Background: The regulation of PHD expression and function under inflammatory conditions in the nucleus pulposus is unknown.
Results: Expression of PHD3 is regulated by TNF-α and IL-1β. PHD3 controls TNF-α activity by modulating NF-κB signaling.
Conclusion: PHD3 promotes the catabolic effects of TNF-α on nucleus pulposus cells.
Significance: PHD3 may play an important role in pathogenesis of disc disease.
Keywords: Cartilage, Cartilage Biology, Hypoxia-inducible Factor (HIF), NF-kappa B (NF-KB), Tumor Necrosis Factor (TNF), HIF-Prolyl Hydroxylase, Nucleus Pulposus
Abstract
Recent studies suggest a differential role of prolyl hydroxylase (PHD) isoforms in controlling hypoxia-inducible factor (HIF)-α degradation and activity in nucleus pulposus (NP) cells. However, the regulation and function of PHDs under inflammatory conditions that characterize disc disease are not yet known. Here, we show that in NP cells, TNF-α and IL-1β induce PHD3 expression through NF-κB. Lentiviral delivery of Sh-p65 and Sh-IKKβ confirms that cytokine-mediated PHD3 expression is NF-κB-dependent. It is noteworthy that although both cytokines induce HIF activity, mechanistic studies using Sh-HIF-1α and PHD3 promoter/enhancer constructs harboring well characterized hypoxia response element (HRE) show lack of HIF involvement in cytokine-mediated PHD3 expression. Loss-of-function studies clearly indicate that PHD3 serves as a co-activator of NF-κB signaling activity in NP cells; PHD3 interacts with, and co-localizes with, p65. We observed that when PHD3 is silenced, there is a significant decrease in TNF-α-induced expression of catabolic markers that include ADAMTS5, syndecan4, MMP13, and COX2, and at the same time, there is restoration of aggrecan and collagen type II expression. It is noteworthy that hydroxylase function of PHDs is not required for mediating cytokine-dependent gene expression. These findings show that by enhancing the activity of inflammatory cytokines, PHD3 may serve a critical role in degenerative disc disease.
Introduction
The intervertebral disc is a complex tissue that permits a range of motions between adjacent vertebrae and accommodates high biomechanical forces. The blood vessels originating in the vertebral body penetrate the superficial region of the endplates; none of these vessels infiltrate the central aggrecan-rich gel-like tissue, the nucleus pulposus. With respect to the annulus, this tissue is avascular except for small discrete capillary beds in the dorsal and ventral surfaces; in no case does the annulus vasculature enter the nucleus pulposus (1–4). Thus, the nucleus pulposus is completely devoid of any vasculature and is hypoxic.
We have previously shown that prolyl-4-hydroxylases (PHDs)2 1, 2, and 3, members of the 2-oxoglutarate/Fe2+-dependent dioxygenase superfamily, are expressed in the hypoxic nucleus pulposus (5, 6). These enzymes hydroxylate specific prolyl residues in the oxygen-dependent degradation domain of hypoxia-inducible factor (HIF)-α subunits and target the protein for proteasomal degradation. In the nucleus pulposus, expression of all three PHD isoforms is regulated by hypoxia in either HIF-1-dependent or HIF-2-dependent fashion (5). We have shown that only PHD2 partially controls the oxygen-dependent degradation of HIF-1α (5, 6). In contrast, although PHD3 plays no role in either HIF-1α or HIF-2α degradation, it controls HIF-1α transcriptional activity in hypoxia (6). Recent studies suggest that there is increased HIF expression during osteoarthritis, a degenerative condition similar to the one that afflicts the intervertebral disc (7, 8). Under degenerative conditions, it is not known whether expression of PHDs is under the control of HIF or other signaling pathways.
The degenerate disc is characterized by elevated levels of pro-inflammatory cytokines including TNF-α, IL-1β, IL-6, and IL-8 as well as down-regulation of the important matrix molecules aggrecan and collagen type II (9–12). TNF-α and IL-1β stimulate production of NGF, BDNF, and VEGF, molecules associated with nerve ingrowth and angiogenesis by nucleus pulposus cells (13). Recent work has shown that both TNF-α and IL-1β promote NF-κB signaling in the nucleus pulposus and control expression of syndecan4, a heparan sulfate proteoglycan involved in ADAMTS5/aggrecanase-2 activation (14).
The major objective of the study was to determine whether expression of PHDs is regulated by TNF-α and IL-1β in cells of the nucleus pulposus. We show for the first time that TNF-α and IL-1β robustly increased PHD3 expression in an NF-κB-dependent fashion. Our findings indicate that by positively controlling NF-κB signaling activity, PHD3 promotes the catabolic effects of the inflammatory cytokines on nucleus pulposus cells. The result suggests that PHD3 may play an important role in pathogenesis of disc disease and may offer a therapeutic target to treat this debilitating and painful degenerative condition.
EXPERIMENTAL PROCEDURES
Plasmids and Reagents
psPAX2 (catalogue number 12260) and pMD2G (catalogue number 12259) developed by Dr. Didier Trono and HRE-Luc (catalogue number 26731) were obtained from Addgene. Lentiviral Sh-PHD3 constructs were from Dr. Kenneth Thirstrup, Denmark (15). Plasmids were kindly provided by Dr. Andree Yeramian, University of Lleida, Spain (lentiviral Sh-HIF-1α, Sh-p65, and Sh-IKKβ) (16), Mark B. Taubman, University of Rochester (NF-κB-responsive luciferase reporter) (17), Dr. Nianli Sang, Philadelphia, PA (pcDNA3.1-PHD3), and Dr. Luis Del Peso, Madrid, Spain (human PHD3 reporter plasmids) (18). As an internal transfection control, vector pRL-TK (Promega) containing Renilla reniformis luciferase gene was used. The amount of transfected plasmid, the pre-transfection period after seeding, and the post-transfection period before harvesting have been optimized for rat nucleus pulposus cells using pSV β-galactosidase plasmid (Promega) (19). p65 null and wild type mouse embryonic fibroblasts (MEFs) were a kind gift from Dr. Denis Guttridge, University of Ohio, Columbus, OH.
Isolation of Nucleus Pulposus Cells, Treatments, and Hypoxic Culture
Rat and human nucleus pulposus cells were isolated and characterized as reported earlier (5, 6, 19). Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) and 10% fetal bovine serum (FBS) supplemented with antibiotics. To investigate the effect of cytokines, cells were treated with IL-1β (10 ng/ml) and TNF-α (25–50 ng/ml) for 4–24 h. Cells were cultured in a Hypoxia workstation (Invivo2 300, Ruskinn, UK) with a mixture of 1% O2, 5% CO2, and 94% N2 for 8–72 h.
Human Tissue Collection and Grading
Disc tissues were collected as surgical waste from individuals undergoing elective spinal surgical procedures. In line with the Thomas Jefferson University Institutional Review Board guidelines, informed consent for sample collection was obtained from each patient. Assessment of the disease state was performed using Pfirrmann grading (20). Nucleus pulposus cells were isolated as reported earlier (5, 19).
Transfections and Dual-Luciferase Assay
Cells were transferred to 48-well plates at a density of 2 × 104 cells/well 1 day before transfection. Lipofectamine 2000 (Invitrogen) was used as a transfection reagent. For each transfection, plasmids were premixed with the transfection reagent. For measuring the effect of TNF-α and IL-1β treatment or hypoxia on HRE-Luc and PHD3 reporter activity, 24 h after transfection, the cells in some wells were treated with TNF-α (25–50 ng/ml) and IL-1β (5–10 ng/ml) or moved to the Hypoxia workstation. The next day, the cells were harvested, and a Dual-LuciferaseTM reporter assay system (Promega) was used for sequential measurements of firefly and Renilla luciferase activities. Quantification of luciferase activities and calculation of relative ratios were carried out using a luminometer (TD-20/20, Turner Designs).
Real Time RT-PCR Analysis
Total RNA was extracted from nucleus pulposus cells using RNeasy mini columns (Qiagen). Before elution from the column, RNA was treated with RNase-free DNase I (Qiagen). The purified, DNA-free RNA was converted to cDNA using SuperScript III reverse transcriptase (Invitrogen). Reactions were set up in triplicate in 96-well plate using 1 μl of cDNA with SYBR Green PCR master mix (Applied Biosystems) to which gene-specific forward and reverse PCR primers were added (see supplemental Table 1, synthesized by Integrated DNA Technologies, Inc.). PCR reactions were performed in a StepOnePlus real time PCR system (Applied Biosystems) according to the manufacturer's instructions. Transcript levels were normalized using β-actin. Melting curves were analyzed to verify the specificity of the RT-PCR reaction and the absence of primer dimer formation.
Protein Extraction, Immunoprecipitation, and Western Blotting
Cells were placed on ice immediately and washed with ice-cold Hanks' balanced salt solution. All the wash buffers and final resuspension buffer included 1× protease inhibitor mixture (Roche Diagnostics), NaF (5 mm), and Na3VO4 (200 μm). Nuclear and cytosolic proteins were prepared using the CelLytic NuCLEAR extraction kit (Sigma-Aldrich). Immunoprecipitation was performed using a commercially available kit (TrueBlot®, eBioscience). Proteins were resolved on 8–12% SDS-polyacrylamide gels and transferred by electroblotting to PVDF membranes (Bio-Rad). The membranes were blocked with 5% nonfat dry milk in TBST (50 mm Tris, pH 7.6, 150 mm NaCl, 0.1% Tween 20) and incubated overnight at 4 °C in 3% nonfat dry milk in TBST with the anti-HIF-1α (1:1000, R&D Systems), anti-p65, or anti-IKKβ or anti-Cox2 (1:1000, Cell Signaling Technology), anti-PHD1 or anti-PHD3 antibody (1:1000, Novus), anti-PHD2 (1:1000, Cell Signaling Technology), anti-SDC4 (1:200, Abcam), and anti-β-tubulin (1:3000, Developmental Studies Hybridoma Bank). Immunolabeling was detected using the ECL reagent (Amersham Biosciences). Relative expression levels were determined by quantitative densitometric analysis using one-dimensional image analysis software (Quantity One, Bio-Rad).
Immunofluorescence Microscopy
Cells were plated in flat bottom 96-well plates (5 × 103/well) and treated with TNF-α or IL-1β for 1–24 h. After incubation, cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100 in PBS for 10 min, blocked with PBS containing 5% FBS, and incubated with antibodies against PHD3 (1:200) and p65 (1:200) at 4 °C overnight. As a negative control, cells were reacted with isotype IgG under similar conditions. After washing, the cells were incubated with Alexa Fluor 488-conjugated anti-rabbit and Alexa Fluor 647-conjugated anti-mouse secondary antibodies (Invitrogen), at a dilution of 1:100 for 1 h at room temperature. Cells were imaged using a laser scanning confocal microscope using 20×/0.4 LCPlanFl objective (Olympus FluoView).
Lentiviral Production and Transduction
HEK 293T cells were seeded in 10-cm plates (60 × 106 cells/plate) in DMEM with 10% heat-inactivated FBS 1 day before transfection. Cells were transfected with 9 μg of Sh-HIF-1α, Sh-p65, Sh-IKKβ, and Sh-PHD3 plasmids along with 6 μg of psPAX2 and 3 μg of pMD2G using Lipofectamine 2000. After 16 h, transfection media were removed and replaced with DMEM with 5% heat-inactivated FBS and penicillin-streptomycin. Lentiviral particles were harvested at 48 and 60 h after transfection. Nucleus pulposus cells were plated in DMEM with 5% heat-inactivated FBS 1 day before transduction. Cells in 10-cm plates were transduced with 5 ml of conditioned media containing viral particles along with 6 μg/ml Polybrene. After 24 h, conditioned media were removed and replaced with DMEM with 5% heat-inactivated FBS. Cells were harvested for protein extraction 5 days after viral transduction. Transduction efficiency between 80–90% was achieved as determined from the number of GFP/YFP-positive cells.
Statistical Analysis
All measurements were performed in triplicate, and data are presented as mean ± S.E. Differences between groups were analyzed by the Student's t test and one-way analysis of variance; *, p < 0.05.
RESULTS
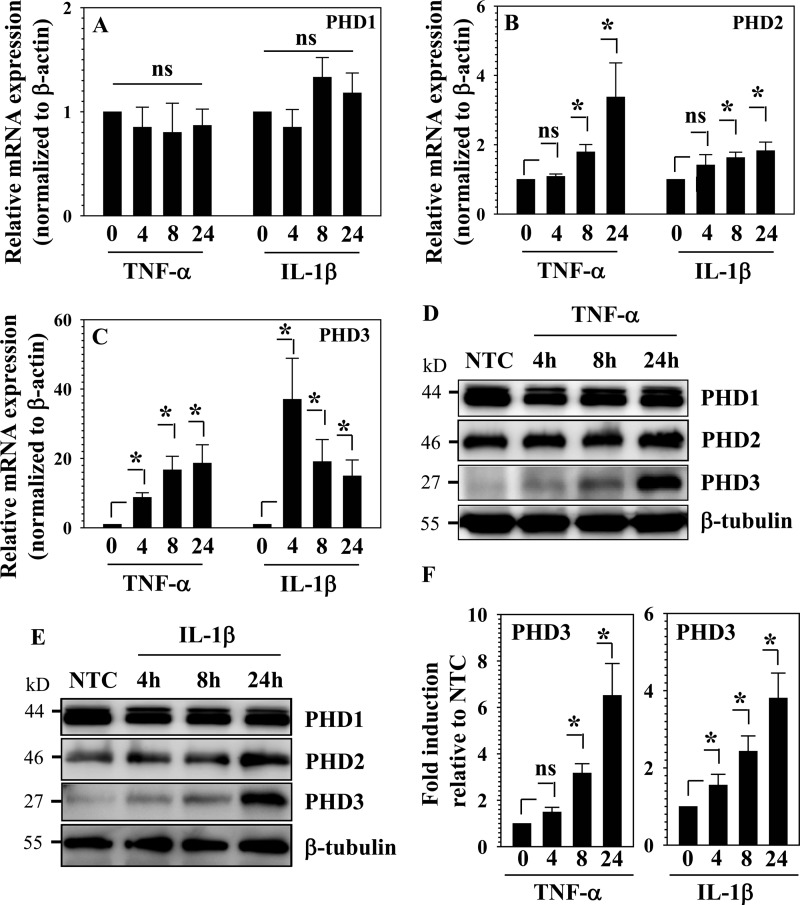
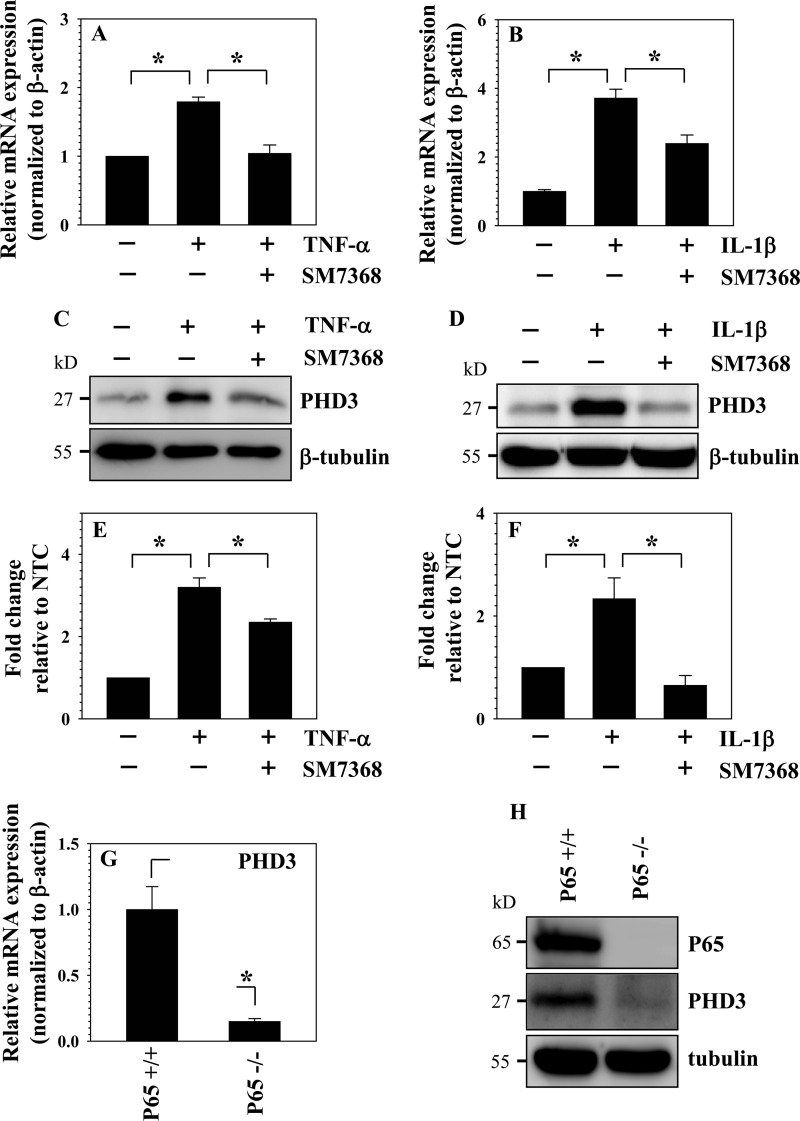
To investigate whether inflammatory cytokines concerned with disc degeneration regulated PHD1–3 expression, rat nucleus pulposus cells were treated with TNF-α and IL-1β, and PHD expression was analyzed. Although mRNA expression of PHD1 is unaffected by this treatment (Fig. 1A), PHD3 and to a lesser extent PHD2 is significantly up-regulated (Fig. 1, B and C). PHD3 mRNA expression is induced ∼10–40-fold by the cytokines; induction is seen as early as 4 h, and levels remain elevated 24 h after treatment. In the case of IL-1β, at 24 h, a decline in PHD3 level is seen when compared with 4 h. Western blot and densitometric analysis show that PHD3 protein levels are strikingly increased as a result of cytokine treatment with 24-h time point exhibiting the highest induction (Fig. 1, D–F). To investigate the role of NF-κB in the regulation of PHD3 expression, we performed loss-of-function studies. When cells are treated with the NF-κB signaling inhibitor SM7368, at 24 h, both TNF-α-mediated and IL-1β-mediated induction in PHD3 mRNA expression is blocked (Fig. 2, A and B). Western blot and densitometric analysis confirmed that inhibitor treatment significantly suppresses cytokine-dependent induction of PHD3 protein expression (Fig. 2, C–F). To further clarify the role of NF-κB in PHD3 regulation and to determine whether this mechanism is cell type-specific, we measured the base-line expression of PHD3 in RelA/p65 null and wild type MEFs. When compared with the wild type, basal expression of PHD3 is significantly lower in p65 null cells (Fig. 2G). Western blot analysis confirmed that PHD3 protein expression is much lower than the wild type cells (Fig. 2H).
FIGURE 1.
Cytokine-mediated regulation of PHD expression in NP cells. A–C, real time RT-PCR analysis of PHD1–3 expression in rat NP cells treated with TNF-α and IL-1β up to 24 h. A, there was no significant change in PHD1 mRNA expression by both TNF-α and IL-1β. B, PHD2 expression level was significantly induced by 8 h when treated with both cytokines. C, PHD3 was robustly induced by both cytokines at all time points. D and E, Western blot analysis of PHDs expression in NP cells treated with TNF-α (D) and IL-1β (E) for 24 h. PHD1 expression level is not changed. The expression level of PHD2 and PHD3 is induced by both cytokines. The induction of PHD3 protein level at 24 h is striking. NTC, nontreated control. F, densitometric analysis of multiple blots from the experiment described in D and E above shows a significant induction in PHD3 protein levels by both TNF-α and IL-1β treatment. Values shown are mean ± S.E. of three independent experiments; *, p < 0.05; ns, not significant.
FIGURE 2.
Effect of NF-κB inhibition on cytokine-mediated induction of PHD3 expression. A and B, real time RT-PCR analysis of PHD3 mRNA expression following TNF-α (A) and IL-1β (B) treatment for 24 h of rat NP cells with or without NF-κB signaling inhibitor SM7368. Inhibitor treatment blocked the cytokine-mediated induction of PHD3 mRNA expression. C and D, Western blot analysis shows that TNF-α- (C) and IL-1β- (D) mediated induction of PHD3 protein expression is dramatically changed following treatment with SM7368. NTC, nontreated control. E and F, densitometric analysis of multiple blots from the experiment described in C and D above shows significant suppression in TNF-α- (E) and IL-1β-dependent (F) induction of PHD3 protein levels by the NF-κB inhibitor. G, real time RT-PCR analysis shows that PHD3 mRNA expression is significantly lower in p65−/− than p65+/+ MEFs. H, Western blot analysis shows that protein expression level of PHD3 is significantly lower in p65−/− when compared with p65+/+ MEFs. Data are represented as mean ± S.E. of three independent experiments performed in triplicate (n = 3); *, p < 0.05.
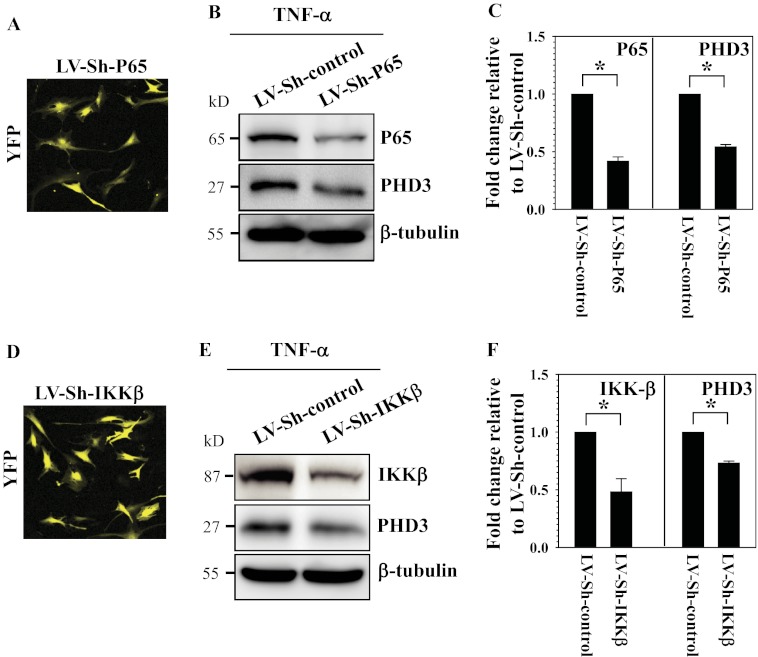
To further investigate the role of NF-κB signaling, we transduced human nucleus pulposus cells with lentivirus co-expressing YFP and p65 shRNA or IKKβ shRNA or scrambled shRNA. Infection efficiency is high (80–90%) for both the lentiviruses (Fig. 3, A and D). Western blot analysis confirmed that there is a decrease in expression of both p65 (Fig. 3B) and IKKβ (Fig. 3E) in cells transduced with respective shRNA. Moreover, we found that when treated with TNF-α, Sh-p65-, and Sh-IKKβ-transduced cells show decreased PHD3 protein expression (Fig. 3, B and E) when compared with cells transduced with scrambled Sh-control. Densitometric analysis confirmed that the decrease in PHD3 is correlated with the suppression of p65 or IKKβ (Fig. 3, C and F).
FIGURE 3.
Involvement of NF-κB in TNF-α-mediated induction of PHD3 expression in human NP cells. A, immunofluorescence analysis of NP cells transduced with lentivirus co-expressing Sh-p65 (LV-Sh-P65) and YFP shows 80–90% transduction efficiency. Original magnification ×20. B, Western blot analysis of cells transduced with LV-Sh-control or LV-Sh-P65 treated with TNF-α. p65 expression level was suppressed by LV-Sh-p65 when compared with cells transduced with control lentivirus (LV-Sh-control). Note that the reduction of PHD3 was seen in cells transduced by LV-Sh-P65. C, densitometric analysis of multiple blots from the experiment described in B above. PHD3 expression is significantly suppressed in LV-Sh-P65-transduced NP cells when compared with control. D, immunofluorescence analysis of NP cells transduced with lentivirus Sh-IKKβ (LV-Sh-IKKβ) with YFP shows high transduction efficiency. Original magnification ×20. E, Western blot analysis of cells transduced with LV-Sh-control or LV-Sh-IKKβ and treated with TNF-α. The suppression of IKKβ was confirmed in LV-Sh-IKKβ-transduced NP cells when compared with cells transduced with control lentivirus (LV-Sh-control). The decrease of PHD3 was seen in the cells transduced by LV-Sh-IKKβ. F, densitometric analysis of multiple blots from the experiment described in E above. Note that reduction of PHD3 expression is correlated with suppression of IKKβ. Data represent mean ± S.E. of three independent experiments performed in triplicate (n = 3); *, p < 0.05.
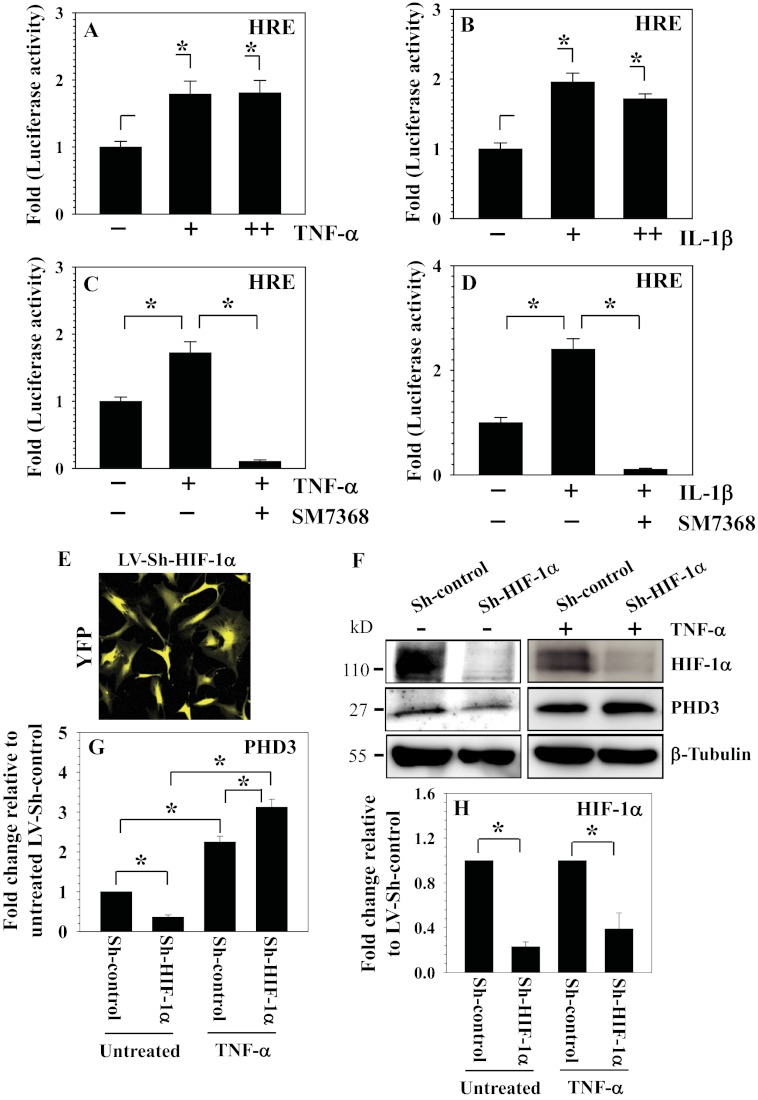
Because HIF-1α controls PHD3 expression in nucleus pulposus cells (5), we determined whether HIF signaling contributes to regulation of PHD3 expression by the inflammatory cytokines. First, we evaluated the effect of TNF-α and IL-1β on HIF signaling in nucleus pulposus cells by measuring the activity of a HIF-responsive reporter (HRE-Luc). Fig. 4, A and B, show that the activity of the HRE reporter is significantly induced by both the cytokines. Interestingly, induction is completely abolished by the NF-κB inhibitor, SM7368 (Fig. 4, C and D). Next, we treated human nucleus pulposus (NP) cells transduced with lentiviral Sh-HIF-1α with TNF-α and measured the expression of PHD3 by Western blot analysis. Once again, we achieved 80–90% transduction efficiency of lentiviral Sh-HIF-1α (Fig. 4E). Similar to cells transduced with a control scrambled shRNA, TNF-α induces expression of PHD3 in the HIF-1α-silenced cells (Fig. 4F). Densitometric analysis confirms that a significant induction in PHD3 by TNF-α is evident in both control and HIF-1α-silenced cells. Moreover, there is a small, but significant increase in PHD3 protein levels in TNF-α-treated HIF-1α-silenced cells when compared with corresponding control cells (Fig. 4G). As expected, silenced cells display significantly lower levels of HIF-1α protein (Fig. 4, F and H).
FIGURE 4.
Cytokine-mediated induction of PHD3 expression is not dependent on HIF activity in NP cells. A and B, treatment with increasing concentration of TNF-α (25–50 ng/ml) (A) and IL-1β (5–10 ng/ml) (B) significantly induced the activity of HRE-Luc in rat NP cells. C and D, activity of HRE-Luc was measured following TNF-α (C) and IL-1β (D) treatment of NP cells with or without NF-κB signaling inhibitor SM7368. Inhibitor treatment abolished the cytokine-mediated induction of HRE-Luc activity. E, immunofluorescence analysis of human NP cells transduced with lentivirus co-expressing Sh-HIF-1α (LV-Sh-HIF-1α) with YFP shows 80–90% transduction efficiency. Original magnification ×20. F, Western blot analysis of cells transduced with LV-Sh-control or LV-Sh-HIF-1α with or without TNF-α treatment. The suppression of HIF-1α was confirmed in LV-Sh-HIF-1α-transduced NP cells when compared with cells transduced with control lentivirus (Sh-control). Note that TNF-α-mediated induction of PHD3 was not altered in the cells transduced by LV-Sh-HIF-1α when compared with control cells. G and H, densitometric analysis of PHD3 (G) and HIF-1α (H) from multiple blots from the experiment described in E above. Note that induction of PHD3 expression by TNF-α is independent of HIF-1α. Data represent mean ± S.E. of three independent experiments performed in triplicate (n = 3); *, p < 0.05.
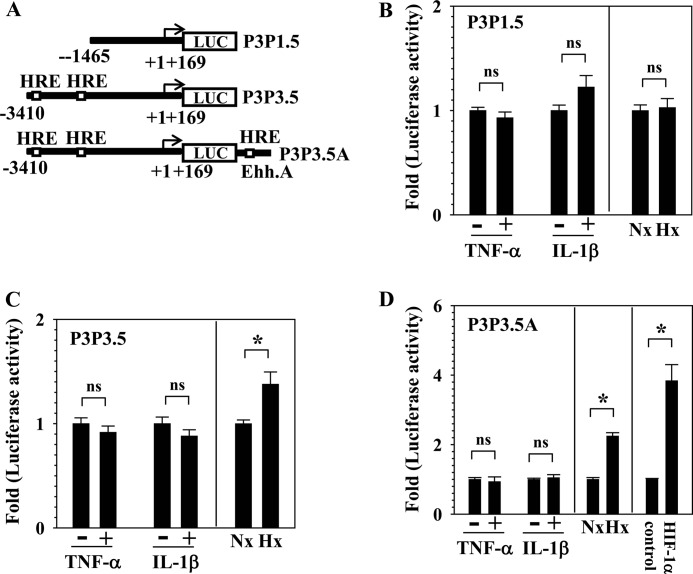
To further determine whether HIF signaling contributes to cytokine-dependent PHD3 expression, we measured the activity of PHD3 promoter and/or enhancer following TNF-α and IL-1β treatment. These reporters contain well characterized HRE motifs that control HIF-dependent transcription of PHD3 (5). We used 3.5-kb (P3P3.5) and 1.5-kb (P3P1.5) promoter of PHD3 that contains either two or no HRE sites and a reporter containing 3.5-kb promoter with an upstream enhancer region (12 kb from transcription site) containing a single HRE site (P3P3.5) (Fig. 5A) (18). Fig. 5B shows that P3P1.5 is not responsive to cytokine treatment, and as expected, to hypoxia. We observed that although the activity of both P3P3.5 and P3P3.5A is induced by hypoxia and HIF-1α, it is not affected by treatment of TNF-α or IL-1β (Fig. 5, C and D).
FIGURE 5.
Influence of cytokine treatment on the promoter and enhancer activity of PHD3 in NP cells. A, schematic of PHD3 reporters (P3P1.5, P3P3.5, and P3P3.5A) used for transfections. B, neither cytokines nor the hypoxia (Hx) significantly changed the activity of 1.5-kb promoter with no HRE sites (P3P1.5). Nx, normoxia. C, the 3.5-kb promoter activity with two putative HRE (P3P3.5) sites was not affected by either cytokine, whereas the promoter activity was significantly induced in hypoxia when compared with normoxia. D, although the activity of 3.5-kb promoter with enhancer A containing one HRE site (P3P3.5A) was significantly increased by hypoxia and HIF-1α, this activity is not changed by the cytokines. Data are represented as mean ± S.E. of three independent experiments performed in triplicate (n = 3); *, p < 0.05; ns, not significant.
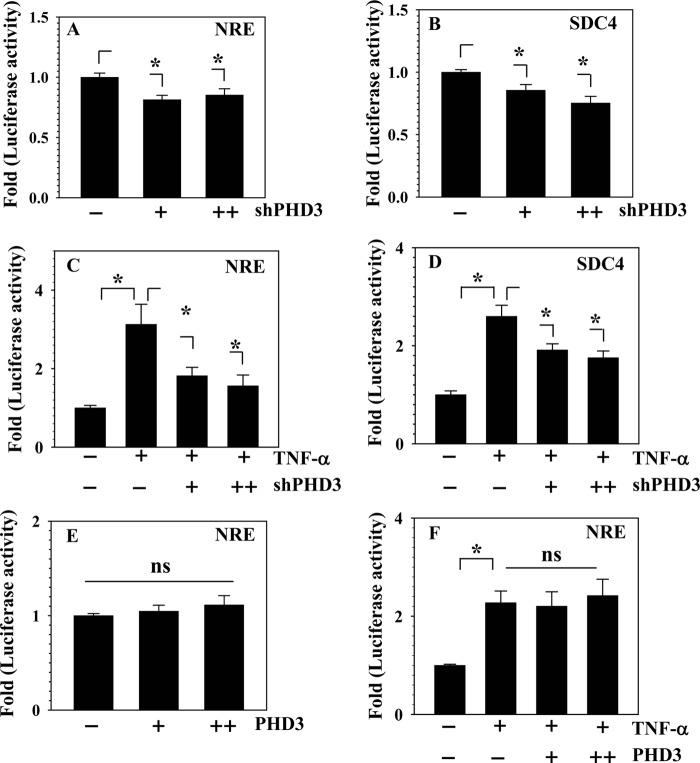
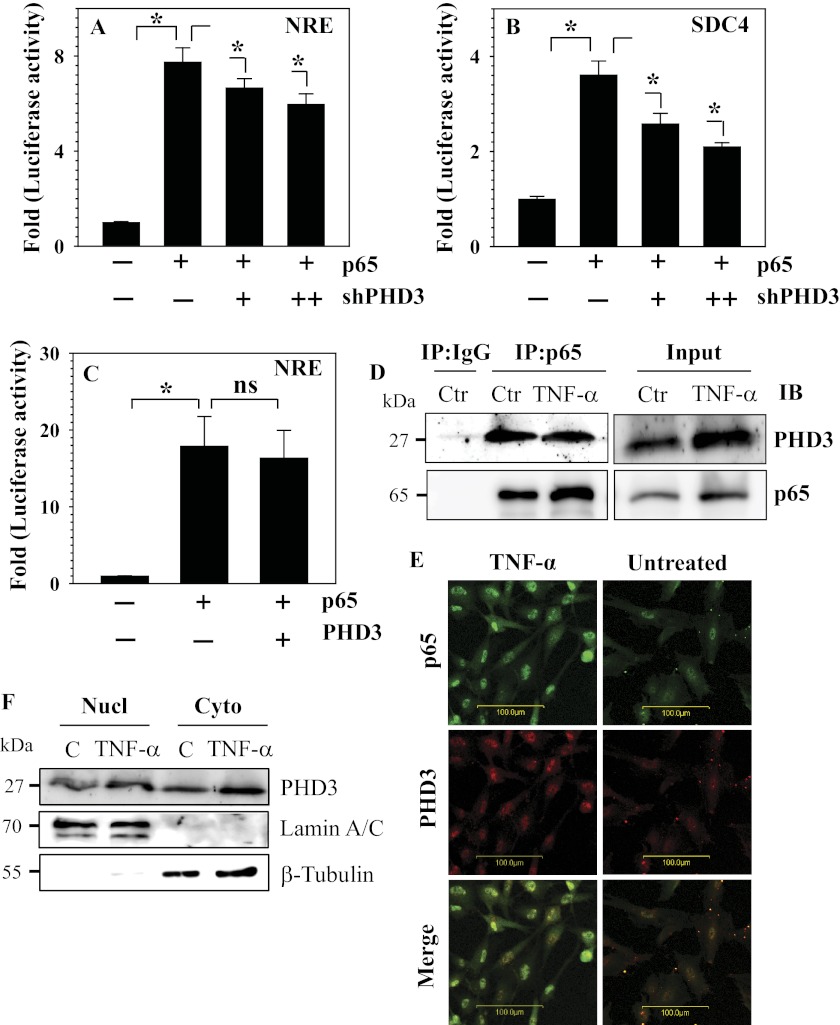
Next, we investigated whether PHD3 participates in TNF-α-dependent signaling and target gene expression using both loss-of-function and gain-of-function experiments. For these studies, a TNF-α/NF-κB-responsive reporter (NRE-Luc) (17) as well as SDC4 promoter regulated by TNF-α in an NF-κB-dependent manner was used (14). Fig. 6, A and B, show that when PHD3 expression is silenced, a small but significant suppression in basal activities of both NRE-Luc (Fig. 6A) and SDC4 promoter (Fig. 6B) is seen. Moreover, silencing of PHD3 also suppresses a TNF-α-dependent increase in both reporter activities (Fig. 6, C and D). However, PHD3 overexpression did not enhance the basal (Fig. 6E) as well as the inductive effect of TNF-α on NRE-Luc activity (Fig. 6F). We then explored the mechanism by which PHD3 regulates TNF-α signaling. First, we investigated whether NF-κB/p65 transcriptional activity was controlled by PHD3. Fig. 7, A and B, show that suppression of PHD3 in rat nucleus pulposus cells partially decreases the inductive effect of p65 on NRE and SDC4 reporter activities, respectively. However, co-transfection of PHD3 with p65 did not result in synergistic activation of NRE-Luc when compared with p65 (Fig. 7C). Next, we used immunoprecipitation/Western blot to determine whether PHD3 interacted with p65. Pulldown of p65 results in co-precipitation of PHD3 (Fig. 7D). Confocal microscopy confirmed that following TNF-α treatment, p65 translocated to the nucleus and showed co-localization with PHD3 (Fig. 7E). Next, using Western blot analysis, we evaluated the expression of PHD3 in nuclear and cytoplasmic fractions of rat nucleus pulposus cells treated with TNF-α. Fig. 7F shows that in agreement with the confocal studies, a robust expression of PHD3 is seen in the nuclear fraction of treated cells. It is noteworthy that both the nuclear and the cytoplasmic fraction of PHD3 evidence induction following cytokine treatment (Fig. 7F).
FIGURE 6.
PHD3 silencing modulates the activity of NF-κB-responsive reporter (NRE-Luc) and syndecan4 (SDC4) promoter in NP cells. A and B, the activity of both NRE-Luc and SDC4 promoter was significantly suppressed when co-transfected with increasing concentration of Sh-PHD3 (100–200 ng). C and D, TNF-α-dependent induction of both NRE and SDC4 reporter activity was significantly suppressed when PHD3 was silenced (+; 100 ng, ++; 200 ng). The effect of PHD3 overexpression on the activity of NRE-Luc in NP cells was determined. E and F, the activity of NRE-Luc was not significantly affected by co-transfection with an increasing dose of PHD3 in the absence (E) or presence (F) of TNF-α (+; 100 ng, ++; 200 ng). Data are represented as mean ± S.E. of three independent experiments performed in triplicate (n = 3); *, p < 0.05; ns, not significant.
FIGURE 7.
PHD3 controls p65 activity in NP cells. A and B, silencing of PHD3 with an increasing dose of Sh-PHD3 (100–200 ng) resulted in suppression of p65-dependent induction in both NRE (A) and SDC4 (B) reporter activity. C, co-transfection of p65 and PHD3 showed no additive effect on NRE reporter activity when compared with p65 alone. D, immunoprecipitation (IP) of p65 from TNF-α-treated rat NP cells followed by a Western blot analysis using PHD3 antibody indicated association between PHD3 and p65 in the treated cells. When an isotype IgG was used in place of the p65 antibody, PHD3 was not co-precipitated. Ctr, control. E, confocal microscopy was used to examine co-localization of PHD3 with p65 in TNF-α-treated NP cells. Cytokine treatment promoted nuclear localization of p65 as well as robustly increasing PHD3 levels in both the nucleus and the cytoplasm. The merged image (yellow signal) shows increased co-localization of these proteins in the treated cells. F, Western blot analysis of cellular localization of PHD3 in NP cells treated with TNF-α for 24 h. PHD3 is present in both the nuclear (Nucl) and the cytoplasmic (Cyto) protein fraction, and expression is induced in both compartments by cytokine treatment. Lane C indicates control. Quantitative data are represented as mean ± S.E. of three independent experiments performed in triplicate (n = 3); *, p < 0.05; ns, not significant.
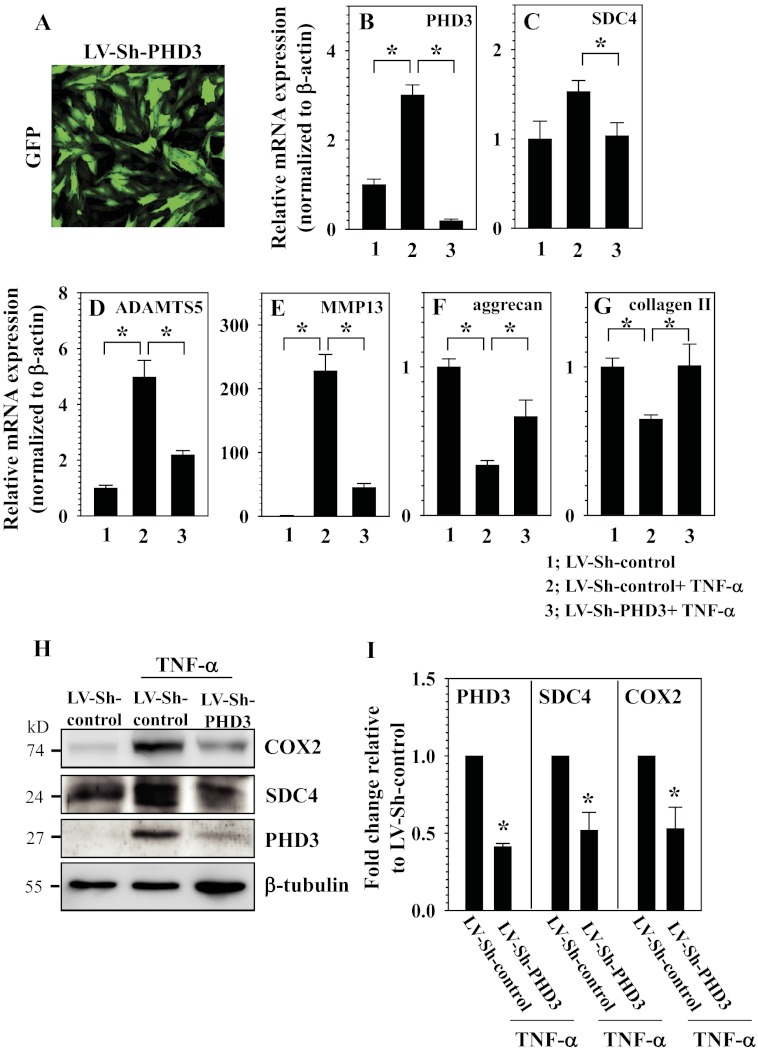
Next, to examine the role of PHD3 in controlling TNF-α-dependent target gene expression, we transduced rat nucleus pulposus cells with lentivirus expressing Sh-PHD3. Assessment of GFP-positive cells confirmed that the transduction efficiency was more than 80% (Fig. 8A). Real time RT-PCR (Fig. 8B) and Western blot analysis (Fig. 8H) indicate that there is robust suppression of PHD3 expression. Moreover, silencing studies show that there are significantly lower levels of PHD3 in TNF-α-treated nucleus pulposus cells (Fig. 8, B and H). It is noteworthy that TNF-α-dependent induction in Sdc4 (Fig. 8C), Adamts5 (Fig. 8D), and Mmp13 (Fig. 8E), catabolic marker genes that are the hallmark of disc degeneration are all significantly reduced in PHD3-silenced cells. Furthermore, in concert with this finding, we evaluated the effect of PHD3 silencing on the expression of important matrix genes aggrecan and collagen type II following TNF-α treatment. Although silencing of PHD3 partially reverses TNF-α-mediated reduction in aggrecan gene expression (Fig. 8F), collagen type II expression is completely rescued (Fig. 8G). Western blot analysis confirmed that the TNF-α-mediated induction in Sdc4 and Cox2 expression is significantly lower in PHD3-silenced cells (Fig. 8, H and I).
FIGURE 8.
PHD3 is an important regulator of catabolic effects of TNF-α on NP cells. A, immunofluorescence analysis of NP cells transduced with lentivirus co-expressing Sh-PHD3 (LV-Sh-PHD3) and GFP indicates high transduction efficiency. Original magnification ×20. B, real time RT-PCR analysis confirmed that TNF-α-mediated induction of PHD3 mRNA expression is completely abolished in NP cells transduced with LV-Sh-PHD3. C–E, TNF-α-induced mRNA expression of Sdc4 (C), Adamts5 (D), and Mmp13 (E) is significantly suppressed in NP cells transduced with LV-Sh-PHD3. F and G, TNF-α-dependent suppression of aggrecan (F) and collagen type II (G) mRNA expression is partially or completely restored in NP cells transduced with LV-Sh-PHD3. H, Western blot analysis of cells transduced by LV-Sh-control or LV-Sh-PHD3 with or without TNF-α treatment. The induction of COX2 and SDC4 protein levels is abolished in PHD3-silenced cells. I, densitometric analysis of multiple blots from the experiment described in H above. Note that silencing of PHD3 results in blocking induction of Sdc4 and Cox2 by TNF-α. Data are represented as mean ± S.E. of three independent experiments performed in triplicate (n = 3); *, p < 0.05.
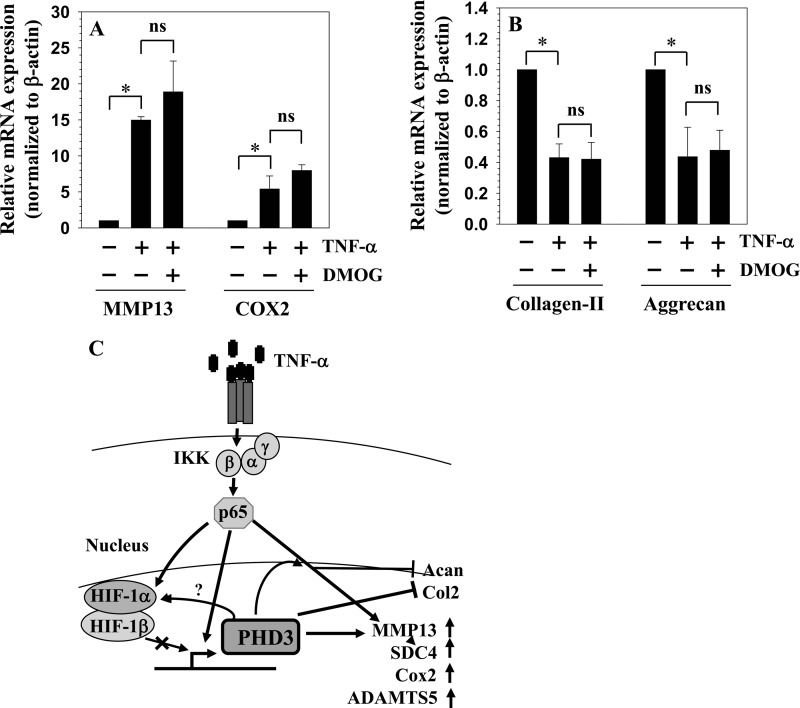
Finally, we determined whether hydroxylase activity of PHD3 is important in mediating TNF-α effects on nucleus pulposus cells. We treated cells with TNF-α in the presence or absence of a well characterized inhibitor of PHD function, dimethyloxalylglycine, and measured gene expression. Fig. 9 clearly shows that induction of Mmp13 and Cox2 (Fig. 9A) and suppression of aggrecan and collagen type II (Fig. 9A) are unaffected by dimethyloxalylglycine.
FIGURE 9.
Hydroxylase function of PHD3 is not required for mediating effects of TNF-α on NP cells. A and B, real time RT-PCR analysis demonstrates that TNF-α-mediated induction of Mmp13 and Cox2 (A) and suppression of aggrecan (B) and collagen type II (B) mRNA expression are unaffected by treatment with dimethyloxalylglycine (DMOG) (1 mm), an inhibitor of PHD enzymatic activity. Data are represented as mean ± S.E. of three independent experiments performed in triplicate (n = 3); *, p < 0.05; ns, not significant. C, schematic showing relationship between TNF-α, NF-κB/p65, HIF-1, and PHD3. In the presence of TNF-α, PHD3 forms a regulatory circuit with p65 in NP cells. Expression of PHD3 is controlled by p65 in a HIF-1-independent fashion; PHD3 in turn regulates p65 activity. It is noteworthy that independent of its hydroxylase activity, PHD3 mediates TNF-α-dependent induction of catabolic genes and suppression of anabolic genes by NP cells.
DISCUSSION
The experiments described in this investigation demonstrated for the first time that in nucleus pulposus cells, expression of PHD3 was controlled by the inflammatory cytokines TNF-α and IL-1β. Our studies also revealed that although the cytokines induced HIF activity, NF-κB and not HIF controlled cytokine-dependent PHD expression. A second major observation was that PHD3 promoted cytokine-induced NF-κB/p65 signaling activity. Most importantly, this interaction promoted the expression of a phenotype linked to disc degeneration. These findings lend strong support to the hypothesis that PHD3 is part of a regulatory circuit, with NF-κB enhancing the impact of the inflammatory cytokines, a critical step in the pathogenesis of the degenerative disc disease.
We showed that PHD3 and to a lesser extent PHD2 expression was responsive to TNF-α and IL-1β. Because both the cytokines promoted NF-κB signaling in nucleus pulposus cells (14), we determined whether they modulated PHD3 expression. Indeed, suppression of NF-κB activity blocked cytokine-dependent PHD3 expression. From a mechanistic viewpoint, because cytokine treatment of p65- and IKKβ-silenced cells caused a decrease in PHD3 levels, it was clear that components of the NF-κB pathway controlled gene expression. Further support for this hypothesis was the observation that MEFs from p65/RelA null mice displayed decreased PHD3 expression and failed to induce PHD3 expression even when treated with cytokines. Together, the results of these functional studies indicate that by controlling the expression of PHD3, NF-κB and especially p65 signaling regulates the activity of PHD-dependent pathways in nucleus pulposus cells.
We and others have previously reported that hypoxic regulation of PHD3 is dependent on HIF activity (5, 18, 21–23). To ascertain whether PHD3 regulation by cytokines involved the HIF signaling pathway, loss-of-function studies were performed. These studies clearly showed that both TNF-α and IL-1β increased HIF activity in nucleus pulposus cells in an NF-κB-dependent fashion. Interestingly, because elevated HIF transcriptional activity was not accompanied by a significant change in HIF-1 protein levels, it is possible that this is mediated by the elevation in PHD3 (5). Results of these experiments support previous findings that indicate that both cytokines induce HIF-1α activity, although they do so by increasing its protein levels. At the same time, these results also suggest that the regulation is cell type-specific (24–26). On the other hand, cytokine-induced PHD3 expression in HIF-1α-silenced nucleus pulposus cells indicates that PHD3 expression is regulated independently of HIF activity. In support of these findings, mechanistic studies using PHD3 promoter/enhancer reporter constructs harboring characterized HRE motifs failed to show an induction in activity when treated with cytokines. Hence, it must be concluded that cytokine-mediated PHD3 expression is distinct from that of hypoxic regulation (5). Moreover, the results strongly suggest that in nucleus pulposus cells, regulation of PHD3 expression is context-dependent. It should be acknowledged that although analysis using the JASPAR database indicates that both 1.5-kb and 3.5-kb PHD3 promoter fragments contain either one or two of the putative IκB response elements located at −736/−727 (GGGACATACC) and −2785/−2776 (GTAAATTCCC), they were nonresponsive to cytokine treatment (27). One possible explanation for this finding is that NF-κB motif(s) outside of the 4-kb promoter region may control transcription.
In nucleus pulposus cells, we have recently shown that in hypoxia, PHD3 functions as a transcriptional co-activator of HIF-1 signaling, whereas playing no role in oxygen-dependent degradation of HIF-α (5, 6). These results are in agreement with studies by Luo et al. (28) that showed that PHD3 and PKM2 interaction was required for HIF-1α binding to p300 to promote HIF-1 transcriptional activity. Important to this discussion are two recent studies that suggest a role of PHD3 in controlling NF-κB transcriptional activity (17, 29). Fu and Taubman (17) noted that PHD3 mediated myogenic differentiation by inhibiting NF-κB signaling, whereas Xue et al. (29) showed that PHD3 inhibited NF-κB signaling by blocking phosphorylation of IKKβ, independent of hydroxylation. In contrast to these studies, and in line with its role in maintenance of HIF-1α transcriptional activity (5), silencing studies suggest that PHD3 partially promotes TNF-α-dependent NF-κB signaling in nucleus pulposus cells. Although the exact mechanism by which PHD3 controls p65 activity is still unclear, our studies indicate that regulation is at the p65 and not the IKKβ level. It is noteworthy that the synergistic induction of NRE reporter activity was lacking when PHD3 was overexpressed in the presence of TNF-α or p65. Moreover, only a small increase in association between PHD3 and p65 is seen in the presence of TNF-α. Thus, it is plausible that due to high basal level of PHD3, further increments in PHD3 protein levels do not enhance NF-κB signaling activity. Taken together, one important outcome of the investigation is an indication that the role of PHD3 in partially controlling NF-κB signaling activity is cell type-specific and possibly related to the inflammatory state of the nucleus pulposus.
Relating the results of this study to the etiology of disc disease, it is clear that the degenerative process involves elevated cytokine activity and matrix degradation (9–12). Indeed, PHD3 loss-of-function studies showed a significant decrease in TNF-α-mediated induction of SDC4, ADAMTS5, MMP13, and COX2 (12, 14) catabolic molecules concerned with degenerative state. Moreover, suppression of PHD3 and not function significantly blocked TNF-α-mediated suppression of aggrecan and collagen type II, matrix genes critical for maintenance of the nucleus pulposus tissue. Of these catabolic genes, expression of Sdc4, Adamts5, and Mmp13 is regulated through NF-κB (14, 30), whereas the mechanism of TNF-α-dependent suppression of aggrecan and collagen type II has not been fully elucidated. It is therefore not unreasonable to assume that in addition to controlling RelA/p65 activity, PHD3 may modulate the function of other components of the TNF-α-signaling pathway, independent of its hydroxylase activity. Taken together our findings clearly suggest that in the nucleus pulposus, PHD3 enhances the activity of inflammatory cytokines, and therefore, this molecule plays a central role in the pathogenesis of degenerative disc disease.
Supplementary Material
Acknowledgment
We thank Dr. Dessislava Markova for help with human tissue collection and cell isolation.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-AR050087 and R01-AR055655 (to M. V. R.).

This article contains supplemental Table 1.
- PHD
- prolyl-4-hydroxylase domain
- HIF
- hypoxia-inducible factor
- HRE
- hypoxia response element
- IKK
- IκB kinase
- MEF
- mouse embryonic fibroblast
- NP
- nucleus pulposus
- LV
- lentivirus
- NRE
- NF-κB-responsive reporter.
REFERENCES
- 1. Gruber H. E., Ashraf N., Kilburn J., Williams C., Norton H. J., Gordon B. E., Hanley E. N. (2005) Vertebral endplate architecture and vascularization. Application of micro-computerized tomography, a vascular tracer, and immunocytochemistry in analyses of disc degeneration in the ageing sand rat. Spine 30, 2593–2600 [DOI] [PubMed] [Google Scholar]
- 2. Hassler O. (1969) The human intervertebral disc. A microangiographical study on its vascular supply at various ages. Acta Orthop. Scand. 40, 765–772 [DOI] [PubMed] [Google Scholar]
- 3. Rudert M., Tillmann B. (1993) Lymph and blood supply of the human intervertebral disc. Cadaver studies of correlations to discitis. Acta Orthop. Scand. 64, 37–40 [DOI] [PubMed] [Google Scholar]
- 4. Bartels E. M., Fairbank J. C., Winlove C. P., Urban J. P. (1998) Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine 23, 1–7; discussion 8 [DOI] [PubMed] [Google Scholar]
- 5. Fujita N., Markova D., Anderson D. G., Chiba K., Toyama Y., Shapiro I. M., Risbud M. V. (2012) Expression of prolyl hydroxylases (PHDs) is selectively controlled by HIF-1 and HIF-2 proteins in nucleus pulposus cells of the intervertebral disc: distinct roles of PHD2 and PHD3 proteins in controlling HIF-1α activity in hypoxia. J. Biol. Chem. 287, 16975–16986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fujita N., Chiba K., Shapiro I. M., Risbud M. V. (2012) HIF-1α and HIF-2α degradation is differentially regulated in nucleus pulposus cells of the intervertebral disc. J. Bone Miner. Res. 27, 401–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saito T., Fukai A., Mabuchi A., Ikeda T., Yano F., Ohba S., Nishida N., Akune T., Yoshimura N., Nakagawa T., Nakamura K., Tokunaga K., Chung U. I., Kawaguchi H. (2010) Transcriptional regulation of endochondral ossification by HIF-2α during skeletal growth and osteoarthritis development. Nat. Med. 16, 678–686 [DOI] [PubMed] [Google Scholar]
- 8. Yang S., Kim J., Ryu J. H., Oh H., Chun C. H., Kim B. J., Min B. H., Chun J. S. (2010) Hypoxia-inducible factor-2α is a catabolic regulator of osteoarthritic cartilage destruction. Nat. Med. 16, 687–693 [DOI] [PubMed] [Google Scholar]
- 9. Shamji M. F., Setton L. A., Jarvis W., So S., Chen J., Jing L., Bullock R., Isaacs R. E., Brown C., Richardson W. J. (2010) Proinflammatory cytokine expression in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 62, 1974–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weiler C., Nerlich A. G., Bachmeier B. E., Boos N. (2005) Expression and distribution of tumor necrosis factor α in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine 30, 44–53; discussion 54 [DOI] [PubMed] [Google Scholar]
- 11. Le Maitre C. L., Freemont A. J., Hoyland J. A. (2005) The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res. Ther. 7, R732–R745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Séguin C. A., Pilliar R. M., Roughley P. J., Kandel R. A. (2005) Tumor necrosis factor-α modulates matrix production and catabolism in nucleus pulposus tissue. Spine 30, 1940–1948 [DOI] [PubMed] [Google Scholar]
- 13. Lee J. M., Song J. Y., Baek M., Jung H. Y., Kang H., Han I. B., Kwon Y. D., Shin D. E. (2011) Interleukin-1β induces angiogenesis and innervation in human intervertebral disc degeneration. J. Orthop. Res. 29, 265–269 [DOI] [PubMed] [Google Scholar]
- 14. Wang J., Markova D., Anderson D. G., Zheng Z., Shapiro I. M., Risbud M. V. (2011) TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thromobospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J. Biol. Chem. 286, 39738–39749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johansen J. L., Sager T. N., Lotharius J., Witten L., Mørk A., Egebjerg J., Thirstrup K. (2010) HIF prolyl hydroxylase inhibition increases cell viability and potentiates dopamine release in dopaminergic cells. J. Neurochem. 115, 209–219 [DOI] [PubMed] [Google Scholar]
- 16. Yeramian A., Santacana M., Sorolla A., Llobet D., Encinas M., Velasco A., Bahi N., Eritja N., Domingo M., Oliva E., Dolcet X., Matias-Guiu X. (2011) Nuclear factor-κB2/p100 promotes endometrial carcinoma cell survival under hypoxia in a HIF-1α-independent manner. Lab. Invest. 91, 859–871 [DOI] [PubMed] [Google Scholar]
- 17. Fu J., Taubman M. B. (2010) Prolyl hydroxylase EGLN3 regulates skeletal myoblast differentiation through an NF-κB-dependent pathway. J. Biol. Chem. 285, 8927–8935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pescador N., Cuevas Y., Naranjo S., Alcaide M., Villar D., Landázuri M. O., Del Peso L. (2005) Identification of a functional hypoxia-responsive element that regulates the expression of the EGL nine homologue 3 (egln3/phd3) gene. Biochem. J. 390, 189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Risbud M. V., Guttapalli A., Stokes D. G., Hawkins D., Danielson K. G., Schaer T. P., Albert T. J., Shapiro I. M. (2006) Nucleus pulposus cells express HIF-1 α under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J. Cell Biochem. 98, 152–159 [DOI] [PubMed] [Google Scholar]
- 20. Pfirrmann C. W., Metzdorf A., Zanetti M., Hodler J., Boos N. (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 26, 1873–1878 [DOI] [PubMed] [Google Scholar]
- 21. Metzen E., Stiehl D. P., Doege K., Marxsen J. H., Hellwig-Bürgel T., Jelkmann W. (2005) Regulation of the prolyl hydroxylase domain protein 2 (phd2/egln-1) gene. Identification of a functional hypoxia-responsive element. Biochem. J. 387, 711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D'Angelo G., Duplan E., Boyer N., Vigne P., Frelin C. (2003) Hypoxia up-regulates prolyl hydroxylase activity. A feedback mechanism that limits HIF-1 responses during reoxygenation. J. Biol. Chem. 278, 38183–38187 [DOI] [PubMed] [Google Scholar]
- 23. Marxsen J. H., Stengel P., Doege K., Heikkinen P., Jokilehto T., Wagner T., Jelkmann W., Jaakkola P., Metzen E. (2004) Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-α-prolyl-4-hydroxylases. Biochem. J. 381, 761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jung Y. J., Isaacs J. S., Lee S., Trepel J., Neckers L. (2003) IL-1β-mediated up-regulation of HIF-1α via an NFκB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 17, 2115–2117 [DOI] [PubMed] [Google Scholar]
- 25. van Uden P., Kenneth N. S., Rocha S. (2008) Regulation of hypoxia-inducible factor-1α by NF-κB. Biochem. J. 412, 477–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nam S. Y., Ko Y. S., Jung J., Yoon J., Kim Y. H., Choi Y. J., Park J. W., Chang M. S., Kim W. H., Lee B. L. (2011) A hypoxia-dependent up-regulation of hypoxia-inducible factor-1 by nuclear factor-κB promotes gastric tumor growth and angiogenesis. Br. J. Cancer 104, 166–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wasserman W. W., Sandelin A. (2004) Applied bioinformatics for the identification of regulatory elements. Nat. Rev. Genet. 5, 276–287 [DOI] [PubMed] [Google Scholar]
- 28. Luo W., Hu H., Chang R., Zhong J., Knabel M., O'Meally R., Cole R. N., Pandey A., Semenza G. L. (2011) Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145, 732–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xue J., Li X., Jiao S., Wei Y., Wu G., Fang J. (2010) Prolyl hydroxylase-3 is down-regulated in colorectal cancer cells and inhibits IKKβ independent of hydroxylase activity. Gastroenterology 138, 606–615 [DOI] [PubMed] [Google Scholar]
- 30. Séguin C. A., Bojarski M., Pilliar R. M., Roughley P. J., Kandel R. A. (2006) Differential regulation of matrix degrading enzymes in a TNF-α-induced model of nucleus pulposus tissue degeneration. Matrix Biol. 25, 409–418 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.