Abstract
The Amplex Red assay, a fluorescent assay for the detection of H2O2, relies on the reaction of H2O2 and colorless, nonfluorescent Amplex Red with a 1:1 stoichiometry to form colored, fluorescent resorufin, catalyzed by horseradish peroxidase (HRP). We have found that resorufin is artifactually formed when Amplex Red is exposed to light. In the absence of H2O2 and HRP, the absorption and fluorescence spectra of Amplex Red changed during exposure to ambient room light or instrumental excitation light, clearly indicating that the fluorescent product resorufin had formed. This photochemistry was initiated by trace amounts of resorufin that are present in Amplex Red stock solutions. ESR spin-trapping studies demonstrated that superoxide radical was an intermediate in this process. Oxygen consumption measurements further confirmed that superoxide and H2O2 were artifactually produced by the photooxidation of Amplex Red. The artifactual formation of resorufin was also significantly increased by the presence of superoxide dismutase or HRP. This photooxidation process will result in a less sensitive assay for H2O2 under ambient light exposure and potentially invalid measurements under high energy exposure such as UVA irradiation. In general, precautions should be taken to minimize exposure to light during measurement of oxidative stress with Amplex Red.
Keywords: Amplex Red, Resorufin, Photooxidation, Free radical, Superoxide, Hydrogen peroxide
Introduction
Fluorescent probes provide a convenient, sensitive and versatile means for detecting reactive oxygen species (ROS) in cells and tissues. Usually these compounds are nonfluorescent or weakly fluorescent and yield highly fluorescent products upon reaction with ROS, which can be measured with a spectrophotometer, microplate reader, confocal microscope or flow cytometer. Confocal microscopy also offers the possibility of observing the subcellular distribution of ROS generation. While these probes can be highly sensitive, there are concerns about their specificity [1, 2] and the potential for artifactual formation of ROS caused by addition of the fluorescent probe to a biological sample in the presence of light [3, 4].
Amplex Red is a colorless and nonfluorescent compound that is used as a probe for the measurement of extracellular H2O2, but because H2O2 is freely diffusible, this measurement is an indication of cellular H2O2 production. Amplex Red reacts with H2O2 at 1:1 stoichiometic ratios catalyzed by horseradish peroxidase (HRP) to form the colored and highly fluorescent compound resorufin [5]. Amplex Red can also be used to measure peroxidase activity and can detect as little as 1 × 10−5 U/mL HRP or, for H2O2, as little as 50 nM H2O2 [5]. However, at high H2O2 concentrations the concentration of Amplex Red becomes limiting and resorufin can be further oxidized by HRP to nonfluorescent, colorless product(s) [6–8]. In addition, the concentration of H2O2 can be underestimated if a biological sample contains high levels of other peroxidase substrates, which may be endogenous compounds or exogenous compounds such as drugs, as these will compete with Amplex Red for oxidation by H2O2/HRP, resulting in less H2O2 available for the oxidation of Amplex Red [8].
HRP catalyzes the oxidation of Amplex Red via two one-electron oxidation steps in which an Amplex Red radical intermediate is formed [9]. The concentration of H2O2 can be underestimated if ascorbate or reduced superoxide reductase is present as they can inhibit resorufin formation by reacting with the Amplex Red radical intermediate, leading to the formation of ascorbyl radical and oxidized superoxide reductase, respectively [10]. In contrast, the presence of reductants such as NADH and GSH in a biological sample can also result in erroneously high resorufin formation because the radicals NAD• and GS• can be formed, which can then react with oxygen in air-saturated solutions to produce superoxide radical (O2• −), which can in turn dismutate, leading to artifactual generation of H2O2 [3, 11]. Minchin and co-workers also observed this effect with NADPH, but this accounted for only a minor proportion (2–5%) of the fluorescence in their Amplex Red assay [12]. Of greater concern was the exponentially increasing fluorescence generated in the presence of NADPH and NADPH-cytochrome P450 reductase when the Amplex Red assay was measured continuously but not when single measurements were taken [12]. This was attributed to the ability of resorufin to redox cycle with NADPH-cytochrome P450 reductase [13, 14]. However, one obvious difference between continuous measurements and single measurements is the duration of light exposure from a fluorescence spectrometer or platereader, which suggests that photochemistry is occurring in the Amplex Red samples subjected to continuous measurements. Such photochemistry is possible and has been described by our previous work [3]. Resorufin (RSF) in the presence of NADH and exposure to light is reduced to RSF• −, which can react with dissolved oxygen leading to the regeneration of resorufin and the formation of O2 • −.
In the current work, we have investigated the effect of exposing Amplex Red to light in the presence and absence of HRP and H2O2. Our results clearly show that resorufin is formed by exposing Amplex Red to light and that this photochemistry is initiated by trace amounts of resorufin present in Amplex Red stock solutions. This effect is dependent on the duration of exposure to light, the intensity of light and the presence of oxygen in solution. O2 • − was identified as an intermediate in this process. We also investigated the potential problems arising from this photochemistry by assessing the efficacy of the Amplex Red assay in measuring H2O2 released by cells exposed to UVA irradiation.
Materials and methods
Chemicals
Amplex Red, Amplex Red hydrogen peroxide/peroxidase assay kit and resorufin were purchased from Invitrogen Co. (Carlsbad, CA). Diethylenetriaminepentaacetic acid (DTPA) and catalase (from bovine liver, 40,200 U/mg) were purchased from Sigma-Aldrich, Inc. (St. Louis, MO). Superoxide dismutase (from bovine kidney, 11,000 U/mg) was purchased from Calzyme Laboratories, Inc. (San Luis Obispo, CA). The spin trap dimethyl-1-pyrroline N-oxide (DMPO) was purchased from Dojindo Molecular Technologies, Inc. (Japan) and was used as received. Chelex-100 resin was purchased from Bio-Rad Laboratories (Hercules, CA). All the samples were freshly prepared in 100 mM phosphate buffer at pH 7.4 except for the samples for ESR measurements, which were prepared at a higher pH. The buffer was treated with Chelex-100 resin for 24 h to remove traces of transition metal ions. After filtration, 25 µM DTPA was added to minimize the possibility of trace metal interference.
Light absorption measurements
The solution containing 10 µM Amplex Red was irradiated in an open quartz cuvette with continuous stirring. The absorbance measurements were carried out on an Agilent 8453 UV-visible spectrophotometer (Agilent Technologies, Inc., Santa Clara, CA) every minute. A 450 W xenon-mercury lamp was used as the light source. Various glass filters were used to cut off wavelengths below 300, 400, and 520 nm according to the requirements of the experiments.
Fluorescence measurements
For continuous measurements, the fluorescence emission at 585 nm was continuously measured with an excitation wavelength at 520 nm in an open quartz cuvette on an FL3-22 spectrofluorometer (Horiba, Edison, NJ).
Oxygen consumption experiments
Oxygen consumption measurements were made using a Clark-type oxygen electrode fitted to a 1.8 ml Gilson sample cell with a stirrer and monitored with a Model 53 Biological oxygen monitor (Yellow Springs Instrument Co., Yellow Springs, OH). Oxygen concentration in the samples as a function of time was recorded by a PC interfaced to the oxygen monitor with a Data Translation DT2801 data acquisition board. All experiments were performed at room temperature, and the incubation conditions were as described in the figure legends.
Electron spin resonance (ESR) spectroscopy
All ESR spectra were recorded at room temperature in a quartz flat cell on a Bruker EMX ESR spectrometer equipped with a super high-Q cavity (Bruker, Billerica, MA). The instrument settings and conditions were as described in the figure legends. Illumination of the sample was carried out directly inside the microwave cavity of the spectrometer using a 340 W slide projector lamp. The fluence rate was 13 mW/cm2 as measured with an SPR-4001 Spectroradiometer (Luzchem Research Inc., Ottawa, Ontario, Canada).
Amplex Red Assay
Reactions containing 50 µM Amplex Red reagent, 0.1 U/mL HRP, H2O2 and 0.5 U/mL catalase in 50 mM sodium phosphate buffer, pH 7.4, as indicated in the figure legends, were prepared in a darkroom under a red light. White ELISA plates containing the samples (100 µL/well) in triplicate were either kept in the dark for 30 and 60 min or under ambient laboratory light. The fluorescence (excitation 535 nm, emission 595 nm) was measured on a GENios platereader (Tecan, Durham, NC, USA). The limit of detection (LoD) for each standard curve was determined by first calculating the limit of blank (LoB) as follows:
LoB = meanblank + 1.645(SDblank)
LoD = LoB + 1.645(SDlow concentration sample)
where the sample containing a low concentration of analyte was 0.1 µM H2O2.
Cell culture
HaCaT keratinocytes, a transformed epidermal human cell line, were grown at 37°C in DMEM containing 10% fetal bovine serum (FBS) to ~95% confluence in 96-well plates in an atmosphere of 95% air/5% CO2. After incubation with 50 µM Amplex Red in PBS for 1 h at 37°C in the dark, cells were irradiated using UVA (315–400 nm) from four fluorescent PUVA lamps (Houvalite F20T12BL-HO; National Biological Co., Twinsburg, OH) for 15 minutes (3.75 J/cm2) and 30 minutes (7.5 J/cm2). Control cells were kept in the dark. Controls without cells were treated with the same conditions. After exposure, the fluorescence (excitation 535 nm, emission 595 nm) was recorded using a plate reader (Spectrafluor Plus, Tecan US, RTP, NC).
Results
Light absorption measurements
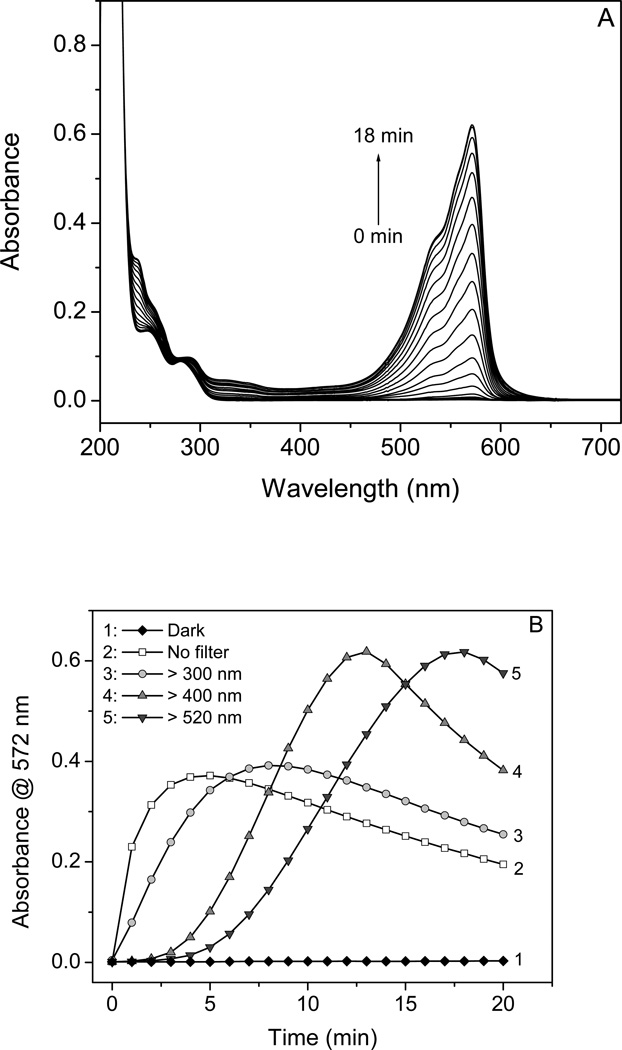
The basis of the Amplex Red assay is the reaction of colorless, nonfluorescent Amplex Red with H2O2 in the presence of HRP to form the colored, fluorescent product resorufin. However, in the absence of H2O2 and HRP, resorufin was also generated when Amplex Red was exposed to a xenon-mercury light (Fig. 1A), as shown by the increase in absorbance at 572 nm. When long-pass filters cutting off at different wavelengths of light were used, the generation of resorufin was approximately proportional to the absorption of Amplex Red (Fig. 1B, line 3–5, >300 nm, >400 nm, >520 nm). The Amplex Red exposed to unfiltered light showed the fastest generation of resorufin (Fig. 1B, line 2), and resorufin was not formed if the Amplex Red was kept in the dark (Fig. 1B, line 1).
Figure 1.
Photo-induced oxidation of Amplex Red with production of resorufin during irradiation as measured by UV-vis absorption. (A) Absorption spectra of 10 µM AR solution at pH 7.4 after irradiation (> 520 nm) for every minute from 0 to 18 min. The arrow indicates direction of the changes. (B) Plot of peak absorbance of RSF at 572 nm against irradiation time (1, dark; 2, no filter; 3, > 300 nm; 4, > 400 nm, and 5, > 520 nm). The fluence rate (from 250 to 850 nm) was 0, 0.392, 0.329, 0.322, and 0.271 W/cm2 as measured with a SPR-4001 Spectroradiometer (Luzchem Research Inc., Ottawa Ontario, Canada).
In each case, the absorbance at 572 nm attributed to resorufin accumulated and then declined with the longer exposure times, suggesting the formation of a photo-bleached oxidation product; this decline occured when Amplex Red was largely consumed and resorufin acts as a peroxidase substrate [6–8].
Fluorescence measurements
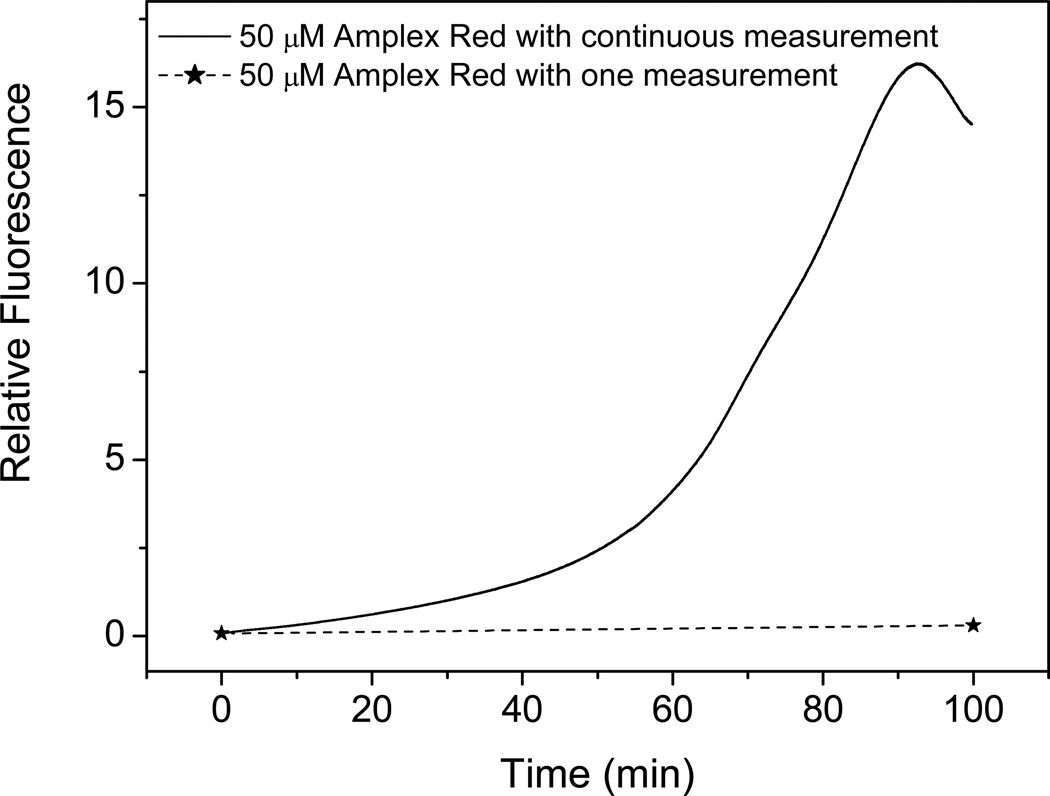
When Amplex Red was exposed to light of 520 nm (excitation wavelength) in a fluorescence spectrometer in the absence of HRP and H2O2, the fluorescence at 585 nm increased, indicating the formation of resorufin when Amplex Red was exposed continuously to light (Fig. 2, solid line). There was no increase in fluorescence when Amplex Red was kept in the dark except for a measurement at the start and end time points (Fig. 2, dashed line). Therefore, resorufin, measured by light absorption (Fig. 1) or fluorescence spectroscopy (Fig. 2), was formed when Amplex Red was exposed to ambient or instrumental light. The rate of this resorufin formation was exponential, suggesting that this process was autocatalytic.
Figure 2.
Fluorescence intensity at 585 nm of 50 µM Amplex Red solution during continuous measurement (solid line) and one measurement at the time of 100 min (dotted line) with an excitation wavelength of 520 nm.
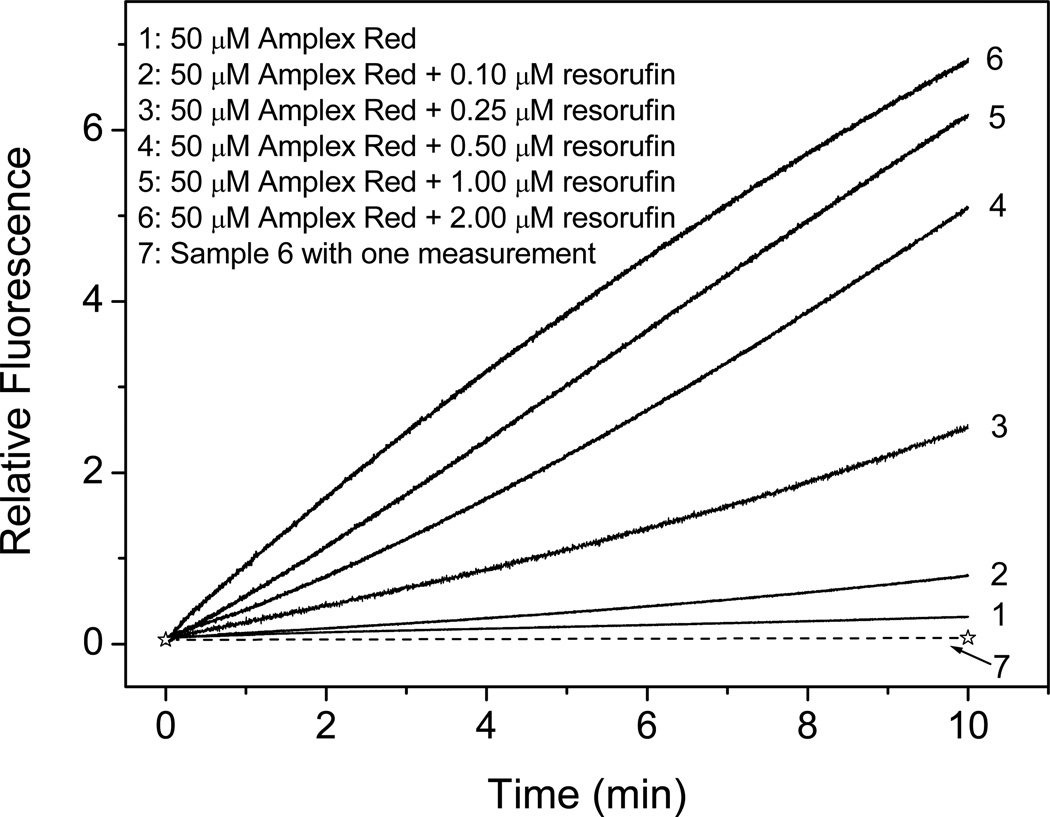
To test the hypothesis that trace amounts of resorufin catalyze the conversion of Amplex Red to resorufin upon exposure to light, we mixed a fixed concentration of Amplex Red with varying low concentrations of resorufin and exposed it to instrumental excitation light, measuring the fluorescence continuously to monitor resorufin formation. The added resorufin increased the rate of Amplex Red photooxidation in proportion to the initial resorufin concentration (Fig. 3). Amplex Red mixed with resorufin but kept in the dark with one measurement at the end time point did not generate more resorufin, indicating that the autooxidation of Amplex Red is dependent on the presence of light and the presence of resorufin.
Figure 3.
Effect of initial concentration of resorufin on Amplex Red (50 µM) fluorescence intensity during continuous measurement (Excitation/Emission: 520 nm/585 nm). Lines 1 to 6 correspond to [RSF] = 0, 0.10, 0.25, 0.50, 1.00, and 2.00 µM. Line 7 is a single measurement at the time of 10 min (dotted line) for a solution containing 50 µM AR and 2.00 µM RSF. The fluorescence intensities for lines 2 to 7 had the background of added resorufin individually subtracted.
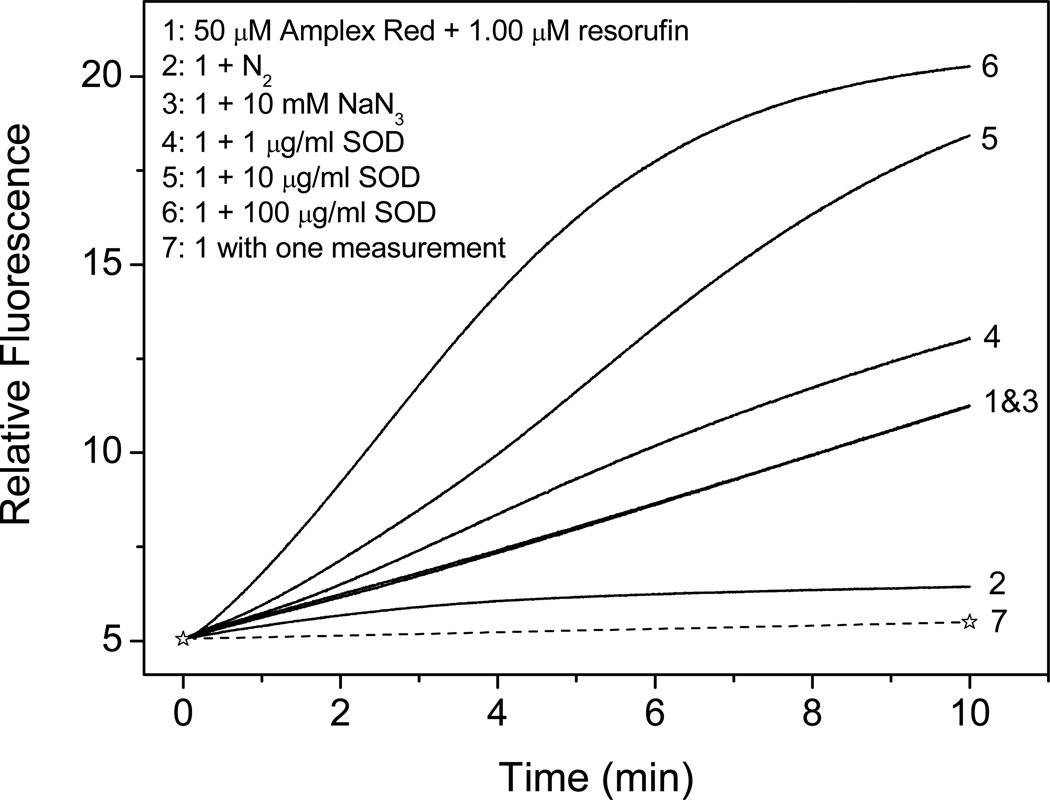
To investigate the role of oxygen, present in air-saturated solutions, in the photooxidation of Amplex Red, we purged the Amplex Red solution with nitrogen and exposed it to light. The removal of oxygen suppressed Amplex Red autooxidation (Fig. 4, compare lines 2 and line 1). Adding azide, which is a classical singlet oxygen quencher, to air-saturated solutions of Amplex Red had no effect on the rate of resorufin formation (Fig. 4, compare lines 1 and 3). Adding superoxide dismutase (SOD), which catalyzes the conversion of O2 • − to H2O2, dramatically increased the oxidation of Amplex Red, and the increase was dependent on the concentration of SOD (Fig. 4, lines 4–6). Taken together, these results suggest that O2• − formed from dissolved oxygen is the intermediate product during the oxidation of Amplex Red.
Figure 4.
Effect of dissolved oxygen, SOD, NaN3 (10 mM, Sample 3), and excitation light on Amplex Red fluorescence intensity during continuous measurement (Excitation/Emission: 520 nm/585 nm). Sample 1, continuous measurement of fluorescence intensity of solution containing 50 µM AR and 1 µM resorufin. Sample 2, same as sample 1, except for removing oxygen by bubbling the solution with N2. Sample 3, same as sample 1, except for the addition of 10 mM NaN3. Samples 4–6, same as sample 1, except for the addition of 1, 10, and 100 µg/ml SOD, respectively. Sample 7, same as sample 1, but with a single measurement at the time of 10 min (dotted line).
ESR measurement of O2• − production
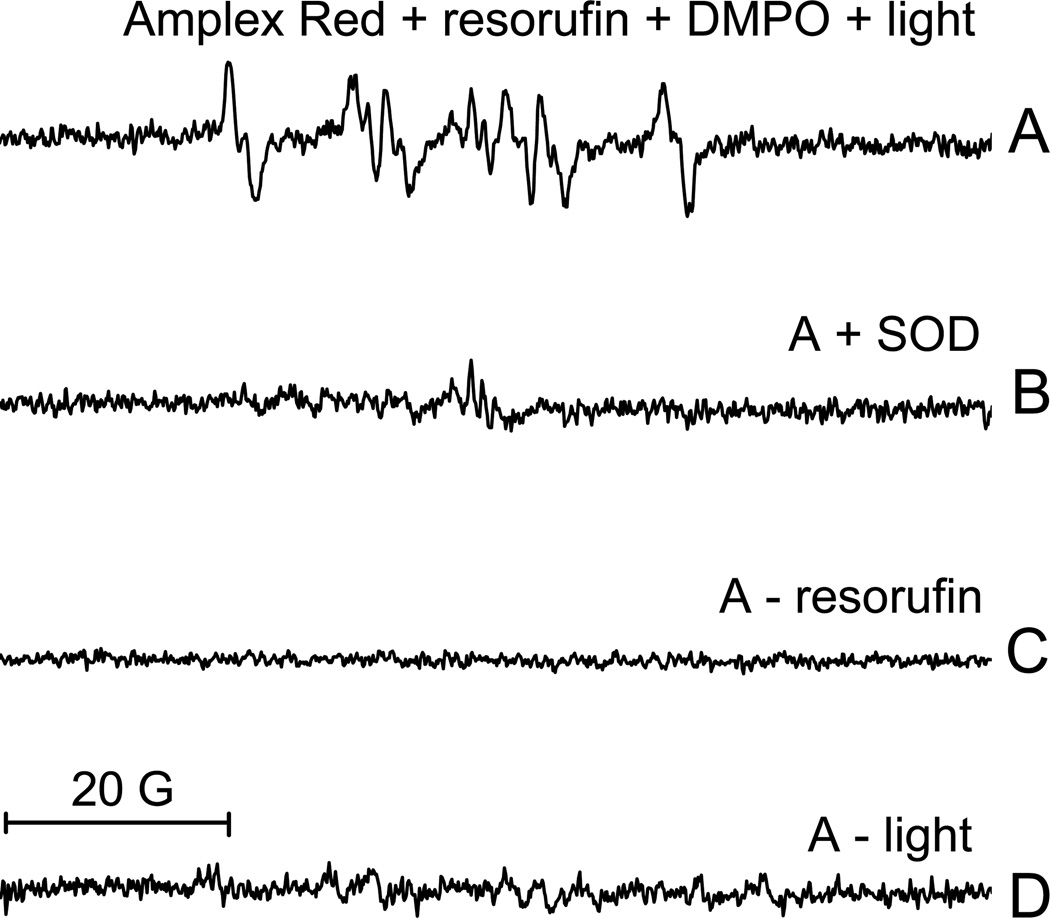
Using ESR and DMPO as a spin trap, we further confirmed that O2 • − is an intermediate during the photooxidation of Amplex Red. When Amplex Red, resorufin and DMPO were exposed to light, the spectrum was characteristic of the DMPO/•OOH radical adduct (Fig. 5A)[15]. This adduct was not detected when samples were prepared in the presence of SOD (Fig. 5B), but adding catalase did not alter the formation of this adduct (data not shown). This indicates that O2 • − but not H2O2 is required for the production of the DMPO/•OOH radical adduct. This adduct was not detected if resorufin was omitted from the mixture (Fig. 5C) or if the mixture was kept in the dark (Fig. 5D). This indicates that the same conditions necessary for the formation of O2 • − are necessary for the photooxidation of Amplex Red, which is consistent with O2 • − being an intermediate in this process.
Figure 5.
ESR spectra of the DMPO-superoxide adduct detected during the irradiation of an aerobic solution containing Amplex Red and resorufin. (A) The ESR spectra obtained from a solution containing 1000 µM Amplex Red , 200 µM resorufin and 100 mM DMPO during the irradiation. (B) Same as A, but with the addition of 100 µg/ml SOD. (C) Same as A, but with the omission of resorufin. (D) Same as A, but with the omission of light illumination. The instrument settings were as follows: microwave power, 20 mW; modulation amplitude, 1 G; time constant, 328 ms; scan time, 336 s; scan range, 100 G; and receiver gain, 1 × 105.
Oxygen consumption measurements of Amplex Red exposed to light
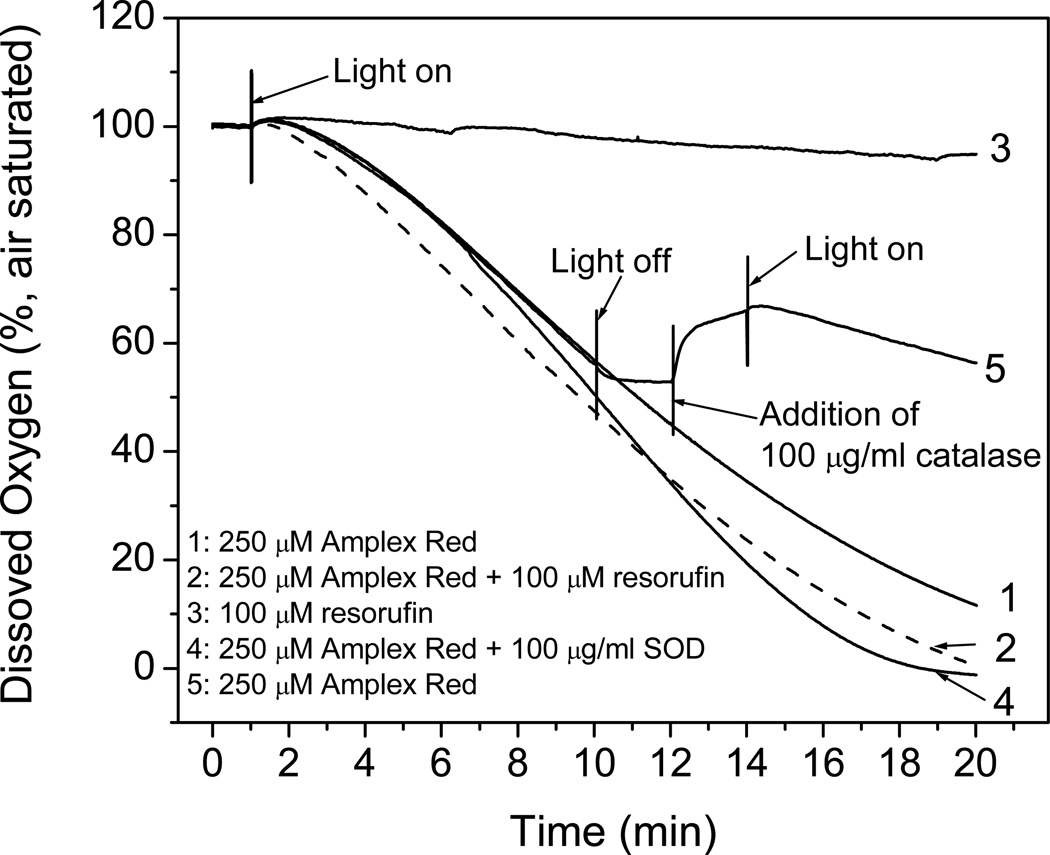
Because removing oxygen from Amplex Red solutions suppressed Amplex Red photooxidation (Fig. 4, line 2 with line 1) and O2 • − was identified as an intermediate (Fig. 5), oxygen consumption experiments were carried out to provide a more complete understanding of this mechanism. Oxygen was rapidly consumed when 250 µM Amplex Red was exposed to light (Fig. 6, line 1). After 20 min of exposure, almost all the oxygen had been consumed (oxygen concentration in air-saturated solutions is ca. 240 –M). The oxygen was consumed more rapidly if a small amount of resorufin was also added (Fig. 6, line 2). The addition of SOD increased the rate even further (Fig. 6, line 4), which is consistent with the fluorescence measurements showing that SOD greatly increased the photooxidation of Amplex Red (Fig. 4, lines 4–6). Oxygen consumption was undetectable when resorufin was exposed to light in the absence of Amplex Red (Fig.6, line 3).
Figure 6.
Oxygen consumption by Amplex Red and the effects of added resorufin, SOD and catalase during illumination. (1) 250 µM AR in 100 mM phosphate buffer at pH 7.4. (2) Same as Line 1, except for the addition of 100 µM resorufin. (3) Same as Line 2, but with the omission of Amplex Red. (4) Same as Line 1, except for the addition of 100 µg/ml SOD (1,100 units/ml). (5) Same as Line 1, except for the light’s being off from the time of 10 to 14 min and the addition of 100 µg/ml catalase (6,030 units/ml) after 12 min.
Amplex Red continuously exposed to light consumes O2, but O2 consumption ceased when the light was turned off, further confirming that this process is dependent on light (Fig. 6, line 5). When catalase was added to this mixture, O2 evolution was detected immediately, indicating that H2O2 had been present and converted to oxygen and water by catalase. With catalase present and further exposure to light, the rate of oxygen consumption was half of that in the absence of catalase. These results indicate that oxygen is converted to O2 • − during the photooxidation of Amplex Red. This O2 • − can spontaneously dismutate in the absence of superoxide dismutase, forming H2O2 or alternatively, SOD can accelerate the formation of H2O2 from O2 • − when present.
Amplex Red exposed to light in the presence of HRP and H2O2
The light-induced formation of resorufin from Amplex Red in the absence of H2O2 and HRP (Figs. 1–6) represents a potential confounding factor in the use of the Amplex Red assay. As the Amplex Red assay also contains HRP and H2O2, the effect of light was investigated under these conditions.
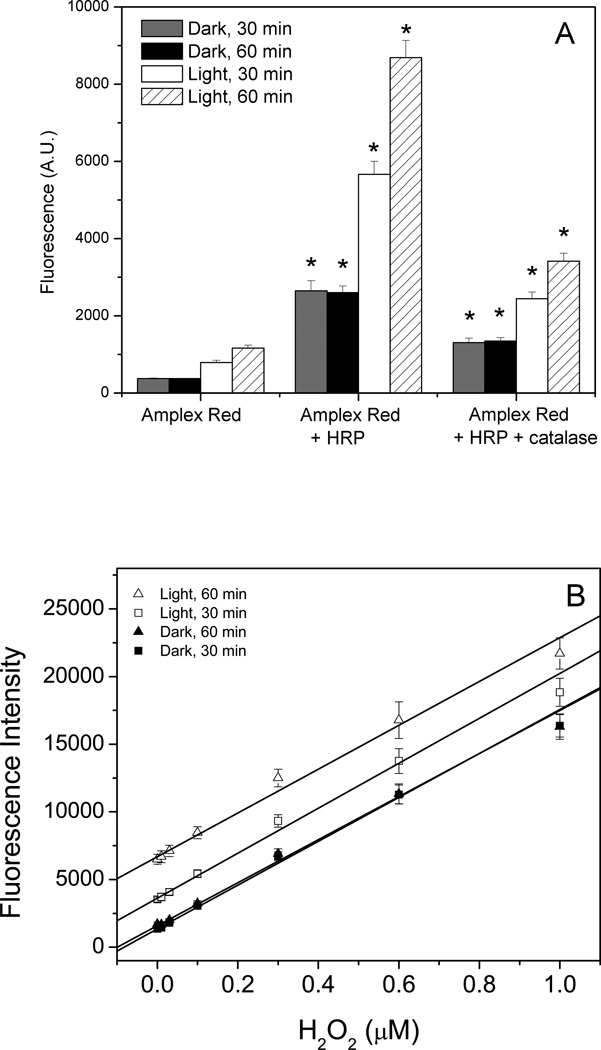
Since the ESR (Fig. 5) and oxygen consumption experiments (Fig. 6) showed that the photooxidation of Amplex Red involved O2 • − as an intermediate (Fig. 5), this raised the possibility that O2 •−, dismutated to H2O2, could serve as a substrate for HRP when Amplex Red was exposed to light in the presence of HRP. To test this possibility, Amplex Red was incubated with HRP in the absence of H2O2 and exposed to ambient room light (Fig. 7A). The presence of HRP greatly increased the fluorescence compared to Amplex Red alone, which indicated that the photooxidation of Amplex Red in the context of an Amplex Red assay is more problematic than that which occurs with Amplex Red alone. Adding catalase to Amplex Red and HRP partially suppressed this increase in fluorescence, but higher concentrations of catalase could not be used because the color of the catalase interfered with the assay. This suggested that the resorufin resulting from the exposure of Amplex Red and HRP to light was formed in two ways: the photooxidation of Amplex Red and the artifactual generation of H2O2 serving as a substrate for HRP. Incubated in the dark, Amplex Red and HRP had higher fluorescence than Amplex alone although this was far less than that seen with exposure to laboratory lighting (Fig. 7A). This might be attributed to the photooxidation of Amplex Red from the red light (> 600 nm) in the darkroom during sample preparation.
Figure 7.
The effect of light on the the Amplex Red assay. Reactions containing 50 µM Amplex Red and 0.1 U/mL HRP in 50 mM sodium phosphate buffer, pH 7.4, were incubated with and without catalase (panel A) or with H2O2 (panel B) for 30 and 60 min under ambient laboratory lighting or in the dark. The significance of adding HRP to Amplex Red and the significance of adding catalase to mixtures containing Amplex Red and HRP was assessed using the Student’s t-test (* p < 0.05).
Therefore, mixtures containing Amplex Red, HRP and varying concentrations of H2O2 were prepared in a darkroom and then incubated in 96-well plates either in the dark or under normal laboratory lights for 30 minutes or 60 minutes before the fluorescence was measured with a fluorescence plate reader (Fig. 7B). The mixtures incubated in the dark for 30 and 60 min yielded overlapping standard curves, indicating that the Amplex Red assay, when incubated in the dark, is an endpoint assay. In contrast, the mixtures exposed to the light had consistently higher fluorescence measurements than those incubated in the dark, and the final fluorescence measurement was dependent on the length of exposure to light, indicating that the Amplex Red assay incubated in the light is not an endpoint assay.
However, the standard curves for the mixtures incubated in the light were parallel to those obtained in the dark, indicating that the generation of resorufin due to photooxidation of Amplex Red is constant over this range of H2O2 concentrations despite varying amounts of resorufin being formed from the HRP-catalyzed reaction of Amplex Red and H2O2 (Fig.7B). This is different from the experiments done in a fluorescence spectrometer, where small initial differences in resorufin concentration made a large difference in the amount of resorufin formed from the photooxidation of Amplex Red (Fig. 3). This difference was attributed to the lower intensity of laboratory lighting compared to the light from the fluorescence spectrometer.
The limit of detection of H2O2 was calculated for each standard curve and was 19 nM for the samples incubated for 30 min in the dark and 30 nM for samples incubated for 60 min in the dark. The higher limit of detection for the 60-min dark samples, despite overlapping standard curves, was due to the larger standard deviation (error bars) for the 60-min-dark standard curve compared to the 30-min dark standard curve. The limit of detection was 31 nM for the 30-min light standard curve and 61 nM for the 60-min light standard curve, with the larger standard deviations (error bars) for increased time and exposure to light. Thus, it can be seen that exposure to light and extended incubation times (the manufacturer’s instructions indicate an incubation time of 30 min) result in a less sensitive and less accurate assay for H2O2.
Measuring H2O2 production in cells exposed to UVA irradiation
The Amplex Red assay has been used previously to measure H2O2 released from cells exposed to UV light because H2O2 has been implicated as a signaling molecule [16, 17]. To ascertain whether this assay should be used in this system, Amplex Red and HRP were added to cells and then exposed to UVA irradiation (315–400 nm). One set of controls was Amplex Red and HRP added to cells and kept in the dark. The other controls were Amplex Red and HRP exposed to UVA irradiation in the absence of cells.
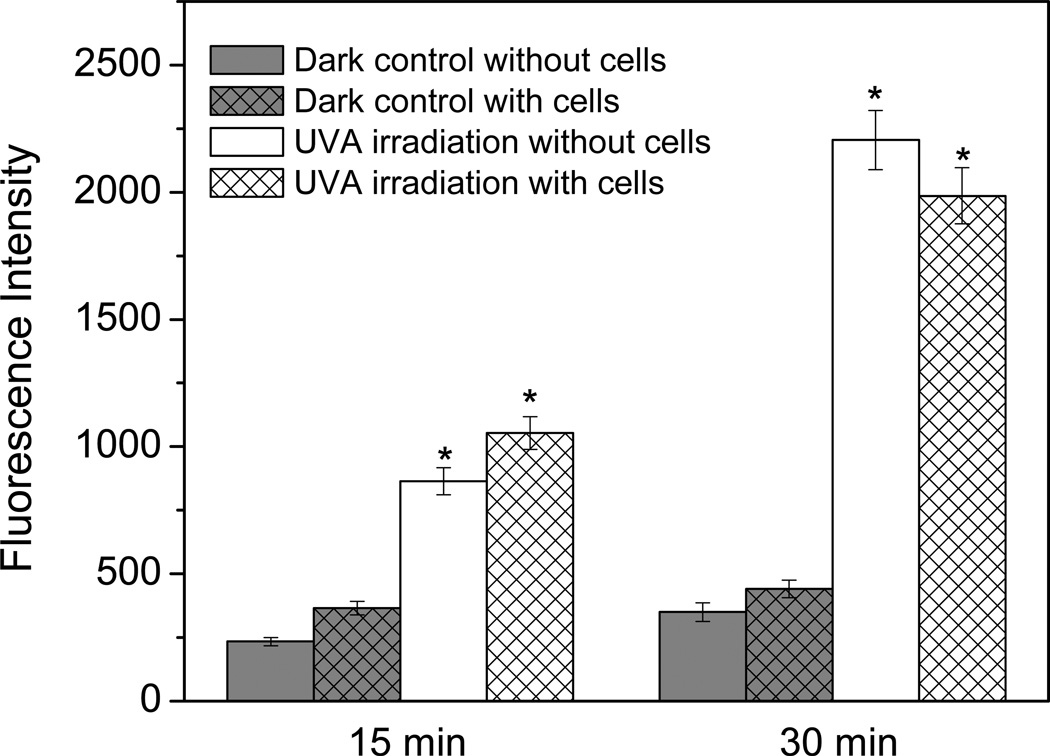
Cells exposed to UVA irradiation had higher fluorescent measurements than cells kept in the dark, and this fluorescence was dependent on the length of exposure to UVA (Fig. 8). However, the same thing occurred in the Amplex Red and HRP exposed to UVA light in the absence of cells, indicating that the majority of the fluorescence from irradiated cells was due to UVA irradiation of Amplex Red and HRP and not due to H2O2 release from the cells. This large artifactual resorufin formation occurred despite the poor absorption of Amplex Red and resorufin in the UVA region (Fig. 1A, 315–400 nm), which was most likely due to the high energy and intensity of the UVA light. This shows that Amplex Red was not useful for measuring H2O2 release from the cells due to UV exposure because the UV irradiation caused such a large amount of artifactual resorufin formation.
Figure 8.
Fluorescence of HaCaT keratinocytes exposed to UVA in the presence of 50 µM Amplex Red. Detection of autooxidation of Amplex Red with cells irradiated with 0, 15, and 30 min UVA and control without cells. The significance of the UVA irradiated sample without cells to its dark control and the significance of the UVA irradiated sample with cells to its dark control was assessed using the Student’s t-test (*p < 0.05).
Discussion
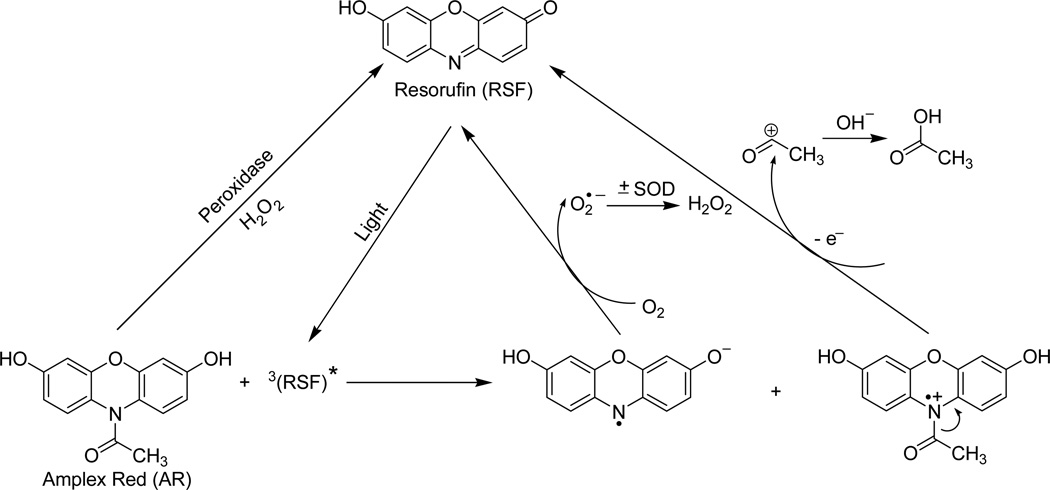
The Amplex Red assay is based on the stoichiometric oxidation of Amplex Red with H2O2 to resorufin catalyzed by HRP. Our results clearly showed that Amplex Red itself can also be oxidized to resorufin after exposure to light in the absence of HRP and H2O2, and that this photochemistry is initiated by trace amounts of resorufin present in Amplex Red stock solutions. This effect is dependent on the duration of light exposure (Figs.1–8), the energy of the light (Fig. 1B), and the presence of oxygen in the solutions (Fig. 4). O2 • − was identified as an intermediate (Figs. 4–6) and H2O2 as a final product (Fig. 6) in this process. So, based on these results, we propose the following photochemical mechanism (Scheme 1). After exposure to light, resorufin is activated to form a triplet excited state. An electron transfer from Amplex Red to the resorufin triplet generates an Amplex Red cation radical and resorufin anion radical. With oxidation, the Amplex Red cation radical can deacetylate to form resorufin. The resorufin anion radical can react with oxygen to produce O2 • − and, in the process, resorufin is regenerated. During exposure to light, this redox cycling continuously oxidizes the Amplex Red to resorufin with the formation of O2 • − and, subsequently, H2O2.
Scheme 1.
Proposed mechanism of photo-induced oxidation of Amplex Red.
When Amplex Red is exposed to light in the presence of HRP, O2 • − can spontaneously dismutate to H2O2 and serve as a substrate for HRP (Scheme 1). The addition of catalase will break down this H2O2, and artifactual resorufin formation can be inhibited (Fig. 7B). This is noteworthy because catalase can be added to a biological system to confirm that H2O2 is the species being produced, so it is important to realize that catalase can break down H2O2 being released from a biological sample but can also inhibit artifactual resorufin formation, thus mimicking the former situation.
Thus, our studies provide some cautions and guidelines for the use of the Amplex Red Assay. Exposure of Amplex Red to light will result in photooxidation of Amplex Red to form resorufin. The impact of this photochemistry on the experiment varies; for example, in Figure 7 the assay is less sensitive when carried out under ambient light than incubated in the dark, which could be problematic if the concentrations of H2O2 to be measured are near the limit of detection of the assay. It is noteworthy that this experiment was carried out in a 96-well plate, so all the samples were exposed to the same amount of light. Nonetheless, it would be prudent to minimize exposure to light because, as well as increasing the background fluorescence, the photochemical formation of O2 • − may alter an enzymatic system or biological response under study, which may change the H2O2 formed by that system. In addition, the amount of photochemistry occurring may not be the same between control samples and treated samples; for instance, the amount of photochemistry in a hypoxia treatment would be expected to be less than in normoxic controls since this photochemistry is dependent on the presence of dissolved oxygen (Figs. 4 and 6). The levels of H2O2 in pulmonary and coronary smooth muscle cells have been reported to be lower in hypoxia than in normoxia [18], but there have been conflicting reports from other groups [19–21], underscoring the need to carefully control for potentially confounding variables.
Inappropriate experimental design may result in invalid measurements such as in Figure 8, where cells, Amplex Red and HRP were exposed to UV irradiation to measure H2O2 from cells produced in response to UV irradiation. However, the irradiated Amplex Red generated large amounts of artifactual resorufin formation, and this high background fluorescence obscured any H2O2 produced from the cells. Even if the sample containing irradiated cells, Amplex Red and HRP had given a higher fluorescence than that containing irradiated Amplex Red and HRP, subtracting the test fluorescence from the control fluorescence would not be a valid method for determining H2O2 release from the cells because Amplex Red photooxidation generates O2 • −, which is also likely to have a biological effect on the cells and thus would not be representative of H2O2 release from cells exposed to light in the absence of Amplex Red. Not only would the H2O2 measurements be inaccurate, but they might divert the researcher’s attention away from more significant findings.
In general, precautions should be taken to minimize the exposure to light, such as using a darkroom or low-energy red light for lab work and incubating the samples in the dark, in order to minimize any effects resulting from the photooxidation of Amplex Red. Single measurements of fluorescence are preferable to continuous measurements as this minimizes exposure to light from the fluorescence spectrometer or plate reader. The H2O2 levels produced by isolated mitochondria are typically measured with continuous measurements using a fluorescence spectrophotometer [22–24]. The light from a spectrophotometer under our conditions was enough to cause photochemical reactions (Figs. 2–4). An exponentially increasing fluorescence can indicate photochemical reactions (Fig. 2), but a linear increase in fluorescence cannot be taken as evidence of an absence of photochemical reactions (Fig. 3). If continuous measurements are necessary to determine the rate of H2O2 production, single measurements could also be taken as a comparison to see if the same effect is produced by inhibitors or substrates and, if this is not the case, the photochemistry described here may be the cause. It may be more convenient to compare single measurements and continuous measurements by performing the experiments in a 96-well plate [12, 25].
Confocal microscopy of Amplex Red samples has been done previously [26]. The cells appeared fluorescent against a fluorescent background, but it should be noted that Amplex Red is cell-impermeable and is designed as an extracellular assay. Confocal microscopy should be avoided because the laser light source is very powerful and will certainly induce this photochemistry. Moreover, it is almost impossible to standardize the exposure of light between samples because, in looking at different parts of a slide under a microscope, the beam of light will fall on different areas for different lengths of time. In addition, if attempts are made to use it in cells, such as if a cell-permeable version of Amplex Red is developed, the high intracellular concentrations of glutathione and NADH [3, 11] and enzymatic redox cycling [13, 14]are likely to result in artifactual production of O2 • − and H2O2. If the above precautions are taken, the Amplex Red assay is, in general, a very sensitive, specific and robust assay for the measurement of H2O2.
Resorufin is artifactually formed when Amplex Red is exposed to light
Superoxide and H2O2 are also artifactually formed during this photochemistry
The rate is increased by the presence of superoxide dismutase or HRP
Minimizing laboratory and instrumental light exposure is necessary to avoid errors
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health and the National Institute of Environmental Health Sciences 52 (Z01 ES050139-13). The authors are indebted to Mrs. Mary Mason and Dr. Ann Motten for their critical reading of the manuscript.
Abbreviations
- AR
Amplex Red
- DMPO
5,5-dimethy-1-pyrroline N-oxide
- ESR
electron spin resonance
- HRP
horseradish peroxidase
- ROS
reactive oxygen species
- RSF
resorufin
- SOD
superoxide dismutase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kalyanaraman B, Darley-Usmar V, Davies KJA, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, II, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic. Biol. Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic. Biol. Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 3.Zhao B, Ranguelova K, Jiang J, Mason RP. Studies on the photosensitized reduction of resorufin and implications for the detection of oxidative stress with Amplex Red. Free Radic. Biol. Med. 2011;51:153–159. doi: 10.1016/j.freeradbiomed.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchesi E, Rota C, Fann YC, Chignell CF, Mason RP. Photoreduction of the fluorescent dye 2'-7'-dichlorofluorescein: a spin trapping and direct electron spin resonance study with implications for oxidative stress measurements. Free Radic. Biol. Med. 1999;26:148–161. doi: 10.1016/s0891-5849(98)00174-9. [DOI] [PubMed] [Google Scholar]
- 5.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal. Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 6.Mohanty JG, Jaffe JS, Schulman ES, Raible DG. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J. Immunol. Methods. 1997;202:133–141. doi: 10.1016/s0022-1759(96)00244-x. [DOI] [PubMed] [Google Scholar]
- 7.Towne V, Will M, Oswald B, Zhao Q. Complexities in horseradish peroxidase-catalyzed oxidation of dihydroxyphenoxazine derivatives: appropriate ranges for pH values and hydrogen peroxide concentrations in quantitative analysis. Anal. Biochem. 2004;334:290–296. doi: 10.1016/j.ab.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 8.Reszka KJ, Wagner BA, Burns CP, Britigan BE. Effects of peroxidase substrates on the Amplex red/peroxidase assay: antioxidant properties of anthracyclines. Anal. Biochem. 2005;342:327–337. doi: 10.1016/j.ab.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Gorris HH, Walt DR. Mechanistic aspects of horseradish peroxidase elucidated through single-molecule studies. J. Am. Chem. Soc. 2009;131:6277–6282. doi: 10.1021/ja9008858. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues JV, Gomes CM. Enhanced superoxide and hydrogen peroxide detection in biological assays. Free Radic. Biol. Med. 2010;49:61–66. doi: 10.1016/j.freeradbiomed.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Votyakova TV, Reynolds IJ. Detection of hydrogen peroxide with Amplex Red: interference by NADH and reduced glutathione auto-oxidation. Arch. Biochem. Biophys. 2004;431:138–144. doi: 10.1016/j.abb.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Mishin V, Gray JP, Heck DE, Laskin DL, Laskin JD. Application of the Amplex red/horseradish peroxidase assay to measure hydrogen peroxide generation by recombinant microsomal enzymes. Free Radic. Biol. Med. 2010;48:1485–1491. doi: 10.1016/j.freeradbiomed.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balvers WG, Boersma MG, Vervoort J, Rietjens IMCM. Experimental and theoretical study on the redox cycling of resorufin by solubilized and membrane-bound NADPH-cytochrome reductase. Chem. Res. Toxicol. 1992;5:268–273. doi: 10.1021/tx00026a019. [DOI] [PubMed] [Google Scholar]
- 14.Dutton DR, Reed GA, Parkinson A. Redox cycling of resorufin catalyzed by rat liver microsomal NADPH-cytochrome P450 reductase. Arch. Biochem. Biophys. 1989;268:605–616. doi: 10.1016/0003-9861(89)90328-7. [DOI] [PubMed] [Google Scholar]
- 15.Kalyanaraman B, Perez-Reyes E, Mason RP. Spin-trapping and direct electron spin resonance investigations of the redox metabolism of quinone anticancer drugs. Biochim. Biophys. Acta. 1980;630:119–130. doi: 10.1016/0304-4165(80)90142-7. [DOI] [PubMed] [Google Scholar]
- 16.Peus D, Meves A, Vasa RA, Beyerle A, O'Brien T, Pittelkow MR. H2O2 is required for UVB-induced EGF receptor and downstream signaling pathway activation. Free Radic. Biol. Med. 1999;27:1197–1202. doi: 10.1016/s0891-5849(99)00198-7. [DOI] [PubMed] [Google Scholar]
- 17.Peus D, Vasa RA, Meves A, Pott M, Beyerle A, Squillace K, Pittelkow MR. H2O2 is an important mediator of UVB-induced EGF-receptor phosphorylation in cultured keratinocytes. J. Invest. Dermatol. 1998;110:966–971. doi: 10.1046/j.1523-1747.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 18.Mehta JP, Campian JL, Guardiola J, Cabrera JA, Weir EK, Eaton JW. Generation of oxidants by hypoxic human pulmonary and coronary smooth-muscle cells. Chest. 2008;133:1410–1414. doi: 10.1378/chest.07-2984. [DOI] [PubMed] [Google Scholar]
- 19.Michelakis ED, Thébaud B, Weir EK, Archer SL. Hypoxic pulmonary vasoconstriction: redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells. J. Mol. Cell. Cardiol. 2004;37:1119–1136. doi: 10.1016/j.yjmcc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Korge P, Ping P, Weiss JN. Reactive oxygen species production in energized cardiac mitochondria during hypoxia/reoxygenation: modulation by nitric oxide. Circ. Res. 2008;103:873–880. doi: 10.1161/CIRCRESAHA.108.180869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waypa GB, Schumacker PT. Hypoxic pulmonary vasoconstriction: redox events in oxygen sensing. J. Appl. Physiol. 2005;98:404–414. doi: 10.1152/japplphysiol.00722.2004. [DOI] [PubMed] [Google Scholar]
- 22.Panov A, Dikalov S, Shalbuyeva N, Taylor G, Sherer T, Greenamyre JT. Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J. Biol. Chem. 2005;280:42026–42035. doi: 10.1074/jbc.M508628200. [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharya A, Muller FL, Liu Y, Sabia M, Liang H, Song W, Jang YC, Ran Q, Van Remmen H. Denervation induces cytosolic phospholipase A2-mediated fatty acid hydroperoxide generation by muscle mitochondria. J. Biol. Chem. 2009;284:46–55. doi: 10.1074/jbc.M806311200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial α-ketoglutarate dehydrogenase complex generates reactive oxygen species. J. Neurosci. 2004;24:7779–7788. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong JS, Whiteman M, Liza Measurement of reactive oxygen species in cells and mitochondria. Methods Cell Biol. 2007;24:355–377. doi: 10.1016/S0091-679X(06)80018-X. [DOI] [PubMed] [Google Scholar]
- 26.DeYulia GJ, Jr, Carcamo JM, Borquez-Ojeda O, Shelton CC, Golde DW. Hydrogen peroxide generated extracellularly by receptor-ligand interaction facilitates cell signaling. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5044–5049. doi: 10.1073/pnas.0501154102. [DOI] [PMC free article] [PubMed] [Google Scholar]