Abstract
Ceramide is a sphingolipid bioactive molecule that induces apoptosis and other forms of cell death, and triggers macroautophagy (referred to below as autophagy). Like amino acid starvation, ceramide triggers autophagy by interfering with the mTOR-signaling pathway, and by dissociating the Beclin 1:Bcl-2 complex in a c-Jun N-terminal kinase 1 (JNK1)-mediated Bcl-2 phosphorylation-dependent manner. Dissociation of the Beclin 1:Bcl-2 complex, and the subsequent stimulation of autophagy have been observed in various contexts in which the cellular level of long-chain ceramides was increased. It is notable that the conversion of short-chain ceramides (C2-ceramide and C6-ceramide) into long-chain ceramide via the activity of ceramide synthase is required to trigger autophagy. The dissociation of the Beclin 1:Bcl-2 complex has also been observed in response to tamoxifen and PDMP (an inhibitor of the enzyme that converts ceramide to glucosylceramide), drugs that increase the intracellular level of long-chain ceramides. However, and in contrast to starvation, over-expression of Bcl-2 does not blunt ceramide-induced autophagy. Whether this autophagy that is unchecked by forced dissociation of the Beclin 1:Bcl-2 complex is related to the ability of ceramide to trigger cell death remains an open question. More generally, the question of whether ceramide-induced autophagy is a dedicated cell death mechanism deserves closer scrutiny.
Keywords: macroautophagy, Bcl-2, Beclin 1, c-Jun N-terminal kinase, cell death, sphingolipids
Sphingolipids are important players in the cell death/survival balance in mammalian cells. The sphingolipid rheostat, ceramide/sphingosine 1-P, has been proposed to orchestrate the balance between cell survival and cell death. Sphingosine 1-phosphate (S1P) is an anti-apoptotic molecule, whereas ceramide is a pro-apoptotic mediator.1,2 More recently, both ceramide and S1P have been shown to stimulate autophagy.3 Ceramide is able to trigger autophagy in the presence of extracellular nutrients,4 and S1P is involved in regulating starvation-induced autophagy.5 The consumption of S1P by the enzyme sphingosine 1-phosphate lyase, which irreversibly terminates the S1P signal,6 blunts starvation-induced autophagy (Codogno P and Levade T, unpublished data), reinforcing the notion that intracellular S1P may be part of the autophagy-signaling mechanism during starvation-induced autophagy. However, S1P can also act extracellularly via its interaction with S1P receptors.7 The possibility that S1P regulates autophagy via S1P receptors cannot therefore be excluded and needs to be explored.
Ceramide and the Induction of Autophagy
Our previous findings demonstrate that ceramide triggers autophagy by interfering with the activation of Akt/PKB upstream of mTOR.4 Recently, we have shown that ceramide activates JNK1 to phosphorylate Bcl-2.8 The phosphorylation of Bcl-2 alleviates the inhibitory effect of Bcl-2 on autophagy as a result of its dissociation from Beclin 1. This effect is replicated by various treatments that increase the level of long-chain ceramides, such as converting exogenous short-chain ceramide analogs into long-chain ceramides, and treating cells with the glucosylceramide synthase inhibitor PDMP or tamoxifen. The phosphorylation of three residues contained in the N-terminal domain of Bcl-2 is required for Bcl-2 to be dissociated from Beclin 1. These results are reminiscent of the role of JNK1 in the dissociation of the Beclin 1:Bcl-2 complex during periods of starvation in stimulating autophagy.9 Interestingly, it has previously been shown that ceramide interferes with amino acid transport at the plasma membrane.10 Following on this observation, Guenther et al. demonstrate that ceramide blocks the entry of amino acids and so starves cells, and stimulates autophagy by downregulating nutrient transporters.11 In a similar manner to the deprivation of extracellular amino acids, ceramide induces inhibition of the mTOR signaling pathway, which is revealed by inhibition of the mTOR substrate, p70S6 kinase. It is tempting to hypothesize that in the model investigated by Guenther et al.11 ceramide may have induced the activation of JNK1, and thereby controlled the dissociation of the Beclin 1:Bcl-2 complex.
We previously reported that ceramide treatment led to an accumulation of Beclin 1 by increasing the level of its mRNA in different cancer cell lines.4 It has recently been demonstrated that ceramide increases the expression of Beclin 1, and stimulates autophagy by activating JNK1/2-dependent transcription of BECN1 via activation of the transcription factor c-Jun in human nasopharyngeal carcinoma and hepatocellular carcinoma cell lines.12
Despite the similarities between starvation-induced autophagy and ceramide-induced autophagy, we noticed a difference in the ability of Bcl-2 to regulate the autophagic response. Previous studies in the human colon carcinoma cell line HT-29 showed that the forced expression of Bcl-2 blocks starvation-induced autophagy,13 whereas ceramide was still able to trigger autophagy in this context.8 In fact the ceramide analog C2-ceramide induces the accumulation of the BH3-only protein BNIP3.14 The protein Beclin 1 contains a BH3 domain, which is involved in the interactions with Bcl-2 and Bcl-xL.15 A BH3-mimetic, ABT747, dissociates the Beclin 1:Bcl-2/Bcl-xL complex.16 Moreover several BH3-only proteins are able to disrupt the Beclin 1:Bcl-2 complex.15 One of them, BNIP3, stimulates autophagy and dissociates the Beclin 1:Bcl-2 complex.17 The BH3-domain-based dissociation of the Beclin 1:Bcl-2 complex is not dependent on the phosphorylation of Bcl-2.15 Thus the possibility cannot be excluded that ceramide may trigger the dissociation of the complex by two non-exclusive mechanisms: (i) activation of JNK1 and subsequent phosphorylation of Bcl-2, and (ii) accumulation of BNIP3- and BH3-dependent dissociation of the Beclin 1:Bcl-2 complex. As summarized in Figure 1, ceramide could trigger autophagy via several distinct pathways.
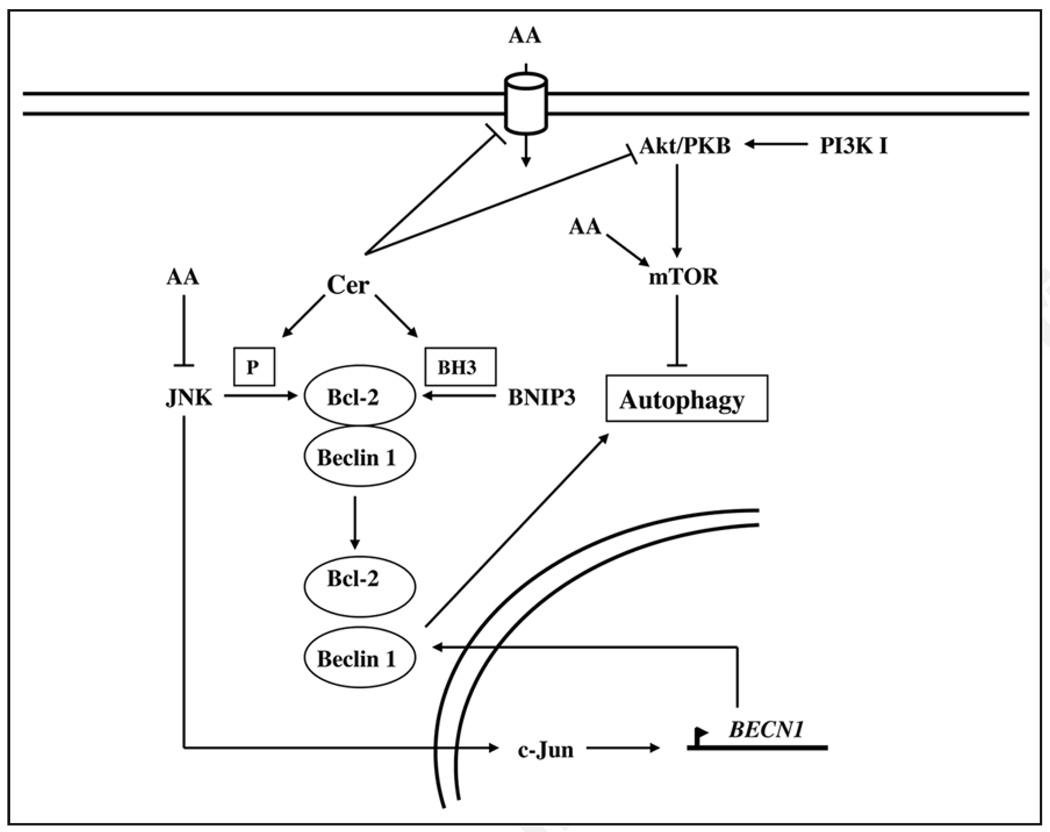
Figure 1.
Regulation of autophagy by ceramide. Ceramide (Cer) impinges both on the signaling pathway of autophagy and on the molecular machinery involved in the early steps of autophagosome formation such as the Beclin 1 complex. Ceramide regulates autophagy signaling by inhibiting Akt/PKB and the entry of nutrients. The low intracellular concentration of nutrients mimics decreases in the activity of the mTORC1 complex (not shown in the figure). Alternatively the low intracellular concentration of amino acids (AA) induced by ceramide may activate JNK1. Like starvation, ceramide favors the phosphorylation of Bcl-2 and its dissociation from Beclin 1 (Boxed P). Ceramide induces the expression of the BH3-only protein BNIP3, which competes with the BH3 domain of Beclin 1 to dissociate the Beclin 1:Bcl-2 complex (Boxed BH3). Finally, JNK1/2 activates the transcription factor c-Jun, leading to the upregulation of Beclin 1, and the stimulation of autophagy.
Ceramide-Induced Autophagy and Cell Death
The role of ceramide as an inducer of cell death is well established. 1 This sphingolipid triggers various forms of cell death, including a caspase-dependent form, a caspase-independent form, and necrosis. Moreover, ceramide-induced cell death through autophagy, either by targeting mitochondria or by increasing the rate of autophagy by upregulating the expression of Beclin 1, has been also reported.12,14 Ceramide-induced blockade of nutrient transport triggers autophagy and cell death by a caspase-independent mechanism.11 Inhibition of autophagy accelerates cell death. In this context, autophagy is an attempt to overcome the bioenergetic catastrophe caused by the loss of nutrient transport. The stimulation of the CD95-dependent pathway in primary rat hepatocytes by bile acid + MEK1/2 inhibitors,18 and in cancer cells by sorafenib (a Raf kinase inhibitor) and vorinostat (a histone deacetylase inhibitor) is dependent on ceramide production to trigger autophagy and apoptosis.19 In both situations, ceramide-dependent autophagy has a cytoprotective effect against the induction of apoptosis. Recent studies show that tamoxifen, which induces autophagic cell death in the estrogen receptor-positive breast cancer cell line MCF-7,20 also triggers autophagy to delay the induction of cell death.21 In this context, it would be interesting to determine the contributions of ceramide to autophagy and cell death, respectively.
The duration and/or the robustness of the signal produced by ceramide probably either determines the impact of autophagy on cell fate, or activates a cell death pathway that autophagy cannot counteract. Interestingly the level of ceramide in the ER is under the control of the class I PI3K/Akt (PKB) signaling pathway that promotes the vesicular transport of ceramide from the ER to the Golgi.22 As this signaling pathway regulates autophagy,23 we cannot exclude the possibility that a fine-tuning mechanism may exist that controls the induction of autophagy by ceramide.
Overall, the regulation of autophagy by sphingolipids is an emerging field. Several issues remain to be investigated in order to better appreciate the function of these lipids in autophagy, and its outcome in terms of cell survival and cell death. For example, the roles of the different molecular species of ceramide as well as those of related metabolites, such as dihydroceramide, sphingosine and ceramide 1-phosphate, remain to be elucidated.24
References
- 1.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 2.Spiegel S, Milstien S. Sphingosine-1-phosphate: An enigmatic signaling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 3.Lavieu G, Scarlatti F, Sala G, Levade T, Ghidoni R, Botti J, Codogno P. Is autophagy the key mechanism by which the sphingolipid rheostat controls the cell fate decision? Autophagy. 2007;3:45–47. doi: 10.4161/auto.3416. [DOI] [PubMed] [Google Scholar]
- 4.Scarlatti F, Bauvy C, Ventruti A, Sala G, Cluzeaud F, Vandewalle A, et al. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and upregulation of beclin 1. J Biol Chem. 2004;279:18384–18391. doi: 10.1074/jbc.M313561200. [DOI] [PubMed] [Google Scholar]
- 5.Lavieu G, Scarlatti F, Sala G, Carpentier S, Levade T, Ghidoni R, et al. Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J Biol Chem. 2006;281:8518–8527. doi: 10.1074/jbc.M506182200. [DOI] [PubMed] [Google Scholar]
- 6.Oskouian B, Saba J. Sphingosine-1-phosphate metabolism and intestinal tumorigenesis: lipid signaling strikes again. Cell Cycle. 2007;6:522–527. doi: 10.4161/cc.6.5.3903. [DOI] [PubMed] [Google Scholar]
- 7.Maceyka MW, Milstien S, Spiegel S. Sphingosine-1-phosphate: the Swiss army knife of sphingolipid signaling. J Lipid Res. 2008 doi: 10.1194/jlr.R800065-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pattingre S, Bauvy C, Carpentier S, Levade T, Levine B, Codogno P. Role of JNK1-dependent Bcl-2 phosphorylation in ceramide-induced macroautophagy. J Biol Chem. 2009;284:2719–2728. doi: 10.1074/jbc.M805920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide downregulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. Faseb J. 2005;19:461–463. doi: 10.1096/fj.04-2284fje. [DOI] [PubMed] [Google Scholar]
- 11.Guenther GG, Peralta ER, Rosales KR, Wong SY, Siskind LJ, Edinger AL. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc Natl Acad Sci USA. 2008;105:17402–17407. doi: 10.1073/pnas.0802781105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li DD, Wang LL, Deng R, Tang J, Shen Y, Guo JF, et al. The pivotal role of c-Jun NH2-terminal kinase-mediated Beclin 1 expression during anticancer agents-induced autophagy in cancer cells. Oncogene. 2009;28:886–898. doi: 10.1038/onc.2008.441. [DOI] [PubMed] [Google Scholar]
- 13.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Daido S, Kanzawa T, Yamamoto A, Takeuchi H, Kondo Y, Kondo S. Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res. 2004;64:4286–4293. doi: 10.1158/0008-5472.CAN-03-3084. [DOI] [PubMed] [Google Scholar]
- 15.Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, et al. Functional and physical interaction between Bcl-XL and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, et al. Mitochondrial autophagy is a HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Zhang G, Park MA, Mitchell C, Walker T, Hamed H, Studer E, et al. Multiple cyclin kinase inhibitors promote bile acid-induced apoptosis and autophagy in primary hepatocytes via p53-CD95-dependent signaling. J Biol Chem. 2008;283:24343–24358. doi: 10.1074/jbc.M803444200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park MA, Zhang G, Martin AP, Hamed H, Mitchell C, Hylemon PB, et al. Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol Ther. 2008;7:1648–1662. doi: 10.4161/cbt.7.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bursch W, Ellinger A, Kienzl H, Torok L, Pandey S, Sikorska M, et al. Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy. Carcinogenesis. 1996;17:1595–1607. doi: 10.1093/carcin/17.8.1595. [DOI] [PubMed] [Google Scholar]
- 21.Samaddar JS, Gaddy VT, Duplantier J, Thandavan SP, Shah M, Smith MJ, et al. A role for macroautophagy in protection against 4-hydroxytamoxifen-induced cell death and the development of antiestrogen resistance. Mol Cancer Ther. 2008;7:2977–2987. doi: 10.1158/1535-7163.MCT-08-0447. [DOI] [PubMed] [Google Scholar]
- 22.Giussani P, Brioschi L, Bassi R, Riboni L, Viani P. Phosphatidylinositol 3-kinase/AKT pathway regulates the endoplasmic reticulum to Golgi traffic of ceramide in glioma cells: A link between lipid signaling pathways involved in the control of cell survival. J Biol Chem. 2008;284:5088–5096. doi: 10.1074/jbc.M808934200. [DOI] [PubMed] [Google Scholar]
- 23.Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P, Ogier-Denis E. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2001;276:35243–35246. doi: 10.1074/jbc.C100319200. [DOI] [PubMed] [Google Scholar]
- 24.Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta. 2006;1758:1864–1884. doi: 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]