glucose Monitoring: A safety net for the artificial pancreas. Diabetes Care. 2010;33:1249–1254. doi: 10.2337/dc09-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassau E, Bequette BW, Buckingham BA, Doyle FJ., III Detection of a Meal Using Continuous Glucose Monitoring. Diabetes Care. 2008a;31:295–300. doi: 10.2337/dc07-1293. [DOI] [PubMed] [Google Scholar]
- Dassau E, Zisser H, Palerm CC, Buckingham BA, Jovanovič L, Doyle FJ., III Modular Artificial β-Cell System: A Prototype for Clinical Research. Diabetes Sci Technol. 2008b;2(5):863–872. doi: 10.1177/193229680800200518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassau E, Palerm CC, Zisser H, Buckingham BA, Jovanovič L, Doyle FJ., III In Silico Evaluation Platform for Artificial Pancreatic β-Cell Development – a Dynamic Simulator for Closed-Loop Control with Hardware-in-the-Loop. Diabetes Technol Ther. 2009;11(3):187–194. doi: 10.1089/dia.2008.0055. [DOI] [PubMed] [Google Scholar]
- Desborough L, Palerm CC, Kaufman F. Characterization of a populations’ blood glucose distribution and its correlation with metrics assessing quality of glycemic control. 4th Int. Conf. on Adv. Technologies & Treatments for Diabetes (ATTD); London, UK. 16–19 Feb, 2011. [Google Scholar]
- Devries JH, Avogaro A, Benesch C, Bruttomesso D, Caldwell K, Cobelli C, Doll W, del Favero S, Heinemann L, Hovorka R, Leelarathna L, Luijf YM, Mader J, Magni L, Nodale M, Place J, Renard E, Toffanin C on behalf of the AP@Home Consortium. Comparison of Two Closed Loop Algorithms with Open Loop Control in Type 1 Diabetes. Diabetes; Oral Paper 224 Presented at the 72nd Scientific Sessions of the American Diabetes Association (ADA); Philadelphia, PA. June 2012; p. A60. [Google Scholar]
- The Diabetes ControlComplications Trial (DCCT) Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insuin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Diabetes Forecast. [Accessed 16 March 2012.];Annual Consumer Guide. 2012 Jan; http://forecast.diabetes.org/january-2012.
- Doyle FJ, III, Jovanovič L, Seborg DE. A tutorial on biomedical process control: glucose control strategies for treating type 1 diabetes mellitus. J Proc Cont. 2007;17(7):572–576. [Google Scholar]
- Doyle FJ., III . Dynamics and Control for the Artificial Pancreas. In: Samad T, Annaswamy AM, editors. The Impact of Control Technology. IEEE Control Systems Society; 2011. Available at www.ieeecss.org. [Google Scholar]
- El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A Bihormonal Closed-Loop Artificial Pancreas for Type 1. Diabetes Sci Transl Med. 2010;2:27ra27. doi: 10.1126/scitranslmed.3000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khatib FH, Jiang J, Gerrity RG, Damiano ER. Adaptive Closed-Loop Control Provides Blood-Glucose Regulation Using Dual Subcutaneous Insulin and Glucagon Infusion in Diabetic Swine. J Diabetes Sci Technol. 2007;1:181–192. doi: 10.1177/193229680700100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Youssef J, Castle J, Ward WK. A Review of Closed-Loop Algorithms for Glycemic Control in the Treatment of Type 1 Diabetes. Algorithms. 2009;2:518–532. [Google Scholar]
- El-Youssef J, Castle JR, Branigan DL, Massoud RG, Breen ME, Jacobs PG, Bequette BW, Ward WK. A Controlled Study of the Effectiveness of an Adaptive Closed-Loop Algorithm to Minimize Corticosteroid-Induced Stress Hyperglycemia in Type 1 Diabetes. J Diabetes Sci Technol. 2011;5(6):1312–1326. doi: 10.1177/193229681100500602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleri D, Allen JM, Dunger DB, Hovorka R. Closed-loop in children with type 1 diabetes: specific challenges. Diabetes Research & Clinical Practice. 2011a Aug;93(Supplement 1):pS131–S135. doi: 10.1016/S0168-8227(11)70029-8. [DOI] [PubMed] [Google Scholar]
- Elleri D, Allen JM, Nodale M, Wilinska ME, Mangat JS, Larsen AMF, Acerini CL, Dunger DB, Hovorka R. Automated overnight closed-loop glucose control in young children with type 1 diabetes. Diabetes Technology & Therapeutics. 2011b;13(4):419–424. doi: 10.1089/dia.2010.0176. [DOI] [PubMed] [Google Scholar]
- Elleri G, Allen JM, Nodale M, Wilinska ME, Acerini CL, Dunger DB, Hovorka R. Suspended insulin infusion during overnight closed-loop glucose control in children and adolescents with Type 1 diabetes. Diabetic Medicine. 2010;27:480–484. doi: 10.1111/j.1464-5491.2010.02964.x. [DOI] [PubMed] [Google Scholar]
- Ellingsen C, Dassau E, Percival MW, Zisser H, Jovanovic L, Doyle FJ., III Safety constraints in artificial beta cell: An implementation of model predictive control with insulin-on-board. J Diabetes Sci Technol. 2009;3(3):536–544. doi: 10.1177/193229680900300319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren-Oruklu M, Cinar A, Quinn L, Smith D. Adaptive control strategy for regulation of blood glucose levels in patients with type 1 diabetes. J Process Cont. 2009;19:1333–1346. [Google Scholar]
- Finan DA, Zisser H, Jovanovi3 L, Bevier WC, Seborg DE. Practical issues in the identification of empirical models from simulated type 1 diabetes data. Diabetes Technol Ther. 2007;9(5):438–450. doi: 10.1089/dia.2007.0202. [DOI] [PubMed] [Google Scholar]
- Finan DA, Doyle FJ, III, Palerm CC, Bevier WC, Zisser HC, Jovanovic L, Seborg DE. Experimental Evaluation of a Recursive Model Identification Technique for Type 1 Diabetes. J Diabetes Sci Technol. 2009;3:1192–1202. doi: 10.1177/193229680900300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan DA, Zisser H, Jovanovic L, Bevier WC, Seborg DE. Automatic detection of stress states in type 1 diabetes subjects in ambulatory conditions. Ind Eng Chem Res. 2010;49:7843–7848. doi: 10.1021/ie901891c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S, Brazg R, Bailey TS, Buckingham BA, Slover RH, Klonoff DC, Shin J, Welsh JB, Kaufman FR. Reduction in duration of hypoglycemia by automatic suspension of insulin delivery: the in-clinic ASPIRE study. Diabetes Technology and Therapeutics. 2012;14(3):205–209. doi: 10.1089/dia.2011.0292. [DOI] [PubMed] [Google Scholar]
- Gopakumaran B, Duman HM, Overholser DP, Federiuk IF, Quinn MJ, Wood MD, Ward WK. A novel insulin delivery algorithm in rats with type 1 diabetes: the fading memory proportional-derivative method. Artificial Organs. 2005;29(8):599–607. doi: 10.1111/j.1525-1594.2005.29096.x. [DOI] [PubMed] [Google Scholar]
- Grosman B, Dassau E, Zisser HC, Jovanovic L, Doyle FJ., III Zone model predictive control: A strategy to minimize hyper- and hypoglycemic events. J Diabetes Sci Technol. 2010;4:961–75. doi: 10.1177/193229681000400428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RA, Wang Y, Grosman B, Percival MW, Bevier W, Finan DA, Zisser H, Seborg DE, Jovanovic L, Doyle FJ, III, Dassau E. The quest for the artificial pancreas: Combining technology with treatment. IEEE Eng Med Biol. 2010 Mar-Apr;:53–62. doi: 10.1109/MEMB.2009.935711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RA, Dassau E, Zisser HC, Bevier W, Seborg DE, Jovanovic L, Doyle FJ., III Clinically relevant hypoglycemia prediction metrics for event mitigation. Diabetes Technol Ther. 2012;14(8):719–727. doi: 10.1089/dia.2011.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann L, Benesch C, DeVries JH AP@home consortium. AP@home: A novel European approach to bring the artificial pancreas home. J Diabetes Sci Technol. 2011;5(6):1363–1372. doi: 10.1177/193229681100500607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann L, Krinelke L. Insulin infusion set: the Achilles Heel of continuous subcutaneous insulin infusion. J Diabetes Sci Technol. 2012;6(4):954–964. doi: 10.1177/193229681200600429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton KL, Ratner BD, Wisniewski NA. Biomechanics of the sensor-tissue interface—Effects of motion, pressure, and design on sensor performance and the foreign body response—Part I: Theoretical framework. J Diabetes Sci Technol. 2011;5(3):632–646. doi: 10.1177/193229681100500317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton KL, Ratner BD, Wisniewski NA. Biomechanics of the sensor-tissue interface—Effects of motion, pressure, and design on sensor performance and the foreign body response—Part II: Examples and application. J Diabetes Sci Technol. 2011;5(3):647–656. doi: 10.1177/193229681100500318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero P, Calm R, Vehi J, Armengol J, Georgiou P, Oliver N, Tomazou C. Robust fault detection system for insulin pump therapy using continuous glucose monitoring. J Diabetes Sci Technol. 2012;6(5):1131–1141. doi: 10.1177/193229681200600518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch IB. Treatment of patients with severe insulin deficiency: what we have learned over the past 2 years. Am J Med. 2004;116(3A):17S–22S. doi: 10.1016/j.amjmed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Hirsch IB. Low glucose suspend: ready for primer time? Diabetes Technol Ther. 2012;14(3):201–202. doi: 10.1089/dia.2012.0036. [DOI] [PubMed] [Google Scholar]
- Hovorka R, Allen JM, Elleri D, Chassin LJ, Harris J, Xing D, Kollman C, Hovorka T, Larsen AMF, Nodale M, De Palma A, Wilinska ME, Acerini CL, Dunger DB. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet. 2010;375:743–751. doi: 10.1016/S0140-6736(09)61998-X. [DOI] [PubMed] [Google Scholar]
- Hovorka R. Closed-loop insulin delivery: from bench to clinical practice. Nat Rev Endocrinol. 2011;7(7):385–395. doi: 10.1038/nrendo.2011.32. [DOI] [PubMed] [Google Scholar]
- Hovorka R. The Future of Continuous Glucose Monitoring: Closed Loop. Current Diabetes Reviews. 2008;4:269–279. doi: 10.2174/157339908785294479. [DOI] [PubMed] [Google Scholar]
- Hovorka R, Canonico V, Chassin LJ, Haueter U, Massi-Benedetti M, Federici MO, Pieber TR, Schaller HC, Schaupp L, Vering T, Wilinska ME. Nonlinear model predictive control of glucose concentration insubjects with type 1 diabetes. Physiol Meas. 2004a;25:905–920. doi: 10.1088/0967-3334/25/4/010. [DOI] [PubMed] [Google Scholar]
- Hovorka R, Chassin LJ, Wilinska ME, Canonico V, Akwi JA, Federici O, Massi-Benedetti M, Hutzli I, Zaugg C, Kaufmann H, Both M, Vering T, Schaller HC, Schaupp L, Bodenlenz M, Pieber TR. Closing the loop: the Adicol experience. Diabetes Technol T her. 2004b;6:307–318. doi: 10.1089/152091504774197990. [DOI] [PubMed] [Google Scholar]
- Hughes CS, Patek SD, Breton M, Kovatchev BP. Anticipating the next meal using meal behavioral profiles: A hybrid model-based stochastic predictive control algorithm for T1DM. Comp Meth Prog Biomed. 2011;102(2):138–148. doi: 10.1016/j.cmpb.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CS, Patek SD, Breton MD, Kovatchev BP. Hypoglycemia prevention via pump attenuation and red-yellow-green “traffic” lights using continuous glucose monitoring and infusion pump data. J Diabetes Sci Tech. 2010;4(5):1146–1155. doi: 10.1177/193229681000400513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes-Karvetski C, Patek SD, Breton MD, Kovatchev BP. Historical data enhances safety supervision system performance in T1DM insulin therapy risk management. Comp Methods Prog Biomed. 2012 doi: 10.1016/j.cmpb.2011.12.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–76. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- Kapitza C, Hovelmann U, Nosek L, Kurth H-J, Essenpreis M, Heinemann L. Continuous glucose monitoring during exercise in patients with type 1 diabetes on continuous subcutaneous insulin infusion. J Diabetes Sci Technol. 2010;4(1):123–131. doi: 10.1177/193229681000400116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith-Hynes P, Patek SD, Breton MD, Kovatchev BP. Ambulatory artificial pancreas platform (AAPP): design concepts and prototype demonstration. Presented at the 5th Int. Conf. on Adv. Tech. Treat. Diabetes (ATTD); Barcelona. February 2012. [Google Scholar]
- Klonoff DC. The current status of bolus calculator decision-support software. J Diabetes Sci Technol. 2012;6(5):990–994. doi: 10.1177/193229681200600501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovatchev BP, Breton M, Dalla Man C, Cobelli C. In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes. Diabetes Sci Technol. 2009;3(1):44–55. doi: 10.1177/193229680900300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovatchev B, Cobelli C, Renard E, Anderson S, Breton M, Patek S, Clarke W, Bruttomesso D, Maran A, Costa S, Avogaro A, Dalla Man C, Facchinetti A, Magni L, De Nicolao G, Place J, Farret A. Multinational study of subcutaneous model-predictive closed-loop control in type 1 diabetes mellitus: Summary of results. J Diab Sci Technol. 2010;4:1374–1381. doi: 10.1177/193229681000400611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovatchev B, Patek S, Dassau E, Doyle FJ, III, Magni L, De Nicolao G, Cobelli C the Juvenile Diabetes Research Foundation Artificial Pancreas Consortium. Control to Range for Diabetes: Functionality and Modular Architecture. J Diabetes Sci Technol. 2009;3(5):1058–1065. doi: 10.1177/193229680900300509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke W. Symmetrization of the blood glucose measurement scale and its applications. Diabetes Care. 1997;20(11):1655–8. doi: 10.2337/diacare.20.11.1655. [DOI] [PubMed] [Google Scholar]
- Kowalski AJ. Can we really close the loop and how soon; accelerating the availability of an artificial pancreas: a roadmap to better diabetes outcomes. Diabetes Tech Ther. 2009;11(S1):S113–S119. doi: 10.1089/dia.2009.0031. [DOI] [PubMed] [Google Scholar]
- Kumareswaran K, Evans ML, Hovorka R. Artificial pancreas: an emerging approach to treat Type 1 diabetes. Expert Rev Med Dev. 2009;6(4):401–410. doi: 10.1586/erd.09.23. [DOI] [PubMed] [Google Scholar]
- Landt KW, Campaigne BN, James FW, Sperling MA. Effects of exercise training on insulin sensitivity in adolescents with type 1 diabetes. Diabetes Care. 1985;8(5):461–465. doi: 10.2337/diacare.8.5.461. [DOI] [PubMed] [Google Scholar]
- Lee H, Bequette BW. A Closed-loop Artificial Pancreas based on MPC: human-friendly identification and automatic meal disturbance rejection. Biomedical Signal Processing and Control. 2009;4:347–354. [Google Scholar]
- Lee H, Buckingham BA, Wilson DM, Bequette BW. A Closed-loop Artificial Pancreas Using Model Predictive Control and a Sliding Meal Size Estimator. J Diabetes Sci Tech. 2009;3(5):1082–1090. doi: 10.1177/193229680900300511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutseiko M, Voskanyan G, Keenan DB, Steil GM. Closed-loop insulin delivery utilizing pole placement to compensate for delays in subcutaneous insulin delivery. J Diabetes Sci Technol. 2011;5(6):1342–1451. doi: 10.1177/193229681100500605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magni L, Raimondo DM, Bossi L, Della Man C, De Nicolao G, Kovatchev B, Cobelli C. Model predictive control of type 1 diabetes: an in silico trial. J Diabetes Sci Technol. 2007;1(6):804–12. doi: 10.1177/193229680700100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti G, Barolo M, Jovanovic L, Zisser H, Seborg D. An improved PID switching strategy for type 1 diabetes. IEEE Trans Biomed Eng. 2008;55(3):857–865. doi: 10.1109/TBME.2008.915665. [DOI] [PubMed] [Google Scholar]
- Marchetti G, Barolo M, Jovanovic L, Zisser H, Seborg D. A feedforward-feedback glucose control strategy for type 1 diabetes mellitus. J Process Cont. 2008;18:149–162. doi: 10.1016/j.jprocont.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauseth R, Wang Y, Dassau E, Kircher R, Matheson D, Zisser H, Jovanovic L, Doyle F. Proposed clinical application for tuning fuzzy logic controller of artificial pancreas utilizing a personalization factor. J Diabetes Sci Technol. 2010;4:913–922. doi: 10.1177/193229681000400422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Nimri R, Atlas E, Grunberg EA, Phillip M. Automatic Learning Algorithm for the MD-Logic Artificial Pancreas System. Diabetes Technol Ther. 2011;13(10):983–990. doi: 10.1089/dia.2010.0216. [DOI] [PubMed] [Google Scholar]
- Moser EG, Morris AA, Garg SK. Emerging diabetes therapies and technologies. Diabetes Research and Clinical Practice. 2012;97:16–26. doi: 10.1016/j.diabres.2012.01.027. [DOI] [PubMed] [Google Scholar]
- Nimri R, Atlas E, Ajzensztejn M, Miller S, Oron T, Phillip M. Feasibility study of automated overnight closed-loop glucose control under MD-Logic artificial pancreas in patients with Type 1 Diabetes: The DREAM Project. Diabetes Technology & Therapeutics. 2012;14(8):728–735. doi: 10.1089/dia.2012.0004. [DOI] [PubMed] [Google Scholar]
- O’Grady MJ, John PM, Winn A. [accessed 20 September 2011.];Changes in Medicare Spending for Type 1 Diabetes with the Introduction of the Artificial Pancreas. 2011 Jun 9; http://www.jdrf.org/files/General_Files/APP/Changes_in_Medicare_Spending_for_Type1.pdf.
- Palerm CC, Bequette BW. Hypoglycemia Detection and Prediction Using Continuous Glucose Monitoring- A Study on Hypoglycemic Clamp Data. J Diabetes Sci Technol. 2007;1:617–623. doi: 10.1177/193229680700100505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palerm CC. Physiologic insulin delivery with insulin feedback: a control systems perspective. Comp Meth Prog Biomed. 2011;102(2):130–137. doi: 10.1016/j.cmpb.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Patek SD, Magni L, Dassau E, Karvetski CH, Toffanin C, de Nicolao G, del Favero S, Breton M, Dalla Man C, Renard E, Zisser H, Doyle FJ, III, Cobelli C, Kovatchev BP. Modular closed-loop control of diabetes. IEEE Trans Biomed Eng. 2012 doi: 10.1109/TBME.2012.2192930. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patek SD, Bequette BW, Breton M, Buckingham BA, Dassau E, Doyle FJ, Lum J, Magni L, Zisser H. In Silico Preclinical Trials: Methodology and Engineering Guide to Closed-Loop Control. J Diabetes Sci Technol. 2009;3:269–282. doi: 10.1177/193229680900300207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival MW, Wang Y, Grosman B, Dassau E, Zisser H, Jovanovic L, Doyle FJ., III Development of a multi-parametric model predictive control algorithm for insulin delivery in type 1 diabetes mellitus using clinical parameters. J Process Control. 2011;21(3):391–404. doi: 10.1016/j.jprocont.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival MW, Bevier WC, Wang Y, Dassau E, Zisser H, Jovanovic L, Doyle FJ., III Modeling the Effects of Subcutaneous Insulin Administration and Carbohydrate Consumption on Blood Glucose. J Diabetes Sci Technol. 2010;4(5):1214–1228. doi: 10.1177/193229681000400522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkos A, Arreaza-Rubin G, Heetderks WJ, Irony I, Joffe HV, Schneider B, Zimliki CL. FDA’s proactive role in the development of an artificial pancreas for the treatmentof diabetes mellitus. Drug Discovery Today : Technologies. 2007;4(1):25–28. doi: 10.1016/j.ddtec.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Renard E, Place J, Cantwell M, Chevassus H, Palerm CC. Closed-loop insulin delivery using a subcutaneous glucose sensor and intraperitoneal insulin delivery. Diabetes Care. 2010;33(1):121–127. doi: 10.2337/dc09-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell M, Perkins BA. Exercise and glucose metabolism in persons with diabetes mellitus: perspectives on the role for continuous glucose monitoring. J Diabetes Sci Technol. 2009;3(4):914–923. doi: 10.1177/193229680900300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas R, Garcia-Gabin W, Bequette BW. Multivariate Statistical Analysis Approaches for Detection of Insulin Infusion Set Failures. Computer Methods and Programs in Biomedicine. 2012 submitted. [Google Scholar]
- Rojas R, Garcia-Gabin W, Bequette BW. Multivariate Statistical Analysis to Detect Insulin Infusion Set Failure. Proceedings of the 2011 American Control Conference; San Francisco. 2011a. pp. 1952–7. [Google Scholar]
- Rojas R, Garcia-Gabin W, Bequette BW. Mean Glucose Slops – Principal Component Analysis Classification to Detect Insulin Infusion Set Failure. preprints of the 18th IFAC World Congress; Milano. 2011b. pp. 14127–14132. [Google Scholar]
- Roy A, Parker RS. Dynamic modeling of exercise effects on plasma glucose and insulin levels. J Diabetes Sci Technol. 2007;1(3):338–347. doi: 10.1177/193229680700100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz JL, Sherr J, Cengiz E, Carria L, Roy A, Voskanyan G, Tamborlane WV, Weinzimer SA. Effect of insulin feedback on closed-loop glucose control: a crossover study. J Diabetes Sci Technol. 2012;6(5):1123–1130. doi: 10.1177/193229681200600517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaepelynck P, Darmon P, Molines L, Jannot-Lamotte MF, Treglia C, Faccah D. Advances in pump technology: insulin patch pumps, combined pumps and glucose sensors, and implanted pumps. Diabetes & Metabolism. 2011;37:S85–S93. doi: 10.1016/S1262-3636(11)70972-7. [DOI] [PubMed] [Google Scholar]
- Scheiner G, Sobel RJ, Smith DE, Pick A, Kruger D, King J, Green K. Insulin pump therapy: guidelines for successful outcomes. Presented at the American Association of Diabetes Educations 2008 Consensus Summit; September 18, 2008; [assessed 27 April 2012]. Available at: http://www.diabeteseducator.org/export/sites/aade/_resources/pdf/Insulin_Pump_White_Paper.pdf. [Google Scholar]
- Schmidt S, Finan DA, Duun-Henriksen AK, Jorgensen JB, Madsen H, Bengtsson H, Holst JJ, Madsbad S, Norgaard Effects of everyday life events on glucose, insulin and glucagon dynamics in continuous subcutaneous insulin infusion—treated Type 1 diabetes: collection of clinical data for glucose modeling. Diabetes Tech Ther. 2012;14(3):210–217. doi: 10.1089/dia.2011.0101. [DOI] [PubMed] [Google Scholar]
- Skyler JS, Ponder S, Kruger DF, Mathesan D, Parkin CG. Is there a place for insulin pump therapy in your practice? Clinical Diabetes. 2007;25(2):50–56. [Google Scholar]
- Sherr J, Cengiz E, Tamborlane WV. From pumps to prevention: recent advances in the treatment of type 1 diabetes. Drug Discovery Today. 2009;14(19/20):973–981. doi: 10.1016/j.drudis.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Soru P, De Nicolao G, Toffanin C, Dalla Man C, Cobelli C, Magni L. MPC based Artificial Pancreas: strategies for individualization and meal compensation. Annual Reviews in Control. 2012;36:118–128. [Google Scholar]
- Steil GM, Palerm CC, Kurtz N, Voskanyan G, Roy A, Paz S, Kandeel FR. The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab. 2011;96(5):1402–1408. doi: 10.1210/jc.2010-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steil GM, Panteleon AE, Rebrin K. Closed-loop insulin delivery—the path to physiological glucose control. Drug Delivery Reviews. 2004;56:125–144. doi: 10.1016/j.addr.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Tamborlane WV. Closed-loop insulin delivery: we’re “virtually” there. Diabetes Technol Ther. 2012;14(3):203–204. doi: 10.1089/dia.2011.0261. [DOI] [PubMed] [Google Scholar]
- Van Bon A, Jonker LD, Doebrugge R, Koops R, Hoekstra JBL, DeVries JH. Feasibility of a bihormonal closed-loop system to control postexercise and postprandial glucose excursions. J Diabetes Sci Technol. 2012;6(5):1114–1122. doi: 10.1177/193229681200600516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bon AC, Verbitskiy E, von Basum G, Hoekstra JBL, DeVries JH. Exercise in closed-loop control: a major hurdle. J Diabetes Sci Technol. 2011;5(6):1337–1341. doi: 10.1177/193229681100500604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bon AC, Hermanides J, Koops JBL, Hoekstra JH, DeVries Postprandial glycemic excursions with the use of a closed-loop platform in subjects with type 1 diabetes: a pilot study. J Diabetes Sci Techol. 2010;4(4):923–928. doi: 10.1177/193229681000400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heusden K, Dassau E, Zisser HC, Seborg DE, Doyle FJ., III Control-relevant models for glucose control using a priori patient characteristics. IEEE Trans Biomed Eng. 2012;59(7):1839–1849. doi: 10.1109/TBME.2011.2176939. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dassau E, Doyle FJ., III Closed-loop control of artificial pancreatic b-cell in type 1 diabetes mellitus using model predictive iterative learning control. IEEE Trans Biomed Eng. 2010;57(2):211–219. doi: 10.1109/TBME.2009.2024409. [DOI] [PubMed] [Google Scholar]
- Ward WK, Castle J, El-Youssef J. Safe glycemic management during closed-loop treatment of type 1 diabetes: the role of glucagon, use of multiple sensors, and compensation for stress hyperglycemia. J Diabetes Sci Technol. 2011;5(6):1373–1380. doi: 10.1177/193229681100500608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward WK, Engle J, Duman HM, Bergstrom CP, et al. The Benefit of Subcutaneous Glucagon During Closed-Loop Glycemic Control in Rats With Type 1 Diabetes. IEEE Sensors. 2008;8:89–96. [Google Scholar]
- Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in prediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31(5):934–939. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- Welsh JB, Vargas S, Williams G, Moberg S. Designing the modern pump: engineering aspects of continuous subcutaneous insulin infusion software. Diabetes Technol Ther. 2010;12(Suppl 1):S-37–S-42. doi: 10.1089/dia.2009.0188. [DOI] [PubMed] [Google Scholar]
- Wilinska ME, Chassin LJ, Acerini CL, Allen JM, Dunger DB, Hovorka R. Simulation Environment to Evaluate Closed-Loop Insulin Delivery Systems in Type 1 Diabetes. J Diabetes Sci Technol. 2010;4:132–144. doi: 10.1177/193229681000400117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilinska ME, Budiman ES, Taub MB, Elleri D, Allen JM, Acerini CL, Dunger DB, Hovorka R. Overnight Closed-Loop Insulin Delivery with Model Predictive Control: Assessment of Hypoglycemia and Hyperglycemia Risk Using Simulation Studies. J Diabetes Sci Technol. 2009;3:1109–1120. doi: 10.1177/193229680900300514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe RR, Nadel ER, Shaw JH, Stephenson LA, Wolfe MH. Role of changes in insulin and glucagon in glucose homeostatis in exercise. J Clin Invest. 1986;77(3):900–907. doi: 10.1172/JCI112388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac J, Shrestha A, Patel P, Poretsky . The main events in the history of diabetes. In: Poretsky L, editor. Principles of Diabetes Mellitus. 2. Vol. 1. Springer; 2010. pp. 3–18. [Google Scholar]
- Zisser H, Robinson L, Bevier W, Dassau E, Ellingsen C, Doyle FJ, III, Jovanovic L. Bolus calculator: a review of four “smart” insulin pumps. Diabetes Technol Ther. 2008;10(6):441–4. doi: 10.1089/dia.2007.0284. [DOI] [PubMed] [Google Scholar]
- Zisser HC. The OmniPod Insulin Management System: the latest innovation in insulin pump therapy. Diabetes Technol Ther. 2010;1(1):10–24. doi: 10.1007/s13300-010-0004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]