Background: Pannexin 1 contains multiple cysteine residues and is highly expressed in cell types rich in nitric oxide species.
Results: S-Nitrosylation of pannexin 1 on cysteines 40 and 346 inhibits channel currents and ATP release.
Conclusion: Pannexin 1 channel function can be regulated by S-nitrosylation.
Significance: Our results provide the first evidence of a reversible post-translational modification on pannexin 1 to regulate channel activity.
Keywords: ATP, Membrane Proteins, Pannexin, Post-translational Modification, S-Nitrosylation
Abstract
S-Nitrosylation is a post-translational modification on cysteine(s) that can regulate protein function, and pannexin 1 (Panx1) channels are present in the vasculature, a tissue rich in nitric oxide (NO) species. Therefore, we investigated whether Panx1 can be S-nitrosylated and whether this modification can affect channel activity. Using the biotin switch assay, we found that application of the NO donor S-nitrosoglutathione (GSNO) or diethylammonium (Z)-1–1(N,N-diethylamino)diazen-1-ium-1,2-diolate (DEA NONOate) to human embryonic kidney (HEK) 293T cells expressing wild type (WT) Panx1 and mouse aortic endothelial cells induced Panx1 S-nitrosylation. Functionally, GSNO and DEA NONOate attenuated Panx1 currents; consistent with a role for S-nitrosylation, current inhibition was reversed by the reducing agent dithiothreitol and unaffected by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, a blocker of guanylate cyclase activity. In addition, ATP release was significantly inhibited by treatment with both NO donors. To identify which cysteine residue(s) was S-nitrosylated, we made single cysteine-to-alanine substitutions in Panx1 (Panx1C40A, Panx1C346A, and Panx1C426A). Mutation of these single cysteines did not prevent Panx1 S-nitrosylation; however, mutation of either Cys-40 or Cys-346 prevented Panx1 current inhibition and ATP release by GSNO. This observation suggested that multiple cysteines may be S-nitrosylated to regulate Panx1 channel function. Indeed, we found that mutation of both Cys-40 and Cys-346 (Panx1C40A/C346A) prevented Panx1 S-nitrosylation by GSNO as well as the GSNO-mediated inhibition of Panx1 current and ATP release. Taken together, these results indicate that S-nitrosylation of Panx1 at Cys-40 and Cys-346 inhibits Panx1 channel currents and ATP release.
Introduction
Pannexin 1 (Panx1)3 is a widely expressed integral membrane protein that is thought to form hexameric plasma membrane channels (1). Since their identification in 2000, Panx1 channels have been characterized as ATP release channels that may play a pivotal role in supporting purinergic signaling in a multitude of cell types (2–14). Importantly, Panx1 channels mediate ATP release from vascular smooth muscle and endothelial cells (7, 14), circulating erythrocytes (11, 15), airway epithelial cells (13), astrocytes (5, 16, 17) and T-cells (6, 8). As purinergic signaling events are critically involved in a number of physiological and pathological processes (18–22), elucidation of the mechanisms controlling the activity of Panx1 channels may provide important insight into how these processes are regulated.
Panx1 channel activation is now known to occur in response to membrane stretch and high extracellular K+ (23); in response to activation of α1D-adrenergic receptors (7), PAR-1 receptors (14), and NMDA receptors (24); and by cleavage of its intracellular C-tail by activated caspases 3 and 7 (9, 25). Because sustained ATP release from cells is detrimental to cell viability, it is crucial that the activity of these channels be tightly regulated to prevent depletion of ATP. Even as novel forms of Panx1 channel activation continue to emerge, mechanisms leading to inhibition of these channels remain poorly understood.
Through the initial characterization of Panx1, evidence has arisen indicating that the channel is post-translationally modified by glycosylation at asparagine 254, an event thought to regulate trafficking of Panx1 channels to the plasma membrane (26, 27). Panx1 channels can also be irreversibly modified during apoptosis by cleavage of the intracellular C-tail by caspases, allowing release of ATP that serves as a “find-me” signal for monocyte recruitment and phagocytosis (9, 25). Whereas recognition of these post-translational modifications has provided critical insight into the regulation of Panx1 trafficking and the role of Panx1 in apoptosis, there are currently no known reversible post-translational modifications involved in the regulation of Panx1 channel activity at the plasma membrane.
Protein S-nitrosylation is a reversible post-translational modification in which nitric oxide (NO) moieties are covalently bound to reactive cysteine thiols via an S-nitrosothiol bond. S-Nitrosylation can have profound effects on protein function with modification of even a single cysteine residue dramatically altering protein activity (28, 29). This modification is known to regulate the activity of several membrane channels including, among others, connexin43 gap junctions and hemichannels (28, 30), the cardiac slowly activating delayed rectifier potassium channel KCNQ1 (31), the transient receptor potential channel TRPC5 (32), and the ryanodine receptor type 1 (29). Notably, connexin43, a constituent of gap junction channels with a membrane topology and oligomerization state similar to those of Panx1, is modified by S-nitrosylation at a single cysteine residue in small arteries, leading to an increase in channel permeability to second messengers (28).
Panx1 contains several cysteine residues that may play important roles in the regulation of channel function. As Panx1 is highly expressed in tissues rich in NO, such as the vasculature and nervous system, we sought to determine whether Panx1 can be post-translationally modified by S-nitrosylation and whether this modification can affect channel activity.
EXPERIMENTAL PROCEDURES
Chemicals and Reagents
Reduced l-glutathione (GSH), dl-dithiothreitol (DTT), N-ethylmaleimide, and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) were purchased from Sigma. Diethylammonium (Z)-1–1(N,N-diethylamino)diazen-1-ium-1,2-diolate (DEA NONOate) was purchased from Cayman Chemicals and prepared by dissolving in 10 mm NaOH (all DEA solutions were adjusted to pH 7.4). S-Nitrosoglutathione (GSNO) was prepared by incubating reduced GSH with sodium nitrate. Carbenoxolone (CBX) was purchased from Fisher. N-[6-(Biotinamido)hexyl]-3′-(2′-pyridyldithio)-propionamide and EZ-Link sulfosuccinimidyl-6-[biotin-amido]hexanoate were purchased from Thermo Scientific.
Cell Culture and Transfections
Human embryonic kidney (HEK) 293T cells were cultured in Dulbecco's modified eagle medium (DMEM) high glucose (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 1% penicillin/streptomycin, 1% l-glutamine, and 1% non-essential amino acids and maintained at 37 °C in a humidified 5% CO2 incubator. All cells were used for experiments at passage ≤20. Primary mouse aortic endothelial cells (mAECs) were purchased from Cell Biologics, cultured in M1168 Mouse endothelial cell medium, and maintained at 37 °C in a humidified 5% CO2 incubator. All mAECs were used for experiments at passage ≤8 according to the manufacturer's recommendations.
Cells at 80–90% confluence were transfected with plasmids or siRNAs using the Lipofectamine 2000 reagent (Invitrogen) following the manufacturer's protocol. For electrophysiology experiments, cells were co-transfected with the Panx1 pcDNA3.1 plasmid and green fluorescent protein (2.5 μg of Panx1 plasmid with 0.5 μg of pEGFP) and plated onto poly-l-lysine-coated glass coverslips 24 h later. The cells were returned to the incubator and allowed to adhere for at least 1 h prior to use. Recordings were conducted within 1 day of plating.
Plasmid Generation and Site-directed Mutagenesis
Full-length murine Panx1 coding region was amplified by PCR from a Panx1-EGFP-N1 plasmid, which was described previously (33), using the following primers: forward, 5′-caaatgggcggtaggcgtgt-3′; and reverse, 5′-cttgtggccgtttacgtcgc-3′. The PCR product was digested (HindIII and BamHI) and ligated into pcDNA3.1. The final construct was sequenced to confirm proper insertion. Single cysteine-to-alanine mutations were performed in the Panx1 pcDNA3.1 construct using the QuikChange II site-directed mutagenesis kit (Agilent Technologies), and double cysteine-to-alanine mutations were constructed using the QuikChange multisite-directed mutagenesis kit (Agilent Technologies) following the manufacturer's protocol.
Biotin Switch Assay
The biotin switch assay was performed as described previously (28, 34). Briefly, cell monolayers were treated with 100 μm GSNO, 50 μm DEA NONOate, or vehicle for 10 min at 37 °C. Monolayers were then washed with PBS, cells were lysed in radioimmune precipitation assay buffer containing protease inhibitors, and protein was quantified using the Bradford technique. Proteins were precipitated with acetone and pelleted by centrifugation for 5 min at 10,000 × g. Pellets were resuspended, and free cysteine thiols were blocked with N-ethylmaleimide for 20 min at 50 °C. Proteins were precipitated as described above to remove excess N-ethylmaleimide, and S-nitrosylated cysteines were reduced with 1 mm ascorbate in the presence of 1 mm Cu2+ and biotinylated with N-[6-(Biotinamido)hexyl]-3′-(2′-pyridyldithio)-propionamide for 1 h at room temperature. Biotinylated proteins were then pulled down with streptavidin-agarose beads for 1 h at room temperature and subjected to SDS-PAGE and Western blotting for detection of S-nitrosylated Panx1. For negative controls, ascorbate was omitted from the assay, which prevented reduction of S-nitrosothiols and subsequent biotinylation.
Electrophysiology
Whole cell voltage clamp recordings were performed as described (25). Recordings were obtained at room temperature with 3–5-megaohm Sylgard-coated borosilicate glass patch pipettes and an Axopatch 200B amplifier (Molecular Devices). The internal solution contained 30 mm tetraethylammonium chloride, 100 mm CsMeSO4, 4 mm NaCl, 1 mm MgCl2, 0.5 mm CaCl2, 10 mm HEPES, 10 mm EGTA, 3 mm ATP-Mg, and 0.3 mm GTP-Tris (pH 7.3). The bath solution was composed of 140 mm NaCl, 3 mm KCl, 2 mm MgCl2, 2 mm CaCl2, 10 mm HEPES, and 10 mm glucose (pH 7.3). Bath solutions containing 50 μm CBX, 1 mm DTT, or 100 μm DEA NONOate (bubbled with 100% O2) were flowed over the cells at a rate of ∼2 ml/min, whereas 100 μm GSNO, 100 μm GSH, or 100 μm GSH with 100 μm H2O2 were pipetted directly into the bath under stop-flow conditions. Ramp voltage clamp commands were applied at 5-s intervals using pCLAMP software and a Digidata 1322A digitizer (Molecular Devices). Peak currents were taken at +80 mV, and percent inhibition was calculated by dividing the decrease in peak current by the total Panx1 current (defined by its CBX sensitivity). We quantified the DTT-reversible component of GSNO/DEA inhibited current to define the fraction of current inhibition that was due to S-nitrosylation.
ATP Release Assay
HEK293T cells or mAECs were plated in 24-well plates coated with 0.01% poly-l-lysine or 0.2% gelatin, respectively. HEK293T cells at 80–90% confluence were transfected with plasmids encoding Panx1WT or Panx1 cysteine mutants for 24 h. mAECs were either allowed to grow to confluence or transfected at 80–90% confluence with two siRNAs targeting Panx1 (Ambion) (7) for 48 h to knock down endogenous Panx1. The medium was removed from each well, and cells were carefully washed two times with warm Krebs-HEPES buffer containing 2 mm Ca2+ and 1% BSA. Cells were then incubated in fresh Krebs-HEPES buffer containing 2 mm Ca2+ and 1% BSA for 30 min at 37 °C to allow degradation of any ATP released as a result of mechanical stimulation imparted by changing the medium. Ectonucleotidases were inhibited by incubating cell monolayers with 300 μm ARL 67156 (Tocris) for 30 min at 37 °C. HEK293T cells were stimulated by depolarizing the cells with 100 mm KCl for 1 min, and mAECs were stimulated with 1 unit/ml mouse thrombin for 5 min as described previously (14). To evaluate the effect of NO donors on ATP release, cells were pretreated with 100 μm GSNO or 50 μm DEA NONOate for 10 min. For controls, cells were pretreated with 100 μm GSH or 50 μm CBX. Following stimulation of the cells, 75 μl of the cell supernatant was collected and placed immediately on ice. All samples were centrifuged at 5000 × g for 2 min, and 50 μl of each sample was transferred to a 96-well plate. Using a FluoStar Omega luminometer, 50 μl of luciferin-luciferase reagent (ATP bioluminescence assay kit HSII, Roche Applied Science) was injected into each well, and luminescence was immediately recorded. The ATP concentration in each sample was calculated from a standard curve for all experiments. Data are expressed as percent change in ATP release from control conditions (i.e. unstimulated cells) or percent inhibition of ATP release by GSNO for experiments on HEK293T cells expressing Panx1 cysteine mutants.
Cell Surface Protein Biotinylation
HEK293T cells were transfected to express Panx1WT or Panx1 cysteine mutants as described above and grown to confluence in 6-well plates. For experiments examining the effect of NO donors on Panx1 membrane expression, confluent monolayers were treated for various times (0–10 min) with GSNO. Cells were washed once with cold PBS and then incubated with cold DMEM (without FBS) and 50 μm CBX at 4 °C for 30 min. CBX was added to prevent biotin from passing through Panx1 channels, which would label intracellular proteins. Cells were then washed with PBS and incubated at 4 °C for 1 h in cold PBS (1.5 ml/dish) containing EZ-Link sulfosuccinimidyl-6-[biotin-amido]hexanoate (1 mg/ml) and CBX (50 μm). The cells were washed again with PBS and lysed in PBST (PBS + 0.5–1% Triton X-100) containing protease inhibitors. Total protein was quantified using the Bradford technique, and equal amounts of protein were incubated with streptavidin-agarose beads for 2 h at 4 °C to pull down biotinylated proteins. Beads were washed five times with PBST and spun down, and bound proteins were eluted by incubation with Laemmli buffer. Eluted proteins were subjected to SDS-PAGE and Western blotting for detection of Panx1.
Immunofluorescence Microscopy
Transfected HEK cells were fixed in 4% paraformaldehyde for 15 min and subjected to standard immunocytochemistry as described previously (7). Images were obtained with an Olympus Fluoview 1000 laser scanning confocal microscope.
cGMP Assay
Transfected HEK cells were incubated with ODQ (10 μm) for 20 min prior to treatment with GSNO (100 μm) or vehicle for 10 min at 37 °C. Cells were then isolated and lysed in buffer provided in the cGMP XP® assay kit (Cell Signaling Technology), and the assay was performed according to the manufacturer's protocol. A standard curve of known cGMP concentrations was constructed and used to calculate cGMP concentrations in the experimental samples.
Data Analysis
Results are presented as means ± S.E. Statistical significance was determined by p < 0.05 using a Mann-Whitney U test or Kruskal-Wallis test followed by Dunn's test for multiple comparisons.
RESULTS
Panx1 Can Be S-Nitrosylated
To determine whether Panx1 can be S-nitrosylated, we transfected HEK293T cells with a plasmid encoding murine Panx1 and treated the cells with two independent NO donors, GSNO and DEA NONOate. This provides a useful model system for examination of Panx1 modifications because we have found that endogenous Panx1 is undetectable in these cells (35). In addition, Western blot analysis did not reveal expression of any nitric-oxide synthase (NOS) isoforms in these cells (data not shown), providing a clean model system with little to no background NO from endogenous sources.
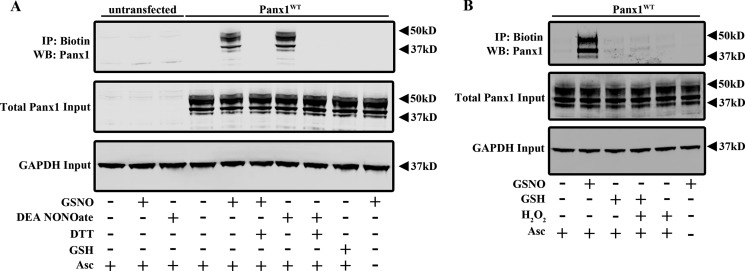
Application of 100 μm GSNO or 50 μm DEA NONOate for 10 min induced S-nitrosylation of Panx1 as detected by the biotin switch assay (Fig. 1). Treating cells with a reducing agent (DTT; 1 mm) immediately following GSNO or DEA NONOate treatment to reduce S-nitrosothiols prevented biotinylation in this assay. In addition, S-nitrosylation of Panx1 was not observed in untransfected cells or when the ascorbate step was omitted to prevent unmasking of S-nitrosylated cysteines for subsequent biotinylation. The biotin switch assay can detect proteins with cysteine modifications in addition to S-nitrosylation, and GSNO is capable of also modifying cysteine thiols by S-glutathionylation. Therefore, we treated Panx1WT-expressing HEK cells with reduced GSH (100 μm). Importantly, we did not observe Panx1 modification by GSH in these cells (Fig. 1A). Protein S-glutathionylation occurs more readily under conditions of oxidative stress, but we did not observe any Panx1 cysteine modification when Panx1WT-expressing cells were exposed to reduced GSH in the presence of 100 μm H2O2. This further supports a role for Panx1 S-nitrosylation over S-glutathionylation (Fig. 1B). Taken together, these results indicate that Panx1 can be S-nitrosylated.
FIGURE 1.
S-Nitrosylation of Panx1 by GSNO and DEA NONOate. A, biotin switch assay on untransfected and Panx1WT-expressing HEK cells treated with 100 μm GSNO ± 1 mm DTT, 50 μm DEA NONOate ± 1 mm DTT, or 100 μm GSH. Ascorbate (Asc−) was omitted from the biotin switch assay for the negative control. B, biotin switch assay on Panx1WT-expressing HEK cells treated with 100 μm GSNO, 100 μm GSH ± 100 μm H2O2, or 100 μm H2O2 alone. IP, immunoprecipitation; WB, Western blot.
S-Nitrosylation Inhibits Panx1 Channel Function
To determine the functional consequences of S-nitrosylation on Panx1 channel activity, we examined the effects of GSNO and DEA NONOate on whole cell currents from Panx1WT-expressing HEK cells. The peak current evoked by voltage ramp protocols (at +80 mV) was strongly inhibited by GSNO (Fig. 2, A and B). Importantly and as expected for an effect mediated by S-nitrosylation, GSNO-mediated current inhibition was completely reversed by the reducing agent DTT (Fig. 2, A and B). The current restored by DTT was inhibited by the Panx1 channel blocker CBX and displayed a current-voltage relationship characteristic of Panx1 (Fig. 2B); note that GSNO-mediated inhibition was evident over the entire voltage range, including at rest potentials. We quantified the DTT-reversible component of GSNO-inhibited current relative to the CBX-sensitive current as a measure of the percentage of Panx1 current that was reduced by S-nitrosylation (∼63%; Fig. 2C). The DTT-reversible inhibition was significantly greater following GSNO treatment by comparison with that following GSH or GSH with H2O2 application, consistent with the biotin switch data that suggested that the channel was modified by S-nitrosylation rather than S-glutathionylation. We also observed DTT-reversible current inhibition following application of DEA NONOate (data not shown). However, the response with DEA NONOate was less consistent than with GSNO (n = 2 of 6 cells tested), and effects of the compound were not more evident when applied at higher concentrations (up to 1 mm) or at elevated temperature (30 °C). There was no significant activation of current by DTT in Panx1-expressing cells that had not been treated with NO donors, and there were no detectable CBX-sensitive currents in untransfected cells (data not shown).
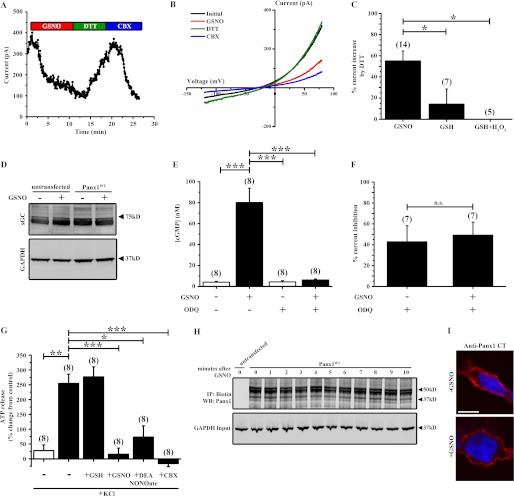
FIGURE 2.
GSNO inhibits Panx1 currents and ATP release. A, time series showing peak Panx1 whole cell current amplitude during application of 100 μm GSNO, 1 mm DTT, and 50 μm CBX. B, current-voltage curves of Panx1 currents from Panx1WT-expressing HEK cells under control conditions (black trace) and following application of 100 μm GSNO (red trace), 1 mm DTT (green trace), and 50 μm CBX (blue trace). C, summary data showing the percentage of Panx1 current inhibition by GSNO, GSH, or GSH with H2O2 that was reversible by DTT. D, Western blot for sGC expression in untransfected or Panx1WT-expressing HEK cells following treatment with 100 μm GSNO or vehicle. E, cGMP assay of Panx1WT-expressing HEK cells treated with 100 μm GSNO or vehicle and the sGC inhibitor ODQ. F, effect of sGC inhibition by ODQ on Panx1 current inhibition by GSNO. G, ATP release assay from untransfected (white bar) and Panx1WT-expressing (black bars) HEK cells. Data represent cells treated with 100 mm KCl following pretreatment with 100 μm GSNO, 50 μm DEA NONOate, 100 μm GSH, or 50 μm CBX. all data are presented as percent change in ATP release compared with control (−KCl). H, cell surface biotinylation assay of Panx1 from Panx1WT-expressing HEK cells following treatment with 100 μm GSNO for 0–10 min. I, immunofluorescence micrographs of Panx1WT-expressing HEK cells treated with or without 100 μm GSNO for 10 min. Red indicates Panx1, and blue indicates DAPI-stained nuclei. Scale bar in all images, 10 μm. n values are indicated in parentheses. *, p < 0.05; **, p < 0.001; ***, p < 0.0001; n.s., not significant. IP, immunoprecipitation; WB, Western blot; CT, C terminus.
In addition to S-nitrosylation, increases in NO can activate soluble guanylate cyclase (sGC) and promote downstream cGMP-dependent phosphorylation cascades. Therefore, we examined whether GSNO treatment activated sGC in our cells and whether this contributed to GSNO-mediated Panx1 current inhibition. Western blot analysis revealed endogenous expression of sGC in our HEK cells that was not affected by transfection with the murine Panx1 plasmid or by treatment with GSNO (Fig. 2D). Although GSNO did not affect the expression of sGC, we observed a significant increase in intracellular cGMP concentration following GSNO treatment in Panx1WT-expressing HEK cells that could be blocked by pretreatment with the sGC inhibitor ODQ (Fig. 2E). To rule out the possibility that GSNO was exerting its functional effects on Panx1 currents by activation of cGMP-dependent signaling cascades, we pretreated Panx1WT-expressing HEK cells with 20 μm ODQ and examined the effect of GSNO on Panx1 currents. Importantly, ODQ had no effect on Panx1 current inhibition by GNSO (Fig. 2F). Taken together, these data indicate that NO can inhibit Panx1 currents by a mechanism distinct from cGMP signaling, likely by S-nitrosylation of the channel.
Although our electrophysiology experiments provide strong evidence that Panx1 channel currents can be inhibited by S-nitrosylation, we also sought to determine whether ATP release from Panx1WT-expressing HEK cells is functionally affected by S-nitrosylation. Based on previous reports indicating the ability of Panx1 channels to release ATP in response to high extracellular K+ (23), we treated Panx1WT-expressing HEK cells with 100 mm KCl and observed a significant increase in ATP release into the extracellular medium that was strongly inhibited by CBX (Fig. 2G). There was no change in ATP release from untransfected HEK cells following KCl stimulation, indicating that Panx1 expression was required for ATP release in these cells. Importantly, treatment of Panx1WT-expressing HEK cells with 100 μm GSNO or 50 μm DEA NONOate attenuated the KCl-evoked ATP release, consistent with our observations on the effects of these NO donor molecules on Panx1 channel currents. Moreover, pretreatment with GSH had no effect on ATP release from these cells (Fig. 2G).
To examine the possibility that the reduction in Panx1 current and ATP release following treatment with our NO donors was due to reduced expression of these channels at the plasma membrane, we performed a membrane biotinylation assay on Panx1WT-expressing HEK cells treated with GSNO for a time course from 0 to 10 min, a time frame consistent with the observed inhibitory effects on Panx1 currents. Treatment of cells with GSNO did not affect Panx1 expression at the plasma membrane (Fig. 2H). Moreover, treatment of Panx1WT-expressing HEK cells with GSNO for 10 min had no effect on Panx1 membrane localization as assessed by immunofluorescence microscopy (Fig. 2I). Together, these data indicate that the inhibitory effect of S-nitrosylation on Panx1 channels is unlikely to reflect decreased plasma membrane expression of the channel.
Multiple Cysteine Residues Contribute to Panx1 Current Inhibition by S-Nitrosylation
To determine which cysteines are modified by S-nitrosylation and contribute to Panx1 inhibition, we generated several cysteine-to-alanine mutations in Panx1 by site-directed mutagenesis. A topology map of Panx1 indicating the cysteines in which we made alanine substitutions (red circles) is shown in Fig. 3A. We identified target cysteines through a combination of experimental and computational evidence. Previous studies have reported increased Panx1 channel currents upon mutation of Cys-40 or Cys-346, suggesting that these residues may be intimately involved in channel regulation (29–31). The C-terminal tail, which contains an additional cysteine (Cys-426), may also be important for channel regulation as removal of the C-tail increases channel activity (19). Additionally, we used the scan-x post-translational modification prediction method (36) to rank the highest probability S-nitrosylation sites, which pointed to Cys-40 as the most likely S-nitrosylation site. Therefore, we constructed single cysteine-to-alanine substitutions at these sites (Panx1C40A, Panx1C346A, and Panx1C426A).
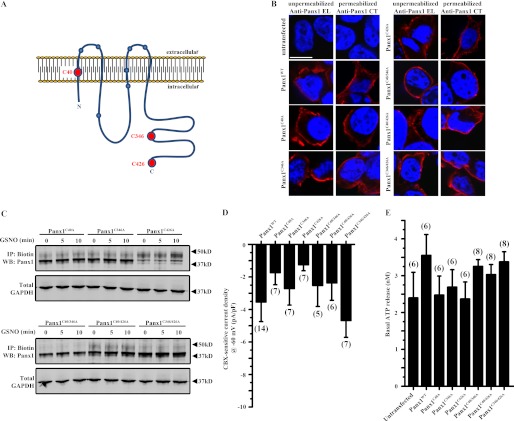
FIGURE 3.
Trafficking of Panx1 cysteine mutants to the plasma membrane is unaffected by GSNO. A, topology map of Panx1 showing the predicted location of cysteine residues (circles) within the Panx1 protein. The red circles indicate the three cysteines that were mutated to alanines within the Panx1 polypeptide (C40A, C346A, and C426A). B, immunofluorescence micrographs of HEK cells depicting Panx1 mutants at the plasma membrane. Samples were processed for immunofluorescence either under non-permeabilizing conditions where they were labeled with an antibody directed against the second extracellular loop of Panx1 (anti-Panx1 EL) or under permeabilizing conditions where they were labeled with an antibody against the C terminus of Panx1 (anti-Panx1 CT). Red indicates Panx1, and blue indicates DAPI-stained nuclei. C, cell surface biotinylation assay of Panx1 from HEK cells expressing Panx1 single or double cysteine mutants following treatment with 100 μm GSNO for 0, 5, or 10 min. GAPDH was used as a loading control. D, Panx1 holding currents at a potential of −60 mV from HEK cells expressing WT and Panx1 cysteine mutants. E, basal ATP release from unstimulated (−KCl) HEK cells expressing WT and Panx1 cysteine mutants. Scale bar in all images, 10 μm. n values are indicated in parentheses. IP, immunoprecipitation; WB, Western blot.
To ensure that mutation of these single cysteines did not affect trafficking to the plasma membrane, we performed immunofluorescence microscopy on mutant-expressing cells under non-permeabilizing conditions with an antibody directed against the second extracellular loop of Panx1 (anti-Panx1 EL) and under permeabilizing conditions with an antibody directed against the intracellular C-tail (anti-Panx1 CT). We found that all Panx1 cysteine mutants were able to reach the plasma membrane (see Fig. 3B and recordings of Cys-substituted channels below). Moreover, we performed cell surface biotinylation assays on HEK cells expressing each of these Panx1 mutants and found that all localize to the membrane and that their expression was not affected by treatment with GSNO (Fig. 3C). In addition, there was no significant difference in Panx1 holding current or basal ATP release between WT and the Panx1 cysteine mutant channels (Fig. 3, D and E, respectively).
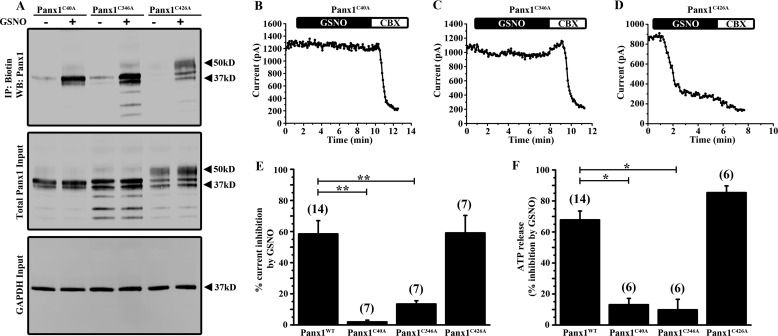
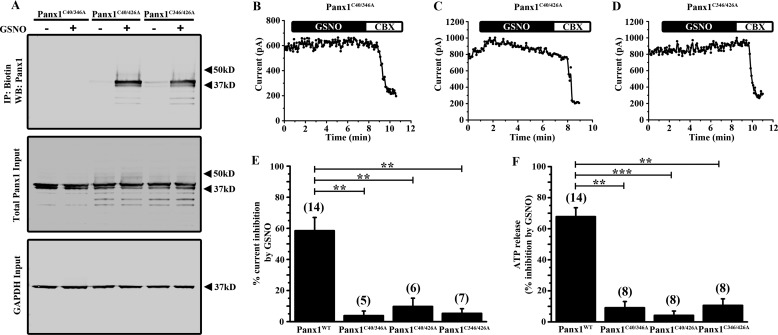
To determine which cysteine residue(s) is modified by S-nitrosylation, we treated HEK cells expressing Panx1 single mutant channels with 100 μm GSNO and performed biotin switch assays. Interestingly, S-nitrosylation of Panx1 was preserved in all three constructs with individual cysteine-to-alanine substitutions (Fig. 4A). It should be noted that mutation of Cys-40 or Cys-346 resulted in decreased representation of the higher molecular weight glycosylation species of Panx1 in this assay (Fig. 4A). Although all three single Cys mutant channels retained the ability to be S-nitrosylated, the inhibitory effects of GSNO on Panx1 currents were abolished specifically in Panx1C40A- (Fig. 4B) and Panx1C346A (Fig. 4C)-expressing cells; GSNO-mediated inhibition was preserved in Panx1C426A-expressing cells (Fig. 4D). Summary data for each Panx1 single cysteine mutant is shown in Fig. 4E indicating that mutation of Cys-40 or Cys-346, but not Cys-426, significantly prevented Panx1 current inhibition by GSNO. Consistent with a lack of current inhibition by GSNO in the Panx1C40A and Panx1C346A mutants, ATP release from cells expressing these two Panx1 mutants was not affected by GSNO with the Panx1C426A mutant exhibiting strong inhibition (Fig. 4F). The ability of Panx1 to be S-nitrosylated in each Panx1 single cysteine mutant with a loss of current inhibition and inhibition of ATP release by GSNO only in the Panx1C40A and Panx1C346A mutants suggested that S-nitrosylation at multiple cysteine residues may be required for channel inhibition.
FIGURE 4.
Single mutation of Cys-40 or Cys-346 prevents Panx1 current inhibition and ATP release by GSNO. A, biotin switch assay on HEK cells expressing each of the Panx1 single cysteine mutants (Panx1C40A, Panx1C346A, and Panx1C426A). Panx1 mutant-expressing cells were treated with or without 100 μm GSNO. B–D, time series of the peak Panx1 current amplitudes from HEK cells expressing Panx1C40A (B), Panx1C346A (C), and Panx1C426A (D) mutant constructs and treated with 100 μm GSNO. Transfected cells were treated with 50 μm CBX near the end of each recording protocol to demonstrate that currents could be blocked. E, summary data showing the percentage of Panx1 current inhibition by GSNO from HEK cells expressing the Panx1 single cysteine mutant constructs. F, ATP release assay from HEK cells expressing Panx1 single cysteine mutants. Data are presented as a percent inhibition of ATP release by GSNO. n values are indicated in parentheses. *, p < 0.05; **, p < 0.001; ***, p < 0.0001. IP, immunoprecipitation; WB, Western blot.
To determine whether multiple cysteines can be S-nitrosylated, we generated double cysteine-to-alanine substitutions in Panx1 (Panx1C40A/C346A, Panx1C40A/C426A, and Panx1C346A/C426A) and tested the ability of GSNO to S-nitrosylate and inhibit the double mutant channels. Each of the Panx1 double cysteine mutants trafficked to the plasma membrane, and expression was unaffected by GSNO (Fig. 3C). The GSNO-induced S-nitrosylation was preserved in Panx1 constructs that retained either Cys-40 or Cys-346 but was lost in Panx1 channels missing both Cys-40 and Cys-346 (Panx1C40A/C346A), indicating that these two cysteines are the sites of modification (Fig. 5A). All double mutant constructs generated CBX-sensitive Panx1 currents, but GSNO-mediated current inhibition was not observed in any of the double mutant constructs, each of which had a substitution at either Cys-40 or Cys-346 (Fig. 5, B–E). Consistent with this, GSNO was unable to inhibit ATP release from cells expressing any of the Panx1 double mutant constructs (Fig. 5F). Together, these data support the idea that both Cys-40 and Cys-346 can be S-nitrosylated and that modification at both sites is required for GSNO-mediated inhibition of Panx1.
FIGURE 5.
S-Nitrosylation of both Cys-40 and Cys-346 is required to inhibit Panx1 currents and ATP release. A, biotin switch assay on HEK cells transfected with each Panx1 double cysteine mutant construct (Panx1C40A/C346A, Panx1C40A/C426A, and Panx1C346A/C426A). Panx1 mutant-expressing cells were treated with or without 100 μm GSNO. B–D, time series of the peak Panx1 current amplitudes from HEK cells expressing Panx1C40A/C346A (B), Panx1C40A/C426A (C), and Panx1C346A/C426A (D) and treated with 100 μm GSNO. Transfected cells were treated with 50 μm CBX near the end of each recording protocol. E, summary data of the percentage of Panx1 current inhibition by GSNO from HEK cells expressing the Panx1 double cysteine mutant constructs. F, ATP release assay from HEK cells expressing Panx1 single cysteine mutants. Data are presented as a percent inhibition of ATP release by GSNO. n values are indicated in parentheses. **, p < 0.001; ***, p < 0.0001. IP, immunoprecipitation; WB, Western blot.
S-Nitrosylation of Panx1 in Endothelial Cells Inhibits Channel Function
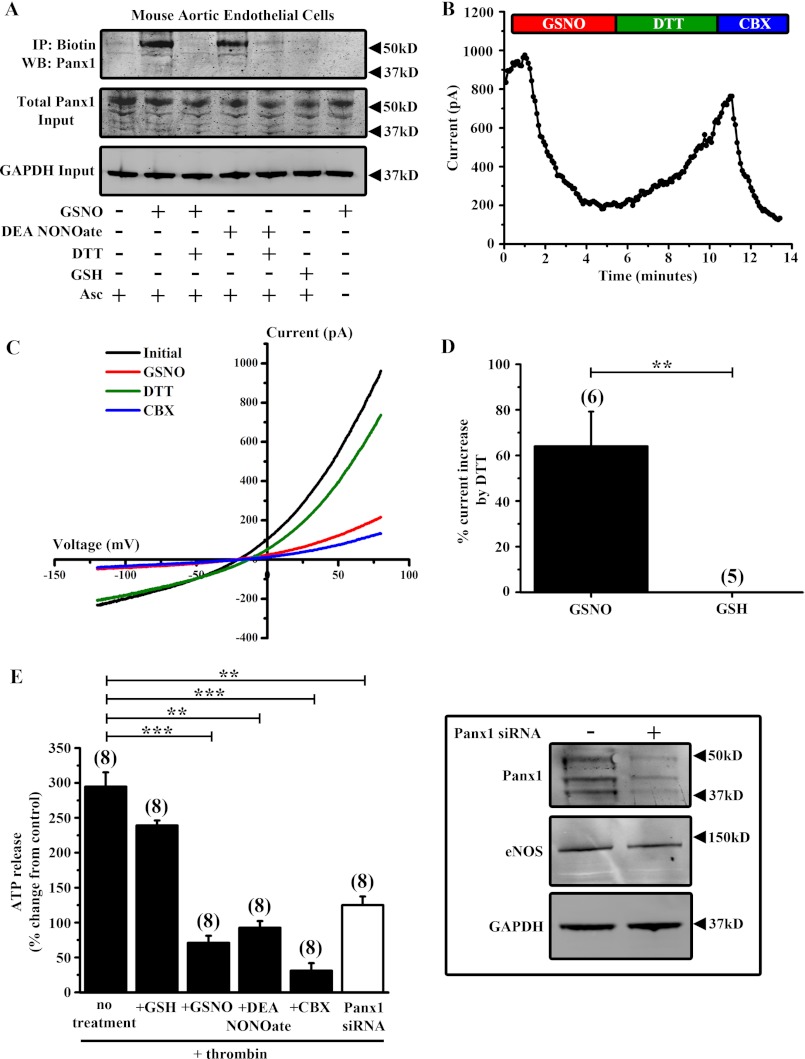
Panx1 is highly expressed in endothelial cells across the arterial tree (35). Because these cells express endothelial NOS and are exposed to large amounts of NO, we sought to determine whether Panx1 can be modified by S-nitrosylation in a native cell. We utilized primary cultures of mAECs, which express endothelial NOS and the endothelial cell markers vascular endothelial cadherin (Cdh5) and PECAM-1 (data not shown). Treatment of mAECs with GSNO or DEA NONOate for 10 min induced S-nitrosylation of endogenous Panx1 (Fig. 6A). Consistent with our HEK cell data, S-nitrosylation of Panx1 in mAECs by GSNO or DEA NONOate was reversed by treatment with DTT. Moreover, treatment with GSH alone had no effect on Panx1 cysteine modification.
FIGURE 6.
S-Nitrosylation inhibits Panx1 currents and ATP release from mouse aortic endothelial cells. A, biotin switch assay on primary mAECs treated with 100 μm GSNO ± 1 mm DTT, 50 μm DEA NONOate ± 1 mm DTT, or 100 μm GSH. Ascorbate (Asc−) was omitted from the assay as a negative control. B, time series showing peak Panx1 whole cell current amplitude from mAECs during application of GSNO, DTT, and CBX. C, current-voltage curves of Panx1 currents from primary mAECs under control conditions (black trace) and following application of 100 μm GSNO (red trace), 1 mm DTT (green trace), and 50 μm CBX (blue trace). C, summary data showing the percentage of Panx1 current inhibition by GSNO or GSH that was reversible by DTT. D, ATP release assay from mAECs stimulated with 1 unit/ml mouse thrombin. Cells were pretreated with 100 μm GSNO, 50 μm DEA NONOate, 100 μm GSH, or 50 μm CBX. A subset of cells was transfected with siRNA against mouse Panx1 to knock down the endogenous protein. All data are presented as percent change in ATP release compared with control (−thrombin). E, inset, Panx1 Western blot of untransfected and Panx1 siRNA-transfected mAECs. Endothelial NOS (eNOS) and GAPDH were used as loading controls. n values are indicated in parentheses. **, p < 0.001; ***, p < 0.0001.
To determine whether S-nitrosylation of Panx1 in mAECs inhibits channel function, we conducted whole cell patch clamp recordings and ATP release assays on mAECs. Single mAECs were patched in the whole cell configuration, and Panx1 currents were identified by their sensitivity to CBX and their characteristic current-voltage relationship (Fig. 6, B and C). Consistent with our previous observations, peak Panx1 currents at +80 mV were significantly inhibited by GSNO, and this inhibition was substantially reversed by treatment with DTT (Fig. 6, B–D). Treatment with GSH or DTT alone did not affect Panx1 current in these cells, indicating that S-nitrosylation of endogenous Panx1 causes potent inhibition of channel currents. We next sought to determine whether ATP release from Panx1 channels in mAECs was affected by S-nitrosylation. Because thrombin is a stimulus for ATP release from Panx1 channels in endothelial cells (14), we stimulated mAECs with 1 unit/ml thrombin for 5 min. Thrombin stimulation significantly increased ATP release that was inhibited by both CBX and siRNA knockdown of endogenous Panx1 (Fig. 6E). Moreover, GSNO and DEA NONOate significantly attenuated ATP release from mAECs, indicating that S-nitrosylation inhibits endogenous Panx1 channel function, confirming our findings on HEK cells.
DISCUSSION
In this study, we identify a novel inhibitory mechanism for Panx1 channels: we show that Panx1 can be modified by S-nitrosylation and that this post-translation modification leads to inhibition of Panx1-mediated currents and ATP release. In brief, we used a biotin switch assay to show that GSNO and DEA NONOate, two NO donor molecules, induce a DTT-sensitive Panx1 modification that is consistent with S-nitrosylation; also, it was not observed when GSH was substituted for GSNO in control or oxidative conditions, ruling out an alternative glutathionylation mechanism. Likewise, inhibition of whole cell Panx1 currents by GSNO and DEA NONOate was reversed by DTT, an action that was also not mimicked by GSH. In addition, GSNO-mediated current inhibition was not dependent on sGC activity, supporting S-nitrosylation over other mechanisms that can contribute to NO actions. In support of a direct inhibitory effect on the channel, substitution of either Cys-40 or Cys-346 in Panx1 prevented GSNO-mediated channel inhibition, whereas mutation of both Cys-40 and Cys-346 blocked S-nitrosylation of the channel. In sum, these data demonstrate that modification at these two critical cysteines is required for Panx1 channel inhibition by S-nitrosylation, and they suggest a functional role for NO in regulating the activity of Panx1 channels.
The role of Panx1 channels in ATP release from cells has been a growing area of investigation with multiple studies identifying mechanisms by which Panx1 channels can be activated. By contrast, there is little understanding of how Panx1 channels are inhibited. The results of our current study provide a novel mechanism by which Panx1 channels can be inhibited, supporting a functional role for NO in controlling the activity of Panx1 channels at the plasma membrane of cells and suggesting a way to negatively regulate ATP release from cells.
Although our results indicate an inhibitory role imparted by NO on Panx1 channels through S-nitrosylation, a recent study has implicated this reactive oxygen species in Panx1 channel activation (37). In that work, it was suggested that NO generated during oxygen-glucose deprivation can activate Panx1 channels expressed in cultured hippocampal neurons. Although it is possible that NO signaling may result in differential Panx1 channel regulation in different physiological contexts, it is important to point out that the aforementioned study used calcein dye leakage as a functional readout for Panx1 channel activity. Because cultured hippocampal neurons express connexin proteins, which are both permeable to calcein dye and activated by S-nitrosylation (28, 30), it is possible that the dye leakage observed in this study represented activity of connexins. Moreover, it is possible that cultured hippocampal neurons express other pannexin isoforms, such as Panx2, which is highly expressed in the central nervous system (38, 39). As such, other pannexin isoforms may be regulated differently by NO. In our work, we assessed S-nitrosylation and recorded whole cell currents as well as ATP release from recombinant Panx1 channels, providing direct evidence for inhibition of Panx1 by NO.
We performed cysteine mutagenesis of Panx1 to identify the specific residues required for S-nitrosylation and channel inhibition. Interestingly, the two sites that we identified as critical for this inhibitory modification, Cys-40 and Cys-346, were previously reported to enhance activity of Panx1 channels in mutagenesis studies (40, 41). In that other work, serine substitution at either Cys-40 (41) or Cys-346 (40) produced “leaky” or constitutively active channels. Together with our results, these observations suggest that Cys-40 and Cys-346 may be localized to regions that are important for dynamic up- and down-regulation of Panx1 channel activity.
We noted that mutation of either Cys-40 or Cys-346 affected the banding pattern of Panx1 on a Western blot. It is known that Panx1 exists in three forms: a core unglycosylated species (Gly0), a high mannose species (Gly1), and a complex glycosylated form (Gly2), each contributing to the characteristic triple banding pattern on a Western blot. Mutation of Cys-40 or Cys-346 resulted in a marked reduction in the Gly2 species with the protein detected mainly as Gly0 and Gly1 forms. Because glycosylation has been implicated in trafficking of Panx1 channels to the plasma membrane, we examined the cellular localization of the cysteine mutants and found that each mutant appeared at the plasma membrane. Although these cysteine substitutions may have affected Panx1 glycosylation status, it is important to point out that Panx1 currents and ATP release were detected for all mutant constructs, indicating that they could form functional channels at the plasma membrane. This is consistent with previous work indicating that all Panx1 Gly species can reach the plasma membrane (26). Also, a previous study identified a loss in the Gly2 species in functional, plasma membrane-localized Panx1C346S (40).
S-Nitrosylation of Panx1 channels may function physiologically as a negative feedback mechanism mediating inhibition of channel activity following their activation to prevent chronic release of ATP. In the vasculature, endothelial cells that line the blood vessel lumen express functional Panx1 channels at the plasma membrane, and multiple studies have emerged implicating these channels in ATP release (7, 11, 14, 15, 35). Recently, it was suggested that thrombin, by activation of endothelial cell PAR-1 receptors, promotes ATP release from Panx1 channels into the extracellular compartment (14). Our data utilizing mouse aortic endothelial cells indicate that thrombin-induced ATP release from these cells can be significantly inhibited by S-nitrosylation of the channel. This novel regulatory mechanism may play an important role in controlling the extent of ATP release from the vascular endothelium and therefore modulate purinergic signaling events in the vasculature. In addition, circulating erythrocytes release ATP via Panx1 into the blood vessel lumen during conditions of low oxygen tension and membrane deformation, conditions that arise in small arterioles and capillaries (11, 15). Released ATP activates endothelial cell P2Y receptors causing generation of NO, which diffuses to and relaxes adjacent smooth muscle cells (42). It is possible that the endothelially derived NO could also diffuse into the blood vessel lumen and S-nitrosylate erythrocyte Panx1 channels, inhibiting further ATP release and preventing excess vasodilation. Regulation of Panx1 channels by S-nitrosylation in these cells could therefore play an important role in the matching of blood flow to tissue oxygen demand.
Although the inhibitory effect of S-nitrosylation on Panx1 channel function was demonstrated, the mechanism by which this modification elicits its effects on the channel is not as clear. In this respect, our data indicate that S-nitrosylation has no effect on Panx1 cell membrane localization, suggesting the possibility that channel activity is directly inhibited. Recent evidence has indicated that the C-terminal tail may form a portion of the Panx1 channel pore (43) and that proteolytic cleavage of the C-terminal tail increases channel currents by its removal from the Panx1 pore (25). It is possible that S-nitrosylation of Cys-346 and Cys-40 promotes a conformation that stabilizes the C terminus-pore interaction and thus the channel closed state. Future studies utilizing structural and biophysical techniques may reveal the precise mechanism by which Panx1 S-nitrosylation inhibits the channel. Nonetheless, our data have provided novel evidence for a post-translational mechanism inhibiting Panx1 channels at the plasma membrane.
Acknowledgments
We thank Daniel Schwartz (University of Connecticut) for help with the scan-x prediction method, Robert K. Nakamoto (University of Virginia) for initial help with plasmid generation, and Dr. Stefanie Gödecke (Heinrich-Heine-University, Düsseldorf, Germany) for advice on thrombin application to endothelial cells.
This work was supported, in whole or in part, by National Institutes of Health Grants HL088554 (to B. E. I.), HL107963 (to B. E. I.), HL112904-1 (to A. C. S.), and NS033583 (to D. A. B.). This work was also supported by an American Heart Association postdoctoral fellowship (to M. B.) and an American Heart Association predoctoral fellowship (to A. W. L.).
- Panx1
- pannexin 1
- GSH
- l-glutathione
- ODQ
- 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- DEA NONOate
- diethylammonium (Z)-1–1(N,N-diethylamino)diazen-1-ium-1,2-diolate
- GSNO
- S-nitrosoglutathione
- CBX
- carbenoxolone
- mAEC
- mouse aortic endothelial cell
- sGC
- soluble guanylate cyclase.
REFERENCES
- 1. Ambrosi C., Gassmann O., Pranskevich J. N., Boassa D., Smock A., Wang J., Dahl G., Steinem C., Sosinsky G. E. (2010) Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J. Biol. Chem. 285, 24420–24431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dando R., Roper S. D. (2009) Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J. Physiol. 587, 5899–5906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reigada D., Lu W., Zhang M., Mitchell C. H. (2008) Elevated pressure triggers a physiological release of ATP from the retina: possible role for pannexin hemichannels. Neuroscience 157, 396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li S., Bjelobaba I., Yan Z., Kucka M., Tomic M., Stojilkovic S. S. (2011) Expression and roles of pannexins in ATP release in the pituitary gland. Endocrinology 152, 2342–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iwabuchi S., Kawahara K. (2011) Functional significance of the negative-feedback regulation of ATP release via pannexin-1 hemichannels under ischemic stress in astrocytes. Neurochem. Int. 58, 376–384 [DOI] [PubMed] [Google Scholar]
- 6. Woehrle T., Yip L., Manohar M., Sumi Y., Yao Y., Chen Y., Junger W. G. (2010) Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors. J. Leukoc. Biol. 88, 1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Billaud M., Lohman A. W., Straub A. C., Looft-Wilson R., Johnstone S. R., Araj C. A., Best A. K., Chekeni F. B., Ravichandran K. S., Penuela S., Laird D. W., Isakson B. E. (2011) Pannexin1 regulates α1-adrenergic receptor-mediated vasoconstriction. Circ. Res. 109, 80–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Woehrle T., Yip L., Elkhal A., Sumi Y., Chen Y., Yao Y., Insel P. A., Junger W. G. (2010) Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood 116, 3475–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chekeni F. B., Elliott M. R., Sandilos J. K., Walk S. F., Kinchen J. M., Lazarowski E. R., Armstrong A. J., Penuela S., Laird D. W., Salvesen G. S., Isakson B. E., Bayliss D. A., Ravichandran K. S. (2010) Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis. Nature 467, 863–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ransford G. A., Fregien N., Qiu F., Dahl G., Conner G. E., Salathe M. (2009) Pannexin 1 contributes to ATP release in airway epithelia. Am. J. Respir. Cell Mol. Biol. 41, 525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sridharan M., Adderley S. P., Bowles E. A., Egan T. M., Stephenson A. H., Ellsworth M. L., Sprague R. S. (2011) Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am. J. Physiol. Heart Circ. Physiol. 299, H1146–H1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schenk U., Westendorf A. M., Radaelli E., Casati A., Ferro M., Fumagalli M., Verderio C., Buer J., Scanziani E., Grassi F. (2008) Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci. Signal. 1, ra6. [DOI] [PubMed] [Google Scholar]
- 13. Seminario-Vidal L., Okada S. F., Sesma J. I., Kreda S. M., van Heusden C. A., Zhu Y., Jones L. C., O'Neal W. K., Penuela S., Laird D. W., Boucher R. C., Lazarowski E. R. (2011) Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J. Biol. Chem. 286, 26277–26286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gödecke S., Roderigo C., Rose C. R., Rauch B. H., Gödecke A., Schrader J. (2012) Thrombin-induced ATP release from human umbilical vein endothelial cells. Am. J. Physiol. Cell Physiol. 302, C915–C923 [DOI] [PubMed] [Google Scholar]
- 15. Locovei S., Bao L., Dahl G. (2006) Pannexin 1 in erythrocytes: function without a gap. Proc. Natl. Acad. Sci. U.S.A. 103, 7655–7659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silverman W. R., de Rivero Vaccari J. P., Locovei S., Qiu F., Carlsson S. K., Scemes E., Keane R. W., Dahl G. (2009) The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J. Biol. Chem. 284, 18143–18151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iglesias R., Dahl G., Qiu F., Spray D. C., Scemes E. (2009) Pannexin 1: the molecular substrate of astrocyte “hemichannels.” J. Neurosci. 29, 7092–7097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lohman A. W., Billaud M., Isakson B. E. (2012) Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc. Res. 95, 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burnstock G. (2002) Purinergic signaling and vascular cell proliferation and death. Arterioscler. Thromb. Vasc. Biol. 22, 364–373 [DOI] [PubMed] [Google Scholar]
- 20. Rumjahn S. M., Yokdang N., Baldwin K. A., Thai J., Buxton I. L. (2009) Purinergic regulation of vascular endothelial growth factor signaling in angiogenesis. Br. J. Cancer 100, 1465–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burnstock G., Ulrich H. (2011) Purinergic signaling in embryonic and stem cell development. Cell. Mol. Life Sci. 68, 1369–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burnstock G. (2011) Purinergic signaling in the gastrointestinal tract. World J. Gastrointest. Pathophysiol. 2, 31–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bao L., Locovei S., Dahl G. (2004) Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 572, 65–68 [DOI] [PubMed] [Google Scholar]
- 24. Weilinger N. L., Tang P. L., Thompson R. J. (2012) Anoxia-induced NMDA receptor activation opens pannexin channels via Src family kinases. J. Neurosci. 32, 12579–12588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sandilos J. K., Chiu Y. H., Chekeni F. B., Armstrong A. J., Walk S. F., Ravichandran K. S., Bayliss D. A. (2012) Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region. J. Biol. Chem. 287, 11303–11311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Penuela S., Bhalla R., Nag K., Laird D. W. (2009) Glycosylation regulates pannexin intermixing and cellular localization. Mol. Biol. Cell 20, 4313–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boassa D., Ambrosi C., Qiu F., Dahl G., Gaietta G., Sosinsky G. (2007) Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J. Biol. Chem. 282, 31733–31743 [DOI] [PubMed] [Google Scholar]
- 28. Straub A. C., Billaud M., Johnstone S. R., Best A. K., Yemen S., Dwyer S. T., Looft-Wilson R., Lysiak J. J., Gaston B., Palmer L., Isakson B. E. (2011) Compartmentalized connexin 43 S-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arterioscler. Thromb. Vasc. Biol. 31, 399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun J., Xin C., Eu J. P., Stamler J. S., Meissner G. (2001) Cysteine-3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc. Natl. Acad. Sci. U.S.A. 98, 11158–11162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Retamal M. A., Cortés C. J., Reuss L., Bennett M. V., Sáez J. C. (2006) S-Nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc. Natl. Acad. Sci. U.S.A. 103, 4475–4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Asada K., Kurokawa J., Furukawa T. (2009) Redox- and calmodulin-dependent S-nitrosylation of the KCNQ1 channel. J. Biol. Chem. 284, 6014–6020 [DOI] [PubMed] [Google Scholar]
- 32. Yoshida T., Inoue R., Morii T., Takahashi N., Yamamoto S., Hara Y., Tominaga M., Shimizu S., Sato Y., Mori Y. (2006) Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2, 596–607 [DOI] [PubMed] [Google Scholar]
- 33. Penuela S., Bhalla R., Gong X. Q., Cowan K. N., Celetti S. J., Cowan B. J., Bai D., Shao Q., Laird D. W. (2007) Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J. Cell Sci. 120, 3772–3783 [DOI] [PubMed] [Google Scholar]
- 34. Jaffrey S. R., Snyder S. H. (2001) The biotin switch method for the detection of S-nitrosylated proteins. Sci. STKE 2001, pl1. [DOI] [PubMed] [Google Scholar]
- 35. Lohman A. W., Billaud M., Straub A. C., Johnstone S. R., Best A. K., Lee M., Barr K., Penuela S., Laird D. W., Isakson B. E. (2012) Expression of pannexin isoforms in the systemic murine arterial network. J. Vasc. Res. 49, 405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwartz D., Chou M. F., Church G. M. (2009) Predicting protein post-translational modifications using meta-analysis of proteome scale data sets. Mol. Cell. Proteomics 8, 365–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang L., Deng T., Sun Y., Liu K., Yang Y., Zheng X. (2008) Role for nitric oxide in permeability of hippocampal neuronal hemichannels during oxygen glucose deprivation. J. Neurosci. Res. 86, 2281–2291 [DOI] [PubMed] [Google Scholar]
- 38. Swayne L. A., Sorbara C. D., Bennett S. A. (2010) Pannexin 2 is expressed by postnatal hippocampal neural progenitors and modulates neuronal commitment. J. Biol. Chem. 285, 24977–24986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ray A., Zoidl G., Wahle P., Dermietzel R. (2006) Pannexin expression in the cerebellum. Cerebellum 5, 189–192 [DOI] [PubMed] [Google Scholar]
- 40. Bunse S., Schmidt M., Prochnow N., Zoidl G., Dermietzel R. (2010) Intracellular cysteine 346 is essentially involved in regulating Panx1 channel activity. J. Biol. Chem. 285, 38444–38452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bunse S., Schmidt M., Hoffmann S., Engelhardt K., Zoidl G., Dermietzel R. (2011) Single cysteines in the extracellular and transmembrane regions modulate pannexin 1 channel function. J. Membr. Biol. 244, 21–33 [DOI] [PubMed] [Google Scholar]
- 42. Ellsworth M. L., Ellis C. G., Goldman D., Stephenson A. H., Dietrich H. H., Sprague R. S. (2009) Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology 24, 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang J., Dahl G. (2010) SCAM analysis of Panx1 suggests a peculiar pore structure. J. Gen. Physiol. 136, 515–527 [DOI] [PMC free article] [PubMed] [Google Scholar]