Background: Sialyl-Lewis X (sLeX) is a fucosylated oligosaccharide that plays critical roles in cell adhesion.
Results: 5-thiofucose is metabolized by cells to produce a new nucleotide sugar that impairs sLeX biosynthesis and cell adhesiveness.
Conclusion: 5-thiofucose blocks fucose transfer within treated cells.
Significance: 5-thiofucose should be useful in further elucidating the biological roles of sLeX.
Keywords: Carbohydrate Metabolism, Carbohydrate Processing, Glycoconjugate, Glycoprotein Biosynthesis, Glycosylation, Glycosylation Inhibitors, Glycosyltransferases, Cell Surface Glycosylation, Fucosyltransferase, Sialyl-Lewis X
Abstract
Sialyl-Lewis X (sLeX) is a tetrasaccharide that serves as a ligand for the set of cell adhesion proteins known as selectins. This interaction enables adhesion of leukocytes and cancer cells to endothelial cells within capillaries, resulting in their extravasation into tissues. The last step in sLeX biosynthesis is the α1,3-fucosyltrasferase (FUT)-catalyzed transfer of an L-fucose residue to carbohydrate acceptors. Impairing FUT activity compromises leukocyte homing to sites of inflammation and renders cancer cells less malignant. Inhibition of FUTs is, consequently, of great interest, but efforts to generate glycosyltransferase inhibitors, including FUT inhibitors, has proven challenging. Here we describe a metabolic engineering strategy to inhibit the biosynthesis of sLeX in cancer cells using peracetylated 5-thio-L-fucose (5T-Fuc). We show that 5T-Fuc is taken up by cancer cells and then converted into a sugar nucleotide analog, GDP-5T-Fuc, that blocks FUT activity and limits sLeX presentation on HepG2 cells with an EC50 in the low micromolar range. GDP-5T-Fuc itself does not get transferred by either FUT3 or FUT7 at a measurable rate. We further demonstrate that treatment of cells with 5T-Fuc impaired their adhesive properties to immobilized adhesion molecules and human endothelial cells. 5T-Fuc, therefore, is a useful probe that can be used to modulate sLeX levels in cells to evaluate the consequences of inhibiting FUT-mediated sLeX formation. These data also reveal the utility of using sugar analogues that lead to formation of donor substrate analogues within cells as a general approach to blocking glycosyltransferases in cells.
Introduction
Variations in the levels and identities of cell-surface glycans serve both to communicate the internal state of cells to adjacent tissues and to confer distinctive biochemical properties to cells. One carbohydrate structure that has received considerable attention because it was discovered as a ligand mediating cell-cell adhesion during inflammation is sialyl-Lewis X (sLeX)3 (1, 2). sLeX is a tetrasaccharide comprised of Neu5Ac(α2–3)Gal(β1–4)[Fuc(α1–3)]GlcNAc (Fig. 1A) and is found appended to both the N- and O-linked glycans of cell surface proteins as well as on glycolipids. Both terminal sugars of sLeX, neuraminic acid (Neu5Ac) and fucose (Fuc), are essential features recognized by cell adhesion molecules, particularly those belonging to the selectin family (3, 4). In this capacity, sLeX enables homing of leukocytes to sites of inflammation (5). More recently, sLeX-containing glycans have been shown to promote selectin-mediated cell adhesion and thereby mediate tumor metastasis. Indeed, many types of carcinomas (6–8) display increased levels of sLeX, and, within tumors, sLeX levels often correlate with malignancy and patient survival rates (9–11). This correlation presumably exists because to invade tissues, metastasizing cells must first adhere to and subsequently penetrate through the vascular endothelial layer.
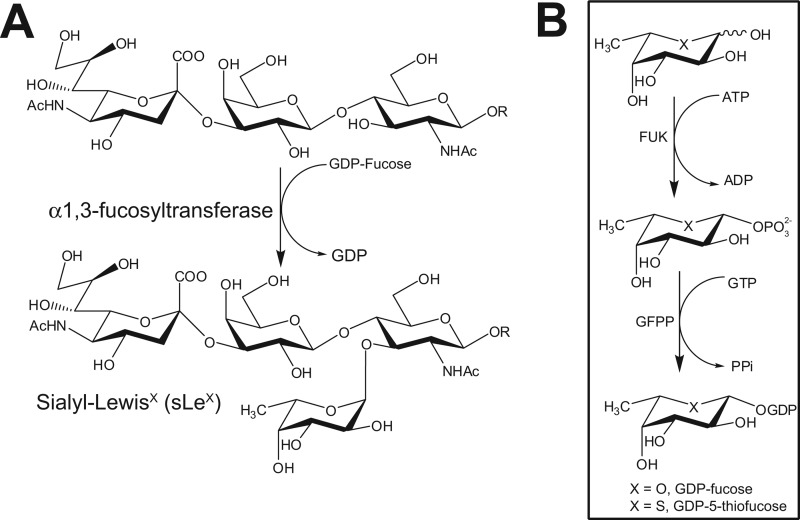
FIGURE 1.
The biosynthesis of sLeX and GDP-Fuc. A, the last and usually rate-limiting step in sLeX biosynthesis is the transfer of a Fuc residue onto a sialylated type 2 glycan precursor by FUTs. B, GDP-Fuc (X = O), the donor sugar for FUTs, is biosynthesized by cells de novo from GDP-mannose in a process requiring the sequential action of GDP-mannose 4,6-dehydratase (GMD) and GDP-keto-6-deoxymannose 3,5-epimerase (GDE). Alternatively, a salvage pathway converts free cytosolic fucose into GDP-Fuc upon its phorphorylation by fucose kinase (FUK) followed by the reversible condensation of fucose-1-phosphate with GTP by GDP-Fuc pyrophosphorylase (GFPP). The first steps of both pathways are subjected to feedback inhibition by GDP-Fuc. We hypothesize that 5T-Fuc (X = S) will be activated as GDP-5T-Fuc by the fucose salvage pathway and, if it is a poor substrate for FUTs, such as those that make sLeX, it may lead to the feedback inhibition of GDP-Fuc.
Because sLeX has been shown to play important roles in both inflammation and cancer, there has been considerable effort dedicated to validating approaches to disrupting selectin-sLeX interactions. One strategy that has been pursued is to generate sLeX mimics as antagonists of the selectins (12). Another approach has been to disrupt the biosynthesis of sLeX by using compounds that block early steps in the biosynthesis of sLeX-containing glycans (13). A promising alternative toward disrupting sLeX biosynthesis involves diverting the efforts of relevant glycosyltransferases by using acceptor decoys (14, 15). Finally, metabolic engineering of cells using modified sugar analogues that get incorporated into the growing structure and block completion of the biosynthesis of sLeX have also shown promise (16, 17). Such approaches to perturbing glycan structure at early stages or diverting glycosyltransferase activity, however, can significantly alter the structures of glycans produced by cells (16) and, in some cases, induce other metabolic side effects (17).
An alternate approach gaining current attention (18) is to use small molecules to inhibit the last step in the biosynthesis of sLeX, which is the transfer of fucose by one of five functionally related α1,3-fucosyltransferases (FUTs) to sialylated N-acetyl-lactosamine-containing glycans (Fig. 1A) (19). Because Fuc is generally present as a terminal sugar residue within glycans, including sLeX, these α1,3-specific FUTs are of interest because their blockade may be less generally disruptive than targeting early biosynthetic steps. Small molecule inhibitors would be useful probes to propel our understanding of the functional role of FUTs in diverse biological processes including, for example, fertilization (20) and cell surface receptor signaling (21). Several lines of evidence also support α1,3-specific FUTs as promising targets for therapeutic intervention. Notable in this regard is that genetic deficiencies in FUT activity lead to an inability to mount a robust inflammatory response (22). Furthermore, sLeX overexpressing tumors often exhibit increased transcription of α1,3-FUTs (23–25), and the increased expression of FUTs is linked to the metastatic ability of cancer cells (26, 27). Consistent with α1,3-FUTs playing an important role in cancer, antisense-mediated decreases in FUTs, including FUT3, impair tumor growth (28) and limit metastasis (29).
The potential utility of small molecule FUT inhibitors as research tools and therapeutics has stimulated identification of inhibitors that act in vitro. However, none have been shown to act in cells. FUTs, including FUT3 and FUT7 use a high-energy nucleotide sugar substrate, GDP-fucose (GDP-Fuc), to form glycosidic bonds. Several GDP-Fuc analogues are known that inhibit these enzymes in vitro (30), but the charged nature of these compounds limits their use in cells. Recently, we found a solution to this problem for the glycosyltransferase known as O-GlcNAc transferase. We found that peracetylated 5-thio-N-acetylglucosamine was taken up by a wide variety of cells and assimilated by the GlcNAc salvage pathway to form the nucleotide thiosugar analog of UDP-GlcNAc, which acted in cells to inhibit O-GlcNAc-transferase (31). This result suggested to us that a metabolic feeding approach might be more widely applicable. Consistent with this view, in vitro studies with milk α1,3-FUT showed that this enzyme transferred GDP-5-thiofucose (GDP-5T-Fuc) at a very low rate when compared with GDP-Fuc (33). On the basis of this observation and our previous findings with 5T-GlcNAc, we hypothesized that feeding cells with 5T-Fuc could lead to the formation of GDP-5T-Fuc via its activation through the Fuc salvage pathway (Fig. 1B). Intracellular GDP-5T-Fuc in turn could inhibit FUTs and thereby block sLeX biosynthesis and presentation. Here we show that this metabolic Trojan horse approach to FUT blockade works efficiently. 5T-Fuc does not become incorporated into glycans at detectable levels but rather generates GDP-5T-Fuc, which in turn leads to diminished sLeX levels and cellular adhesiveness. Following completion of the work we describe here, a related strategy using a different Trojan horse inhibitor, 2-deoxy-2-fluoro-fucopyranose, was reported independently (32), which demonstrates the validity of this approach to decreasing cell surface sLeX by blocking fucosyltransferases.
EXPERIMENTAL PROCEDURES
Synthesis of 5T-Fuc and GDP-5T-Fuc
5T-Fuc was synthesized following an established procedure (34). Only peracetylated 5T-Fuc was tested in cells because previous work has established that acetate-protected monosaccharides are more amenable to cell uptake than the parent sugars (15, 31). GDP-5T-Fuc was chemoenzymatically prepared from (deprotected) 5T-Fuc, ATP, and GTP with the enzyme L-fucokinase-GDP-L-fucose pyrophosphorylase (FKP) (35), which was used according to a procedure published previously (36), with some modifications. GDP-5T-Fuc was purified by ion exchange and ion-paired reverse-phase HPLC exactly as described previously (31) and converted into its disodium salt with AG 50W-X8 (Na+ form) ion exchange resin. GDP-Fuc was also prepared using this procedure. See the supplemental data for complete experimental details and compound characterization.
Cell Culture
HepG2 and CHO K1 cells were grown in DMEM (Lonza) supplemented with 20% FBS (Invitrogen), and DMEM:Ham's F12 (Invitrogen) containing 10% FBS, respectively. HL-60 cells were grown in RPMI 1640 medium (Invitrogen) containing 10% FBS, whereas HUVECs were grown on collagen-coated plates (BD Biosciences) (5 μg/ml) in Endothelial Cell Growth Media. (Lonza) medium containing SingleQuot (Lonza) supplements. Cells were routinely subpassaged twice per week using trypsin/EDTA (Sigma) or, in the case of HL-60 cells, by resuspending cell pellets in fresh media. All experiments involving HUVECs used cells at passage 4 or 5. Except where indicated, cells were incubated in a humidified atmosphere containing 5% CO2 at 37 °C.
Nucleotide Sugar Extraction
HepG2 cells were treated with varying doses (0–100 μm) of 5T-Fuc for 24 h before nucleotide sugars were purified by solid-phase extraction exactly as described previously (31).
Capillary Electrophoresis (CE) Analysis of Nucleotide Sugars
HepG2-derived nucleotide sugars were analyzed by CE as described previously (31). Optimal resolution between UDP-GlcNAc and GDP-5T-Fuc or GDP-Man required an applied voltage of −25 kV (37). To ensure correct peak determination and allow for the accurate measurement of GDP-5T-Fuc in samples obtained from 5T-Fuc-treated cells, nucleotide sugar extracts were sequentially treated with UDP-GlcNAc pyrophosphorylase (AGX1) and FKP, each in the presence of 200 mm pyrophosphate (Sigma). Please refer to the supplemental data for a more detailed description of the optimization and use of these enzymes to simplify nucleotide sugar analysis.
List of Antibodies and Lectins
sLeX was detected using the mouse monoclonal IgM CSLEX-1 (BD Biosciences), which was used at dilutions of 1:2500 and 1:300 in immunoblot and flow cytometry experiments, respectively. An isotype-matched control antibody (for CSLEX-1) was also obtained from BD Biosciences. The anti-sLeX and anti-LeX antibodies SNH3 and FH-2, respectively, were generous gifts from Sen-itiroh Hakomori. SNH3 was used for immunoblot analysis at a dilution of 1:4000, whereas FH-2 was used at concentration of 1.7 μg/ml in flow cytometry experiments. LeX was also detected with the mouse monoclonal IgM TG-1 (Santa Cruz Biotechnology), which was used at a dilution of 1:25. Actin was detected with mouse IgM JLA20 (Developmental Studies Hybridoma Bank), which was diluted 1:4000 before use. The following biotinylated lectins were purchased from Vector Laboratories: Aleuria aurantia (AAL), Maackia amurensis II, and Datura stramonium. These were typically used at concentrations of 0.85, 5, and 3 μg/ml, respectively. AAL was used at a concentration of 20 μg/ml for flow cytometry. Biotinylated Sambucus nigra agglutinin, wheat germ agglutinin, and Lens culinaris agglutinin (LCA) were purchased from EY Laboratories, Inc. Sambucus nigra agglutinin and wheat germ agglutinin were used at 0.12 μg/ml for immunoblotting, whereas LCA was used at a concentration of 20 μg/ml for flow cytometry. The following secondary reagent were used for detection: goat anti-mouse IgM-HRP conjugate (Santa Cruz Biotechnology) (1:20,000), streptavidin-HRP conjugate (Pierce) (1:20,000), goat anti-mouse IgM/G-FITC-conjugated (Jackson ImmunoResearch Laboratories, Inc.) (15 μg/ml), and streptavidin-FITC conjugate (Sigma) (2 μg/ml).
Lectin and Immunoblot Analysis of Cell Lysates
Cells were grown in the presence or absence of peracetylated 5T-Fuc for 48 h before they were harvested. Several stock solutions of 5T-Fuc in dimethyl sulfoxide were prepared so that cells could be exposed to varying amounts of the compound while maintaining the concentration of dimethyl sulfoxide to which they were exposed to 0.1% v/v. In all instances, cells were exposed to 5T-Fuc in the presence of FBS. When the analysis of secreted glycoproteins was desired, FBS was omitted from the culture medium. Cells were harvested by removing the media, washing monolayers twice with cold PBS, and scraping them off the culture plates in PBS containing 0.5% SDS. Cell lysates were prepared by sonication (4 °C, 2 × 15-s blasts, 20% duty) using a Sonic Dismembrator (Fisher Scientific), and cell debris was removed by centrifugation (4 °C, 10 min, 14,000 × g). When secreted glycoproteins were analyzed, the media from 5T-Fuc-treated cells were saved, gently mixed with a protease inhibitor mixture (Roche), and centrifuged (4 °C, 10 min, 100 × g) to remove dead cells. The clarified medium was then concentrated using centrifugal filters (Millipore, nominal molecular weight cut off 3000 Da). If samples were not to be used immediately, they were quickly frozen and stored at −20 °C. For all experiments, the protein concentrations of the samples were measured using the DC protein assay (Bio-Rad) before they were mixed with 5× SDS-PAGE loading buffer, boiled, and resolved through 8% polyacrylamide gels. The volume of each sample was adjusted so that an equal amount of protein was added to each lane of a gel. Resolved proteins were electrophoretically transferred to nitrocellulose membranes (Bio-Rad), which were then blocked with 2.5% BSA in PBS containing 0.1% Tween 20 (Sigma, PBS-T) at room temperature and incubated overnight at 4 °C with primary antibodies that were diluted in PBS-T + 2.5% BSA. Blotted membranes were washed with four changes of PBS-T over 1 h, reblocked for 30 min with 1% BSA, and probed for 1 h with an appropriate HRP-conjugated secondary antibody at room temperature. After further washing with PBS-T (1 h), membranes were developed with ECL Prime (GE Healthcare) and exposed to Amersham Biosciences Hyperfilm (GE Healthcare). Lectin blots were performed using the same procedure with the omission of BSA from all steps (38). Biotinylated lectins were detected using HRP-conjugated streptavidin. When blots were to be reprobed, they were first stripped by incubating them with agitation at room temperature in 62.5 mm Tris (pH 6.8) containing 2% SDS and 0.008% β-mercaptoethanol for 1 h. Blots were washed extensively with PBS-T before reprobing. Some samples were digested with PNGaseF (New England Biolabs) before immunoblot analysis. In these cases, cells were harvested as described above, and lysates were mixed with DTT (to 40 mm) and boiled for 10 min before the addition of NP-50 (Fluka, to 1%) and PNGaseF (50 mU/ml). Samples were digested overnight at 37 °C. For the EC50 determination of 5T-Fuc by immunoblot/densitometry, films were scanned and quantified using Scion Image (Scion Corp.).
Flow Cytometry
HepG2 or HL-60 cells were grown in the presence or absence of 50 μm 5T-Fuc for 3 or 4 days before they were harvested, pelleted by centrifugation (4 °C, 5 min, 90 × g), and resuspended in 100 μl cold PBS + 3% BSA + 0.5% NaN3 containing appropriate antibodies or lectins. Background staining was assessed using isotype-matched controls (for CSLEX-1, FH-2, and TG-1 mAbs) or streptavidin-FITC alone (for AAL). Typically, 5 × 104 HepG2 and 5 × 105 HL-60 cells were used for each condition tested. After 30 min at 4 °C, primary reagents were removed, and cells were incubated for an additional 30 min at 4 °C with FITC-conjugated secondary antibodies or streptavidin before they were washed and analyzed using a Guava easyCyte 8HT (Millipore) flow cytometer. Typically, 5000 or 7500 events (HepG2) and 10,000 events (HL-60) were counted for each condition tested. Data were analyzed and quantified using FlowJo Version 7.6.5 (TreeStar, Inc.) software, and for each sample the mean fluorescent intensity of cells was determined. For each condition, the mean fluorescent intensity of three separate samples was determined, and the means ± S.E. were reported. Statistical significance was determined by means of an unpaired, two-tailed Student's t test. In some instances, cells were treated with neuraminidase to destroy sLeX antigens prior to analysis. These samples were incubated with 0.3 mg/ml neuraminidase in 50 mm NaOAc (pH 5.5), for 30 min at 37 °C prior to the addition of primary mAbs. A test reaction demonstrated that under these conditions, essentially all cell surface Neu5Ac residues were cleaved.
Adhesion Assays
Cells were grown for 4 days in the presence or absence of 50 μm 5T-Fuc before they were harvested and labeled with 5 μm calcein (Molecular Probes) for 20–30 min at 37 °C in phenol red-free DMEM (Invitrogen) containing 1% FBS. 100 μl of this cell suspension was added to each well (HepG2, 5 × 104 cells/well; HL-60, 5 × 105 cells/well) of a NuncTM black 96-well plates (VWR International) that had been coated for at least 18 h at 4 °C with 4 μg/ml E-selectin or 5 μg/ml P-selectin (both recombinant human proteins were obtained from R&D Systems) and preblocked for 40 min with 1% BSA in PBS. Cells were allowed to settle for 25 min at room temperature. In the case of E-selectin, plates were agitated gently for the last 15 min of this period. The fluorescence intensity (485/520 nm excitation/emission) for each well was measured using an fmax fluorimeter (Molecular Devices). Plates were then washed. E-selectin plates were immersed upside-down in PBS for 10s, after which wells were aspirated and washed with another 2 × 100 μl PBS. For P-selectin-coated plates, the immersion step was omitted. 100 μl DMEM +1% FBS was added back to each well, and the fluorescence was measured a second time. For each well, the percentage of bound cells was calculated on the basis of the fraction of the initial fluorescence remaining after the wells were washed. Controls included adding cells to blank wells to which no adhesion molecules had been adsorbed and cells in which surface sLeX moieties had been degraded by neuraminidase digestion exactly as described above. Quadruplicate wells were measured for each condition, and the mean ± S.E. was reported. Statistic significance was determined by means of an unpaired, two-tailed Student's t test.
The adhesion of 5T-Fuc-treated cells to activated human endothelial cells was assessed by adding calcein-labeled cells to the wells of black 96-well plates (Perkin-Elmer) in which HUVEC cells (seeded at 3 × 104 cells/well) had been grown for 2 days. HUVEC cells were stimulated with 30 ng/ml tumor necrosis factor-α (TNF-α, Sigma) for 4 h before the onset of the adhesion assay. Cells were allowed to settle on to monolayers of TNF-α-stimulated HUVECs at room temperature for 20 min before wells were washed with 3 × 100 μl PBS. The fraction of bound cells was determined as described for the E/P-selectin adhesion assays. Controls included cells that had been treated with neuraminidase or wells in which TNF- α had been omitted.
Fucosyltransferase Assays
See the supplemental datafor experimental details.
RESULTS AND DISCUSSION
Chemoenzymatic Synthesis of GDP-5T-Fuc
Despite the potential utility of glycosyltransferase inhibitors as research tools and therapeutics, remarkably few compounds have been identified that can permeate through cellular membranes to inhibit intracellular targets such as α1,3-specific FUTs. Previous studies, however, have shown that the Fuc salvage pathway enables metabolic engineering of cells to display cell surface Fuc residues bearing chemically reactive moieties (39). We speculated that metabolic feeding of a Fuc analog in which the endocyclic ring oxygen is replaced by sulfur (5T-Fuc) could lead to the intracellular formation of GDP-5T-Fuc which, in turn, could inhibit cellular FUTs and thereby decrease sLeX levels.
As a first step toward evaluating this proposal, we chemoenzymatically prepared GDP-5T-Fuc using synthetic 5T-Fuc in conjunction with recombinant bifunctional Bacteroides fragilis fucose kinase:GDP-Fuc pyrophosphorylase (FKP) (supplemental Figs. S1A–S4) (35). FUTs 7 and 3 are reported to be the important FUTs catalyzing installation of α1,3-linked Fuc residues onto glycoconjugates, including sLeX, (Fig. 1A) in both HL-60 (40) and HepG2 (41) cells, respectively. We used CE with laser-induced fluorescence detection as a sensitive analytical method to evaluate the ability of these enzymes to use GDP-5T-Fuc as a substrate in vitro to form a sulfur-containing sLeX analog (supplemental Fig. S12 and S13). We found that neither of these enzymes transferred 5T-Fuc to fluorescently labeled glycans at detectable levels. These results indicate that if GDP-5T-Fuc is formed within cells it should inhibit these enzymes and thereby impede the biosynthesis of sLeX.
GDP-5T-Fuc Is Produced by the Fucose Salvage Pathway within 5T-Fuc-treated Cells
As a next step, we set out to determine whether5T-Fuc could be assimilated by cells to form GDP-5T-Fuc. We elected to first study HepG2 cells because this human hepatocellular carcinoma-derived cell line has been shown to present sLeX on glycoproteins (41, 42). We extracted the nucleotide sugars from HepG2 cells grown in the presence or absence of 100 μm 5T-Fuc and analyzed them by CE. We observed a newly prominent peak in electropherograms obtained only from 5T-Fuc-treated cells (Fig. 2A). This species comigrated with the synthetic GDP-5T-Fuc standard. Complicating analysis, however, we found that endogenously occurring GDP-mannose (GDP-Man) also comigrated with GDP-5T-Fuc (supplemental Fig. S1B). We confirmed the identity of this newly prominent peak as GDP-5T-Fuc by running FKP in reverse to cleave GDP-5T-Fuc. After treating the extracts with FKP we found that the prominent peak having a migration time matching GDP-5T-Fuc was digested (Fig. 2B and supplemental Fig. S5) and is therefore GDP-5T-Fuc and not GDP-Man, which cannot be processed by FKP. Further, by comparing samples before and after FKP digestion, we were able to quantify the relative levels of GDP-Man and GDP-5T-Fuc (Fig. 2C). No significant changes in GDP-Man levels were observed when cells were treated with 5T-Fuc, although GDP-5T-Fuc was found to accumulate at the expense of endogenous GDP-Fuc. These data show that the human enzymes of the Fuc-salvage pathway accept 5T-Fuc as a substrate to generate intracellular GDP-5T-Fuc.
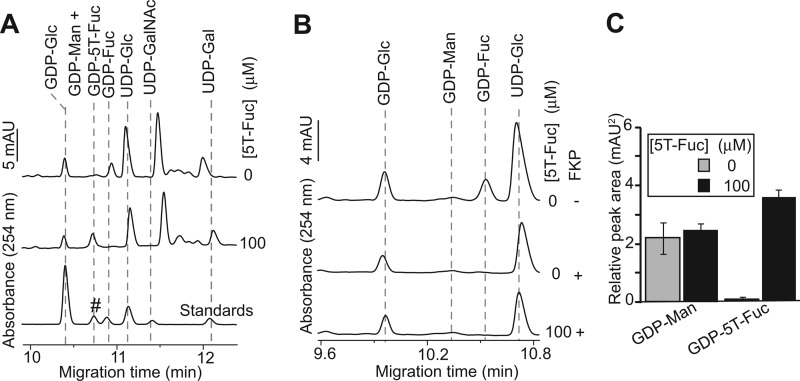
FIGURE 2.
5T-Fuc is converted into GDP-5T-Fuc within HepG2 cells. A, nucleotide sugars were extracted from control and 5T-Fuc-treated cells and analyzed by CE. All samples were spiked with GDP-Glc before extraction and further processed with UDP-GlcNAc pyrophosphorylase (AGX1) in the presence of exogenous pyrophosphate (resulting in the elimination of this nucleotide sugar from electropherograms) to allow for the precise quantification of GDP-5T-Fuc. Notice that in cells treated with 5T-Fuc a new peak appears that comigrates with chemoenzymatically prepared GDP-5T-Fuc (#). Concomitantly, treatment of HepG2 cells with the thiosugar causes a dramatic reduction in GDP-Fuc levels. B, GDP-5T-Fuc and GDP-Man comigrate and therefore samples were treated with FKP in the presence of pyrophosphate to convert GDP-Fuc/5T-Fuc into their corresponding sugar phosphates. The FKP sensitivity of the new nucleotide sugar extracted from 5T-Fuc-treated cells establishes that it was indeed GDP-5T-Fuc and not an accumulation of the comigrating GDP-Man. C, GDP-5T-Fuc and GDP-Man levels could be quantified by subtracting the peak areas obtained upon the CE analysis of samples before and after treatment with FKP. Averaged peak areas are reported ± S.E. (n = 3).
5T-Fuc Decreases the Levels of sLeX Found on N-linked Glycoproteins
Having established that 5T-Fuc was tolerated by the enzymes of the Fuc-salvage pathway, we next investigated by immunoblotting the effects of this compound on the biosynthesis of sLeX on protein-linked glycans. The exposure of HepG2 cells to 5T-Fuc for 24 h resulted in a dose-dependent decrease in sLeX immunoreactivity, observed when protein blots were probed with sLeX-specific mAbs CSLEX-1 (6) and SNH3 (8, 43) (Fig. 3A). Treating cell lysates with peptide:N-glycanase F (PNGaseF) prior to immunoblotting reduced their immunoreactivity, indicating that most glycoprotein-borne sLeX epitopes produced by HepG2 cells are present within N-linked glycans (Fig. 3B). Time course studies (supplemental Fig. S7A) indicated a lag of more than 8 h between the addition of 5T-Fuc to cells and decreased expression of sLeX epitopes on glycoproteins. In contrast, we observed that recovery of sLeX expression begins within 2 h after removing 5T-Fuc (supplemental Fig. S7B). Using densitometry we established an EC50 value for 5T-Fuc mediated decrease of sLeX of 21 ± 1 μm in HepG2 cells (supplemental Fig. S6).
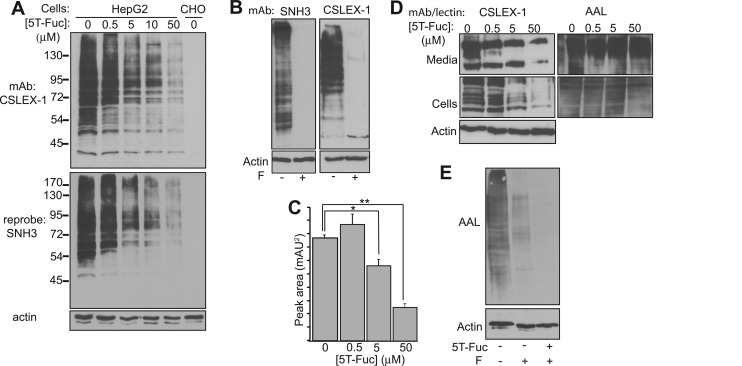
FIGURE 3.
5T-Fuc prevents the formation of sLeX on glycoproteins in HepG2 cells. A, the immunoblot analysis of HepG2 lysates prepared from cells exposed to increasing concentrations of 5T-Fuc demonstrated that 5T-Fuc inhibits sLeX expression on glycoproteins in a dose-dependent manner. Blots were probed with two sLeX-specific antibodies, first with CSLEX-1 and then, after stripping, with SNH3. Chinese hamster ovary (CHO) cell lysates were used as a negative control because these cells do not express the FUTs necessary to produce sLeX. B, PNGaseF-treatment (F) of HepG2 cell lysates indicates that the CSLEX-1 and SNH3 antibodies are reactive against sLeX contained within N-glycans. C, the relative concentration of GDP-Fuc decreased as cells were exposed to increasing concentrations of 5T-Fuc. CE peak areas (n = 3) are reported as mean ± S.E. *, p < 0.05; **, p < 0.0005. D, 5T-Fuc also treatment also decreased sLeX levels on glycoproteins secreted by HepG2 cells although glycoprotein fucosylation did not appear to be greatly affected when blots were probed with AAL. E, when cell lysates were treated with PNGaseF (F), those derived from cells exposed to 50 μm 5T-Fuc exhibited a reduced ability to bind to AAL.
Given our in vitro data, direct inhibition of cellular FUTs by GDP-5T-Fuc is the most likely explanation for 5T-Fuc-induced decreases in sLeX presentation. However, it is worth considering alternative mechanisms by which 5T-Fuc could act in this way through a FUT-independent processes. One mechanism could involve depletion of GDP-Fuc (Fig. 3C) caused by feedback inhibition (44) from GDP-5T-Fuc accumulation. Although feedback inhibition of GDP-Fuc biosynthesis is certainly likely, this depletion scenario being the chief driver of decreased FUT activity, however, seems less likely, given that at 5T-Fuc concentrations bracketing the EC50 value significant GDP-Fuc remains present within cells and that core fucosylation appears unaffected by 5T-Fuc treatment. Regardless, at this time we cannot conclude unambiguously which mechanism is operative. Indeed, it is possible that both factors contribute to the observed decreases in sLeX levels.
A second mechanism by which sLeX levels on proteins could be indirectly decreased is by 5T-Fuc causing large changes in the N-glycan structures (16, 17). To address this question, we subjected both the intracellular and secreted (42) glycoproteins, which we also find show decreased sLeX presentation upon treatment with 5T-Fuc (Fig. 3D), to lectin blot analysis (Fig. 3D and supplemental Fig. S8). We observe no decreases in the extent of binding of any Neu5Ac- or GlcNAc-specific lectins to glycoproteins obtained from 5T-Fuc-treated cells, indicating that 5T-Fuc does not cause any gross changes in protein glycosylation. The lack of a clear correlation between sLeX levels and the binding of the terminal Fuc-specific AAL (45) was surprising because we had expected that the AAL reactivity of 5T-Fuc-treated cells would decrease (Fig. 3D). AAL, however, detects the total pool of glycoproteins containing terminal Fuc, of which the α1,3-linked Fuc residues contained within sLeX structures may only be a small fraction. In support of this view, we note a large decrease in AAL binding to proteins from HepG2 cells lysates when they are treated with PNGaseF (Fig. 3E). PNGaseF removes all N-glycans, including those having α1,6-linked “core” Fuc residues, indicating that most Fuc is contained within N-linked glycans. The further drop in AAL binding to lysates from 5T-Fuc-treated cells digested with PNGaseF suggests that 5T-Fuc also blocks α1,3-Fuc presentation on O-linked glycoproteins (Fig. 3E). We corroborated these lectin blot data by the direct analysis of HepG2-derived N-glycans by CE (supplemental Fig. S9), which revealed only small changes in the glycans obtained from cells treated with 5T-Fuc, and these changes were limited solely to the terminal sugars Fuc and Neu5Ac.
5T-Fuc Reduces Cell Surface sLeX Expression but Does Not Affect Core Fucosylation
Because sLeX is also found on glycolipids, which are not assayed by immunoblotting, we used flow cytometry to assess the effects of 5T-Fuc on total cell surface sLeX levels. 5T-Fuc treatment of HepG2 cells leads to a 4-fold decrease in sLeX-bearing glycoconjugates relative to untreated controls (Fig. 4, A and B). Interestingly, in contrast to lectin blot analysis (Fig. 3D), we observed that binding of AAL to 5T-Fuc-treated cells was also decreased (Fig. 4, A and B). The basis for the differences between immunoblotting and flow cytometry is worth considering. We speculated that these differences stem from 5T-Fuc affecting predominantly α1,3-linked Fuc, which is the only form of Fuc present on glycolipids in these cells. To assess this question, we probed HepG2 cells using the α1,6-Fuc-specific lectin LCA and found no effect from 5T-Fuc treatment (Fig. 4, A and B). Additionally, AAL-binding is also unaffected in cell lines such as A549 or COS-7 that lack readily detectable levels of α1,3Fuc-containing structures such as sLeX or LeX (supplemental Fig. S8B). At the concentrations used here, 5T-Fuc therefore appears to have no great effect on core fucosylation (α1,6) but significantly reduces sLeX (α1,3) on both protein (observed by immunoblot and flow cytometry) and glycolipids (observed by flow cytometry).
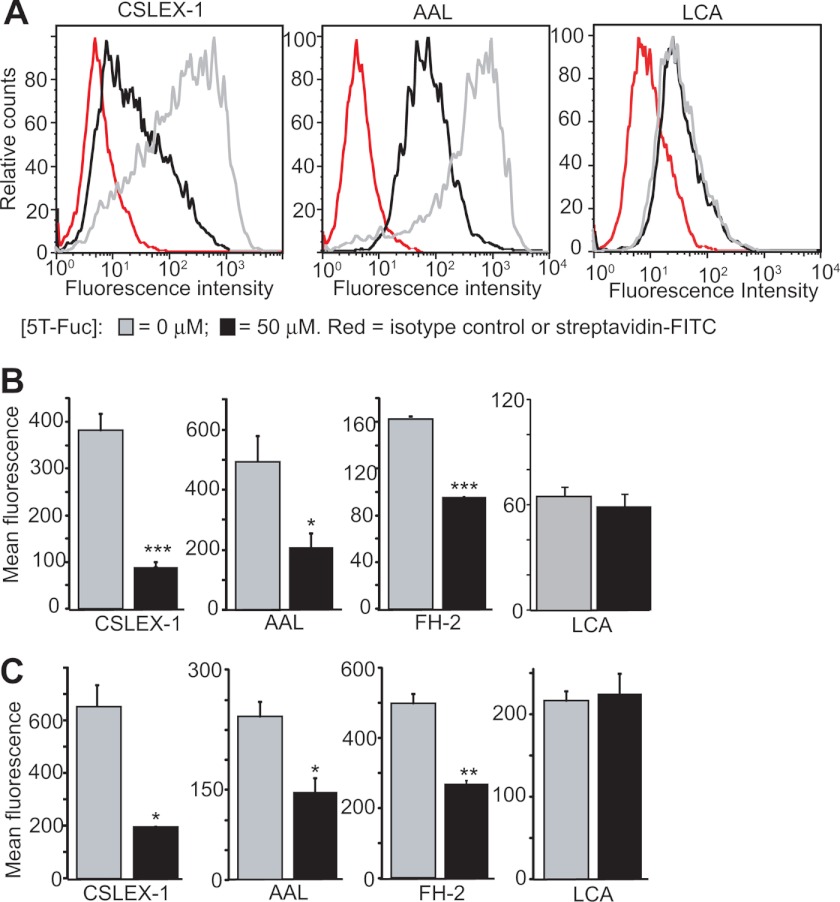
FIGURE 4.
5F-Fuc reduces cell surface sLeX expression. A, HepG2 cells treated with (black) or without (gray) 50 μm 5T-Fuc were incubated in the presence of anti-sLeX antibodies (CSLEX-1) or isotype-matched controls (red) followed by fluorescein-conjugated anti-IgM or streptavidin. The number of positively stained cells was assessed by flow cytometry, and the mean fluorescence intensity (MFI) for each sample was calculated (B). Values shown (n = 3) are mean ± S.E. 5T-Fuc causes a significant decrease in the average MFI of 5T-Fuc-treated cells. *, p < 0.05; **p < 0.01; ***, p < 0.0005. 5T-Fuc also significantly reduced the expression of fucosylated glycoconjugates (AAL) and LeX (FH-2) but not core fucosylated ones (LCA) in HepG2 cells (B). Similar results were obtained with HL-60 cells (C). Histograms for all conditions are reported in the supplemental information.
Given that HepG2 lysates show fucosyltransferase activity toward non-sialylated acceptors (46), we investigated the effect of 5T-Fuc treatment on cell surface LewisX (LeX) levels. We found that LeX is indeed expressed on HepG2 cells and, using the LeX-specific mAb FH-2 (47), we found that its levels are reduced by 5T-Fuc treatment (Fig. 4B). Neuraminidase treatment of cells, which abolished CSLEX-1 immunoreactivity (supplemental Fig. S10A) by converting sLeX into LeX (48), also showed 5T-Fuc treatment reduced LeX levels as measured using the anti-LeX mAbs FH-2 and TG-1 (supplemental Fig. S10B). The FH-2 mAb binds equally well to LeX and a synthetic LeX analog in which a sulfur is present in the pyranose ring of fucose (49). The parallel drop in sLeX and LeX levels observed on 5T-Fuc treatment is therefore in accord with the in vitro data indicating that 5T-Fuc is not transferred by FUTs to form a sulfur analog of LeX or sLeX. Variation in the changes of sLeX observed on proteins upon 5T-Fuc treatment in immunoblots as compared with overall cell surface sLeX measured by flow cytometry likely stem from the presence of sLeX on glycolipids, which are not observed by immunoblotting but are detected during flow cytometry. Furthermore, we found, using both CE (supplemental Fig. S9) and flow cytometry (supplemental Fig. S11), that N-glycans from both treated and untreated HepG2 cells were sensitive to α-fucosidase digestion. Because 5T-glycosides are known to be resistant to glycosidase-catalyzed digestion (50) these experiments further indicate that 5T-Fuc is not transferred in cells at detectable levels.
Finally, to examine the generality of these observations, we evaluated the effect of 5T-Fuc treatment on HL-60 cells, which is a human leukocyte cell line expressing both LeX (48) and sLeX (15, 16). 5T-Fuc treatment of these cells also reduced their reactivity toward Fuc (AAL)-, sLeX (CSLEX-1)-, and LeX-specific (FH-2) lectins and mAbs (Fig. 4C), illustrating the general utility of 5T-Fuc as a probe for perturbing α1,3FUTs. Again, as observed with HepG2 cells, we observed no effect on LCA binding in HL-60 cells treated with 5T-Fuc, further suggesting that at these doses core fucosylation of N-glycans remained unaffected.
5T-Fuc Inhibits the Adhesion of Cells to Selectins and Activated Endothelial Cells
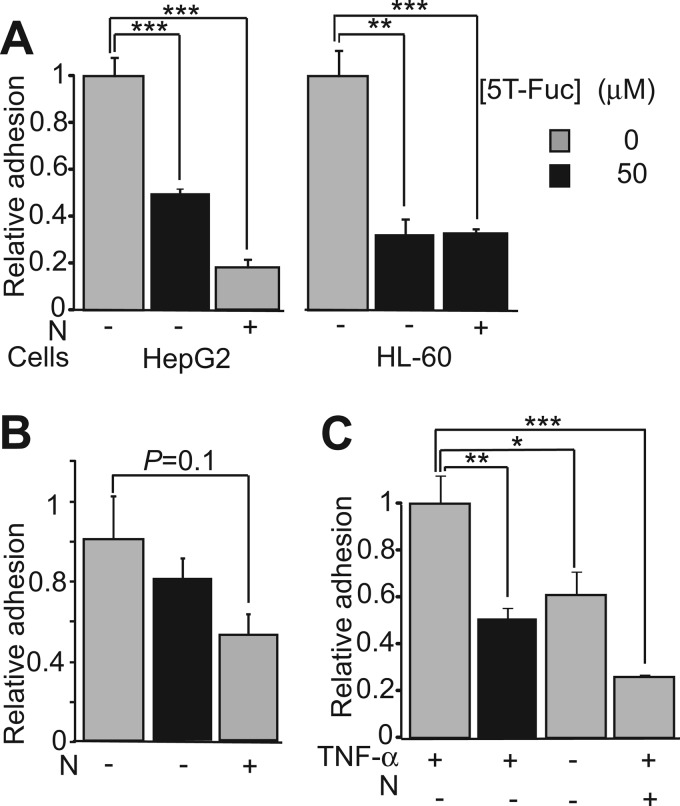
Given the robust decreases observed in both sLeX levels on both glycoproteins and the cell surface, we hypothesized that 5T-Fuc treatment would reduce cellular adhesiveness. Consistent with this view, we find that 5T-Fuc treatment significantly reduced adhesion of both HepG2 and HL-60 cells to E-selectin-coated plates in static adhesion assays (Fig. 5A). To evaluate whether some sialylated E-selectin ligands remained on 5T-Fuc-treated HepG2 cells, we digested these cells using neuraminidase. We found that neuraminidase digestion leads to a greater loss of adhesiveness than 5T-Fuc-treatment alone, indicating that some E-selectin ligands remained after 5T-Fuc treatment. In contrast, HL-60 cells show no further loss of adhesiveness when treated with neuraminidase, suggesting that 5T-Fuc alone abrogated all sLeX-mediated adhesion. We also observed a trend toward reduced adhesiveness of 5T-Fuc-treated HL-60 cells to P-selectin but no reduction for treated HepG2 cells (Fig. 5B) in accord with previous reports that the latter do not significantly bind to P-selectin (41, 51). This finding suggests that P-selectin ligands are somewhat more resistant to 5T-Fuc treatment, perhaps because they have a slower cell surface turnover rate. The relative insensitivity of P-selectin ligand levels in leukocytes to fucose availability as compared with E-selectin ligand levels is also consistent with these results (52), although other mechanisms may be operative. Furthermore, we note that others have also observed that E-selectin adherence in HL-60 cells treated with Fuc analogues is impaired to a greater extent than P-selectin binding (32). To evaluate the effects of 5T-Fuc treatment in a traditional model where E-selectin is presented at physiologically relevant levels, we measured the adherence of HL-60 cells to TNF-α-activated HUVECs. We found that 5T-Fuc treatment reduced adhesion to a level comparable with that obtained when using non-stimulated HUVECs (Fig. 5C).
FIGURE 5.
5T-Fuc decreases the affinity of treated cells to adhesion molecules. A, both HepG2 and HL-60 cells exhibited an impaired ability to adhere to E-selectin-coated plates when pretreated with 5T-Fuc, whereas P-selectin adhesion (B) was only slightly reduced for HL-60 cells. C, the ability of HL-60 cells to adhere to TNF-α-stimulated HUVECs was also significantly reduced by 5T-Fuc. For each assay, the number of calcein-labeled cells binding to 96-well plates coated with adhesion molecules or activated endothelial cells was expressed as a percentage of the total number of cells added per well. All data were normalized to the values obtained for non-treated cells. In all assays, cells in which sLeX-containing glycans were digested with neuraminidase (N) were used as negative controls. Values reported (n = 4) are mean ± S.E. *, p < 0.05; **, p < 0.01; ***p < 0.001).
CONCLUSIONS
To summarize, the conserved biosynthetic Fuc salvage pathway tolerates replacement of the endocyclic ring oxygen of Fuc with sulfur. The product of 5T-Fuc assimilation by this pathway, GDP-5T-Fuc, is not transferred to generate sulfur-containing LeX or sLeX analogues. Instead, accumulation of GDP-5T-Fuc within cells leads to decreased levels of cell surface sLeX and LeX in different cell lines, with no detectable changes in core glycosylation. Notably, this GDP-5T-Fuc-mediated blockade of fucosyltransferases leads to functionally significant impairment in sLeX levels, as assayed using static cell adhesion models.
Because Fuc-containing glycans have been found to be important players in various biological processes, FUT inhibitors are a topic of significant interest. The ability to block FUTs using small molecule inhibitors to alter cell surface glycosylation, in a dose- and time-dependent manner, within different models should speed recognition and understanding of the biological roles played by this epitope (20). Small molecule inhibitors will also present the opportunity to validate FUTs as an attractive therapeutic target given the roles played by fucosylated glycoconjugates such as sLeX in mediating processes like tumor metastasis and chronic inflammation. FUT inhibitors have been identified that act in vitro (18). Yet, to our knowledge, none of these has been shown to be active within live cells. With regard to sLeX, because its biosynthesis can occur by different pathways and be catalyzed by different FUTs, its blockade in biological systems may require concomitant inhibition of multiple fucosyltransferases. In this regard it is interesting to note that because GDP-5T-Fuc mimics the natural donor sugar GDP-Fuc, it is likely to inhibit multiple fucosyltransferases with potencies paralleling dissociation constants of GDP-Fuc for each FUT. The apparent pan-specificity of the Trojan horse inhibitor 5T-Fuc for α1,3 fucosyltransferases responsible for sLeX biosynthesis consequently offers a significant potential benefit for disrupting LeX and sLeX presentation. Thus, 5T-Fuc should prove to be a useful tool to study sLeX, and further study is merited to determine the inhibitory properties of GDP-5T-Fuc against the repertoire of mammalian fucosyltransferases.
An interesting future line of inquiry is to rationally engineer selective inhibitors of FUTs. Given that the fucose biosynthetic pathway has been shown to tolerate groups appended to the methyl group of fucose (39), one can also envision the potential to generate allele-specific 5T-Fuc-based FUT inhibitors using a bump and hole strategy. More generally, the findings reported here in conjunction with earlier observations (31) indicate that monosaccharide analogues in which the endocyclic ring oxygen is replaced with sulfur, as well as other analogues that form incompetent donor sugars, will be a general approach to inhibition of other families of glycosyltransferases.
Supplementary Material
Acknowledgments
We thank Dr. Laurie Comstock (Harvard Medical School) for providing the FKP plasmid and Dr Sen-itiroh Hakomori (University of Washington) for providing the SNH3 and FH-2 antibodies. We also thank Dr. Jonathan Choy (Simon Fraser University) for providing HUVEC cells, Dr. Andrew Bennett for the Micromonospora viridifaciens neuraminidase and Dr. Mark Brockman (Simon Fraser University) for training and access to a flow cytometer.
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC).

This article contains supplemental Figs. S1–S13, Experimental Procedures, and References.
- sLEX
- sialyl-Lewis X
- Neu5Ac
- neuraminic acid
- Fuc
- fucose
- FUT
- α1,3-fucosyltransferases
- FKP
- L-fucokinase-GDP-L-fucose pyrophosphorylase
- HUVEC
- human umbilical vein endothelial cell
- CE
- capillary electrophoresis
- AAL
- Aleuria aurantia
- LCA
- Lens culinaris agglutinin
- TNF-α
- tumor necrosis factor α
- PGNaseF
- peptide:N-glycanase F.
REFERENCES
- 1. Tiemeyer M., Swiedler S. J., Ishihara M., Moreland M., Schweingruber H., Hirtzer P., Brandley B. K. (1991) Carbohydrate ligands for endothelial-leukocyte adhesion molecule 1. Proc. Natl. Acad. Sci. U.S.A. 88, 1138–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. (1990) ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-LeX. Science 250, 1130–1132 [DOI] [PubMed] [Google Scholar]
- 3. Rinnbauer M., Ernst B., Wagner B., Magnani J., Benie A. J., Peters T. (2003) Epitope mapping of sialyl LewisX bound to E-selectin using saturation transfer difference NMR experiments. Glycobiology 13, 435–443 [DOI] [PubMed] [Google Scholar]
- 4. Somers W. S., Tang J., Shaw G. D., Camphausen R. T. (2000) Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to sLeX and PSGL-1. Cell 103, 467–479 [DOI] [PubMed] [Google Scholar]
- 5. Malý P., Thall A., Petryniak B., Rogers C. E., Smith P. L., Marks R. M., Kelly R. J., Gersten K. M., Cheng G., Saunders T. L., Camper S. A., Camphausen R. T., Sullivan F. X., Isogai Y., Hindsgaul O., von Andrian U. H., Lowe J. B. (1996) The α(1,3) fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 86, 643–653 [DOI] [PubMed] [Google Scholar]
- 6. Fukushima K., Hirota M., Terasaki P. I., Wakisaka A., Togashi H., Chia D., Suyama N., Fukushi Y., Nudelman E., Hakomori S. (1984) Characterization of sialosylated LewisX as a new tumor-associated antigen. Cancer Res. 44, 5279–5285 [PubMed] [Google Scholar]
- 7. Hoff S. D., Matsushita Y., Ota D. M., Cleary K. R., Yamori T., Hakomori S., Irimura T. (1989) Increased expression of sialyl-dimeric LeX antigen in liver metastases of human colorectal carcinoma. Cancer Res. 49, 6883–6888 [PubMed] [Google Scholar]
- 8. Numahata K., Satoh M., Handa K., Saito S., Ohyama C., Ito A., Takahashi T., Hoshi S., Orikasa S., Hakomori S. I. (2002) Sialosyl-LeX expression defines invasive and metastatic properties of bladder carcinoma. Cancer 94, 673–685 [DOI] [PubMed] [Google Scholar]
- 9. Nakagoe T., Fukushima K., Hirota M., Kusano H., Ayabe H., Tomita M., Kamihira S. (1993) Immunohistochemical expression of sialyl LeX antigen in relation to survival of patients with colorectal carcinoma. Cancer 72, 2323–2330 [DOI] [PubMed] [Google Scholar]
- 10. Nakamori S., Kameyama M., Imaoka S., Furukawa H., Ishikawa O., Sasaki Y., Kabuto T., Iwanaga T., Matsushita Y., Irimura T. (1993) Increased expression of sialyl LewisX antigen correlates with poor survival in patients with colorectal carcinoma. Clinicopathological and immunohistochemical study. Cancer Res. 53, 3632–3637 [PubMed] [Google Scholar]
- 11. Narita T., Funahashi H., Satoh Y., Watanabe T., Sakamoto J., Takagi H. (1993) Association of expression of blood group-related carbohydrate antigens with prognosis in breast cancer. Cancer 71, 3044–3053 [DOI] [PubMed] [Google Scholar]
- 12. Kretzschmar G. (1998) Synthesis of novel Sialyl-LewisX glycomimetics as selectin antagonists. Tetrahedron 54, 3765–3780 [Google Scholar]
- 13. Dennis J. W. (1986) Effects of swainsonine and polyinosinic. Polycytidylic acid on murine tumor cell growth and metastasis. Cancer Res. 46, 5131–5136 [PubMed] [Google Scholar]
- 14. Fuster M. M., Brown J. R., Wang L., Esko J. D. (2003) A disaccharide precursor of sialyl Lewis X inhibits metastatic potential of tumor cells. Cancer Res. 63, 2775–2781 [PubMed] [Google Scholar]
- 15. Sarkar A. K., Fritz T. A., Taylor W. H., Esko J. D. (1995) Disaccharide uptake and priming in animal cells. Inhibition of sialyl LewisX by acetylated Gal β 1–>4GlcNAc β-O-naphthalenemethanol. Proc. Natl. Acad. Sci. U.S.A. 92, 3323–3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marathe D. D., Buffone A., Jr., Chandrasekaran E. V., Xue J., Locke R. D., Nasirikenari M., Lau J. T., Matta K. L., Neelamegham S. (2010) Fluorinated per-acetylated GalNAc metabolically alters glycan structures on leukocyte PSGL-1 and reduces cell binding to selectins. Blood 115, 1303–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barthel S. R., Antonopoulos A., Cedeno-Laurent F., Schaffer L., Hernandez G., Patil S. A., North S. J., Dell A., Matta K. L., Neelamegham S., Haslam S. M., Dimitroff C. J. (2011) Peracetylated 4-fluoro-glucosamine reduces the content and repertoire of N- and O-glycans without direct incorporation. J. Biol. Chem. 286, 21717–21731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rillahan C. D., Brown S. J., Register A. C., Rosen H., Paulson J. C. (2011) High-throughput screening for inhibitors of sialyl- and fucosyltransferases. Angew. Chem. Int. Ed. Engl. 50, 12534–12537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grabenhorst E., Nimtz M., Costa J., Conradt H. S. (1998) In vivo specificity of human α1,3/4-fucosyltransferases III-VII in the biosynthesis of LewisX and sialyl LewisX motifs on complex-type N-glycans. J. Biol. Chem. 273, 30985–30994 [DOI] [PubMed] [Google Scholar]
- 20. Pang P. C., Chiu P. C., Lee C. L., Chang L. Y., Panico M., Morris H. R., Haslam S. M., Khoo K. H., Clark G. F., Yeung W. S., Dell A. (2011) Human sperm binding is mediated by the sialyl-LewisX oligosaccharide on the zona pellucida. Science 333, 1761–1764 [DOI] [PubMed] [Google Scholar]
- 21. Wang X., Inoue S., Gu J., Miyoshi E., Noda K., Li W., Mizuno-Horikawa Y., Nakano M., Asahi M., Takahashi M., Uozumi N., Ihara S., Lee S. H., Ikeda Y., Yamaguchi Y., Aze Y., Tomiyama Y., Fujii J., Suzuki K., Kondo A., Shapiro S. D., Lopez-Otin C., Kuwaki T., Okabe M., Honke K., Taniguchi N. (2005) Dysregulation of TGF-β receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 102, 15791–15796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Etzioni A., Frydman M., Pollack S., Avidor I., Phillips M. L., Paulson J. C., Gershoni-Baruch R. (1992) Recurrent severe infections caused by a novel leukocyte adhesion deficiency. New Eng. J. Med. 327, 1789–1792 [DOI] [PubMed] [Google Scholar]
- 23. Barthel S. R., Gavino J. D., Wiese G. K., Jaynes J. M., Siddiqui J., Dimitroff C. J. (2008) Analysis of glycosyltransferase expression in metastatic prostate cancer cells capable of rolling activity on microvascular endothelial (E)-selectin. Glycobiology 18, 806–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Togayachi A., Kudo T., Ikehara Y., Iwasaki H., Nishihara S., Andoh T., Higashiyama M., Kodama K., Nakamori S., Narimatsu H. (1999) Up-regulation of Lewis enzyme (Fuc-TIII) and plasma-type α1,3Fucosyltransferase (Fuc-TVI) expression determines the augmented expression of sialyl LewisX antigen in non-small cell lung cancer. Int. J. Cancer 83, 70–79 [DOI] [PubMed] [Google Scholar]
- 25. Trinchera M., Malagolini N., Chiricolo M., Santini D., Minni F., Caretti A., Dall'olio F. (2011) The biosynthesis of the selectin-ligand sialyl LewisX in colorectal cancer tissues is regulated by fucosyltransferase VI and can be inhibited by an RNA interference-based approach. Int. J. Biochem. Cell Biol. 43, 130–139 [DOI] [PubMed] [Google Scholar]
- 26. Barthel S. R., Wiese G. K., Cho J., Opperman M. J., Hays D. L., Siddiqui J., Pienta K. J., Furie B., Dimitroff C. J. (2009) α 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proc. Natl. Acad. Sci. U.S.A. 106, 19491–19496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ohyama C., Tsuboi S., Fukuda M. (1999) Dual roles of sialyl LewisX oligosaccharides in tumor metastasis and rejection by natural killer cells. EMBO J. 18, 1516–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hiller K. M., Mayben J. P., Bendt K. M., Manousos G. A., Senger K., Cameron H. S., et al. (2000) Transfection of α(1,3)fucosyltransferase antisense sequences impairs the proliferative and tumorigenic ability of human colon carcinoma cells. Mol. Carcinogenesis 27, 280–288 [PubMed] [Google Scholar]
- 29. Weston B. W., Hiller K. M., Mayben J. P., Manousos G. A., Bendt K. M., Liu R., Cusack J. C., Jr. (1999) Expression of human α(1,3)fucosyltransferase antisense sequences inhibits selectin-mediated adhesion and liver metastasis of colon carcinoma cells. Cancer Res. 59, 2127–2135 [PubMed] [Google Scholar]
- 30. Norris A. J., Whitelegge J. P., Strouse M. J., Faull K. F., Toyokuni T. (2004) Inhibition kinetics of carba- and C-fucosyl analogues of GDP-fucose against fucosyltransferase V. Implication for the reaction mechanism. Bioorg. Med. Chem. Lett. 14, 571–573 [DOI] [PubMed] [Google Scholar]
- 31. Gloster T. M., Zandberg W. F., Heinonen J. E., Shen D. L., Deng L., Vocadlo D. J. (2011) Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells. Nat. Chem. Biol. 7, 174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rillahan C. D., Antonopoulos A., Lefort C. T., Sonon R., Azadi P., Ley K., Dell A., Haslam S. M., Paulson J. C. (2012) Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 8, 661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsuruta O., Yuasa H., Hashimoto H., Sujino K., Otter A., Li H., Palcic M. M. (2003) Synthesis of GDP-5-thiosugars and their use as glycosyl donor substrates for glycosyltransferases. J. Org. Chem. 68, 6400–6406 [DOI] [PubMed] [Google Scholar]
- 34. Hashimoto H., Fujimori T., Yuasa H. (1990) Synthesis of 5-thio-L-fucose and its inhibitory effect on fucosidase. J. Carb. Chem. 9, 683–694 [Google Scholar]
- 35. Coyne M. J., Reinap B., Lee M. M., Comstock L. E. (2005) Human symbionts use a host-like pathway for surface fucosylation. Science 307, 1778–1781 [DOI] [PubMed] [Google Scholar]
- 36. Zhao G., Guan W., Cai L., Wang P. G. (2010) Enzymatic route to preparative-scale synthesis of UDP-GlcNAc/GalNAc, their analogues and GDP-fucose. Nat. Protoc. 5, 636–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zandberg W. F., Gao N., Kumarasamy J., Lehrman M. A., Seidah N. G., Pinto B. M. (2012) 5-Thiomannosides block the biosynthesis of dolichol-linked oligosaccharides and mimic class I congenital disorders of glycosylation. Chem. Biochem. 13, 392–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gravel P., Walker J. M. (2002) in The Protein Protocols Handbook (Walker J. M., ed) pp. 779–793, Humana Press, Totowa, NJ [Google Scholar]
- 39. Sawa M., Hsu T. L., Itoh T., Sugiyama M., Hanson S. R., Vogt P. K., Wong C. H. (2006) Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc. Natl. Acad. Sci. U.S.A. 103, 12371–12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Clarke J. L., Watkins W. (1996) 1,3-L-Fucosyltransferase expression in developing human myeloid cells. J. Biol. Chem. 271, 10317–10328 [DOI] [PubMed] [Google Scholar]
- 41. St Hill C. A., Baharo-Hassan D., Farooqui M. (2011) C2-O-sLeX glycoproteins are E-selectin ligands that regulate invasion of human colon and hepatic carcinoma cells. PLoS ONE 6, e16281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Higai K., Shibukawa K., Muto S., Matsumoto K. (2003) Targeted proteo-glycomics analysis of sialyl LewisX antigen expressing glycoproteins secreted by human hepatoma cell line. Anal. Sci. 19, 85–92 [DOI] [PubMed] [Google Scholar]
- 43. Muroi K., Suda T., Nojiri H., Ema H., Amemiya Y., Miura Y., Nakauchi H., Singhal A., Hakomori S. (1992) Reactivity profiles of leukemic myeloblasts with monoclonal antibodies directed to sialosyl-LeX and other lacto-series type 2 chain antigens:absence of reactivity with normal hematopoietic progenitor cells. Blood 79, 713–719 [PubMed] [Google Scholar]
- 44. Sullivan F. X., Kumar R., Kriz R., Stahl M., Xu G. Y., Rouse J., et al. (1998) Molecular cloning of human GDP-mannose 4,6-dehydratase and reconstitution of GDP-fucose biosynthesis in vitro. J. Biol. Chem. 273, 8193–8202 [DOI] [PubMed] [Google Scholar]
- 45. Yamashita K., Kochibe N., Ohkura T., Ueda I., Kobata A. (1985) Fractionation of L-fucose-containing oligosaccharides on immobilized Aleuria aurantia lectin. J. Biol. Chem. 260, 4688–4693 [PubMed] [Google Scholar]
- 46. Johnson P., Donald A., Clarke J., Watkins W. (1995) Purification, properties and possible gene assignment of an α1,3-fucosyltransferase expressed in human liver. Glycoconj. J. 12, 879–893 [DOI] [PubMed] [Google Scholar]
- 47. Akamatsu S., Yazawa S., Zenita K., Matsumoto H., Tachikawa T., Kannagi R. (1996) Elevation of an α(1,3) fucosyltransferase activity correlated with apoptosis in the human colon adenocarcinoma cell line, HT-29. Glycoconj. J. 13, 1021–1029 [DOI] [PubMed] [Google Scholar]
- 48. Gadhoum S. Z., Sackstein R. (2008) CD15 expression in human myeloid cell differentiation is regulated by sialidase activity. Nat. Chem. Biol. 4, 751–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tsuruta O., Yuasa H., Hashimoto H., Kurono S., Yazawa S. (1999) Affinity of 5-thio-L-fucose-containing lewisX (LeX) trisaccharide analogs to anti-LeX monoclonal antibody. Bioorg. Med. Chem. Lett. 9, 1019–1022 [DOI] [PubMed] [Google Scholar]
- 50. Mehta S., Andrews J. S., Svensson B., Pinto B. M. (1995) Synthesis and enzymic activity of novel glycosidase inhibitors containing sulfur and selenium. J. Am. Chem. Soc. 117, 9783–9790 [Google Scholar]
- 51. Kannagi R. (1997) Carbohydrate-mediated cell adhesion involved in hematogenous metastasis of cancer. Glycoconj. J. 14, 577–584 [DOI] [PubMed] [Google Scholar]
- 52. Lühn K., Marquardt T., Harms E., Vestweber D. (2001) Discontinuation of fucose therapy in LADII causes rapid loss of selectin ligands and rise of leukocyte counts. Blood 97, 330–332 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.