Abstract
Objectives
Panax ginseng has been extensively used as an adaptogen and is among the top 10 selling herbal supplements in the United States over the past decade. However, there have been few reports about the toxicity of P. ginseng in human studies. Given the lack of toxicological studies in human, this study investigated whether P. ginseng administration causes any noticeable toxic effects in healthy volunteers.
Methods
This study was designed as a randomized, double-blind, placebo-controlled, and parallel group trial in healthy volunteers. The subjects were required to be healthy, free from any significant disease, as assessed at screening by physical examination, medical history, and laboratory (hematological and biochemical) tests. Eligible subjects received P. ginseng extract (1 g/day or 2 g/day) or placebo over a 4-week period.
Results
Although mild adverse events, such as dyspepsia, hot flash, insomnia, and constipation, were reported in both P. ginseng and placebo group, no serious untoward reactions were reported following P. ginseng administration. Nonsignificant changes were observed in hematological and biochemical tests.
Conclusions
P. ginseng administration for 4 weeks was shown to be safe, tolerable, and free of any untoward toxic effect in healthy male and female volunteers. Future results from ongoing multicenter collaborative efforts to evaluate short- and long-term effects of P. ginseng may contribute to our current understanding of safety and tolerability of this herbal product.
Introduction
Ginseng has been used medicinally for thousands of years in Korea, China, and Japan,1 and it is widely used in Western herbal preparations as an adaptogen.2,3 Ginseng has been among the top 10 selling herbal supplements in the United States over the past decade.4 The term ginseng refers to the dried root of several species in the plant genus Panax, which belongs to the Araliaceae family. It comprises two commonly used ginseng species (i.e., P. ginseng C.A. Meyer (Asian ginseng) and P. quinquefolius L. (North American ginseng).5
P. ginseng (PG) contains triterpene glycosides as its major active compounds, commonly referred to as ginsenosides or saponins. Up to 40 distinct ginsenosides have been identified from PG.3,6 Modern therapeutic studies claim a wide range of pharmaceutical activity of PG such as vitality, immune function, cancer, cardiovascular disease, cognitive and physical performance, and sexual function.7–14
PG is generally considered to be very safe and well tolerated in animals,15–18 whereas there have been few reports about the toxicity of PG in human studies. In spite of the worldwide use of PG, there has been concern about the lack of clinical toxicology information of PG.7 This study aimed to examine whether the 4-week administration of PG exhibits any noticeable toxic effect in healthy volunteers in a randomized controlled trial.
Materials and Methods
Study design and participants
This study was designed as a randomized, double-blind, placebo-controlled, and parallel group study in healthy Koreans at a single center (Oriental Hospital of Daejeon University). The subjects were required to be in satisfactory health, free from any significant cardiac, hepatic, renal, pulmonary, neurological, gastrointestinal, and hematological disease, as assessed at screening by physical examination, medical history, and laboratory (hematological and biochemical) tests. Volunteers between the ages of 18 and 60 years with a body–mass index of 16–31 kg/m2 were eligible for participation. Eligible female volunteers enrolled in this study were not pregnant. All participants gave their written informed consent and were able to comprehend fully the protocol, including the nature and purpose of the study as well as the possible risks and side-effects. Subjects were not eligible if they met any of the following criteria applied at the time of screening (pre-study): clinical abnormalities (including abnormal hematological and biochemical tests), participation in other clinical trials simultaneously or within the last 4 weeks, previous history of hypersensitivity to ginseng preparations, history of any acute/chronic diseases, history of drug dependence or chronic alcohol, history of chronic medication within the past 6 weeks, regular smokers who smoke more than 20 cigarettes weekly, and night workers.
Ethics aspects
This study was approved by the ethical committee of Daejeon University Hospital (authorization number: DJOMC-33-1) and was conducted in accordance with ethical standards for human experimentation established by the Declaration of Helsinki (1965) with subsequent revisions (Tokyo, 1975, Venice, 1983, Hong Kong, 1989, and Somerset, 1996), and current standards for Good Clinical Practice in clinical trials.
Preparation of ginseng extract and placebo
A 20% ethanol extract of 4-year-old PG root was prepared by Guryoung Pharmaceutical Company Ltd. (Cheorwon, Korea) according to over-the-counter Korean monographs. Lyophilized PG extract (13.2% [w/w]) was obtained. Placebo control material was carefully prepared to match the appearance, volume, weight, color, flavor, and taste of ginseng in a formulation of starch (99.68%, Daesang Co., Korea), artificial ginseng flavor (0.3%, Hanbit flavor & Fragrance Co, Korea), and caramel color (0.02%, Namyoung Food Co., Korea) in a capsule. The 100% PG extract, 50% PG extract with 50% placebo material, or 100% placebo material was contained in a soft capsule as 250 mg in each.
Fingerprinting using high-performance thin-layer chromatography
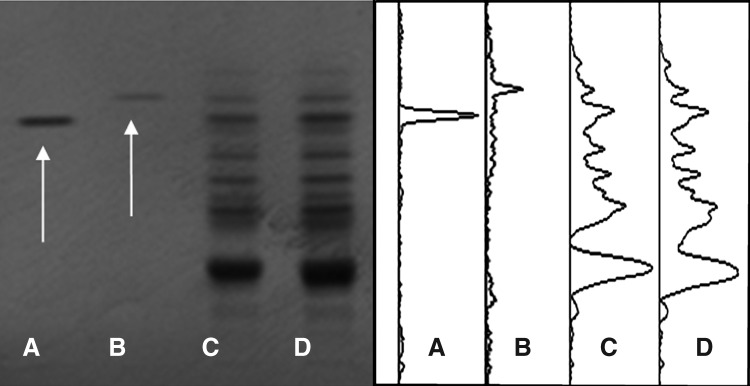
Gensenoside Rg1 (2 mg/mL), ginsenoside Rg3 (10 mg/mL), and PG (150, 300 mg/mL) were dissolved in 90% methanol, sonicated for 1 hour, and centrifuged for 15 minutes at 3000 rpm. The supernatant was applied onto prewashed silica gel 60 F254 thin-layer chromatography plates (20×10 cm; 0.2-mm thickness) (Merck, Darsttadt, Germany) using an automated applicator, Linomat IV (CAMAG, Mu cenz, Switzerland). The constituent ingredients of PG were separated with mobile phase (chloroform: ethyl acetate:methanol:water [17:46:25:12]) in automated multiple development AMD2 (CAMAG) and visualized by a Reprostar 3 mounted digital camera (CAMAG) under white light after derivatization with 10% sulfuric acid. Images were captured by Win CATS software. Densitograms were generated by the VideoScan software (Fig. 1).
FIG. 1.
High-performance thin-layer chromatography (TLC)–based fingerprint of Panax ginseng (PG). Two micrograms of ginsenoside Rg1 (A), 10 μg of ginsenoside Rg3 (B), 150 μg of PG (C), and 300 μg of PG (D) were applied onto prewashed silica gel 60 F254 TLC plates, then separated with mobile phase (chloroform:ethyl acetate:methanol:water [17:46:25:12]). The migrated components were visualized under white light after derivatization with 10% sulfuric acid solution (left), and the densitograms were generated (right). The arrows appeared in the first lane was ginsenoside Rg1 and ginsenoside Rg3 in the second lane.
Intervention
Eligible subjects were randomly assigned to receive PG extract 500 mg twice a day (PG 500 twice a day), PG extract 1000 mg twice a day (PG 1000 twice a day), or placebo for 4 weeks. A computer‐generated randomization schedule was used to assign subjects to the study groups. PG was presented as 250-mg tablets, with placebo to match. All participants were administered four identical 250 mg capsules twice a day (9:00 am and 9:00 pm) according to their allocation. No medication other than PG was allowed except under exceptional conditions only with the permission of the investigator. There was no restriction placed on normal and routine activity or diet during the study period. Venous whole blood was sampled at baseline, and at 4 weeks after randomization with all treatment regimens (PG 500 twice a day, PG 1000 twice a day, placebo). All participants fasted overnight and were alcohol‐ and caffeine‐free for 12 hours prior to, and during, assessment days. To test the intactness of the blind at the end of the study, participants were asked to guess which treatment (PG 500 twice a day, PG 1000 twice a day, placebo) they received. Compliance was confirmed at the 2nd and 4th week by checking each subject's medication intake and the number of remaining capsules.
Assessment of adverse events
All subjects were monitored for adverse events (including adverse drug reactions and illnesses with onset during the study). All of the adverse events were forwarded in a blinded fashion to the primary investigator to be rated as mild, moderate, severe and undesirable, life threatening or disabling, or death-related based on criteria suggested by the National Institutes of Health.19 The primary investigator was alerted of potentially serious adverse events immediately so that appropriate steps could be taken, including notification of appropriate regulatory bodies. If an adverse event was still ongoing at the end of the study (week 4), suitable medical support was provided.
Laboratory tests
Safety laboratory tests (including hematology, chemistry, and urinalysis) were performed in laboratories at Oriental Hospital of Daejeon University at baseline and after 4-week treatment using standardized procedures.
Hematology tests include white blood cells, segmented cells, monocytes, lymphocytes, red blood cells, hemoglobin, hematocrit, erythrocyte sedimentation rate, platelet, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and red blood cell distribution width.
Chemistry tests include total protein, albumin, albumin/globulin ratio, total bilirubin, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, γ‐glutamyl transpeptidase, cholesterol, triglycerides, HDL‐cholesterol, glucose, blood urea nitrogen and creatinine.
Urinalysis includes glucose, bilirubin, ketones, specific gravity, blood, pH, protein, urobilinogen, nitrites, and leukocytes.
Statistical methods
Means and standard deviations were reported for continuous scaled variables and number and percentages for categorical variables. The baseline characteristics were compared between the intervention groups using χ2 tests for categorical and dichotomous variables and one‐way analysis of variance, followed by Duncan's multirange analyses for continuous variables. Pre- and post-treatment data were compared by t-test. Differences at the level of p<0.05 were regarded as statistically significant. This study was designed to have 90% statistical power at a 5% significance level to detect differences between treatments. Data processing and analysis were performed with SPSS for Windows, version 13 (SPSS Japan, Tokyo, Japan).
Results
Subject disposition and demographic characteristics
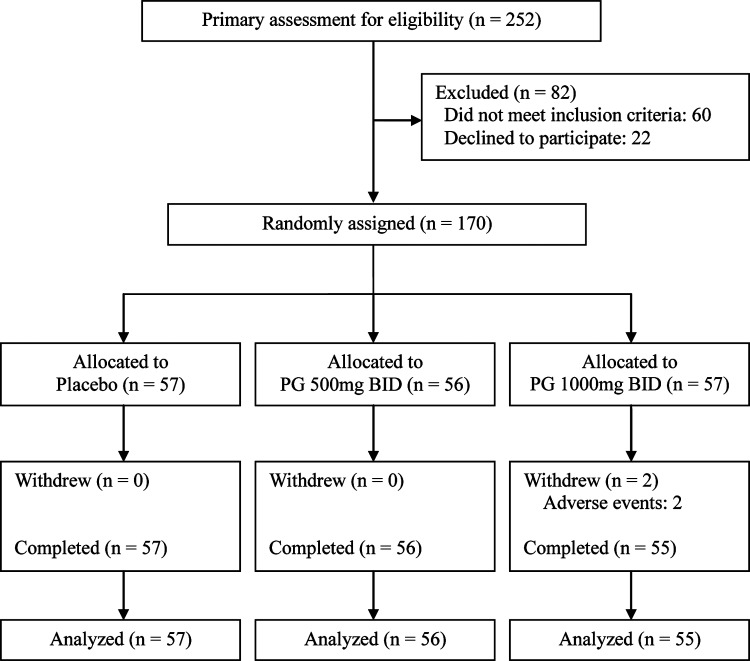
Recruitment identified 252 volunteers. Participants were of Asian (Korean) background. After excluding 60 ineligible persons, 192 subjects were screened; 22 declined to participate after receiving the study information. After 22 subjects were excluded, 170 persons (41 males and 129 females; age range, 18–60 years; body–mass index 16.1–30.3 kg/m2) were randomly assigned to PG 500 twice a day (n=56), PG 1000 twice a day (n=57) or placebo (n=57) (Fig. 2). Baseline demographics characteristics among study groups are shown in Table 1.
FIG. 2.
Trial flow chart. Primary recruitment identified 252 volunteers. After excluding 60 ineligible persons and 22 subjects declined to participate in this trial, the authors randomly assigned 170 persons to groups as follows: placebo (n=57), Panax ginseng (PG) 500 mg twice a day (BID) (n=56), or PG 1000 mg BID (n=57).
Table 1.
Baseline Demographics
| Characteristics | Placebo (n=57) | PG 500 mg BID (n=56) | PG 1000 mg BID (n=57) |
|---|---|---|---|
| Age (yr) | |||
| Mean±SD | 40.8±11.3 | 39.8±9.3 | 41.3±10.3 |
| Range | 18–60 | 19–59 | 19–60 |
| Race, n | |||
| White | 0 | 0 | 0 |
| Asian | 57 | 56 | 57 |
| Black | 0 | 0 | 0 |
| Mixed | 0 | 0 | 0 |
| Male gender | |||
| n (%) | 13 (22.8) | 14 (25.0) | 14 (24.6) |
| Weight, kg | |||
| Mean±SD | 59.7±10.7 | 58.7±10.1 | 58.2±10.1 |
| Range | 42.6–86.8 | 41.9–78.7 | 42.9–88.3 |
| Height, kg | |||
| Mean±SD | 161.4±8.6 | 161.5±7.0 | 162.3±8.3 |
| Range | 147.0–185.0 | 148.0–179.0 | 146.0–184.0 |
| BMI, kg/m2 | |||
| Mean±SD | 22.8±2.8 | 22.4±2.7 | 22.1±3.0 |
| Range | 18.3–29.1 | 17.0–28.7 | 16.1–30.3 |
One hundred and seventy (170) participants were randomly assigned to placebo, PG 500 BID, or PG 1000 BID.
PG, Panax ginseng; BID, twice a day; SD, standard deviation; BMI, body–mass index.
Adverse effects
There were no deaths or serious adverse events in any of the study groups (Table 2). The four most frequently reported adverse events were dyspepsia, hot flash, insomnia, and constipation.
Table 2.
Adverse Events
| |
Placebo (n=57) |
PG 500 mg BID (n=56) |
PG 1000 mg BID (n=57) |
|---|---|---|---|
| Subjects with adverse events, n (%) (p=0.895) | 18 (31.6) | 19 (33.9) | 17 (29.8) |
| Symptoms and signs, n | 20 | 20 | 19 |
| Dyspepsia, n | 4a | 4a | 3 |
| Hot flash, n | 4 | 3 | 4 |
| Insomnia, n | 3 | 1a | 2a |
| Constipation, n | 1b | 4 | 1 |
| Low energy, n | 1 | 2 | 2 |
| Headache, n | 2a | 2 | 1 |
| Skin disorders, n | 2b | 0 | 2b |
| Dizziness, n | 1 | 1 | 1 |
| Nausea, n | 1 | 0 | 1b |
| Diarrhea, n | 1 | 1 | 0 |
| Abdominal pain, n | 0 | 1 | 1 |
| Epistaxis, n | 0 | 1 | 0 |
| Rapid heartbeat, n | 0 | 0 | 1a |
| Committee results, overall | |||
| Severity | |||
| Mild, n/N(%) | 20/20 (100.0) | 20/20 (100.0) | 19/19 (100.0) |
| Moderate, n | 0 | 0 | 0 |
| Severe, n | 0 | 0 | 0 |
Panax ginseng (PG) was administered (500 mg twice a day [BID] or 1000 mg BID) for 4 weeks. During the study period, all the adverse events were documented. χ2 statistics were used for calculating p-values.
Events in same patient.
Events in same patient.
Nineteen (19; 33.9%) subjects in the PG 500 mg twice a day group showed adverse events such as low energy, insomnia, hot flash, headache, dizziness, abdominal pain, dyspepsia, diarrhea, constipation, and epistaxis. Seventeen (17; 29.8%) participants in the PG 100 mg twice a day group reported adverse events (low energy, insomnia, hot flash, headache, dizziness, abdominal pain, nausea, dyspepsia, constipation, rapid heartbeat, and skin disorders). Eighteen (18; 31.6%) subjects in the placebo group reported adverse events (low energy, insomnia, hot flash, headache, dizziness, nausea, dyspepsia, constipation, and skin disorders). The difference in the number of subjects with adverse events among each of the three treatment arms was nonsignificant (p=0.895).
Numbers of total adverse events were similar in each group (20 in PG 500 mg twice a day, 19 in PG 1000 mg twice a day and 20 in placebo). Of the 59 adverse events, the masked primary investigator rated all events as mild (i.e., self‐resolving). The scheduled interim and final data safety and monitoring committee reviews recommended no changes to the study.
In total, 168 of 170 participants completed the intervention with good compliance. Discontinuation for adverse events occurred in 2 female subjects in the PG 1000 mg twice a day group. One (1) subject (age 54 years; weight 44.8 kg) experienced rapid heartbeat and insomnia from days 2 to 5, while a second subject (age 38 years; weight 61.9 kg) experienced rash and nausea from days 3 to 7. Even though their adverse events were rated to be mild, the participants wanted to discontinue this study protocol. Their adverse events resolved spontaneously after discontinuation of PG.
Laboratory tests results
The hematological and biochemical test results were within the normal range both at baseline and after 4 weeks of PG administration, and there were no statistical differences (p>0.05) between clinical laboratory tests performed at screening (prestudy), and at final assessment (poststudy), and across three treatment arms (Tables 3–6).
Table 3.
Hematological Test Result of Male Subjects After 4 Weeks Administration of Panax ginseng (PG)
| Variables | Treatment | Placebo | PG 500 mg BID | PG 1000 mg BID | Reference range |
|---|---|---|---|---|---|
| WBC (102/μL) | Before | 61.4±14.6 | 67.6±17.1 | 58.7±14.7 | 45–110 |
| After | 62.5±14.8 | 70.4±17.2 | 57.8±13.2 | ||
| Segmented (%) | Before | 56.1±9.6 | 60.6±8.2 | 58.4±8.7 | 40–80 |
| After | 56.6±10.2 | 63.2±6.9 | 58.6±9.3 | ||
| Monocyte (%) | Before | 3.3±0.9 | 3.1±0.9 | 2.9±0.6 | 2–10 |
| After | 2.6±0.8 | 2.6±0.7 | 2.9±1.1 | ||
| Lymphocyte (%) | Before | 40.7±8.9 | 36.3±8.1 | 38.7±8.6 | 15–45 |
| After | 40.8±10.2 | 34.2±6.8 | 38.5±8.4 | ||
| RBC (104/μL) | Before | 491.2±35.0 | 488.4±37.3 | 487.7±54.8 | 450–650 |
| After | 496.0±39.0 | 497.4±36.3 | 487.4±54.6 | ||
| Hemoglobin (g/dL) | Before | 15.6±1.0 | 15.6±0.8 | 14.9±0.8 | 13–17 |
| After | 15.5±0.8 | 15.8±0.8 | 14.6±1.1 | ||
| Hematocrit (%) | Before | 45.2±2.6 | 45.5±2.1 | 43.6±2.4 | 38–52 |
| After | 45.5±2.3 | 46.2±2.5 | 43.4±2.9 | ||
| ESR (mm/hr) | Before | 5.8±5.4 | 3.1±1.9 | 4.6±3.7 | 0–10 |
| After | 4.8±4.5 | 3.4±2.7 | 4.5±4.2 | ||
| Platelet (104/μL) | Before | 24.3±3.9 | 22.1±3.1 | 23.9±5.1 | 15–45 |
| After | 23.1±4.5 | 21.5±2.8 | 24.9±4.7 | ||
| MCV (fl) | Before | 92.1±4.0 | 93.4±5.3 | 90.3±7.6 | 80–94 |
| After | 92.1±4.9 | 93.2±5.6 | 89.8±8.1 | ||
| MCH (pg) | Before | 31.9±1.3 | 32.0±1.8 | 30.8±3.0 | 26–33 |
| After | 31.4±1.6 | 31.9±2.0 | 30.2±2.8 | ||
| MCHC (%) | Before | 34.6±0.6 | 34.3±0.7 | 35.5±5.9 | 32–37 |
| After | 34.1±0.3 | 34.2±0.4 | 33.6±0.8 | ||
| RDW (%) | Before | 12.9±0.4 | 12.9±0.6 | 12.8±0.7 | 11.5–16.5 |
| After | 12.7±0.5 | 12.4±0.5 | 12.8±0.8 |
Data are expressed as mean±standard deviation.
BID, twice a day; WBC, white blood cells; RBC, red blood cells; ESR, erythrocyte sedimentation rate; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width.
Table 6.
Biochemistry Test Result of Female Subjects After 4 Weeks Administration of Panax ginseng (PG)
| Variables | Treatment | Placebo BID | PG 500 mg BID | PG 1000 mg BID | Reference range |
|---|---|---|---|---|---|
| Total protein (g/dL) | Before | 7.6±0.4 | 7.5±0.3 | 7.5±0.3 | 6.4–8 3 |
| After | 7.6±0.4 | 7.6±0.4 | 7.6±0.3 | ||
| Albumin (g/dL) | Before | 4.6±0.2 | 4.5±0.2 | 4.6±0.2 | 3.8–5.1 |
| After | 4.5±0.2 | 4.5±0.2 | 4.5±0.2 | ||
| A/G ratio | Before | 1.5±0.2 | 1.5±0.2 | 1.6±0.2 | |
| After | 1.5±0.2 | 1.5±0.2 | 1.5±0.1 | ||
| T. bilirubin (mg/dL) | Before | 0.8±0.3 | 0.8±0.3 | 0.7±0.2 | 0.1–1.2 |
| After | 0.8±0.3 | 0.8±0.2 | 0.8±0.3 | ||
| AST (IU/L) | Before | 19.8±3.2 | 21.7±6.1 | 20.8±5.4 | 0–40 |
| After | 20.9±8.0 | 21.6±6.4 | 20.7±4.7 | ||
| ALT (IU/L) | Before | 14.8±6.8 | 17.5±8.0 | 16.3±8.3 | 0–40 |
| After | 16.3±10.2 | 17.7±9.0 | 16.2±5.8 | ||
| ALP (IU/L) | Before | 57.5±12.7 | 59.1±14.3 | 59.8±19.9 | 30–120 |
| After | 57.4±14.2 | 59.1±15.8 | 59.4±19.7 | ||
| r‐GTP (IU/L) | Before | 15.1±6.7 | 16.7±7.8 | 15.5±6.1 | 0–64 |
| After | 14.3±5.0 | 17.5±14.9 | 15.1±5.8 | ||
| Cholesterol (mg/dL) | Before | 189.2±31.0 | 195.9±31.3 | 197.9±41.8 | 140–271 |
| After | 188.0±25.5 | 194.1±29.5 | 200.8±47.0 | ||
| Triglycerides (mg/dL) | Before | 103.0±68.1 | 108.0±65.2 | 96.6±64.3 | 130–220 |
| After | 112.0±70.3 | 117.3±77.4 | 107.6±64.3 | ||
| HDL‐Chol (mg/dL) | Before | 55.5±11.4 | 53.8±11.4 | 55.4±10.6 | 42–88 |
| After | 54.0±11.1 | 53.6±11.0 | 54.9±10.2 | ||
| Glucose (mg%) | Before | 86.9±7.6 | 86.5±7.4 | 87.2±9.2 | 70–115 |
| After | 89.3±8.1 | 88.8±7.3 | 89.4±9.3 | ||
| Creatinine (mg/dL) | Before | 0.7±0.1 | 0.7±0.1 | 0.7±0.1 | 0.4–1.5 |
| After | 0.8±0.1 | 0.8±0.1 | 0.8±0.1 | ||
| BUN (mg/dL) | Before | 12.5±3.3 | 12.5±2.7 | 12.6±3.0 | 5–24 |
| After | 12.4±3.2 | 11.4±2.4 | 12.8±3.4 |
Data are expressed as mean±standard deviation.
A/G, albumin to globulin ratio; T. bilirubin, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; r-GTP, gamma-glutamyl transpeptidase; HDL-Chol, high-density lipoprotein cholesterol; BUN, blood urea nitrogen.
Table 4.
Hematological Test Result of Female Subjects After 4 Weeks Administration of Panax ginseng (PG)
| Variables | Treatment | Placebo | PG 500 mg BID | PG 1000 mg BID | Reference range |
|---|---|---|---|---|---|
| WBC (102/μL) | Before | 52.2±12.3 | 55.0±14.9 | 53.9±14.4 | 45–110 |
| After | 55.5±17.3 | 58.4±16.9 | 57.3±18.2 | ||
| Segmented (%) | Before | 55.0±8.2 | 56.6±8.8 | 56.4±6.3 | 40–80 |
| After | 56.7±9.8 | 58.6±7.9 | 57.6±9.6 | ||
| Monocyte (%) | Before | 3.2±0.9 | 3.4±1.0 | 3.1±1.0 | 2–10 |
| After | 3.1±1.0 | 2.9±0.7 | 3.1±1.0 | ||
| Lymphocyte (%) | Before | 41.8±8.3 | 40.0±8.5 | 40.5±6.3 | 15–45 |
| After | 40.2±9.5 | 38.5±7.7 | 39.3±9.3 | ||
| RBC (104/μL) | Before | 419.9±32.8 | 428.0±27.7 | 429.5±29.3 | 400–600 |
| After | 424.2±29.7 | 427.7±29.9 | 429.1±31.4 | ||
| Hemoglobin (g/dL) | Before | 12.7±1.5 | 13.3±1.2 | 12.9±1.2 | 12–16 |
| After | 12.7±1.5 | 13.1±1.1 | 12.8±1.4 | ||
| Hematocrit (%) | Before | 37.8±3.6 | 39.1±2.9 | 38.4±3.1 | 36–46 |
| After | 38.1±3.8 | 39.0±3.0 | 38.4±3.7 | ||
| ESR (mm/hr) | Before | 14.9±8.5 | 12.0±7.4 | 14.4±8.1 | 0–20 |
| After | 13.9±8.0 | 12.1±7.8 | 11.8±6.1 | ||
| Platelet (104/μL) | Before | 23.9±6.2 | 23.7±5.0 | 23.8±5.7 | 15–45 |
| After | 24.1±5.6 | 23.2±3.9 | 24.1±5.8 | ||
| MCV (fl) | Before | 90.2±7.2 | 91.5±4.9 | 89.7±7.3 | 81–99 |
| After | 90.0±7.3 | 91.4±4.8 | 89.5±7.2 | ||
| MCH (pg) | Before | 30.3±3.1 | 31.1±2.1 | 30.2±3.0 | 26–33 |
| After | 30.0±3.1 | 30.7±2.0 | 30.0±2.9 | ||
| MCHC (%) | Before | 33.6±1.2 | 33.9±0.9 | 33.6±0.9 | 32–37 |
| After | 33.2±1.2 | 33.5±0.9 | 33.4±0.9 | ||
| RDW (%) | Before | 13.0±1.1 | 13.0±0.9 | 13.2±1.1 | 11.5–16.5 |
| After | 12.9±1.1 | 12.7±0.9 | 12.9±1.0 |
Data are expressed as mean±standard deviation.
BID, twice a day; WBC, white blood cells; RBC, red blood cells; ESR, erythrocyte sedimentation rate; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width.
Table 5.
Biochemistry Test Result of Male Subjects After 4 Weeks Administration of Panax ginseng (PG)
| Variables | Treatment | Placebo BID | PG 500 mg BID | PG 1000 mg BID | Reference range |
|---|---|---|---|---|---|
| Total protein (g/dL) | Before | 7.6±0.3 | 7.5±0.4 | 7.5±0.3 | 6.4–8.3 |
| After | 7.6±0.3 | 7.6±0.4 | 7.7±0.4 | ||
| Albumin (g/dL) | Before | 4.7±0.3 | 4.8±0.2 | 4.7±0.2 | 3.8–5.1 |
| After | 4.7±0.2 | 4.7±0.2 | 4.7±0.2 | ||
| A/G ratio | Before | 1.6±0.2 | 1.7±0.1 | 1.7±0.2 | |
| After | 1.6±0.2 | 1.7±0.2 | 1.6±0.2 | ||
| T. bilirubin (mg/dL) | Before | 1.1±0.4 | 1.0±0.2 | 0.9±0.4 | 0.1–1.2 |
| After | 1.1±0.5 | 0.9±0.3 | 1.0±0.4 | ||
| AST (IU/L) | Before | 24.4±10.6 | 22.2±5.5 | 21.8±4.5 | 0–40 |
| After | 22.9±7.6 | 23.6±6.1 | 21.2±4.6 | ||
| ALT (IU/L) | Before | 26.4±19.3 | 21.4±11.6 | 21.0±9.1 | 0–40 |
| After | 24.6±12.7 | 21.5±9.7 | 19.5±6.4 | ||
| ALP (IU/L) | Before | 65.9±17.3 | 79.7±21.1 | 61.5±9.8 | 30–120 |
| After | 68.8±18.7 | 80.4±21.4 | 63.4±11.0 | ||
| r‐GTP (IU/L) | Before | 28.6±14.1 | 24.6±12.7 | 28.2±17.7 | 0–64 |
| After | 25.4±11.2 | 24.1±11.0 | 24.8±11.8 | ||
| Cholesterol (mg/dL) | Before | 209.4±38.9 | 187.0±42.5 | 201.9±33.7 | 140–271 |
| After | 206.8±47.0 | 188.1±25.3 | 199.6±26.3 | ||
| Triglycerides (mg/dL) | Before | 144.4±111.4 | 130.4±61.1 | 145.6±82.5 | 130–220 |
| After | 153.6±96.4 | 138.0±86.3 | 155.3±59.3 | ||
| HDL‐Chol (mg/dL) | Before | 47.8±11.0 | 48.5±8.7 | 49.1±7.3 | 42–88 |
| After | 45.3±10.5 | 49.3±10.6 | 48.1±7.1 | ||
| Glucose (mg%) | Before | 91.1±7.8 | 88.9±9.3 | 91.6±5.9 | 70–115 |
| After | 96.6±10.7 | 92.3±10.0 | 96.5±10.6 | ||
| Creatinine (mg/dL) | Before | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 0.4–1.5 |
| After | 1.1±0.2 | 1.0±0.1 | 1.0±0.2 | ||
| BUN (mg/dL) | Before | 13.7±3.7 | 13.3±2.8 | 13.7±2.5 | 5–24 |
| After | 12.6±2.4 | 12.7±3.6 | 12.9±3.0 |
Data are expressed as mean±standard deviation.
A/G, albumin to globulin ratio; T. bilirubin, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; r-GTP, gamma-glutamyl transpeptidase; HDL-Chol, high-density lipoprotein cholesterol; BUN, blood urea nitrogen.
The urinalysis results are summarized in Tables 7 and 8. Even though leukocytes, blood, and ketones in females were related, the safety committee decided those were not directly related with this study and were negligible. In addition, there were no signs of toxicity.
Table 7.
Urinalysis Result of Male Subjects After 4 Weeks Administration of Panax ginseng (PG)
| Variables | Treatment | Placebo | PG 500 mg BID | PG 1000 mg BID | Reference range |
|---|---|---|---|---|---|
| Specific gravity | Before | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.003–1.030 |
| After | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | ||
| pH | Before | 6.2±1.0 | 6.0±1.3 | 6.3±1.5 | 5.0–7.0 |
| After | 5.8±0.8 | 6.0±1.1 | 6.0±1.3 | ||
| Albumin (%) | Before | 0 | 0 | 0 | |
| After | 0 | 0 | 0 | ||
| Leukocytes (%) | Before | 0 | 12.5 | 0 | |
| After | 0 | 12.5 | 0 | ||
| Nitrite (%) | Before | 0 | 0 | 0 | |
| After | 0 | 0 | 0 | ||
| Glucose (%) | Before | 0 | 0 | 0 | |
| After | 0 | 0 | 0 | ||
| Urobilinogen (%) | Before | 0 | 0 | 0 | |
| After | 0 | 0 | 0 | ||
| Ketone (%) | Before | 0 | 0 | 0 | |
| After | 0 | 0 | 0 | ||
| Bilirubin (%) | Before | 0 | 0 | 0 | |
| After | 0 | 0 | 0 | ||
| Blood (%) | Before | 0 | 0 | 0 | |
| After | 0 | 12.5 | 0 |
Data are expressed as percentages of dipstick positive except specific gravity and pH.
BID, twice a day.
Table 8.
Urinalysis Result of Female Subjects After 4 Weeks Administration of Panax ginseng (PG)
| Variables | Treatment | Placebo | PG 500 mg BID | PG 1000 mg BID | Reference range |
|---|---|---|---|---|---|
| Specific gravity | Before | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.003–1.030 |
| After | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | ||
| pH | Before | 5.9±1.0 | 5.8±1.4 | 6.1±1.0 | 5.0–7.0 |
| After | 5.9±0.8 | 6.0±1.1 | 5.9±0.9 | ||
| Albumin (%) | Before | 0 | 0 | 0 | |
| After | 0 | 0 | 0 | ||
| Leukocytes (%) | Before | 20.8 | 38.1 | 21.7 | |
| After | 45.8 | 33.3 | 21.7 | ||
| Nitrite (%) | Before | 0 | 0 | 0 | |
| After | 0 | 0 | 0 | ||
| Glucose (%) | Before | 0 | 0 | 0 | |
| After | 0 | 0 | 0 | ||
| Urobilinogen (%) | Before | 0 | 0 | 0 | |
| After | 0 | 0 | 0 | ||
| Ketone (%) | Before | 8.3 | 0 | 0 | |
| After | 4.2 | 0 | 0 | ||
| Bilirubin (%) | Before | 0 | 0 | 0 | |
| After | 0 | 4.8 | 0 | ||
| Blood (%) | Before | 12.5 | 19 | 4.3 | |
| After | 33.3 | 28.6 | 17.4 |
Data are expressed as percentages of dipstick positive except specific gravity and pH.
Discussion
In the present study, PG administration did not significantly alter the hematological and biochemical test results in 170 healthy volunteers. The results showed that 170 healthy volunteers administered PG over a 4-week interval did not show any serious adverse reactions. Adverse events reported by volunteers were mainly mild in severity and included dyspepsia, hot flashes, insomnia, constipation, and so on. Most of the adverse events reported were all transient, resolving without any clinical sequelae. This is in accordance with a recent systematic review.7 This report provides scientific results concerning the safety and tolerability of PG in healthy volunteers.
There is a limitation that needs to be acknowledged and addressed regarding the present study. Subjects who had a previous history of hypersensitivity to ginseng preparations were excluded. Therefore, possible adverse events from ginseng might have been missed.
PG is a long-standing medication used largely for reducing physical, chemical, and biologic stress, while increasing general vitality and immune function, including physical and mental capacity.20–26
In general, ginseng has a good safety record. The root of PG appeared nontoxic to rats, dogs, and humans.16 In mice, a lethal oral dose of purified ginseng was determined to be higher than 5 g/kg, the highest dose that can be orally given to a mouse and considered good practice at the maximal dose volumes without violating animal welfare standards.27 In a 2-year human study, 14 of a total of 133 subjects were reported to experience side-effects attributed to long-term exposure of ginseng when consumed at levels up to 15 g/day.28 Despite its broad clinical use, there have been few toxicological studies performed in humans.
It is well known that the most common side-effects of PG resulting from its overdose are nervousness and excitability. These side-effects usually decrease after the first few days. In inappropriate uses, the most common side-effects are the inability to sleep and high blood pressure.
Other side-effects include mastalgia,29 Stevens-Johnson syndrome,30 psychiatric conditions,31 cerebral arteritis,32 agranulocytosis,33 eye symptoms,34 hypertension,35 and pneumonitis.36 PG may lower blood glucose levels.37 In some cases, active substances of herbs can interact with other herbs' active substances, supplements, or medications. Interactions between ginseng and warfarin, phenelzine, or alcohol have been reported.38–42
For these reasons, herbs should be taken with care, under supervision of a practitioner knowledgeable in the field of botanical medicine.
Conclusions
In summary, PG was shown in the present study to be safe, tolerable, and free of any untoward toxic effect in healthy male and female volunteers, when administered during a 4-week period. Although minor side-effects were reported, the physical, hematological, and biochemical parameters measured were within normal limits. Future results from ongoing multicenter collaborative efforts to evaluate short- and long-term effects of PG may contribute to current understanding of safety and tolerability of this herbal product.
Acknowledgments
This study was supported by a grant from the Korea Science and Engineering Foundation (KOSEF) by the Korean government (# R01-2007-000-11248-0 and 20110003150).
Disclosure Statement
No financial conflict exists.
References
- 1.Radad K. Gille G. Liu L. Rausch WD. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci. 2006;100:175–186. doi: 10.1254/jphs.crj05010x. [DOI] [PubMed] [Google Scholar]
- 2.Duke J. The Green Pharmacy Herbal Handbook: Your Comprehensive Reference to the Best Herbs for Healing. Emmaus, PA: Rodale; 2000. [Google Scholar]
- 3.Blumenthal M. The ABC Clinical Guide to Herbs. New York: Thieme; 2003. [Google Scholar]
- 4.Blumenthal M. Cavaliere C. Rea P. Herbal supplement sales in United States show growth in all channels. HerbalGram. 2008;78:60–63. [Google Scholar]
- 5.Yi SW. Sull JW. Hong JS, et al. Association between ginseng intake and mortality: Kangwha cohort study. J Altern Complement Med. 2009;15:921–928. doi: 10.1089/acm.2008.0296. [DOI] [PubMed] [Google Scholar]
- 6.Lee TK. Johnke RM. Allison RR, et al. Radioprotective potential of ginseng. Mutagenesis. 2005;20:237–243. doi: 10.1093/mutage/gei041. [DOI] [PubMed] [Google Scholar]
- 7.Coon JT. Ernst E. Panax ginseng: A systematic review of adverse effects and drug interactions. Drug Saf. 2002;25:323–344. doi: 10.2165/00002018-200225050-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kim TH. Jeon SH. Hahn EJ, et al. Effects of tissue-cultured mountain ginseng (Panax ginseng CA Meyer) extract on male patients with erectile dysfunction. Asian J Androl. 2009;11:356–361. doi: 10.1038/aja.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J. Li S. Fan Y, et al. Anti-fatigue activity of the water-soluble polysaccharides isolated from Panax ginseng C. A. Meyer. J Ethnopharmacol. 2010;130:421–423. doi: 10.1016/j.jep.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Lee ST. Chu K. Sim JY, et al. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:222–226. doi: 10.1097/WAD.0b013e31816c92e6. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z. Li M. Wu WK, et al. Ginsenoside Rb1 preconditioning protects against myocardial infarction after regional ischemia and reperfusion by activation of phosphatidylinositol-3-kinase signal transduction. Cardiovasc Drugs Ther. 2008;22:443–452. doi: 10.1007/s10557-008-6129-4. [DOI] [PubMed] [Google Scholar]
- 12.Li QF. Shi SL. Liu QR, et al. Anticancer effects of ginsenoside Rg1, cinnamic acid, and tanshinone IIA in osteosarcoma MG-63 cells: Nuclear matrix downregulation and cytoplasmic trafficking of nucleophosmin. Int J Biochem Cell Biol. 2008;40:1918–1929. doi: 10.1016/j.biocel.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 13.Jang HI. Shin HM. Wild Panax ginseng (Panax ginseng C.A. Meyer) protects against methotrexate-induced cell regression by enhancing the immune response in RAW 264.7 macrophages. Am J Chin Med. 2010;38:949–960. doi: 10.1142/S0192415X10008378. [DOI] [PubMed] [Google Scholar]
- 14.Li XT. Chen R. Jin LM. Chen HY. Regulation on energy metabolism and protection on mitochondria of Panax ginseng polysaccharide. Am J Chin Med. 2009;37:1139–1152. doi: 10.1142/S0192415X09007454. [DOI] [PubMed] [Google Scholar]
- 15.Hess FG., Jr Parent RA. Cox GE, et al. Reproduction study in rats or ginseng extract G115. Food Chem Toxicol. 1982;20:189–192. doi: 10.1016/s0278-6915(82)80246-9. [DOI] [PubMed] [Google Scholar]
- 16.Hess FG., Jr Parent RA. Stevens KR, et al. Effects of subchronic feeding of ginseng extract G115 in beagle dogs. Food Chem Toxicol. 1983;21:95–97. doi: 10.1016/0278-6915(83)90275-2. [DOI] [PubMed] [Google Scholar]
- 17.Popov IM. Goldwag WJ. A review of the properties and clinical effects of ginseng. Am J Chin Med (Gard City N Y) 1973;1:263–270. doi: 10.1142/s0192415x73000280. [DOI] [PubMed] [Google Scholar]
- 18.Bittles AH. Fulder SJ. Grant EC. Nicholls MR. The effect of ginseng on lifespan and stress responses in mice. Gerontology. 1979;25:125–131. doi: 10.1159/000212330. [DOI] [PubMed] [Google Scholar]
- 19.NIH. Common Terminology Criteria for Adverse Events. General Characteristics of the CTCAE Grading (Severity) Scale. 2010. https://webapps.ctep.nci.nih.gov/webobjs/ctc/webhelp/Grading_General_Characteristics.htm. [Nov;2010 ]. https://webapps.ctep.nci.nih.gov/webobjs/ctc/webhelp/Grading_General_Characteristics.htm
- 20.Coleman CI. Hebert JH. Reddy P. The effects of Panax ginseng on quality of life. J Clin Pharm Ther. 2003;28:5–15. doi: 10.1046/j.1365-2710.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko H. Nakanishi K. Proof of the mysterious efficacy of ginseng: Basic and clinical trials: Clinical effects of medical ginseng, korean red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004;95:158–162. doi: 10.1254/jphs.fmj04001x5. [DOI] [PubMed] [Google Scholar]
- 22.Murphy LL. Lee TJ. Ginseng, sex behavior, and nitric oxide. Ann N Y Acad Sci. 2002;962:372–377. doi: 10.1111/j.1749-6632.2002.tb04081.x. [DOI] [PubMed] [Google Scholar]
- 23.Gillis CN. Panax ginseng pharmacology: A nitric oxide link? Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 24.Bahrke MS. Morgan WP. Evaluation of the ergogenic properties of ginseng. Sports Med. 1994;18:229–248. doi: 10.2165/00007256-199418040-00003. [DOI] [PubMed] [Google Scholar]
- 25.Wood WB. Roh BL. White RP. Cardiovascular actions of Panax ginseng in dogs. Jpn J Pharmacol. 1964;14:284–294. doi: 10.1254/jjp.14.284. [DOI] [PubMed] [Google Scholar]
- 26.Scott GI. Colligan PB. Ren BH. Ren J. Ginsenosides Rb1 and Re decrease cardiac contraction in adult rat ventricular myocytes: Role of nitric oxide. Br J Pharmacol. 2001;134:1159–1165. doi: 10.1038/sj.bjp.0704377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X. Jia L. The conduct of drug metabolism studies considered good practice (I): Analytical systems and in vivo studies. Curr Drug Metab. 2007;8:815–821. doi: 10.2174/138920007782798153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel RK. Ginseng abuse syndrome: Problems with the panacea. JAMA. 1979;241:1614–1615. [PubMed] [Google Scholar]
- 29.Dukes MN. Ginseng and mastalgia. Br Med J. 1978;1:1621. doi: 10.1136/bmj.1.6127.1621-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dega H. Laporte JL. Frances C, et al. Ginseng as a cause for Stevens-Johnson syndrome? Lancet. 1996;347:1344. doi: 10.1016/s0140-6736(96)91001-6. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Seijo JC. Ramos YM. Lastra I. Manic episode and ginseng: Report of a possible case. J Clin Psychopharmacol. 1995;15:447–448. doi: 10.1097/00004714-199512000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Ryu SJ. Chien YY. Ginseng-associated cerebral arteritis. Neurology. 1995;45:829–830. doi: 10.1212/wnl.45.4.829. [DOI] [PubMed] [Google Scholar]
- 33.Ries CA. Sahud MA. Agranulocytosis caused by Chinese herbal medicines: Dangers of medications containing aminopyrine and phenylbutazone. JAMA. 1975;231:352–355. [PubMed] [Google Scholar]
- 34.Lou BY. Li CF. Li PY. Ruan JP. Eye symptoms due to ginseng poisoning [in Chinese] Yan Ke Xue Bao. 1989;5:96–97. [PubMed] [Google Scholar]
- 35.Hammond TG. Whitworth JA. Adverse reactions to ginseng. Med J Aust. 1981;1:492. doi: 10.5694/j.1326-5377.1981.tb135752.x. [DOI] [PubMed] [Google Scholar]
- 36.Nakagawa A. Yamaguchi T. Takao T. Amano H. [Five cases of drug-induced pneumonitis due to Sho-saiko-to or interferon-alpha or both [in Japanese] Nihon Kyobu Shikkan Gakkai Zasshi. 1995;33:1361–1366. [PubMed] [Google Scholar]
- 37.Reay JL. Kennedy DO. Scholey AB. Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. J Psychopharmacol. 2005;19:357–365. doi: 10.1177/0269881105053286. [DOI] [PubMed] [Google Scholar]
- 38.Janetzky K. Morreale AP. Probable interaction between warfarin and ginseng. Am J Health Syst Pharm. 1997;54:692–693. doi: 10.1093/ajhp/54.6.692. [DOI] [PubMed] [Google Scholar]
- 39.Zhu M. Chan KW. Ng LS, et al. Possible influences of ginseng on the pharmacokinetics and pharmacodynamics of warfarin in rats. J Pharm Pharmacol. 1999;51:175–180. doi: 10.1211/0022357991772105. [DOI] [PubMed] [Google Scholar]
- 40.Shader RI. Greenblatt DJ. Bees, ginseng and MAOIs revisited. J Clin Psychopharmacol. 1988;8:235. [PubMed] [Google Scholar]
- 41.Jones BD. Runikis AM. Interaction of ginseng with phenelzine. J Clin Psychopharmacol. 1987;7:201–202. doi: 10.1097/00004714-198706000-00030. [DOI] [PubMed] [Google Scholar]
- 42.Lee FC. Ko JH. Park JK. Lee JS. Effects of Panax ginseng on blood alcohol clearance in man. Clin Exp Pharmacol Physiol. 1987;14:543–546. doi: 10.1111/j.1440-1681.1987.tb01510.x. [DOI] [PubMed] [Google Scholar]