Background: The CysC-CatB axis affects levels of Aβ from hAPP with familial mutations. How it affects Aβ from wild-type hAPP remains unknown.
Results: Enhancing CatB reduces and deleting CatB elevates levels of Aβ derived from wild-type hAPP.
Conclusion: The CysC-CatB axis regulates Aβ degradation similarly regardless of familial mutations.
Significance: Enhancing CatB activity as an Aβ-lowering strategy might be applicable in familial and sporadic AD.
Keywords: Amyloid, Amyloid Precursor Protein, Animal Models, Cysteine Protease, Lysosomes, Protein Degradation
Abstract
Accumulation of amyloid-β (Aβ), believed to be a key trigger of Alzheimer disease (AD), could result from impaired clearance mechanisms. Previously, we showed that the cysteine protease cathepsin B (CatB) degrades Aβ, most likely by C-terminal truncation, in mice expressing human amyloid precursor protein with familial AD-linked mutations (hAPPFAD). In addition, the Aβ-degrading activity of CatB is inhibited by its endogenous inhibitor, cystatin C (CysC). Reducing CysC expression markedly lowers Aβ levels by enhancing CatB-mediated Aβ degradation in hAPPFAD mice. However, because a vast majority of AD patients do not carry familial mutations, we investigated how the CysC-CatB axis affects Aβ levels in mice expressing wild-type hAPP (hAPPWT). Enhancing CatB activity by CysC deletion significantly lowered total Aβ and Aβ42 levels in hAPPWT mice, whereas CatB deletion increased Aβ levels. To determine whether neuron-derived CatB degrades Aβ in vivo, we generated transgenic mice overexpressing CatB under the control of a neuron-specific enolase promoter. Enhancing neuronal CatB activity in hAPPWT mice significantly lowered Aβ42 levels. The processing of hAPPWT was unaffected by increasing or ablating CatB activity. Thus, the CysC-CatB axis affects degradation of Aβ42 derived from hAPP lacking familial mutations. These findings support the notion that enhancing CatB activity could lower Aβ, especially Aβ42, in AD patients with or without familial mutations.
Introduction
Amyloid-β (Aβ),3 a key pathogenic factor in Alzheimer disease (AD), accumulates and forms toxic oligomers as a result of overproduction or inefficient clearance in the brain (1). Given the importance of catabolic mechanism in controlling Aβ levels, enhancing Aβ degradation and clearance could lead to Aβ-lowering strategies (2). Several Aβ-degrading enzymes have been identified; most are metalloproteases, including neprilysin, insulin-degrading enzymes, endothelin-converting enzymes, and matrix metalloproteinase-9 (3–6). Previously, we showed that cathepsin B (CatB), a cysteine protease, degrades Aβ by truncating Aβ42 at the C terminus (7). In transgenic mice overexpressing human amyloid precursor protein (hAPP) with familial AD (FAD)-linked Swedish and Indiana mutations (hAPPFAD-J20), plaque load was reduced by overexpression of CatB and increased by ablation of CatB. Consistent with the inhibition of CatB activity by its endogenous inhibitor cystatin C (CysC), genetic deletion of CysC enhanced CatB activity in the brain (8) and significantly reduced Aβ levels and Aβ-dependent behavioral and synaptic deficits in hAPP mice carrying FAD-linked mutations (8).
In contrast to our findings in hAPPFAD-J20 mice, deleting CatB was reported to cleave human wild-type APP (referred to as hAPPWT) at the β-secretase site, leading to production of Aβ and β C-terminal fragments (CTFs) (9). Because most AD patients do not carry FAD mutations, it is critical to determine how the CysC-CatB axis affects the production of Aβ and the processing of hAPPWT. To address this question, we deleted CatB or CysC in hAPPWT-I63 mice, which express hAPPWT at levels similar to those produced by hAPPFAD-J20 mice but produce much lower levels of Aβ. Because CatB is expressed in neurons and glia in the brain (10, 11), we also established a transgenic line that overexpresses CatB under the control of a neuron-specific enolase (NSE) promoter to further assess the Aβ-degrading activity of neuronal CatB. To determine how enhanced CatB activity in neurons affects the catabolism of Aβ and the processing of hAPPWT, we crossed NSE-catB mice with hAPPWT mice.
EXPERIMENTAL PROCEDURES
Mice
catB−/− mice (12) and cst3−/− mice (13) were backcrossed to the C57BL/6 background for more than 10 (for catB−/− mice) or 8 (for cst3−/− mice) generations. catB−/− or cst3−/− mice were then crossed with hAPPWT mice (C57BL/6) from line I63 (14). The first cross resulted in hAPPWT/catB+/− or hAPPWT/cst3+/− mice, respectively. hAPPWT/catB+/− mice were crossed with catB+/− mice to generate hAPPWT/catB+/+, hAPPWT/catB+/−, hAPPWT/catB−/−, catB+/+, catB+/−, and catB−/− mice. Similarly, hAPPWT/cst3+/− mice were crossed with cst3+/− mice to generate hAPPWT/cst3+/+, hAPPWT/cst3+/−, hAPPWT/cst3−/−, cst3+/+, cst3+/−, and cst3−/− mice. PCR-based genotyping for the catB and cst3 alleles and the hAPP transgene was performed as described (7). All mouse studies were approved by the Animal Care and Use Committee of the University of California (San Francisco, CA).
Generation of NSE-catB Transgenic Mice
The cDNA encoding full-length mouse CatB was cloned from the pCMV-SPORT6 vector containing mouse CatB (Addgene) by high fidelity PCR using the following primers (containing a HindIII site): 5′-TCT AAG CTT CCA GGA TGT GGT GGT CCT TGA TCC-3′ and 5′-CTC TAA GCT TTT AGA ATC TTC CCC AGT ACT-3′. The PCR fragment was inserted into the NSE vector (15) with blunted HindIII linkers to generate the NSE-CatB plasmid. The orientation and sequence of mouse CatB were confirmed by sequencing. NSE-CatB was linearized by SalI digestion and purified by passing over an Elutip column (Schleicher & Schuell). The concentration of the linearized NSE-CatB construct was adjusted to 2 μg/ml in injection buffer (5 mm Tris, pH 7.5 and 0.2 mm EDTA) and microinjected into the pronuclei of single-cell embryos harvested from C57BL/6 mice. Transgenic offspring were identified by PCR analysis of tail lysates. Two founder lines of NSE-catB transgenic mice were generated. The line with the higher expression level was crossed with the hAPP-I63 line to generate hAPPWT/NSE-catB mice.
Aβ and Soluble Amyloid Precursor Protein-β ELISA
Mice were perfused with 0.9% saline, and their hemi-brains were snap-frozen on dry ice and stored at −80 °C. Hippocampal and cortical Aβ levels were measured by ELISA as described (16). The capture antibodies were 266 (for Aβ1–x) and 21F12 (for Aβ1–42), and the detection antibody was biotin-conjugated 3D6 (Elan, South San Francisco, CA).
To measure soluble amyloid precursor protein-β (s-APPβ) levels, cortices were homogenized in TBS/EDTA buffer (50 mm Tris (pH 7.4), 150 mm NaCl, and 2 mm EDTA) containing 1% Triton X-100 and 0.6% SDS. After ultracentrifugation for 20 min at 350,000 × g, the supernatant was assayed for s-APPβ according to the human s-APPβ ELISA kit protocol (Covance, Princeton, NJ).
CatB Enzymatic Assay
Brain CatB activities were quantified as described (7). Briefly, cortices from cst3−/−, cst3+/+, and cst3+/− mice or NSE-catB mice were lysed in lysis buffer in the absence of proteinase inhibitors, pre-activated with 4 mm cysteine, and incubated with the synthetic substrate benzyloxycarbonyl-Arg-Arg-7-amino-4-methylcoumarin at 37 °C for 30 min. Released free 7-amino-4-methylcoumarin was determined fluorometrically. The enzymatic activities inhibited by CA074 were defined as CatB-specific activities. Protein concentrations were measured with a BCA protein assay kit (Pierce), and CatB activities are shown as fluorescent units/μg of protein.
Western Blot Analysis
Cortical samples were homogenized and sonicated at 4 °C in radioimmune precipitation assay buffer containing 10 mm HEPES (pH 7.4), 150 mm NaCl, 50 mm NaF, 1 mm EDTA, 1 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, 1 mm Na3VO4, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1% SDS. Equal amounts of protein (by BCA assay) were resolved by SDS-PAGE and transferred to nitrocellulose membranes. After blocking, membranes were labeled with rabbit anti-CT15 antibody (1:1000; a kind gift of E. H. Koo, University of California, San Diego, La Jolla, CA) or mouse anti-GAPDH antibody (1:100,000; Millipore, Billerica, MA) and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2000) or goat anti-mouse IgG (1:2000) antibody (Millipore). Bands were visualized by enhanced chemiluminescence, and the densitometry measurements of the bands were acquired from scanned images with Quantity One software (Bio-Rad).
Immunohistochemistry
Sliding microtome sections (30 μm) of NSE-catB transgenic mice were used for immunohistochemistry as described previously with the following modifications (7). Briefly, antigen retrieval was performed by heating the sections in 10 mm citric acid at 90 °C for 30 min. Sections were then incubated with rabbit anti-CatB antibody (1:100; Upstate Biotechnology, Lake Placid, NY) or sheep anti-CatB antibody (1:10; MP Biomedicals, Solon, OH). After overnight incubation with primary antibodies, the sections were incubated with secondary antibodies, including Cy3-labeled donkey anti-rabbit IgG (1:500; Jackson ImmunoResearch Laboratories, West Grove, PA) and Alex Fluor 488-labeled donkey anti-sheep IgG (1:500; Invitrogen). For double staining, the sections were then incubated with mouse anti-NeuN antibody (1:500; Millipore), mouse anti-glial fibrillary acidic protein antibody (1:500; Millipore), or rabbit anti-Iba1 antibody (1:500; Wako Chemicals USA, Richmond, VA), followed by incubation with secondary antibodies, including fluorescein-labeled goat anti-mouse IgG (1:500; Vector Laboratories, Burlingame, CA) and Cy3-labeled donkey anti-rabbit IgG (1:500). Images were acquired using a Eclipse Ti confocal microscope (Nikon, Melville, NY) and analyzed by Micro-Manager software (University of California, San Francisco).
Statistical Analysis
Statistical analyses were conducted with GraphPad Prism 5. Values are expressed as means ± S.E. Differences among multiple means with one variable (catB or cst3 genotype) were evaluated by one-way analysis of variance (ANOVA) and Tukey-Kramer post hoc tests. Differences between two means were evaluated using unpaired Student's t tests. p < 0.05 was considered significant.
RESULTS
CatB Ablation Elevates Aβ Levels without Affecting hAPP Processing in Transgenic Mice overexpressing hAPPWT
CatB degrades Aβ in hAPPFAD-J20 mice without affecting the processing of hAPPFAD (7). To directly determine the effects of CatB deletion on hAPPWT, we crossed catB−/− mice with hAPPWT-I63 mice, which express hAPP at a level similar to hAPPFAD-J20 mice but produce much less Aβ. The levels of total Aβ (Aβ1–x) in the hippocampuses and cortices of hAPPWT mice were slightly increased by deleting one catB allele and significantly increased by complete deletion of catB (Fig. 1, A and C). The levels of Aβ1–42 were also significantly increased in the cortices of hAPPWT/catB−/− mice and were slightly increased in the hippocampuses, although not statistically significantly in the latter (Fig. 1, B and D). Nevertheless, these results suggest that endogenous CatB lowers the level of Aβ derived from hAPPWT. Deleting CatB did not affect the levels of s-APPβ in the cortex, as determined by ELISA (Fig. 1E), indicating that CatB may not function as a β-secretase for hAPPWT. In addition, Western blot analyses with a C terminus-specific antibody revealed that the levels of full-length hAPP (FL-hAPP) and α- and β-CTFs were similar in hAPPWT mice with or without CatB (Fig. 1, F and G), further supporting that neither the β- nor α-cleavage of hAPPWT is affected by CatB deletion.
FIGURE 1.
CatB ablation increases levels of Aβ but does not affect hAPP processing in hAPPWT mice. A and B, ELISA measurements of hippocampal Aβ1–x (A) and Aβ1–42 (B) in 3.5-month-old hAPPWT/catB+/+, hAPPWT/catB+/−, and hAPPWT/catB−/− mice. The levels of Aβ1–x were elevated significantly by CatB deletion (n = 9–13 mice/genotype). **, p < 0.01 (one-way ANOVA with Tukey's post hoc test). C and D, ELISA measurements of cortical Aβ1–x (C) and Aβ1–42 (D) in 3.5-month-old hAPPWT/catB+/+, hAPPWT/catB+/−, hAPPWT/catB−/− mice. The levels of Aβ1–x and Aβ1–42 were elevated significantly by CatB deletion (n = 7–10 mice/genotype). *, p < 0.05; **, p < 0.01 (one-way ANOVA with Tukey's post hoc test). E, ELISA measurement of cortical s-APPβ in 3.5-month-old hAPPWT/catB+/+, hAPPWT/catB+/−, and hAPPWT/catB−/− mice. CatB deletion did not affect s-APPβ levels (n = 6 mice/genotype). F, representative Western blot analyses of hAPP metabolic fragments in the cortices of 3.5-month-old hAPPWT/catB+/+ and hAPPWT/catB−/− mice. G, quantification of the levels of FL-hAPP (normalized to those of GAPDH) and α- and β-CTFs (normalized to those of FL-hAPP). CatB deletion did not affect the levels of hAPP fragments (n = 9–12 mice/genotype). Values are means ± S.E. (A–E and G).
CysC Reduction Lowers Aβ Levels without Affecting hAPPWT Processing
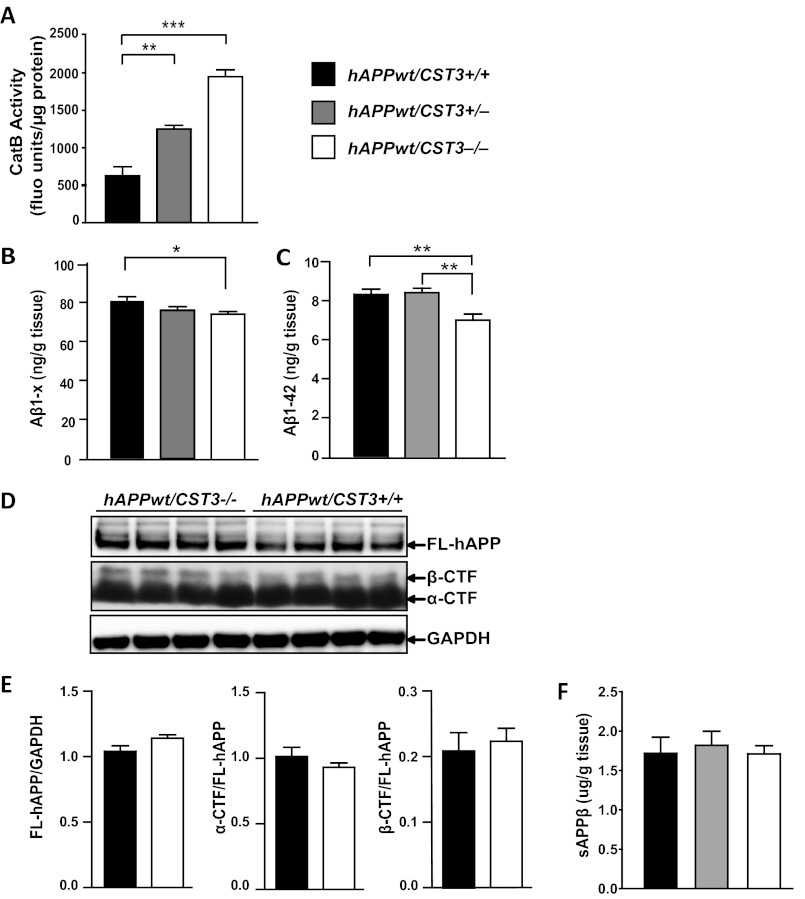
CatB activity is blocked by its endogenous inhibitor, CysC (cst3) (17, 18). To further explore the effects of CatB activity on Aβ and hAPPWT processing, we crossed hAPPWT mice with cst3−/− mice. CysC ablation yielded a gene dose-dependent increase in cortical CatB activity (Fig. 2A). Reducing CysC expression lowered the levels of total Aβ and Aβ1–42 in the hippocampuses of hAPPWT mice (Fig. 2, B and C), in agreement with our previous findings in mice expressing hAPPFAD (8). Thus, elevated CatB activity lowered Aβ levels derived from hAPP with or without FAD mutations. Consistent with our observations in hAPPFAD mice (8), removing CysC did not affect the levels of FL-hAPP, α-CTF, or β-CTF in hAPPWT mice (Fig. 2, D and E). In addition, the levels of s-APPβ were not affected by CysC deletion (Fig. 2F), confirming that enhancing CatB activity, via deleting CysC, does not affect β-cleavage of hAPPWT.
FIGURE 2.
CysC reduction lowers Aβ levels but does not affect hAPP processing in hAPPWT mice. A, enzymatic activity of CatB in cortex lysates from cst3+/+, cst3+/−, and cst3−/− mice (n = 4–6 mice/genotype). **, p < 0.01; ***, p < 0.0001 (one-way ANOVA with Tukey's post hoc test). fluo units, fluorescent units. B and C, ELISA measurements of hippocampal Aβ1–x (B) and Aβ1–42 (C) in 3.5-month-old hAPPWT/cst3+/+, hAPPWT/cst3+/−, hAPPWT/cst3−/− mice. The levels of Aβ1–x and Aβ1–42 were reduced significantly by CysC deletion (n = 12–19 mice/genotype). *, p < 0.05; **, p < 0.01 (one-way ANOVA with Tukey's post hoc test). D, representative Western blot analyses of hAPP metabolic fragments in cortices of 3.5-month-old hAPPWT/cst3+/+ and hAPPWT/cst3−/− mice. E, quantification of levels of FL-hAPP (normalized to those of GAPDH) and α- and β-CTFs (normalized to those of FL-hAPP). CysC deletion did not affect the levels of hAPP fragments (n = 13 mice/genotype). F, ELISA measurement of cortical s-APPβ in 3.5-month-old hAPPWT/cst3+/+ (black bar), hAPPWT/cst3+/− (gray bar), hAPPWT/cst3−/− (white bar) mice. CysC deletion did not affect s-APPβ levels (n = 6 mice/genotype). Values are means ± S.E. (A–C, E, and F).
Neuronal Overexpression of CatB Reduces the Levels of Aβ Derived from hAPPWT
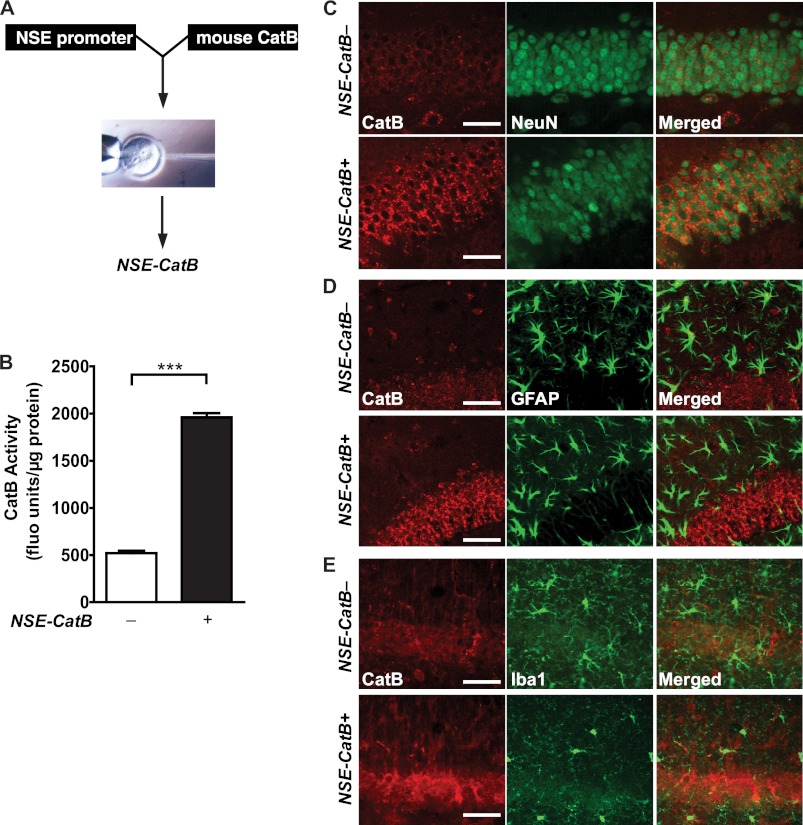
CysC reduction enhances CatB activity in the brain, where CatB is expressed in neurons and glia. To determine whether neuron-derived CatB degrades Aβ in vivo, we established a transgenic mouse line that overexpresses mouse CatB under the control of a neuronal enolase promoter (NSE-catB) (Fig. 3A). CatB activity in the cortex was significantly higher in NSE-catB mice than in non-transgenic controls (Fig. 3B), indicating that overexpressed CatB is functionally active in vivo. Moreover, CatB staining was enhanced in hippocampal neurons expressing NeuN (Fig. 3C) but not in glial fibrillary acidic protein-expressing astroglia (Fig. 3D) or Iba1-positive microglia (Fig. 3E). These results suggest that CatB is increased predominantly in the neurons of NSE-catB mice.
FIGURE 3.
Neuron-specific overexpression of CatB in hAPPWT mice increases CatB activity in the brain. A, schematic of the NSE-CatB construct injected into fertilized eggs from C57BL mice. B, CatB activities in cortex lysates were higher in NSE-catB mice compared with non-transgenic controls (n = 8–25 mice/genotype). ***, p < 0.0001 (unpaired Student's t test). fluo units, fluorescent units. C, compared with non-transgenic mice, elevated CatB immunostaining (red) was localized in neurons identified with an anti-NeuN antibody (green) in the dentate gyrus of NSE-catB mice. D and E, no elevation of CatB immunostaining (red) was observed in astrocytes identified with an anti-glial fibrillary acidic protein antibody (D, green) or in microglia identified with anti-Iba1 antibody (E, green) in brains of NSE-catB mice. Scale bars = 50 μm. Values are means ± S.E. (B).
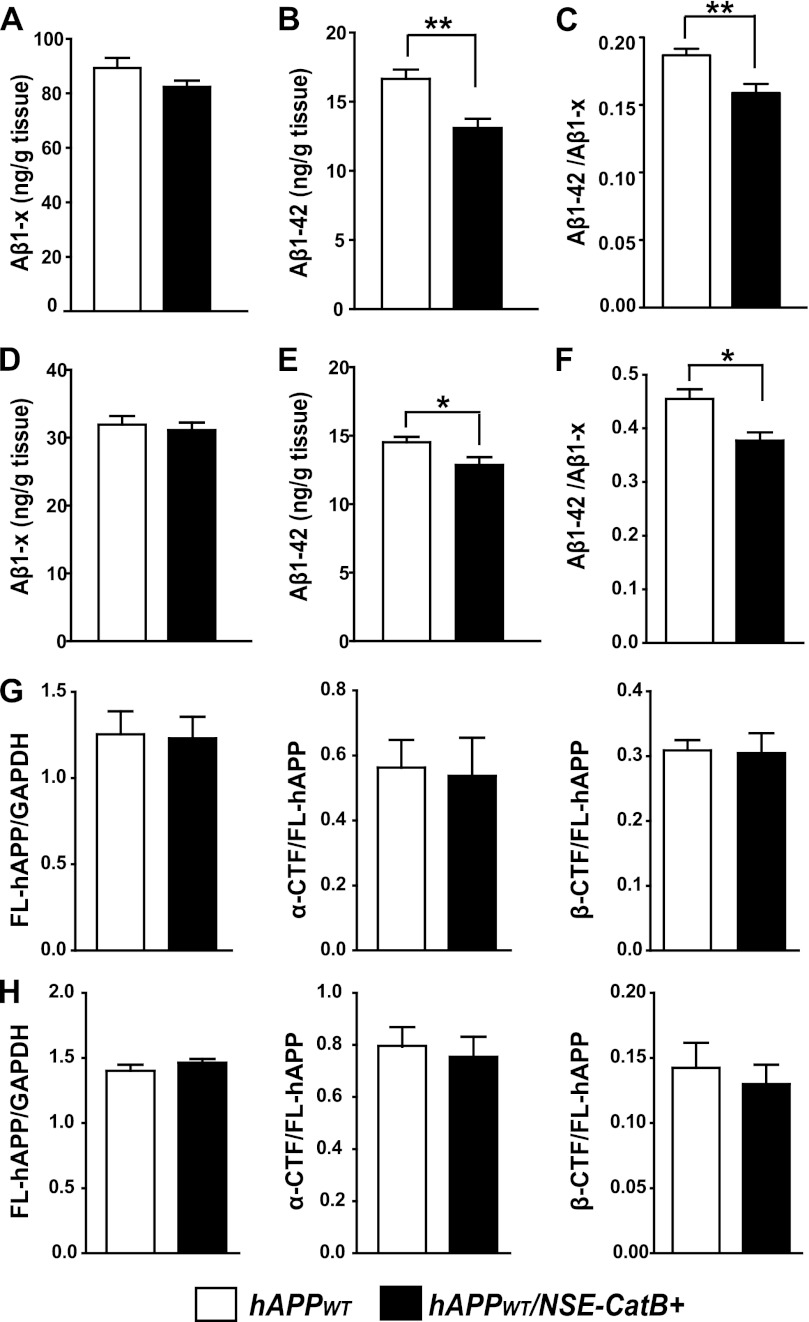
We next crossed NSE-catB mice with hAPPWT mice and determined the effects of elevated neuronal CatB on Aβ and hAPP processing. Enhancing neuronal CatB in hAPPWT/NSE-catB mice significantly lowered the levels of Aβ1–42 in both the hippocampus and cortex (Fig. 4, B and E). The levels of total Aβ were modestly lower in hAPPWT/NSE-catB mice compared with hAPPWT mice (Fig. 4, A and D). Increased CatB activity in neurons also significantly reduced the relative abundance of Aβ42 in both the hippocampus and cortex (Fig. 4, C and F). These results suggest that neuronal CatB reduces Aβ1–42 preferentially, likely by truncating at the C terminus as described previously (7). Neuronal overexpression of CatB did not affect the levels of FL-hAPP, α-CTF, or β-CTF in the hippocampus (Fig. 4G) or in the cortex (Fig. 4H), confirming that neuronal CatB does not affect hAPPWT processing.
FIGURE 4.
Neuron-derived CatB overexpression reduces levels of Aβ but does not affect hAPP processing in hAPPWT mice. A and B, ELISA measurements of hippocampal Aβ1–x (A) and Aβ1–42 (B) in 4-month-old hAPPWT and hAPPWT/NSE-catB mice. C, the Aβ1–42/Aβ1–x ratio was significantly lower in hAPPWT/NSE-catB mice than in hAPPWT mice (n = 8–12 mice/genotype). **, p < 0.01 (unpaired Student's t test). D and E, ELISA measurements of cortical Aβ1–x (D) and Aβ1–42 (E) in 4-month-old hAPPWT and hAPPWT/NSE-catB mice. F, the Aβ1–42/Aβ1–x ratio was significantly lower in hAPPWT/NSE-catB mice than in hAPPWT mice (n = 6–8 mice/genotype). *, p < 0.05 (unpaired Student's t test). G and H, quantification of hippocampal (G) and cortical (H) levels of FL-hAPP (relative to those of GAPDH) and α- and β-CTFs (relative to those of FL-hAPP) in hAPPWT mice by Western blotting (n = 8 mice/genotype). Values are means ± S.E.
DISCUSSION
This study shows that CysC-CatB affects Aβ levels in hAPPWT mice in a similar fashion as in hAPPFAD mice as described in our previous studies (7, 8). CatB removal elevated the levels of Aβ in hAPPWT mice, as in hAPPFAD mice (7). In mice expressing hAPPWT, neuron-derived CatB effectively reduced the levels of Aβ1–42, the most pathogenic of the Aβ peptides in the brain (19). Removing CysC, the endogenous inhibitor of CatB, in hAPPWT mice lowered total Aβ and Aβ1–42 levels. However, hAPPWT processing was unaffected by removal of CatB or CysC. Enhancing neuronal CatB also had no effect on hAPPWT processing. Thus, targeting the CysC-CatB axis to enhance Aβ degradation might be applicable not only in AD patients with FAD mutations but also in sporadic AD cases.
Our results differ from a previous study that showed that deleting CatB reduces the levels of s-APPβ, Aβ1–40, and Aβ1–42 in hAPPWT mice (9). In contrast, we found that total Aβ (Aβ1–x) and Aβ1–42 levels were reduced, whereas the levels of s-APPβ were unaffected. Although the exact reason underlying this discrepancy remains unclear, one key difference between the two studies is the Aβ ELISA used to measure total Aβ. We used a well established ELISA that allowed us to detect all Aβ species starting at position +1 and ending at or after position +23, which would include Aβ1–40, Aβ1–38, and Aβ1–42 among others (16), whereas previous ELISA probing total Aβ levels measured Aβ1–40 specifically (9). Because CatB could convert Aβ42 to Aβ40 and Aβ38, it is conceivable that CatB deficiency could lead to a reduction in Aβ40 and/or Aβ38 because Aβ42 could not be cleaved to generate Aβ40 and/or Aβ38. In support of this notion, a recent study showed that enhancing CatB activity pharmacologically significantly reduced Aβx–42 but increased levels of Aβ1–38 (20).
To determine the effects of the CysC-CatB axis on Aβ derived from hAPPWT, we used three approaches to modulate CatB levels. Besides deleting CatB, we enhanced CatB activity either by deleting CysC or by overexpressing CatB in neurons. In both cases, enhancing CatB activity significantly lowered Aβ1–42 levels. Moreover, the enhanced activity reduced the relative abundance of Aβ1–42, supporting the notion that enhanced CatB activity reduces Aβ1–42 preferentially, most likely by C-terminal truncation. Consistent with these findings, systemic injections of the CatB-enhancing drug benzyloxycarbonyl-Phe-Ala diazomethyl ketone significantly reduced Aβ deposition and Aβx–42 levels in two independent AD models, even at advanced ages (10–12 and 20–22 months) (20). In contrast, deleting CatB in hAPPWT mice led to a modest increase in Aβ levels. One possible explanation is that the physiological concentration of endogenous CysC is much greater than that of CatB (21). Because the Aβ-degrading activity of CatB is normally inhibited strongly by CysC, the effects of removing CatB would be modest. Indeed, CatB ablation had more robust effects on Aβ42 levels when CysC levels were reduced (8).
In contrast to the effects on Aβ levels, modulating CatB or CysC did not affect the levels of s-APPβ, α-CTF, β-CTF, or FL-hAPP, suggesting that CatB is not involved in β- or α-cleavage of hAPP with or without FAD mutations. However, in another study, deleting CatB in hAPPWT mice reduced the levels of Aβ and β-CTF (9). The reason for this discrepancy is unclear. However, it is important to note that CatB activities were altered with three independent genetic approaches in hAPPWT mice in the present study, none of which led to changes in β- or α-CTF. It is thus very unlikely that CatB acts as a major β-secretase on hAPPWT. Indeed, ablation of BACE1 completely abolished processing of the β-secretase site and Aβ generation in PDAPP mice, which overexpress hAPPV717F with no mutations at the β-secretase site (22), further supporting the notion that BACE1, not CatB, is the major β-secretase of hAPP with or without FAD mutations.
Accumulating evidence supports the notion that disruption of substrate proteolysis within lysosomal pathways is a mechanism underlying early pathological changes in AD (23). The importance of lysosomal dysfunction in AD pathogenesis is further supported by the discovery that mutant presenilin-1 linked with early-onset AD disrupts autophagy directly by impeding lysosomal proteolysis (24). Our studies of the CysC-CatB proteolytic axis in hAPP mice provided one of the first examples of lysosomal pathways in Aβ degradation (7, 8). In agreement with our findings, deleting cystatin B, another endogenous cysteine protease inhibitor, rescued autophagic-lysosomal pathology, reduced the accumulation of intraneuronal Aβ and plaque load, and prevented behavioral deficits in hAPP mice (25). Indeed, deleting cystatin B accelerated protein turnover and enhanced the activities of lysosomal enzymes, including CatB, suggesting that the enhanced activities of CatB or other lysosomal enzymes could be responsible for the beneficial effects of cystatin B deletion.
CatB is expressed in various cell types in the brain, including neurons and microglia. Previously, we showed that Aβ and Aβ42 levels in primary neuronal cultures were reduced by enhancing neuronal CatB and elevated by inhibiting CatB (7). By enhancing neuronal CatB in hAPPWT/NSE-catB mice, we showed unequivocally that neuron-derived CatB reduces Aβ42 in vivo. The modest Aβ-lowering effects of neuronal CatB suggest that CatB in non-neuronal cells could also be involved in degrading Aβ. For example, CatB is highly expressed in cultured microglia, and its expression levels are elevated by treatment with Aβ42 in culture (26, 27). Moreover, in cultured microglia, inhibition of CatB prevented the degradation of oligomeric Aβ, whereas inhibitors of neprilysin, matrix metalloproteinases, or insulin-degrading enzymes had no effect (28). In hAPPFAD mouse brain, we previously observed strong CatB immunoreactivity in microglia surrounding the plaques (7). However, in hAPPWT/NSE-catB mice, we did not detect clear CatB immunoreactivity in microglia. One possible explanation is that CatB expression is very low in quiescent microglia in mice with low levels of Aβ42. Indeed, CatB expression is directly up-regulated by Aβ42 in cultured microglia (26, 27), which might help explain the readily detectable CatB in microglia surrounding the plaques but not those away from the plaques (7). Further study is needed to determine the importance of microglial CatB in Aβ degradation and clearance in vivo.
Our results support an important role for neuronal CatB in Aβ degradation. However, where the CysC-CatB axis acts to modulate Aβ degradation at the subcellular level remains unknown. In most pyramidal neurons, mature and proenzyme forms of CatB are present in early endosomes (11, 29). Consistent with this finding, CatB in endosomes catalyzes C-terminal truncations of epidermal growth factor and insulin-like growth factor and inhibits their signaling (30, 31). Although CysC is generated mainly in astroglia, CysC immunoreactivity is found in neurons with a punctate distribution, which co-localize with CatB in AD brains (32). Thus, CysC could be taken up by neurons or microglia into the endocytic pathway and inhibit the Aβ-degrading activity of CatB. Regardless of the specific sites at which the CysC-CatB axis regulates Aβ degradation, our findings support enhancing proteolytic activity of CatB as a potential new Aβ-lowering strategy in sporadic AD.
Acknowledgments
We thank Drs. Hidde Ploegh and Christoph Peters for CatB-null mice; Stephen Ordway, Anna Lisa Lucido, and Gary Howard for editorial review; and Kelley Nelson for administrative assistance.
Note Added in Proof
A recent paper by K. Viswanathan et al. (33) identified new cathepsin B-enhancing small molecules that protect against synaptic deficits in a transgenic model of AD.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 AG030207-01A2 (to L. G.) and Grant CO6 RRO18928 from the National Center for Research Resources (a facilities grant to the J. David Gladstone Institutes). This work was also supported by the California Health Services (to L. G.), Swedish Research Council Grant 05196 (to A. G.), the S. D. Bechtel, Jr. Foundation, and Alden W. and Helen T. Clausen.
- Aβ
- amyloid-β
- AD
- Alzheimer disease
- CatB
- cathepsin B
- hAPP
- human amyloid precursor protein
- FAD
- familial AD
- CysC
- cystatin C
- CTF
- C-terminal fragment
- NSE
- neuron-specific enolase
- s-APPβ
- soluble amyloid precursor protein-β
- ANOVA
- analysis of variance
- FL-hAPP
- full-length hAPP.
REFERENCES
- 1. Tanzi R. E., Moir R. D., Wagner S. L. (2004) Clearance of Alzheimer's Aβ peptide: the many roads to perdition. Neuron 43, 605–608 [DOI] [PubMed] [Google Scholar]
- 2. Miners J. S., Barua N., Kehoe P. G., Gill S., Love S. (2011) Aβ-degrading enzymes: potential for treatment of Alzheimer disease. J. Neuropathol. Exp. Neurol. 70, 944–959 [DOI] [PubMed] [Google Scholar]
- 3. Iwata N., Tsubuki S., Takaki Y., Shirotani K., Lu B., Gerard N. P., Gerard C., Hama E., Lee H. J., Saido T. C. (2001) Metabolic regulation of brain Aβ by neprilysin. Science 292, 1550–1552 [DOI] [PubMed] [Google Scholar]
- 4. Yan P., Hu X., Song H., Yin K., Bateman R. J., Cirrito J. R., Xiao Q., Hsu F. F., Turk J. W., Xu J., Hsu C. Y., Holtzman D. M., Lee J. M. (2006) Matrix metalloproteinase-9 degrades amyloid-β fibrils in vitro and compact plaques in situ. J. Biol. Chem. 281, 24566–24574 [DOI] [PubMed] [Google Scholar]
- 5. Eckman E. A., Reed D. K., Eckman C. B. (2001) Degradation of the Alzheimer's amyloid-β peptide by endothelin-converting enzyme. J. Biol. Chem. 276, 24540–24548 [DOI] [PubMed] [Google Scholar]
- 6. Qiu W. Q., Walsh D. M., Ye Z., Vekrellis K., Zhang J., Podlisny M. B., Rosner M. R., Safavi A., Hersh L. B., Selkoe D. J. (1998) Insulin-degrading enzyme regulates extracellular levels of amyloid-β protein by degradation. J. Biol. Chem. 273, 32730–32738 [DOI] [PubMed] [Google Scholar]
- 7. Mueller-Steiner S., Zhou Y., Arai H., Roberson E. D., Sun B., Chen J., Wang X., Yu G., Esposito L., Mucke L., Gan L. (2006) Anti-amyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer's disease. Neuron 51, 703–714 [DOI] [PubMed] [Google Scholar]
- 8. Sun B., Zhou Y., Halabisky B., Lo I., Cho S. H., Mueller-Steiner S., Devidze N., Wang X., Grubb A., Gan L. (2008) Cystatin C-cathepsin B axis regulates amyloid-β levels and associated neuronal deficits in an animal model of Alzheimer's disease. Neuron 60, 247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hook V. Y., Kindy M., Reinheckel T., Peters C., Hook G. (2009) Genetic cathepsin B deficiency reduces β-amyloid in transgenic mice expressing human wild-type amyloid precursor protein. Biochem. Biophys. Res. Commun. 386, 284–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Banati R. B., Rothe G., Valet G., Kreutzberg G. W. (1993) Detection of lysosomal cysteine proteinases in microglia: flow cytometric measurement and histochemical localization of cathepsins B and L. Glia 7, 183–191 [DOI] [PubMed] [Google Scholar]
- 11. Cataldo A. M., Barnett J. L., Pieroni C., Nixon R. A. (1997) Increased neuronal endocytosis and protease delivery to early endosomes in sporadic Alzheimer's disease: neuropathologic evidence for a mechanism of increased β-amyloidogenesis. J. Neurosci. 17, 6142–6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deussing J., Roth W., Saftig P., Peters C., Ploegh H. L., Villadangos J. A. (1998) Cathepsins B and D are dispensable for major histocompatibility complex class II-mediated antigen presentation. Proc. Natl. Acad. Sci. U.S.A. 95, 4516–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huh C. G., Håkansson K., Nathanson C. M., Thorgeirsson U. P., Jonsson N., Grubb A., Abrahamson M., Karlsson S. (1999) Decreased metastatic spread in mice homozygous for a null allele of the cystatin C protease inhibitor gene. Mol. Pathol. 52, 332–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mucke L., Masliah E., Yu G. Q., Mallory M., Rockenstein E. M., Tatsuno G., Hu K., Kholodenko D., Johnson-Wood K., McConlogue L. (2000) High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 20, 4050–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raber J., Wong D., Buttini M., Orth M., Bellosta S., Pitas R. E., Mahley R. W., Mucke L. (1998) Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: increased susceptibility of females. Proc. Natl. Acad. Sci. U.S.A. 95, 10914–10919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson-Wood K., Lee M., Motter R., Hu K., Gordon G., Barbour R., Khan K., Gordon M., Tan H., Games D., Lieberburg I., Schenk D., Seubert P., McConlogue L. (1997) Amyloid precursor protein processing and Aβ42 deposition in a transgenic mouse model of Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 94, 1550–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Turk B., Bieth J. G., Björk I., Dolenc I., Turk D., Cimerman N., Kos J., Colic A., Stoka V., Turk V. (1995) Regulation of the activity of lysosomal cysteine proteinases by pH-induced inactivation and/or endogenous protein inhibitors, cystatins. Biol. Chem. Hoppe-Seyler 376, 225–230 [DOI] [PubMed] [Google Scholar]
- 18. Cimerman N., Prebanda M. T., Turk B., Popovic T., Dolenc I., Turk V. (1999) Interaction of cystatin C variants with papain and human cathepsins B, H, and L. J. Enzyme Inhib. 14, 167–174 [DOI] [PubMed] [Google Scholar]
- 19. Golde T. E., Eckman C. B., Younkin S. G. (2000) Biochemical detection of Aβ isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer's disease. Biochim. Biophys. Acta 1502, 172–187 [DOI] [PubMed] [Google Scholar]
- 20. Butler D., Hwang J., Estick C., Nishiyama A., Kumar S. S., Baveghems C., Young-Oxendine H. B., Wisniewski M. L., Charalambides A., Bahr B. A. (2011) Protective effects of positive lysosomal modulation in Alzheimer's disease transgenic mouse models. PLoS ONE 6, e20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turk B., Turk D., Salvesen G. S. (2002) Regulating cysteine protease activity: essential role of protease inhibitors as guardians and regulators. Curr. Pharm. Des. 8, 1623–1637 [DOI] [PubMed] [Google Scholar]
- 22. McConlogue L., Buttini M., Anderson J. P., Brigham E. F., Chen K. S., Freedman S. B., Games D., Johnson-Wood K., Lee M., Zeller M., Liu W., Motter R., Sinha S. (2007) Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP transgenic mice. J. Biol. Chem. 282, 26326–26334 [DOI] [PubMed] [Google Scholar]
- 23. Nixon R. A., Yang D. S. (2011) Autophagy failure in Alzheimer's disease–locating the primary defect. Neurobiol. Dis. 43, 38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee J. H., Yu W. H., Kumar A., Lee S., Mohan P. S., Peterhoff C. M., Wolfe D. M., Martinez-Vicente M., Massey A. C., Sovak G., Uchiyama Y., Westaway D., Cuervo A. M., Nixon R. A. (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141, 1146–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang D. S., Stavrides P., Mohan P. S., Kaushik S., Kumar A., Ohno M., Schmidt S. D., Wesson D., Bandyopadhyay U., Jiang Y., Pawlik M., Peterhoff C. M., Yang A. J., Wilson D. A., St George-Hyslop P., Westaway D., Mathews P. M., Levy E., Cuervo A. M., Nixon R. A. (2011) Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer's disease ameliorates amyloid pathologies and memory deficits. Brain 134, 258–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gan L., Ye S., Chu A., Anton K., Yi S., Vincent V. A., von Schack D., Chin D., Murray J., Lohr S., Patthy L., Gonzalez-Zulueta M., Nikolich K., Urfer R. (2004) Identification of cathepsin B as a mediator of neuronal death induced by Aβ-activated microglial cells using a functional genomics approach. J. Biol. Chem. 279, 5565–5572 [DOI] [PubMed] [Google Scholar]
- 27. Halle A., Hornung V., Petzold G. C., Stewart C. R., Monks B. G., Reinheckel T., Fitzgerald K. A., Latz E., Moore K. J., Golenbock D. T. (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 9, 857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang C. N., Shiao Y. J., Shie F. S., Guo B. S., Chen P. H., Cho C. Y., Chen Y. J., Huang F. L., Tsay H. J. (2011) Mechanism mediating oligomeric Aβ clearance by naïve primary microglia. Neurobiol. Dis. 42, 221–230 [DOI] [PubMed] [Google Scholar]
- 29. Tagawa K., Maruyama K., Ishiura S. (1992) Amyloid-β/A4 precursor protein (APP) processing in lysosomes. Ann. N.Y. Acad. Sci. 674, 129–137 [DOI] [PubMed] [Google Scholar]
- 30. Tsujinaka T., Ebisui C., Fujita J., Morimoto T., Ogawa A., Ishidoh K., Kominami E., Yano M., Shiozaki H., Monden M. (1995) Autocatalytic inactivation of lysosomal cathepsins is associated with inhibition of protein breakdown by insulin-like growth factor-1 (IGF-1) in myotubes. Biochem. Biophys. Res. Commun. 208, 353–359 [DOI] [PubMed] [Google Scholar]
- 31. Authier F., Métioui M., Bell A. W., Mort J. S. (1999) Negative regulation of epidermal growth factor signaling by selective proteolytic mechanisms in the endosome mediated by cathepsin B. J. Biol. Chem. 274, 33723–33731 [DOI] [PubMed] [Google Scholar]
- 32. Deng A., Irizarry M. C., Nitsch R. M., Growdon J. H., Rebeck G. W. (2001) Elevation of cystatin C in susceptible neurons in Alzheimer's disease. Am. J. Pathol. 159, 1061–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Viswanathan K., Hoover D. J., Hwang J., Wisniewski M. L., Ikonne U. S., Bahr B. A., Wright D. L. (2012) Nonpeptidic lysosomal modulators derived from A-Phe-Ala-diazomethylketone for treating protein accumulation diseases. ACS Med. Chem. Lett., in press [DOI] [PMC free article] [PubMed] [Google Scholar]