Background: Intestinal commensal bacteria are not ignored by the mucosal immune system, yet the mechanisms ensuring homeostatic communication are poorly defined.
Results: Coating of commensals by SIgA mediates their targeting to Peyer's patch dendritic cells.
Conclusion: SIgA is important for the natural dynamic host-microbiota dialogue.
Significance: Beyond pathogens, immune surveillance function of SIgA applies to the control of commensal bacteria.
Keywords: Antibodies, Dendritic Cells, Intestinal Epithelium, Mouse, Mucosal Immunology, Commensal Bacteria, Immunoglobulin A, Peyer's Patch
Abstract
The mammalian gastrointestinal (GI) tract harbors a diverse population of commensal species collectively known as the microbiota, which interact continuously with the host. From very early in life, secretory IgA (SIgA) is found in association with intestinal bacteria. It is considered that this helps to ensure self-limiting growth of the microbiota and hence participates in symbiosis. However, the importance of this association in contributing to the mechanisms ensuring natural host-microorganism communication is in need of further investigation. In the present work, we examined the possible role of SIgA in the transport of commensal bacteria across the GI epithelium. Using an intestinal loop mouse model and fluorescently labeled bacteria, we found that entry of commensal bacteria in Peyer's patches (PP) via the M cell pathway was mediated by their association with SIgA. Preassociation of bacteria with nonspecific SIgA increased their dynamics of entry and restored the reduced transport observed in germ-free mice known to have a marked reduction in intestinal SIgA production. Selective SIgA-mediated targeting of bacteria is restricted to the tolerogenic CD11c+CD11b+CD8− dendritic cell subset located in the subepithelial dome region of PPs, confirming that the host is not ignorant of its resident commensals. In conclusion, our work supports the concept that SIgA-mediated monitoring of commensal bacteria targeting dendritic cells in the subepithelial dome region of PPs represents a mechanism whereby the host mucosal immune system controls the continuous dialogue between the host and commensal bacteria.
Introduction
A particular feature of the gastrointestinal (GI)2 tract is the presence of a very diverse and dense microbiota comprising as many as 1014 bacterial cells, outnumbering the amount of cells composing the human body by a factor of up to 10 (1). In addition to peacefully co-existing with the host, an equilibrium referred to as commensalism, bacteria residing in the gut exhibit numerous protective and metabolic features essential to the filter function of the epithelial barrier lining mucosal surfaces (2). In contrast to enteropathogens turning on multiple proinflammatory circuits that result in their elimination, commensal bacteria populating the GI tract are not overtly inflammatory, ensuring graded or dampened responses essential to their symbiotic survival (3–5). The absence of a virulence program and the differential perception of microbial-associated molecular patterns by epithelial cells may explain such a dichotomy in the host response (6). On the other hand, in IgA-deficient mice, systemic antibody (Ab) responses against commensal species are increased (7, 8), changes in epithelial cell protective responses occur (9), and survival of specific bacteria is promoted (10), a collection of features all arguing for a relevant function of SIgA in the sensing mechanisms of the mucosal environment.
SIgA in mucosal secretions results from the association during transport across epithelial cells from joining (J) chain-containing polymeric IgA with secretory component (SC) (11). The primary function fulfilled by SIgA is to prevent adhesion of pathogenic antigens to mucosal epithelia, a mechanism known as immune exclusion. In addition, SIgA, alone or associated with antigens, is retrotransported back into Peyer's patches (PPs) across microfold (M) cells (12) in the follicle-associated epithelium (FAE) via a specific receptor expressed at their surface (13). In the subepithelial dome (SED) region, SIgA-based immune complexes associate with dendritic cells (DCs) (14), resulting in the onset of immunomodulatory types of responses associated with quenching of proinflammatory pathways (15–17).
Seminal papers published by the team of John Cebra have demonstrated that commensal bacteria are coated with natural SIgA in the mouse GI tract, a feature instrumental to the maintenance of bacterial homeostasis (18). The use of more sophisticated animal models led to similar conclusions as to the involvement of SIgA in the numerical control of the gut microbiota (8, 19–21). Noteworthy, natural association of commensals with SIgA has also been described in humans at steady-state adult conditions (22). Strikingly, interaction between commensals and SIgA is not only antigen-driven, as natural polyreactive SIgA isolated from human colostrum and mouse hybridomas was shown to associate with commensals in a Fab/Fc-independent, glycan-mediated manner (23). Moreover, complexes made of such SIgA and commensal bacteria potentiate epithelial cell responsiveness to bacteria in vitro (24). The combination of these mechanistic data led us to speculate that SIgA may govern the mucosal sensing of commensals by facilitating communication with partners of the underlying immune system.
To tackle this open issue in vivo, we investigated in ligated ileal loops the effect of the association with SIgA on the translocation and fate of commensal bacteria into PPs of conventionally raised or germ-free mice. We could demonstrate that targeting of DCs within PPs by two main representatives of the microbiota, i.e. Firmicutes (Lactobacillus rhamnosus) (25) and Bacteroidetes (Bacteroides thetaiotaomicron) was substantially mediated by their association with SIgA. Our data underscore the importance of the Ab in the regulation of the dynamic interaction that takes place between the resident microbiota and the mucosal immune system.
EXPERIMENTAL PROCEDURES
Mice
Female BALB/c mice with a conventional microbiota were purchased from Harlan (AD Horst, The Netherlands) and housed in the animal facility of the Centre Hospitalier Universitaire Vaudois under conventional conditions prior to use at the age of 6–8 weeks. Germ-free BALB/c mice were purchased from the French Institute for Agricultural Research (Jouy-en-Josas, France), and kept in sterile incubators at the Nestlé Research Center Animal Facility until use at the age of 6–8 weeks. All experiments were performed upon approval of the State Veterinary Office.
Source of SIgA
Culture supernatants of mouse hybridoma cell clone IgAC5, specific for Shigella flexneri serotype 5a LPS, grown at 37 °C in RPMI 1640 medium complemented with 10% fetal calf serum (FCS) were used as a source of IgA (24); to reconstitute SIgA, equimolar purified polymeric IgAC5 mAb and recombinant mouse SC were combined as described (26).
Bacteria
The commensal strains L. rhamnosus (LPR, CGMCC 1.3724 and B. thetaiotaomicron DSM 2079 (Bt) were cultured as published (24). Fluorescent labeling was performed by incubating 1 × 109 bacteria in 1 ml of PBS containing 50 μg/ml FITC (Sigma-Aldrich) for 30 min at room temperature in the dark. Washed labeled bacteria were resuspended at a concentration of 4 × 108/100 μl and used freshly as such or after association with 2 μg of reconstituted SIgA for immediate administration into mouse ligated ileal loops.
Injection into a Mouse Ligated Ileal Loop
The procedure of Rey et al. was used (12) To comply with Swiss law governing animal experimentation, the investigation protocol had to be limited to 6 h. 100 μl of the solution containing 4 × 108 bacteria associated or not with SIgA was delivered into the lumen of a ligated ileal loop containing a PP. Mice were sacrificed 1, 2, 4, or 6 h later, and ligated ileal loop tissue samples were collected.
Preparation of Tissue Sections and Immunolabeling
Intestinal segments containing a PP were immediately fixed in 1 ml of PBS/4% paraformaldehyde (Fluka) for 2 h at room temperature and further processed as described (14). Frozen sections (average thickness of 7 μm) were generated on a Leica cryostat, washed in PBS, and blocked for 20 min with first 5% FCS and then with purified anti-CD16/32 mAbs (1/100; BD Biosciences) at room temperature. Various cell types in PPs were detected upon incubation in PBS-5% FCS with biotinylated anti-CD11c mAb (1/10; BD Biosciences), purified anti-CD4 rat IgG (1/50; BD Biosciences), or purified anti-CD45R rat IgG (1/50; Invitrogen), followed by Cy3- or Cy5-labeled streptavidin (1/500; both from Amersham Biosciences) or anti-rat IgG conjugated to Alexa Fluor 647 (1/100; Invitrogen). Endogenous SIgA was detected with rabbit anti-mouse SC antiserum at 1/200 dilution (26) and goat anti-rabbit IgG coupled to Alexa Fluor 647 (1/200; Invitrogen). M cells were stained with rhodamine-coupled UEA-1 (Vector Laboratories, Burlingame, CA) used at a concentration of 20 μg/ml. The DNA stain 4′,6′-diamidino-2-phenylindole (DAPI) was used at a concentration of 100 ng/ml. Sections were finally washed with PBS and mounted with anti-fading Vectashield reagent (Vector Laboratories).
Laser Scanning Confocal Microscopy (LSCM)
LSCM images were obtained using a Zeiss LSM 710 Quasar confocal microscope (Zeiss, Göttingen, Germany) in multi-track mode. Raw images were analyzed and processed on the Zen 2009 software. All of the images presented in the paper are representative of 5–10 sections prepared from at least three individual experiments. Bacterial counts in the FAE and the SED region were obtained from three-dimensional acquisitions generated from six individual sections prepared from three mice. Numbering of fluorescent bacteria on sections was blindly carried out by three trained individuals.
Flow Cytometry
Independent mice were administered 4 × 108 FITC-LPR (n = 3) or PBS as control (n = 3) into a ligated ileal loop containing a PP. Mice were sacrificed 6 h later, and the three individual PPs per condition were pooled and digested with Liberase (Roche Applied Science) in RPMI 1640 medium complemented with 2 mm CaCl2 for 30 min at 37 °C. Cells were resuspended in medium containing 2% FCS and 2 mm EDTA prior to labeling with mAbs (all from BD Biosciences) including anti-mouse CD45-PE-Cy7 (1/500 dilution), anti-mouse CD8-Pacific blue (1/200 dilution), anti-mouse CD11c-APC (1/100 dilution), anti-mouse MHC class II-phycoerythrin (1/200 dilution), anti-mouse F4/80-biotinylated (1/100 dilution, Invitrogen), anti-mouse CD11b-biotinylated (1/100 dilution); the last two were followed by strepavidin-PerCP-Cy5.5. Labeled cells were analyzed on a LSR II flow cytometer (BD Biosciences), and data were processed with FlowJo software (BD Biosciences).
Statistics
Statistical analysis was performed using Prism software version 5.0 (GraphPad, La Jolla, CA). Data were analyzed with the Mann-Whitney test.
RESULTS
Preassociation of LPR with SIgA mAb Impacts on the Dynamics of Bacterial Entry into PPs and Subsequent Uptake by Underlying DCs
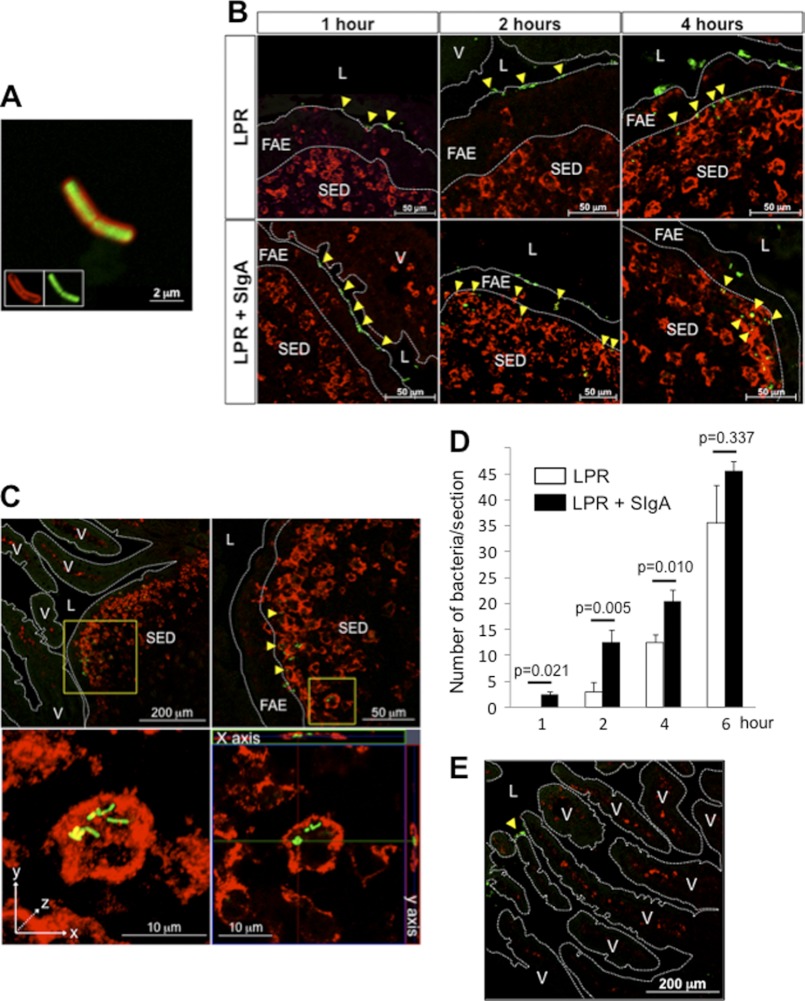
LPR-SIgA complexes were generated by co-incubation of FITC-LPR with purified SIgA molecules. After washing of the excess of free SIgA, the formation of FITC-LPR-SIgA complexes was visualized by LSCM via detection of coating SIgA with anti-mouse SC antiserum and secondary Cy3-labeled Ab (Fig. 1A). The importance of SIgA in driving in vivo bacterial sampling by PPs was evaluated by administering bacteria alone or in the form of complexes with SIgA into a mouse ligated ileal loop. Visualization of bacterial entry was performed by LSCM on tissue sections harvested at time points ranging from 1 to 6 h after administration of FITC-LPR. Despite the existing abundant microbiota in conventional mice, bacterial adsorption on the surface of the epithelium was detected at 1 h for either the bacteria given alone or in complex with SIgA (Fig. 1B). Two h after administration, FITC-LPR-SIgA complexes were observed in the FAE layer, whereas the distribution of bacteria delivered alone remained mostly limited to its surface, as observed for the 1-h time point (Fig. 1B). Four h after administration, bacteria delivered as SIgA-based complexes accumulated in close association with DCs present in the SED region (yellow spots), in contrast to FITC-LPR alone that were mostly observed at the interface between the FAE and the SED region. At 6 h, bacteria given alone (data not shown) or in complex with SIgA (Fig. 1C) located at the surface level and within the cytoplasm of DCs, as demonstrated by magnification of the SED region (top right) and through three-dimensional image reconstruction (bottom left). An orthogonal projection of the three-dimensional acquisition along the x- and y-axes further confirmed the presence of bacteria beneath the cell plasma membrane and hence their unambiguous intracellular localization (bottom right).
FIGURE 1.
Outcome of FITC-LPR and FITC-LPR-SIgA complexes in vivo after administration in a ligated ileal loop comprising a PP. A, visualization by LSCM of the complex formed between FITC-LPR (green) and SIgA (red) after 1 h of in vitro incubation. Insets show the fluorescence emanating from each independent channel. B, representative pictures of the tracking of FITC-LPR, administered alone or in complex with SIgA in a ligated ileal loop, after 1, 2, and 4 h of incubation. DCs are shown in red. LPR is associated with the epithelial surface, the FAE, and in the SED region (yellow arrowheads). C, detection of FITC-LPR-SIgA 6 h after intra-loop administration. Magnifications of the SED region demonstrate surface (yellow) and internal (green) co-localization with red-labeled DCs. Three-dimensional reconstructed image is on the bottom left, and orthogonal projections of the same image along the x- and y-axes are on the bottom right. D, quantification of FITC-LPR in the FAE and SED region of six individual sections obtained from three independent experiments. Numbers are medians ± S.E. (error bars). E, presence of FITC-LPR administered as FITC-LPR-SIgA complexes in the ligated ileal loop restricted to the lumen in areas devoid of PPs. Intraepithelial DCs are visualized in red. V, villus; L, lumen.
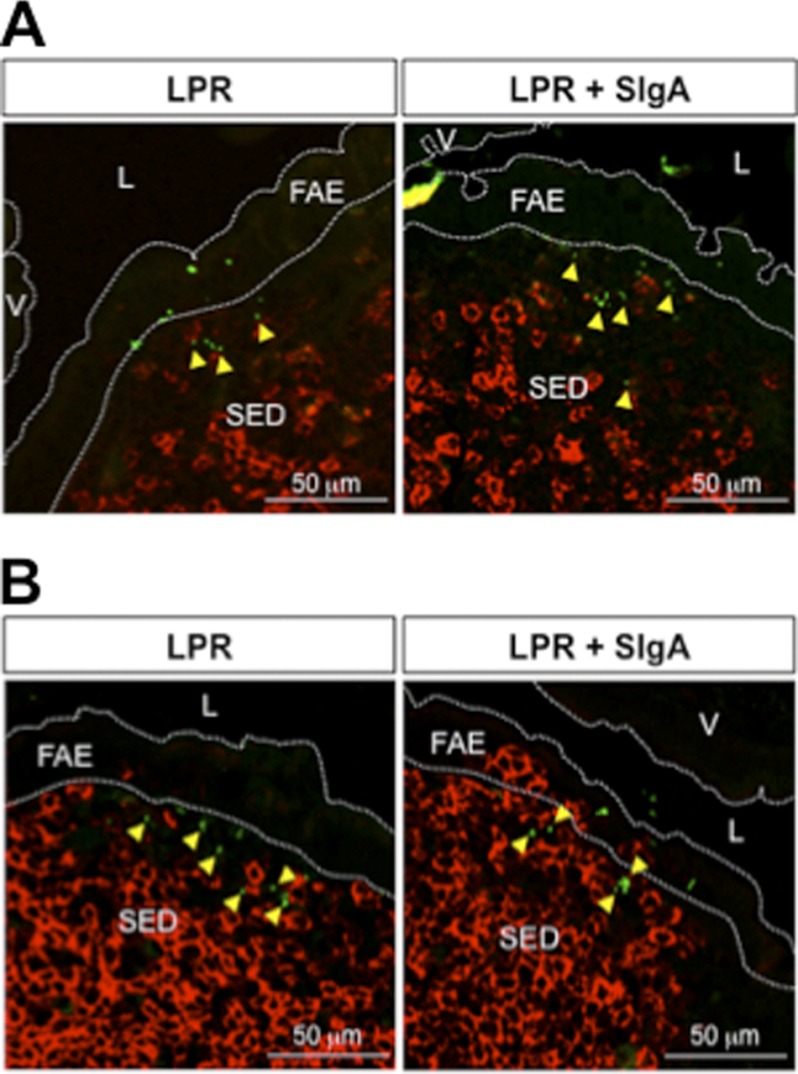
The contribution of SIgA in the entry process was further stressed upon enumeration of FITC-LPR in the FAE and SED regions at short time points (Fig. 1D). Compared with bacteria administered alone, significantly more bacteria were counted at early time points when delivered in complex with SigA. Interestingly, after 6 h, the number of FITC-LPR present in the PPs reached a similar value (approximately 40 bacteria/section) in both conditions, despite the fact that 108 bacteria had been administered, arguing for tightly regulated entry. Moreover, translocation of bacteria appeared to be limited to the PPs; sections devoid of such a structure displayed no green staining within the neighboring lamina propria and villi (Fig. 1E), although occasional FITC-LPR were observed on the luminal surface. Consistent with our previous observations that only DCs in the SED region are capable of internalizing exogenously delivered SIgA molecules (12), we were unable to detect any co-localization after staining of sections with antibodies directed against B220 (B cells) and CD4 (T cells), yet bacteria had reached the SED dome region (Fig. 2, A and B). The different dynamics of entry identified via the experimental setting used offers a first clue to ascribe to SIgA a function in promoting the transport of commensal bacteria into PPs and further implies DCs in the SED region as the privileged target cell type for selective capture.
FIGURE 2.
T and B cells do not co-localize with LPR 4 h after administration in a ligated ileal loop containing a PP. LSCM imaging demonstrates no interaction between free or FITC-LPR-SIgA (green) and red CD4+ T cells (A) or red B220+ B cells (B). Yellow arrows indicate the bacteria located outside the labeled cells. V, villus; L, lumen.
LPR Given Alone Are Coated with Endogenous SIgA before Their Internalization by PPs
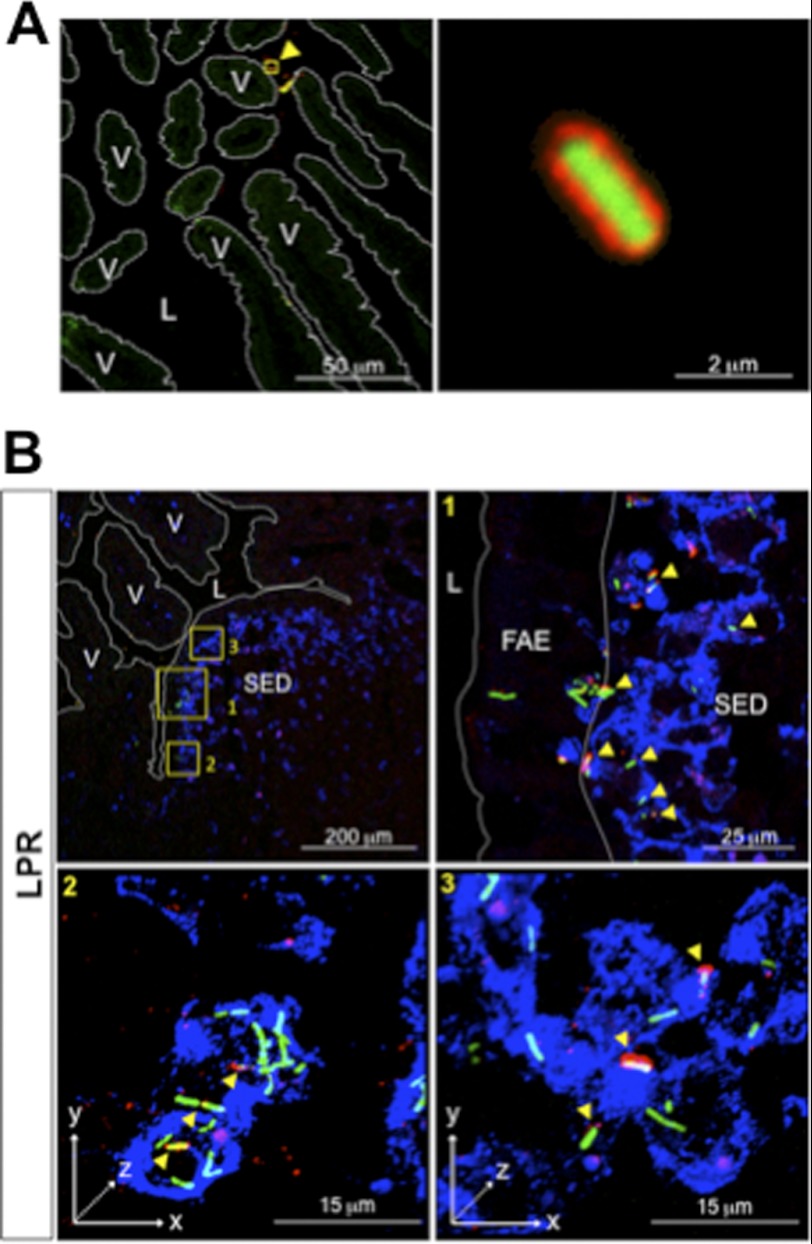
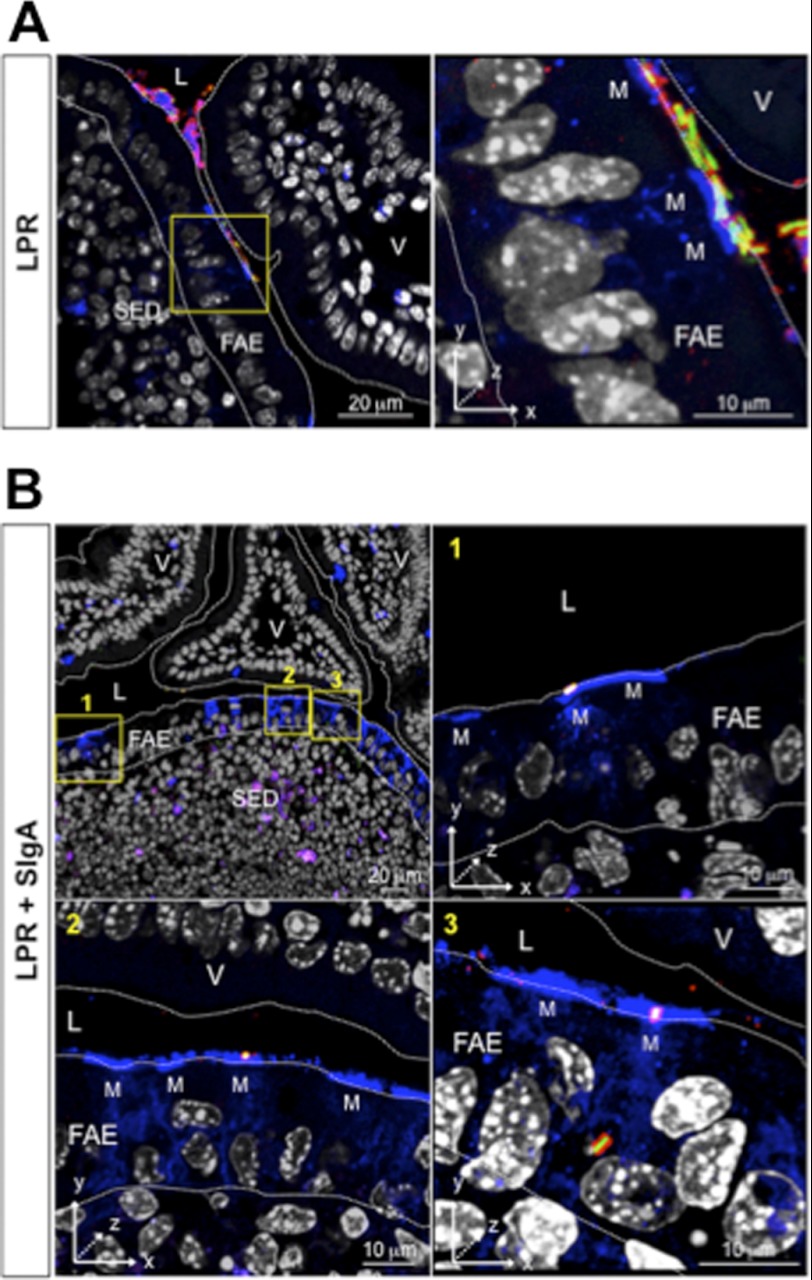
The fact that FITC-LPR administered as such are internalized by PPs as well, although with some delay compared with FITC-LPR-SIgA complexes, led us to speculate that coating by endogenous SIgA may occur under steadily normal conditions and hence participate in limited entry of bacteria. In an attempt to track endogenous IgA in complexes with bacteria, we faced the technical limitation that massive hiding of epitopes on α and κ chains prevented reliable detection with Abs specific for these two polypeptides (data not shown and Ref. 26). In contrast, we established that recognition of bound SC by a specific antiserum (26) remained efficient; this approach further presented the advantage to discriminate between SIgA in luminal secretions from IgA abundantly found in the lamina propria. Following administration of FITC-LPR alone into a ligated ileal loop containing a PP, bound endogenous SIgA was observed by LSCM on sections as soon as 1 h after bacterial delivery (Fig. 3A). Remarkably, despite the high quantity of LPR administered, a significant proportion of bacteria were found coated with SIgA, underlining the presence of sufficient amounts of endogenous SIgA in intestinal secretions to coat the exogenously delivered bacteria.
FIGURE 3.
Endogenous in vivo coating of LPR by intestinal SIgA and subsequent entry into a PP. A, visualization by LSCM of the complex formed in vivo between FITC-LPR and endogenous SIgA 1 h after administration in a ligated ileal loop containing a PP. Right, a ×25 magnification focused on one bacterium. B, representative pictures of the tracking of FITC-LPR 6 h after administration into a ligated ileal loop. Magnification of areas in the first image (yellow squares) shows the heterogeneous state of SIgA coating (arrowheads in panels 1, 2, and 3). Endogenous SIgA are stained in red and DCs in blue. Three-dimensional reconstructed pictures (panels 2 and 3) identify immune complexes on the surface of DCs (white spots) and within DCs (yellow spots). FITC-LPR without SIgA are observed within (green) and at the surface of DCs (turquoise). V, villus; L, lumen.
We then sought to determine whether translocation into the SED region of bacteria injected alone was linked with coating with SIgA. At 6 h after intraluminal administration, most of internalized SIgA detected with anti-SC antiserum co-localized with FITC-LPR in the FAE (Fig. 3B, panel 1), as well as in close contact with the surface of, and within, DCs (Fig. 3B, panels 2 and 3). Noteworthy, various populations of bacteria, i.e. fully, partially, or even not coated with endogenous SigA, may reflect a dynamic removal of the Ab timewise, a phenomenon possibly related to bacterial interaction with DCs, as pinpointed by the appearance of either surface-bound turquoise/white staining and intracellular green rods.
Reduced Natural Entry of LPR into PPs of Germ-free Mice Is Restored by Pre-formation of Complexes with SIgA
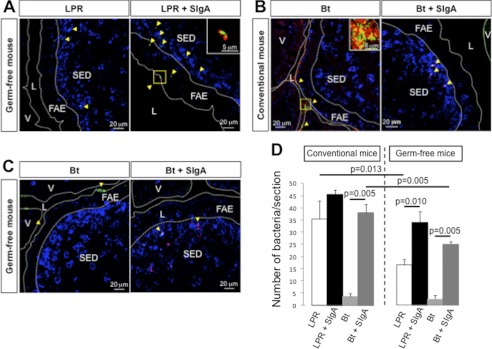
To confirm further the contribution of SIgA to the transport of FITC-LPR into PPs, the same experiments were performed in germ-free mice, known to have much reduced number of IgA secreting cells in the lamina propria (27). In this model, entry of bacteria administered alone into ligated ileal loops was significantly decreased at 6 h compared with the picture obtained at the same time point in conventional mice (Fig. 4, A and D). Noteworthy, low numbers of translocated bacteria confirmed that the integrity of the epithelial barrier was preserved under these germ-free conditions and that entry remained selective. However, when administered in pre-formed complexes with SIgA, entry of FITC-LPR into PPs was restored to levels resembling those determined in conventional mice at 6 h, further emphasizing the role of SIgA in supporting translocation (Fig. 4, A and D). Moreover, although germ-free mice have underdeveloped PPs, the overall bacterial translocation was not substantially different from that assessed in conventional animals, arguing for functional M cells involved in the process.
FIGURE 4.
Entry of LPR and Bt is diminished in germ-free mice and is restored after association with SigA. A, tracking by LSCM of FITC-LPR or FITC-LPR-SIgA after 6 h of incubation in a ligated ileal loop of germ-free mice. Inset, magnification of FITC-LPR-SIgA complex. B, tracking of FITC-Bt or FITC-Bt-SIgA after 6 h of incubation in a ligated ileal loop of conventional mice. Inset, magnification of FITC-Bt-SIgA complex. C, visualization of FITC-Bt administered alone or in pre-formed complex with SIgA 6 h after administration into a ligated ileal loop of germ-free mice. On all panels, bacteria are visualized in green, SIgA in red, and DCs in blue. D, quantification of FITC-bacteria in the FAE and SED region of six individual sections obtained from three independent experiments. Numbers are medians ± S.E. (error bars). V, villus; L, lumen.
Entry of the Gram-negative Bt Commensal Bacterium Requires Association with SIgA as Well
The concept of SIgA-promoted entry of Gram-positive commensal bacteria prompted us to examine whether the prototype Gram-negative commensal bacterium Bt, a prominent member of the normal microbiota of mice and humans, harbors the same characteristics with respect to SIgA-mediated transepithelial transport. We found that although it gets similarly co-localized with endogenous SIgA 6 h after administration in the lumen of a ligated ileal loop (Fig. 4B, inset), FITC-Bt given alone barely entered the FAE and was recovered in very low amount in the SED region (Fig. 4, B and D) at this time point. This shows that under steadily normal conditions, during the permitted experimental time frame, FITC-Bt displays a lower rate of entry compared with LPR, suggesting a possible delay in the process of association with endogenous SIgA. As a matter of fact, when administered as pre-formed SIgA-based complexes, Bt was detected within and on the surface of DCs in the SED region as for LPR (Fig. 4B). The important role of SIgA in governing the passage of the bacterium was again exemplified in germ-free mice as shown by targeting of DCs in the SED region occurring almost exclusively with pre-formed SIgA-bacteria complexes (Fig. 4, C and D). Collectively, data in Figs. 3 and 4 indicate that uptake of SIgA-based complexes in the gut represents a mechanism by which the members of microbiota are sensed by the local immune system via selective targeting of DCs located in the SED region.
Entry of LPR-SIgA Complexes Occurs via M Cells
The controlled entry of SIgA-based complexes at the level of FAE in PPs, together with the knowledge that retrotransport of SIgA is limited to M cells, prompted us to investigate the direct involvement of this cell type in the process of bacterial translocation. LSCM imaging of sections prepared 2 h after administration of FITC-LPR or as FITC-LPR-SIgA complexes showed the presence of bacteria co-localizing with Ulex europaeus agglutinin-1 (UEA-1)-stained M cells. FITC-LPR delivered alone were detected in the form of SIgA-based complexes on the surface of M cells, as well as in close association with neighboring columnar cells in the FAE (Fig. 5A). In agreement with previous data (Fig. 1) showing promotion of passage for pre-formed LPR-SIgA complexes, these latter were detected not only in close interaction with the luminal surface of M cells (Fig. 5B, panels 1 and 2), but also in the cell pocket (Fig. 5, panel 3). The observation of intact bacterium-SIgA complexes within M cells is consistent with the property of these latter to deliver unprocessed antigens to the SED region (28).
FIGURE 5.
Interaction of LPR alone and in complex with SIgA with M cells. A, LSCM image acquired 2 h after administration of FITC-LPR alone in a ligated ileal loop containing a PP. Magnification (×10) of an area rich in M cells demonstrates the association of LPR (green)-SIgA (red) complexes (yellow) with blue-labeled M cell surface. Cell nuclei are white. B, same analysis as in A following administration of pre-formed FITC-LPR-SIgA complexes in a ligated ileal loop. Magnifications of M cell-rich areas demonstrate the presence of LPR-SIgA complexes at the surface and within M cells in three-dimensional reconstructed images (panels 1, 2, and 3). V, villus; L, lumen.
CD11c+CD11b+ DCs and Macrophages in the PP Internalize LPR in Vivo
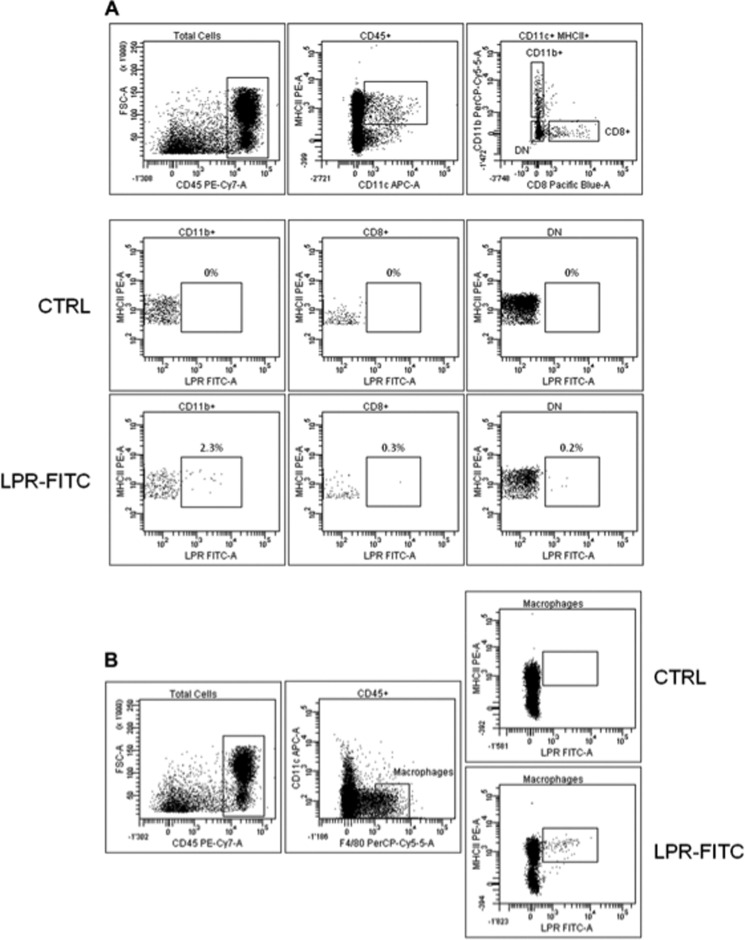
DCs in PPs exhibit three different subsets with defined localization: CD11c+CD11b+CD8− (localized in the SED region), CD11c+CD11b−CD8+ (localized in the interfollicular region), and CD11c+CD11b−CD8− (double negative, localized at both sites) (29). In agreement with previous observations that CD11c+CD11b+CD8− DCs are the exclusive target of SIgA-based complexes (14), we identified preferential interaction of FITC-LPR with this subset (2.3% of LPR-FITC+ cells), as opposed to other DCs displaying barely detectable staining compared with controls (Fig. 6A). A low percentage of internalized FITC-LPR is consistent with LSCM data and further supports the co-localization observed in the SED region with certain DCs solely. We took the opportunity of this approach to address the possible role of macrophages in the process; indeed, although the specific F4/80 mAb does not function on tissue sections, thus precluding the possibility to use it for histologic analyses, it is a valuable reagent in flow cytometry. Fig. 6B indicates that macrophages present in mouse PPs are also able to engulf FITC-LPR (1% of LPR-FITC+ cells), in a proportion that again is consistent with the weak amount of LPR observed within PPs by LSCM.
FIGURE 6.
Analysis by flow cytometry identifies CD11b+ DC subset and macrophages as cells capturing LPR in PPs. A, PP cells from three independent ligated ileal loops incubated with FITC-LPR for 6 h were recovered. Flow cytometry analysis was performed on the three CD45+CD11c+ DC subsets in PPs for both control (CTRL) and experimental settings. B, gating for F4/80+CD11c− macrophages indicates a capacity to take up FITC-LPR.
DISCUSSION
In this work, we have established the so far undefined mechanism by which SIgA is involved in the interplay between the gut-associated lymphoid tissue and bacteria from the intestinal microbiota. By using the technique of ileal loop, the fate of commensal bacteria in adult conventional mice displaying a heterogeneous community of resident microbiota and a mature immune system, or in germ-free mice, was tracked, leading to a series of novel observations including: (i) the selective passage of commensal bacteria across FAE to DCs in the SED region of PPs is related to their association with SIgA in a non-antigen-specific way, as exemplified by the fact that preassociation of bacteria with nonspecific SIgA increased their dynamics of entry and restored the reduced transport observed in germ-free mice; (ii) SIgA-based complexes between endogenous SIgA and bacteria spontaneously form in the intestinal lumen; (iii) immune complexes are detectable in the pocket of M cells at early time points, identifying these latter as the portal of entry and indicating the absence of destructive processing by these cells; (iv) targeting of SIgA-based complexes to DCs in the SED region suggests that this is in this particular form that commensal bacteria are primarily exposed to the antigen-presenting cell.
Interestingly, low quantities of bacteria were found translocated in PPs compared with the hundreds of millions administered. The importance for bacteria to be associated with SIgA to enter PPs may be an intrinsic property of the mouse intestine responsible for their low sampling rate by M cells (14, 22, 30), thus minimizing their interaction with the underlying immune system. First, the differential rates of entry of bacteria alone or in pre-formed complex with nonspecific SIgA into PPs indirectly shed light on the role of the Ab in M cell-mediated sampling; second, experiments performed in germ-free mice further revealed the role of SIgA in the translocation of commensals in the SED region. Moreover, the fact that M cells express a specific receptor for SIgA (13) definitely reinforces the hypothesis of an actively controlled process of intestinal sampling by M cells mediated through SIgA. The contribution of other sampling sites such as isolated lymphoid follicles and villous M cells, or snorkeling DCs present in the lamina propria (31, 32), cannot be excluded at this stage, although addressing them in SIgA-based sampling will be a challenging task in terms of localization and appropriate time of incubation.
Following passage across the FAE, specific targeting of CD11c+CD11b+ DCs in the SED region is fully consistent with the moderate local cellular and humoral responses reported for SIgA working as a carrier for antigens (16). Indeed, this subset produces high levels of IL-10 and induces the differentiation of IL-4/IL-10/TGF-β-producing T cells (33) prone to favor oral tolerance and class switch to IgA secretion (34). The same subset has been involved in cross-tolerance against intestinal antigens (35). The presence of specialized DCs in the SED region that encounter microbiota in a SIgA-mediated manner may be essential to the development of commensal-specific peripheral Treg cells in the gut (36–38) rather than pathogenic effectors. Interestingly, PP macrophages not recognized by SIgA (12) can however engulf LPR; this suggests that SIgA is not a partner of macrophage phagocytic activity and that those cells may do much more than killing translocated commensals in the mesenteric lymph nodes (21). This will represent a topic for further investigation in the light of a recent paper showing that such cells are inflammation adverse (39).
Provision of local SIgA appears to monitor the microbiota in some sort of a self-regulatory loop that would have for ultimate consequence to finely balance the host reaction to its resident microbes and to ensure appropriate mucosal gut homeostasis (40). This appears to be particularly true in the light of the observation that bacteria delivered as such are coated within 1 h with endogenous SIgA. The involvement of both commensal-specific and natural IgA antibodies in maintaining intestinal homeostasis has been reported (41–43). Indeed, as the mouse microbiota comprises Lactobacilli, it is therefore conceivable that part of the endogenous SIgA coating observed in the present study is mediated by cross-reactive Abs recognizing related epitopes on human microbiota-derived LPR. Nonetheless, coating with nonspecific polyreactive SIgA is highly likely to take place in vivo. This is supported by the fact that commensal exclusion remains completely effective in quasimonoclonal neonatal mice, in which the antibody specificity is restrained to the hapten nitrophenol (44). Furthermore, at the biochemical level, carbohydrates carried by SC in SIgA molecules have been shown to account for the Fab-independent association of these latter with a series of commensal species (24). However, whether the manner SIgA associates with bacteria would have an effect on their fate in PPs will have to wait for commensal-specific SIgA reagents.
Unexpectedly, the dynamics of entry of LPR was higher than Bt in conventional mice during the short term protocol, as reflected by the higher number of LPR bacteria in the SED region 6 h after injection in the intestinal loop. Whether this relies on a differential capacity to bind endogenous SIgA in this particular experimental setting, or constitutes effective distinct properties between these two micro-organisms deserves further investigation. Nevertheless, it is worth mentioning that preassociation with exogenous SIgA yielded equivalent results.
As final considerations, it is worthwhile to highlight that experiments performed in germ-free mice may somehow reflect what happens in the underdeveloped epithelium of the newborn; intestinal coating by exogenous SIgA Abs originating from maternal milk during this period may combine the properties of neutralizing newly colonizing bacteria and educate the developing mucosal immune system not to overreact against what will become a symbiont. In support of this, polyreactive SIgA purified from human colostrum have been recently shown to interact strongly with microorganisms isolated from the human GI tract (24). Consequently, the recognition of the perpetual interaction among SIgA, commensals, and the mucosal immune system may find an echo in the correct interpretation of results emanating from germ-free animal models. Indeed, such models only partially reflect the “actual” situation in the GI tract because of the very low endogenous production of SIgA found in those animals, thus impacting on bacterial sampling as deciphered in the present study.
In conclusion, the sum of our data demonstrates that SIgA, in addition to leading to immune exclusion, is involved in the mechanism by which the gut-associated lymphoid tissue permanently checks the commensal content of the intestine. Specific targeting of DCs in the SED region of PPs via the M cell pathway in conventional mice as shown here suggests that SIgA bound to bacteria may indeed serve as the “driving force” to ensure the permanent communication between the content of the gut lumen and components of the neighboring mucosal immune system. The overall goal would be to keep the microbiota confined within the gut lumen, while rare controlled translocation of coated commensal bacteria by natural and/or specific SIgA would thus help the host to trigger an optimal activation of the mucosal immune response (14, 15).
Acknowledgments
Germ-free mice and commensal bacterial strains were kindly provided by the Nestlé Research Center.
This work was supported by Swiss Science Research Foundation Grant 3100-138422 (to B. C.).
- GI
- gastrointestinal
- Bt
- Bacteroides thetaiotaomicron
- DC
- dendritic cell
- FAE
- follicle-associated epithelium
- LPR
- Lactobacillus rhamnosus
- LSCM
- laser scanning confocal microscopy
- M cell
- microfold cell
- PP
- Peyer's patch
- SC
- secretory component
- SED
- subepithelial dome
- SIgA
- secretory IgA.
REFERENCES
- 1. Savage D. C. (1977) Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 31, 107–133 [DOI] [PubMed] [Google Scholar]
- 2. O'Hara A. M., Shanahan F. (2006) The gut flora as a forgotten organ. EMBO Rep. 7, 688–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neish A. S. (2009) Microbes in gastrointestinal health and disease. Gastroenterology 136, 65–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hooper L. V., Macpherson A. J. (2010) Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 10, 159–169 [DOI] [PubMed] [Google Scholar]
- 5. Cerf-Bensussan N., Gaboriau-Routhiau V. (2010) The immune system and the gut microbiota: friends or foes? Nat. Rev. Immunol. 10, 735–744 [DOI] [PubMed] [Google Scholar]
- 6. Sansonetti P. J. (2011) To be or not to be a pathogen: that is the mucosally relevant question. Mucosal Immunol. 4, 8–14 [DOI] [PubMed] [Google Scholar]
- 7. Sait L. C., Galic M., Price J. D., Simpfendorfer K. R., Diavatopoulos D. A., Uren T. K., Janssen P. H., Wijburg O. L., Strugnell R. A. (2007) Secretory antibodies reduce systemic antibody responses against the gastrointestinal commensal flora. Int. Immunol. 19, 257–265 [DOI] [PubMed] [Google Scholar]
- 8. Peterson D. A., McNulty N. P., Guruge J. L., Gordon J. I. (2007) IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe 2, 328–339 [DOI] [PubMed] [Google Scholar]
- 9. Shulzhenko N., Morgun A., Hsiao W., Battle M., Yao M., Gavrilova O., Orandle M., Mayer L., Macpherson A. J., McCoy K. D., Fraser-Liggett C., Matzinger P. (2011) Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat. Med. 17, 1585–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Obata T., Goto Y., Kunisawa J., Sato S., Sakamoto M., Setoyama H., Matsuki T., Nonaka K., Shibata N., Gohda M., Kagiyama Y., Nochi T., Yuki Y., Fukuyama Y., Mukai A., Shinzaki S., Fujihashi K., Sasakawa C., Iijima H., Goto M., Umesaki Y., Benno Y., Kiyono H. (2010) Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc. Natl. Acad. Sci. U.S.A. 107, 7419–7424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaetzel C. S. (2005) The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 206, 83–99 [DOI] [PubMed] [Google Scholar]
- 12. Rey J., Garin N., Spertini F., Corthésy B. (2004) Targeting of secretory IgA to Peyer's patch dendritic and T cells after transport by intestinal M cells. J. Immunol. 172, 3026–3033 [DOI] [PubMed] [Google Scholar]
- 13. Mantis N. J., Cheung M. C., Chintalacharuvu K. R., Rey J., Corthésy B., Neutra M. R. (2002) Selective adherence of IgA to murine Peyer's patch M cells: evidence for a novel IgA receptor. J. Immunol. 169, 1844–1851 [DOI] [PubMed] [Google Scholar]
- 14. Kadaoui K. A., Corthésy B. (2007) Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer's patches with restriction to mucosal compartment. J. Immunol. 179, 7751–7757 [DOI] [PubMed] [Google Scholar]
- 15. Favre L., Spertini F., Corthésy B. (2005) Secretory IgA possesses intrinsic modulatory properties stimulating mucosal and systemic immune responses. J. Immunol. 175, 2793–2800 [DOI] [PubMed] [Google Scholar]
- 16. Corthésy B. (2007) Roundtrip ticket for secretory IgA: role in mucosal homeostasis? J. Immunol. 178, 27–32 [DOI] [PubMed] [Google Scholar]
- 17. Mantis N. J., Rol N., Corthésy B. (2011) Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 4, 603–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cebra J. J., Bos N. A., Cebra E. R., Cuff C. F., Deenen G. J., Kroese F. G., Shroff K. E. (1994) Development of components of the mucosal immune system in SCID recipient mice. Adv. Exp. Med. Biol. 355, 255–259 [DOI] [PubMed] [Google Scholar]
- 19. Suzuki K., Meek B., Doi Y., Muramatsu M., Chiba T., Honjo T., Fagarasan S. (2004) Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc. Natl. Acad. Sci. U.S.A. 101, 1981–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Macpherson A. J., Slack E., Geuking M. B., McCoy K. D. (2009) The mucosal firewalls against commensal intestinal microbes. Semin. Immunopathol. 31, 145–149 [DOI] [PubMed] [Google Scholar]
- 21. Macpherson A. J., Uhr T. (2004) Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303, 1662–1665 [DOI] [PubMed] [Google Scholar]
- 22. van der Waaij L. A., Limburg P. C., Mesander G., van der Waaij D. (1996) In vivo IgA coating of anaerobic bacteria in human faeces. Gut 38, 348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mathias A., Duc M., Favre L., Benyacoub J., Blum S., Corthésy B. (2010) Potentiation of polarized intestinal Caco-2 cell responsiveness to probiotics complexed with secretory IgA. J. Biol. Chem. 285, 33906–33913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mathias A., Corthésy B. (2011) Recognition of Gram-positive intestinal bacteria by hybridoma- and colostrum-derived secretory immunoglobulin A is mediated by carbohydrates. J. Biol. Chem. 286, 17239–17247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hooper L. V., Gordon J. I. (2001) Commensal host-bacterial relationships in the gut. Science 292, 1115–1118 [DOI] [PubMed] [Google Scholar]
- 26. Duc M., Johansen F. E., Corthésy B. (2010) Antigen binding to secretory immunoglobulin A results in decreased sensitivity to intestinal proteases and increased binding to cellular Fc receptors. J. Biol. Chem. 285, 953–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cebra J. J., Periwal S. B., Lee G., Lee F., Shroff K. E. (1998) Development and maintenance of the gut-associated lymphoid tissue (GALT): the roles of enteric bacteria and viruses. Dev. Immunol. 6, 13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neutra M. R., Frey A., Kraehenbuhl J. P. (1996) Epithelial M cells: gateways for mucosal infection and immunization. Cell 86, 345–348 [DOI] [PubMed] [Google Scholar]
- 29. Iwasaki A., Kelsall B. L. (2000) Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3α, MIP-3β, and secondary lymphoid organ chemokine. J. Exp. Med. 191, 1381–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pron B., Boumaila C., Jaubert F., Berche P., Milon G., Geissmann F., Gaillard J. L. (2001) Dendritic cells are early cellular targets of Listeria monocytogenes after intestinal delivery and are involved in bacterial spread in the host. Cell. Microbiol. 3, 331–340 [DOI] [PubMed] [Google Scholar]
- 31. Rescigno M., Urbano M., Valzasina B., Francolini M., Rotta G., Bonasio R., Granucci F., Kraehenbuhl J. P., Ricciardi-Castagnoli P. (2001) Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2, 361–367 [DOI] [PubMed] [Google Scholar]
- 32. Niess J. H., Brand S., Gu X., Landsman L., Jung S., McCormick B. A., Vyas J. M., Boes M., Ploegh H. L., Fox J. G., Littman D. R., Reinecker H. C. (2005) CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258 [DOI] [PubMed] [Google Scholar]
- 33. Iwasaki A., Kelsall B. L. (2001) Unique functions of CD11b+, CD8α+, and double-negative Peyer's patch dendritic cells. J. Immunol. 166, 4884–4890 [DOI] [PubMed] [Google Scholar]
- 34. Cerutti A., Rescigno M. (2008) The biology of intestinal immunoglobulin A responses. Immunity 28, 740–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chung Y., Chang J. H., Kweon M. N., Rennert P. D., Kang C. Y. (2005) CD8α-11b+ dendritic cells but not CD8α+ dendritic cells mediate cross-tolerance toward intestinal antigens. Blood 106, 201–206 [DOI] [PubMed] [Google Scholar]
- 36. Cong Y., Feng T., Fujihashi K., Schoeb T. R., Elson C. O. (2009) A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc. Natl. Acad. Sci. U.S.A. 106, 19256–19261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Round J. L., Mazmanian S. K. (2010) Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. U.S.A. 107, 12204–12209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lathrop S. K., Bloom S. M., Rao S. M., Nutsch K., Lio C. W., Santacruz N., Peterson D. A., Stappenbeck T. S., Hsieh C. S. (2011) Peripheral education of the immune system by colonic commensal microbiota. Nature 478, 250–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith P. D., Smythies L. E., Shen R., Greenwell-Wild T., Gliozzi M., Wahl S. M. (2011) Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 4, 31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Willing B. P., Gill N., Finlay B. B. (2010) The role of the immune system in regulating the microbiota. Gut Microbes 1, 213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bos N. A., Bun J. C., Popma S. H., Cebra E. R., Deenen G. J., van der Cammen M. J., Kroese F. G., Cebra J. J. (1996) Monoclonal immunoglobulin A derived from peritoneal B cells is encoded by both germ line and somatically mutated VH genes and is reactive with commensal bacteria. Infect. Immun. 64, 616–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jiang H. Q., Thurnheer M. C., Zuercher A. W., Boiko N. V., Bos N. A., Cebra J. J. (2004) Interactions of commensal gut microbes with subsets of B- and T-cells in the murine host. Vaccine 22, 805–811 [DOI] [PubMed] [Google Scholar]
- 43. Hapfelmeier S., Lawson M. A., Slack E., Kirundi J. K., Stoel M., Heikenwalder M., Cahenzli J., Velykoredko Y., Balmer M. L., Endt K., Geuking M. B., Curtiss R., 3rd, McCoy K. D., Macpherson A. J. (2010) Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 328, 1705–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harris N. L., Spoerri I., Schopfer J. F., Nembrini C., Merky P., Massacand J., Urban J. F., Jr., Lamarre A., Burki K., Odermatt B., Zinkernagel R. M., Macpherson A. J. (2006) Mechanisms of neonatal mucosal antibody protection. J. Immunol. 177, 6256–6262 [DOI] [PubMed] [Google Scholar]