Background: Rit signaling promotes cell survival.
Results: Rit controls stress-dependent p38-MSK1 signaling and CREB-mediated expression of anti-apoptotic Bcl-2 proteins.
Conclusion: Rit regulates a p38-MSK1-CREB survival cascade in cells adapting to stress.
Significance: Defining how diverse stimuli induce CREB-dependent gene expression is crucial to understanding complex cellular processes ranging from development to disease.
Keywords: CREB, MAP Kinases (MAPKs), Ras, Signal Transduction, Stress, Cell Survival, Mitogen- and Stress-activated Kinase
Abstract
The cAMP response element (CRE)-binding protein (CREB) is a key regulatory factor of gene transcription, and plays an essential role in development of the central nervous system and for neuroprotection. Multiple signaling pathways have been shown to contribute to the regulation of CREB-dependent transcription, including both ERK and p38 mitogen-activated protein (MAP) kinases cascades. Recent studies have identified the Ras-related small G-protein, Rit, as a central regulator of a p38-MK2-HSP27 signaling cascade that functions as a critical survival mechanism for cells adapting to stress. Here, we examine the contribution of Rit-p38 signaling to the control of stress-dependent gene transcription. Using a pheochromocytoma cell model, we find that a novel Rit-p38-MSK1/2 pathway plays a critical role in stress-mediated CREB activation. RNAi-mediated Rit silencing, or inhibition of p38 or MSK1/2 kinases, was found to disrupt stress-mediated CREB-dependent transcription, resulting in increased cell death. Furthermore, ectopic expression of active Rit stimulates CREB-Ser133 phosphorylation, induces expression of the anti-apoptotic Bcl-2 and BclXL proteins, and promotes cell survival. These data indicate that the Rit-p38-MSK1/2 signaling pathway may have an important role in the stress-dependent regulation of CREB-dependent gene expression.
Introduction
Cells are constantly exposed to environmental stresses and sophisticated adaptive mechanisms have evolved to afford protection from stress-mediated injury. A major component of the adaption process is regulation of gene expression. Protein kinases, including the extracellular signal-regulated kinase (ERK), c-Jun N-terminal protein kinase (JNK), and p38 mitogen-activated protein kinase (MAPK) pathways, play central roles in signal propagation to the nucleus in response to both mitogens and cellular stress, coordinating appropriate cellular responses including cell proliferation, differentiation, and survival (1). In particular, the JNK and p38 kinase pathways are activated in response to diverse chemical and physical stresses and are commonly associated with growth inhibition and apoptosis (2, 3). However, the physiological role for p38 signaling appears to be quite broad, functioning in a context-dependent manner to modulate cell proliferation, differentiation, migration, and cell survival (2–4). This is reflected in the diversity of cellular targets modulated by activated p38, including the direct phosphorylation of an array of cellular substrates. Among these targets, p38 regulates the activity of a diverse group of subordinate kinases, which in turn control the activity of additional cellular targets, including a number of transcription factors (5).
Activation of the cyclic AMP response element (CRE)3-binding protein (CREB), a member of the basic leucine zipper family of transcription factors that regulates expression of a large and diverse group of CRE-containing genes, is known to be involved in directing cellular responses to a variety of physiological stimuli, including neurotransmitters, growth factors, and cellular stress (6). In neurons, CREB-dependent gene expression has been implicated in the control of development and plasticity (7), but also contributes to neuroprotection by inducing a variety of target genes, including those that promote cellular survival such as B cell lymphoma 2 (Bcl-2) (8–10). Phosphorylation of CREB at serine 133 (Ser-133) is a critical step in activation, inducing an increase in CREB-dependent transcription by allowing the recruitment of co-activators such as CBP (CREB-binding protein) (6, 11). While cAMP-dependent protein kinase (PKA) was the first identified CREB-Ser-133 kinase, additional CREB kinases have been identified, including members of the calcium/calmodulin-dependent kinase family, and blockade of both ERK and p38 MAPK signaling has been found to inhibit mitogen- and stress-mediated CREB phosphorylation (6, 12). However, CREB phosphorylation is not catalyzed directly by MAPK family members, but instead by subordinate kinases under their control, including both ribosomal S6 kinase (RSK) and mitogen- and stress-activated protein kinase 1/2 (MSK1/2) kinases (13–15). Of these, MSK1/2 has been reported to mediate stress-induced phosphorylation of CREB, and is known to be activated in a p38-dependent fashion in response to cellular stress (13, 16, 17).
Ras-related GTP-binding proteins serve as critical regulators of a wide range of cellular process, functioning as molecular switches to control the activity of a variety of cellular effectors, including both ERK and p38 MAPK signaling pathways (18). Studies in primary neurons first suggested that Rit activates signaling pathways to control axonal and dendritic morphology (19, 20), and pheochromocytoma cell studies demonstrated a role for Rit signaling in the regulation of neural differentiation and survival (21–23). We have recently demonstrated that Rit serves as a central regulator of stress-activated MAPK regulation and pro-survival signaling (22, 24). Rit silencing renders cells susceptible to apoptosis and results in a disruption of stress-dependent p38 and Akt signaling. In part, Rit-dependent survival signaling was shown to involve an ability to couple p38 to the activation of a HSP27-MK2-Akt cascade (22, 24). However, p38 signaling has been implicated in the regulation of additional pro-survival cascades, in particular the activation of a number of transcriptional cascades, including the activation of MEF2 (25), β-catenin (26), and MSK1/2-CREB pathways (13, 16, 17). Here, we investigated the contribution of Rit signal transduction to stress-mediated CREB signaling. These studies identify a role for Rit in the regulation of a p38-MSK-CREB signaling pathway, and suggest that this signaling axis plays a critical role in stress-induced CREB transcriptional activity, particularly of pro-survival Bcl-2 family proteins.
MATERIALS AND METHODS
Plasmids and Reagents
Human Rit, HSP27, MK2, p38α/γ, and their mutants, and small hairpin shRNA reagents against rat Rit, MK2, and HSP27 as well as a control shRNA with no predicted target in the rat genome (shCTR) have been described previously (21, 22). TrueORF-targeted cDNA clones for CREB1b and MSK1 were purchased from Origene (Rockville, MD) and subcloned into either pCMV-Myc (BD-Clontech, San Diego) or p3XFlag-CMV-14 (Sigma) vectors and fully sequenced. Dominant-negative (DN) MSK1 (MSK1CKT-R101A, C-terminal kinase domain truncation (CKT) while bearing a R101A mutation) were made by subcloning followed by site-direct mutagenesis. DN-CREB constructs, K-CREB, and CREBS133A, were purchased from BD-Clontech (San Diego, CA). TNFα (R&D systems); etoposide (ET) (CalBiochem); hydrogen peroxide (H2O2) (Sigma); p38 inhibitor SB203580 (Tocris); MEK1/2 inhibitor PD98059, PI3K inhibitor LY294002, Akt inhibitor V (Triciribine), MSK1/2 inhibitor Ro-31–8220 (CalBiochem); and antibodies against caspase-3, phospho-p38, p38, phospho-ERK1/2, ERK1/2, phospho-Akt (Ser-473 and Thr-308), Akt, phospho-MK2 (Thr-334), MK2, phospho-MSK1 (Thr-581), Phospho-MSK1 (Ser-376), phospho-CREB (Ser-133), CREB, HSP27, Bcl-2, and BclXL (Cell Signaling); phospho-MSK1/2 (Ser-376/360) (R&D Systems); and actin, Flag (Sigma) were purchased.
Cell Culture and Transfections
PC6 cells were cultured and transfected as described previously (21, 22, 27). HEK293T cells were obtained from American Type Culture Collection (ATCC) and maintained in DMEM supplemented with 10% (v/v) FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Primary mouse embryonic fibroblasts (MEFs) cultures were established from E13.5 embryonic Rit-knock-out mice and their WT-littermates, and maintained in DMEM supplemented with 10% FBS and 100 μg/ml streptomycin and 100 units/ml penicillin in a 37 °C incubator with humidified atmosphere containing 5% CO2 as described previously (24). HEK293T cells and MEFs were transfected with TransginTM DNA Transfection Reagent (America Pharma Source (APS-Bio) Gaithersburg, MD) following the manufacturer's instructions.
Protein Expression and Phosphorylation Analysis
Whole cell lysates were prepared using kinase lysis buffer (20 mm Hepes (pH 7.4), 150 mm NaCl, 50 mm KF, 50 mm β-glycerolphosphate, 2 mm EGTA (pH 8.0), 1 mm Na3VO4, 1% Triton X-100, 10% glycerol, and 1× protease inhibitor mixture (Calbiochem)). Protein phosphorylation was determined by immunoblotting with appropriate phospho-specific antibodies as described previously (21, 22, 27). To determine whether Rit is capable of activating MSK1/2 and CREB, PC6 cells were transfected with either empty Flag vector (Flag-EV) or Flag-RitQ79L (2 μg) and enriched by G418 (400 μg/ml) selection prior to lysate preparation. To examine the contribution of Rit to stress-induced signaling cascade activation, PC6 cells were transfected with either shCTR or shRit208 (1.5 μg) in the presence of wild-type (WT) Myc-MSK1 and -CREB1b (0.25 μg, respectively), subjected to G418 selection for 48 h, prior to stimulation. To examine the contribution of signaling pathways to Rit-mediated CREB activation, PC6 cells were transfected with either Flag-EV or Flag-RitQ79L (1.5 μg) in the presence of Myc-MSK1-WT and Myc-CREB1-WTb (0.25 μg, respectively) after pretreatment with appropriate kinase-specific inhibitors, co-transfected with effector-specific DN-mutants (0.5 μg) or effector-specific shRNA (1.5 μg) (see Fig. 3), and transfected cells were enriched by G418 selection. The cells were subsequently starved in serum-free DMEM with or without kinase inhibitors for 5 h prior to cell lysate preparation. Similar experiments were performed in MEFs and HEK293 cells.
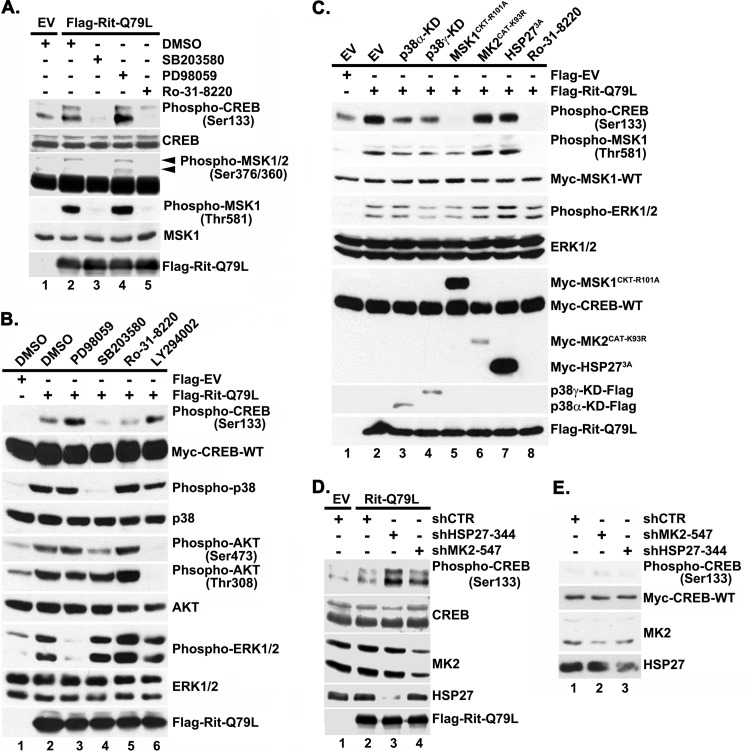
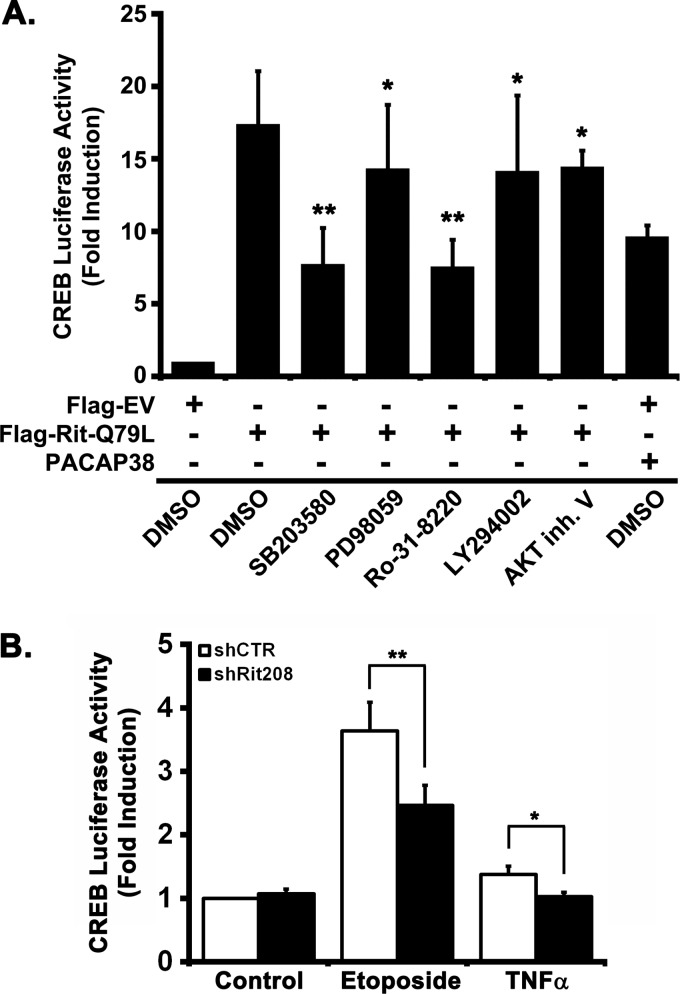
FIGURE 3.
Rit-mediated CREB activation requires p38-MSK1/2 signaling. A, Rit activates MSK1/2 and CREB in a p38-dependent manner. PC6 cells were pretreated with p38 (SB203580, 10 μm), MEK1/2 (PD98059, 10 μm), or MSK1/2 (Ro-31–8220, 2 μm) kinase inhibitors prior to transfection with Flag-RitQ79L (2 μg). Flag-EV transfection was used as control. Cell lysates were prepared after starvation (5 h) in the presence of appropriate inhibitors, and subjected to phospho-specific immunoblotting. The results are representative of three separate experiments. B, Rit-mediated CREB phosphorylation does not rely upon PI3 kinase. PC6 cells were transfected with either Flag-EV or Flag-RitQ79L after pretreatment with SB203580 (10 μm), PD98059 (10 μm), Ro-31–8220 (2 μm), LY294002 (10 μm), or vehicle (DMSO) as control, and cell lysates subjected to phospho-specific immunoblotting. C, CREB activation is inhibited by DN-p38 or DN-MSK1, but not DN-MK2 or DN-HSP27 expression. PC6 cells were transfected with either Flag-EV or Flag-RitQ79L (1.5 μg) together with DN-mutants of p38α-KD, p38γ-KD, MSK1CKT-R101A, MK2CAT-K93R, or HSP273A (0.5 μg). MSK1/2 inhibitor Ro-31-8220 (2 μm, 30 min) pretreatment was used as control. Cell lysates were examined with phospho-specific immunoblotting. Results are representative of four independent experiments. D, MK2 and HSP27 silencing does not inhibit Rit-mediated CREB phosphorylation. PC6 cells were transfected with either Flag-EV or Flag-RitQ79L (1.5 μg) in the presence or absence of shCTR, shMK2–547 or shHSP27–344 (1.5 μg) and subjected to G418 (400 μg/ml) selection. 60 h after transfection, cell lysates were prepared and phosphorylated CREBSer133 levels examined by immunoblotting. Results are representative of three independent experiments. E, PC6 cells were transfected with shCTR, shMK2–547, or shHSP27–344 (1.5 μg) and subjected to G418 selection as in D. Cell lysates were prepared and phosphorylated CREBSer133 levels examined by immunoblotting. Note that MK2 and HSP27 silencing does not stimulate CREB phosphorylation.
To examine the role of Rit on the regulation of Bcl-2 family member expression, HEK293T cells were transfected with either Flag-EV or Flag-RitQ79L (2 μg) and subjected to H2O2 treatment. To explore the signaling pathways contributing to Rit-mediated Bcl-2 expression, HEK293T cells were pretreated with the indicated kinase-specific inhibitors prior to transfection with either Flag-EV or Flag-RitQ79L (2 μg), and cell lysates were prepared at 48 h after transfection. Bcl-2 or BclXL protein abundance was determined by image quantification using ImageJ software (NIH, Bethesda) and fold induction calculated by normalizing to actin.
Caspase-3 Activation Assay
Cell death was examined by caspase-3 cleavage (22). To examine the contribution of MSK1 to Rit-mediated protection, PC6 cells expressing Flag-RitQ79L (1.5 μg) were either co-transfected with DN-MSK1 (MSK1CKT-R101A) or pretreated with the MSK1/2 inhibitor, Ro-31–8220 (2.5 μm), and exposed to etoposide (40 μm, 15 h), TNFα (50 ng/ml, 24 h), or H2O2 (200 μm, 24 h). To examine the contribution of CREB signaling to Rit-dependent survival, PC6 cells were transfected with either Flag-EV or Flag-RitQ79L (1.5 μg) in the presence or absence of two DN-CREB constructs, K-CREB and CREBS133A (0.5 μg each), and cell lysates were prepared following exposure to TNFα (100 ng/ml), H2O2 (200 μm), etoposide (40 μm), or sorbitol (0.2 m) for 6 h. Anti-caspase-3 immunoblotting of total cell lysates was used to detect levels of cleaved caspase-3.
CREB-luciferase Reporter Gene Assay
PC6 cells were transfected with either shCTR or shRit208 (2.0 μg) in the presence of CREB-luciferase reporter system including plasmids pFA2-CREB, pFR-Luci, and pRSV-β-gal, and subjected to starvation with serum-free DMEM for 5 h in the presence or absence of either etoposide (40 μm) or TNFα (50 ng/ml). To examine the contribution of signaling cascades to Rit-mediated CREB transcription, prior to Flag-EV or Flag-RitQ79L (2 μg) transfection, PC6 cells were pretreated with the appropriate kinase inhibitors. Cells were subjected to starvation for 5 h before luciferase activity has analyzed as described previously (28). PACAP38 (20 nm) stimulation was used as a positive control.
RESULTS
Rit Contributes to Stress-mediated MSK1/2 and CREB Activation
We recently demonstrated that Rit controls an evolutionarily conserved p38-dependent signaling pathway that functions as an important survival mechanism in cells responding to stress (22, 24). In particular, these studies identified a role for Rit in controlling a p38-HSP27-Akt pro-survival pathway. However, p38 MAPK signaling has been implicated in the regulation of several survival signaling cascades, including MSK1/2-CREB (13, 16, 17), and expression of an activated Rit mutant (RitQ79L) was used to explore whether Rit also functions to regulate CREB activity. In agreement with earlier studies, expression of activated Rit stimulated both ERK and Akt kinases (21). Activated Rit was also found to promote MSK1/2 activation and stimulate CREB-S133 phosphorylation (Fig. 1A).
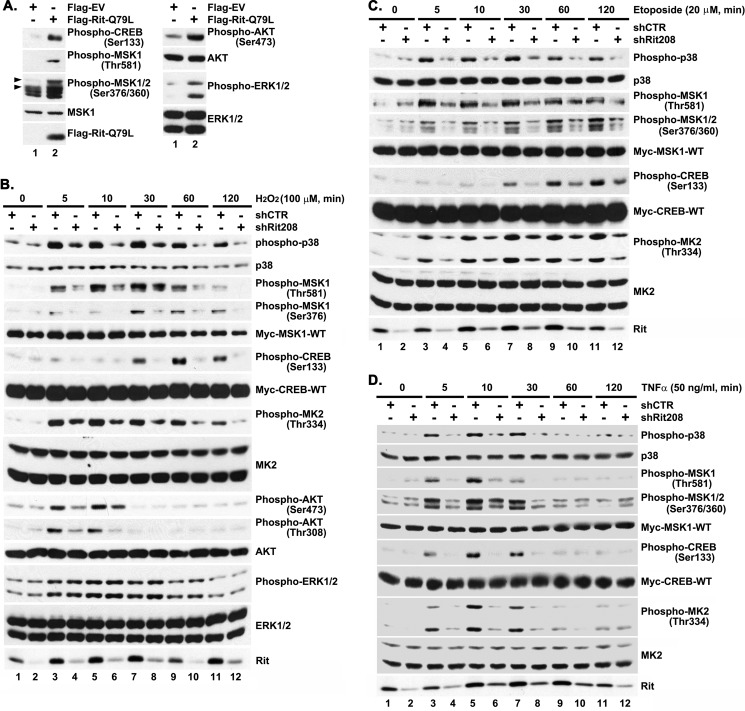
FIGURE 1.
Rit contributes to stress-mediated MSK1/2 and CREB activation. A, active Rit provokes MSK1/2 and CREB activation. PC6 cells expressing either Flag-EV or Flag-RitQ79L (2 μg) were serum-starved for 5 h prior to preparation of total cell lysates. The phosphorylation levels of individual protein were detected by immunoblotting with appropriate phospho-specific antibody as indicated. The results are representative of three individual experiments. B–D, Rit is required for stress-mediated MSK1/2 and CREB activation. PC6 cells were transfected with either shCTR or shRit208 (1.5 μg) in the presence of Myc-CREB-WT and Myc-MSK1-WT (0.25 μg, respectively) and subjected to G418 selection (400 μg/ml) for 60 h. Enriched PC6 cells were serum starved for 5 h prior to H2O2 (100 μm) (B), etoposide (20 μm) (C) or TNFα (50 ng/ml) (D) exposure for 5, 10, 30, 60, or 120 min, and total cell lysates subjected to phospho-specific immunoblotting. The results are representative of 3 to 5 independent experiments.
We next asked if Rit is required for stress-mediated MSK1/2-CREB activation. In agreement with our earlier studies (21), shRNAi-mediated Rit silencing (small hairpin shRit208) was found to dramatically suppress p38 and MK2 kinase activation downstream of a range of cellular stresses, including hydrogen peroxide (Fig. 1B), etopside (Fig. 1C), and tumor necrosis factor α (TNFα) (Fig. 1D). Importantly, Rit knockdown also inhibited both MSK1/2 activation and CREB phosphorylation downstream of these same stress stimuli (Fig. 1, B–D). Thus, Rit appears to serve as an upstream regulator of the MSK-CREB signaling pathway.
Rit-induced Survival Involves MSK1-CREB Signaling
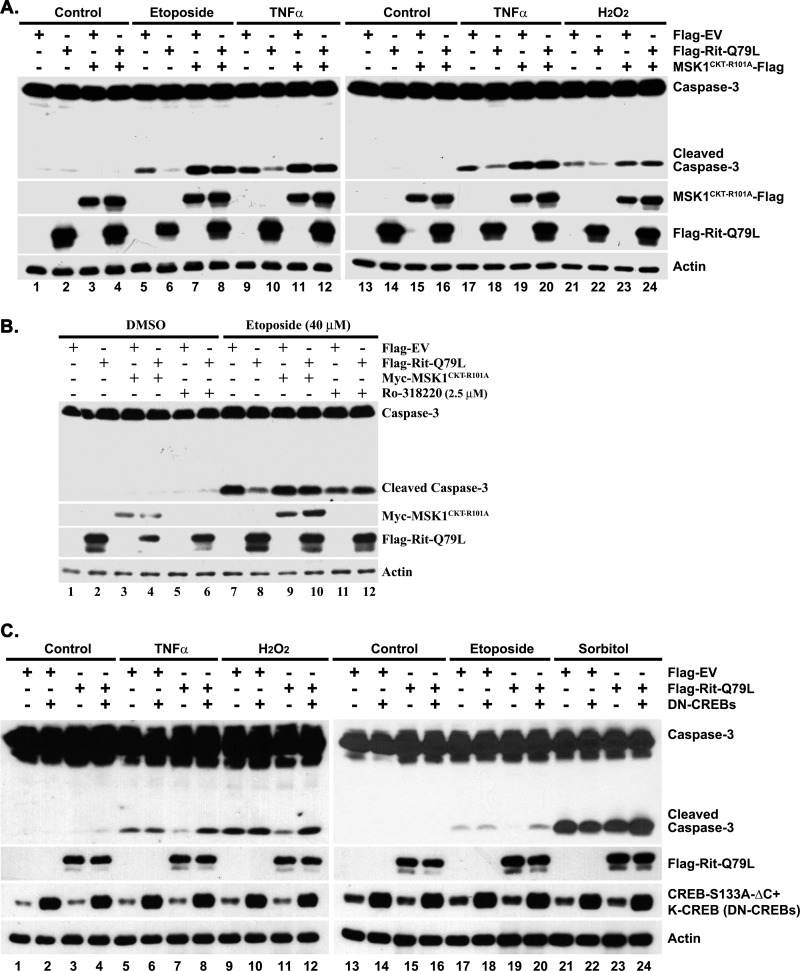
To ascertain the importance of MSK1/2-CREB signaling to Rit-mediated cell survival, PC6 cells were transfected with either a DN-MSK1 mutant (MSK1CKT-R101A) (13), or with a pair inactive CREB mutants (CREBS133A which disrupts phosphorylation-dependent transcription (29), and K-CREB which serves as a dominant repressor by forming an inactive dimer with endogenous CREB (30)) and monitored for cell survival. In agreement with our earlier work (22), expression of RitQ79L was sufficient to promote robust cell survival in response to a variety of stresses, including DNA-damaging agents (etoposide), reactive oxygen species (ROS: hydrogen peroxide, H2O2), and TNFα (Fig. 2A). However, co-expression with DN-MSK1, or exposure to Ro-318220 (2.5 μm), a MSK1/2 inhibitor (13), reversed the RitQ79L-mediated reduction in cleaved caspase-3 levels seen following stress exposure (Fig. 2, A and B). Likewise, co-expression of CREBS133A and K-CREB potently inhibited Rit-mediated survival signaling (Fig. 2C). Taken together, these studies suggest that MSK1/2-CREB signaling is critical for Rit-dependent survival.
FIGURE 2.
MSK1/2 and CREB contribute to Rit-mediated survival signaling. A, DN-MSK1 inhibits Rit-mediated cell survival. PC6 cells were transfected with either Flag-EV or Flag-RitQ79L (1.5 μg) in the presence or absence of DN-MSK1 (MSK1CKT-R101A-Flag) (0.5 μg), and cultured for additional 48 h before etoposide (40 μm, 15 h), TNFα (50 ng/ml, 24 h) or H2O2 (200 μm, 24 h) exposure. Immunoblotting was used to determine levels of cleaved caspase-3. The results are representative of three separate experiments. B, MSK1/2 kinase inhibitor, Ro-31–8220, inhibits Rit-dependent protection. PC6 cells were transfected with either Flag-EV or Flag-RitQ79L (1.5 μg) after pretreatment with Ro-31–8220 (2.5 μm, 30 min), or co-transfected with DN-MSK1 (Myc-MSK1CKT-R101A) (0.5 μg), and subjected to etoposide (40 μm, 15 h) exposure ∼60 h after transfection. Results are representative of three independent experiments. C, Rit-mediated reduction in caspase-3 cleavage is attenuated by CREB inhibition. PC6 cells were transfected with either Flag-EV or Flag-RitQ79L (1.5 μg) in the presence or absence of K-CREB and CREB-S133A (0.5 μg, respectively) and cultured for an additional 72 h before TNFα (100 ng/ml), H2O2 (200 μm), etoposide (40 μm), or sorbitol (0.2 m) stimulation (6 h). Cell death was monitored by anti-caspase-3 immunoblotting. Results are the representative of three independent studies.
Rit-dependent CREB Activation Requires p38 and MSK Signaling
Rit has been found to function as an upstream regulator of both ERK and p38 MAP kinase cascades (22), and these same MAP kinase cascades have been implicated as major cellular regulators of MSK1/2 activation (13). Mitogen signaling appears to activate MSKs predominantly through the ERK pathway, whereas stress stimuli appear to signal through the p38 MAPK cascade (13). Pharmacological inhibitor studies suggest that Rit-dependent MSK1/2 activation required p38 signaling, since SB203580 treatment, but not blockade of MEK/ERK (10 μm PD98059), inhibited RitQ79L-mediated MSK1 activation (Fig. 3A). p38 signaling was also required to couple active Rit to CREB phosphorylation, with either treatment with SB203580 (10 μm) or co-expression of kinase-dead (KD) p38α/γ mutants, but not blockade of MEK/ERK (10 μm PD98059) or PI3-kinase activity (10 μm, LY294002), inhibiting Rit-mediated CREB phosphorylation (Fig. 3, A and B). Incubation with Ro-318220 (2.5 μm), a potent MSK1/2 inhibitor along with other kinases (31), or co-expression of DN-MSK1 disrupted Rit-dependent MSK1 activation, but also inhibited CREB phosphorylation, suggesting that Rit-mediated CREB activation requires MSK1/2 (Fig. 3C).
Although stress-activated p38 transcriptional activation of immediate early genes is thought to predominantly involve MSK-mediated CREB activation, recent work suggests that p38-mediated activation of the MK2/3 kinases can also contribute to stress-induced gene expression (16). Since we have identified a role for Rit in regulating stress-dependent activation of a p38-MK2-HSP27 signaling cascade (22, 24), we next examined whether this pathway contributes to Rit-dependent CREB phosphorylation. As seen in Fig. 3C, co-expression of a DN-MK2 mutant (MK2CAT-K93R) or a HSP27 mutant incapable of undergoing phosphorylation-dependent changes in oligomerization state [HSP273A(S15/78/82A)] and thus compromised in its ability to promote cell survival (32), had no effect on Rit-mediated CREB phosphorylation. Furthermore, rather than blunting CREB activation, RNAi-mediated knockdown of endogenous MK2 (shMK2–547) or HSP27 (shHSP27–344) (Fig. 3D) was found to increase Rit-mediated CREB phosphorylation, but had no effect on CREB phosphorylation in vector control transfected cells (Fig. 3E). Taken together, these data suggest that p38-MSK1/2 signaling plays a prominent role in Rit-mediated CREB phosphorylation.
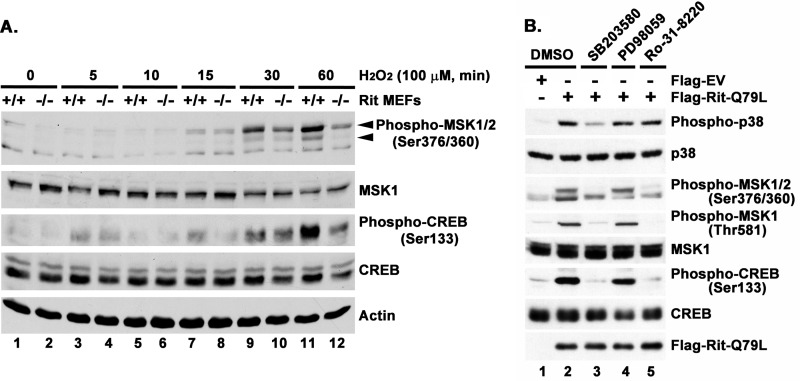
The importance of Rit to MSK-CREB signaling is not restricted to PC6 cells, as similar results were observed in primary MEFs. As seen in Fig. 4A, hydrogen peroxide-mediated activation of MSK-CREB signaling was compromised in Rit-null (Rit−/−) MEFs when compared with wild-type MEFs. Moreover, expression of active Rit promotes p38-MSK1-CREB signaling in immortalized WT-MEFs in a p38 and MSK1/2, but not MEK, -dependent fashion (Fig. 4B). These data support a general role for Rit in the regulation of p38-MSK1/2-CREB signaling.
FIGURE 4.
Rit regulates p38-MSK1/2-CREB signaling in MEFs. A, H2O2-induced MSK1/2 and CREB phosphorylation is blunted in Rit−/− MEFs. Primary MEFs (passage 4) were serum-starved (2 h) prior to H2O2 (100 μm) stimulation for the indicated duration. Cell lysates were subjected to immunoblotting with indicated antibodies. Results are representative of three individual experiments using MEFs established from different litters. B, Rit activates p38-MSK1/2-CREB signaling. Immortalized WT-MEFs were transfected with either Flag-EV or Flag-RitQ79L (2 μg) after pretreated with SB203580 (10 μm), PD98059 (10 μm), or Ro-31–8220 (2.5 μm) for 30 min. Vehicle DMSO pretreatment was used as control. MEFs (60 h post-transfection) were serum-starved (3 h), cell lysates prepared, and subjected to immunoblot analysis. The results are representative of two different experiments.
Rit Signaling Contributes to Stress-mediated CREB Transcriptional Activation
CREB has been identified as a critical stress-mediated transcriptional regulator in a wide range of cell process, and MSK1 has been found to contribute to both neurotrophin- and stress-mediated CREB activation (13, 16, 17, 33). Earlier studies identified a role for Rit in PACAP38-mediated CREB activation (28), so we next examined whether Rit also contributes to stress-mediated CREB activation. As seen in Fig. 5A, PC6 cells co-transfected with a CREB-regulated reporter and active Rit, resulted in a 17.4 ± 3.7-fold maximal induction. As expected, pharmacological inhibition of either p38 (SB203580; 10 μm) (7.7 ± 2.5-fold) or MSK1 (Ro-318220; 2.5 μm) (7.6 ± 1.7-fold) significantly inhibited RitQ79L-dependent CREB activation, while blockade of MEK/ERK (PD98059; 10 μm), PI3-kinase (LY294002; 10 μm), or Akt (Akt Inhibitor V; 2 μm) signaling resulted in more modest reduction, supporting a critical role for p38-MSK1/2 signaling in Rit-mediated CREB activation (Fig. 5A).
FIGURE 5.
Rit contributes to CREB-dependent transcription via p38 and MSK1/2. A, blockade of p38 and MSK1/2 signaling inhibits Rit-mediated CREB transcription. PC6 cells were pretreated (30 min) with SB203580 (10 μm, p38), PD98050 (10 μm, MEK1/2), Ro-31–8220 (2.5 μm, MSK1/2), LY294002 (10 μm, PI3K), or Akt inhibitor V (2 μm) prior to transfection with either Flag-EV or Flag-RitQ79L (2 μg) in the presence of the CREB-luciferase reporter system. Cells were serum-starved in the presence of kinase inhibitors for 5 h prior to analysis of luciferase activity as described under “Materials and Methods.” PACAP38 stimulation (20 nm, 5 h) served as positive control. Results are presented as mean ± S.D. from four independent experiments repeated in quadruplicate. *, p > 0.05; **, p < 0.001. B, Rit silencing attenuates stress-mediated CREB transcription. PC6 cells were transfected with either shCTR or shRit208 (2.0 μg) in the presence of pFA2-CREB, pFR-luci, and pRSV-β-gal. Following transfection (24 h), cells were treated with or without etoposide (40 μm) or TNFα (50 ng/ml) in serum-free DMEM (5 h), and analyzed by luciferase assay as described under “Materials and Methods.” Results are presented as mean ± S.D. calculated from three separate experiments in triplicate. *, p < 0.05; **, p < 0.001.
To test whether Rit contributes to stress-mediated CREB activation, PC6 cells were co-transfected with the CREB reporter and either a control shRNA (shCTR) with no predicted target in the rat genome, or shRit208. Stimulation of shCTR-transfected PC6 cells with either etoposide (40 μm) or TNFα (50 ng/ml) induced expression of the luciferase reporter, with a 3.6- and 1.5-fold induction, respectively (Fig. 5B). In contrast, shRit208-mediated Rit silencing resulted in a potent inhibition of reporter gene activity following etoposide stimulation, while also diminishing TNFα-mediated luciferase activity. Taken together, these data suggest that Rit signaling contributes to both etoposide and TNFα–mediated activation of CREB, through regulation of a p38-MSK1/2 kinase pathway.
p38 and MSK Kinases Contribute to Rit-dependent CREB Regulation
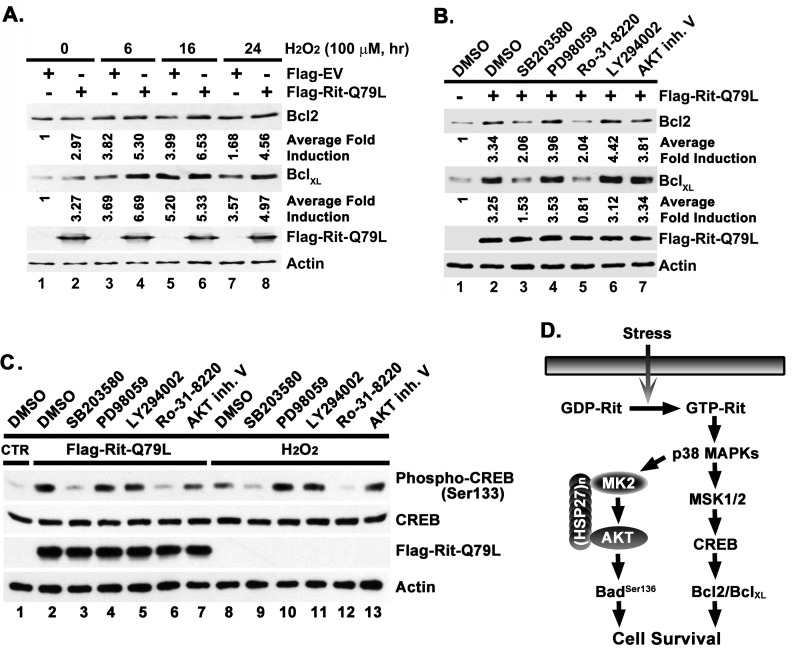
Stress is known to regulate gene expression by activating several transcriptional cascades. Although we have shown that Rit is critical for stress-induced CREB phosphorylation, and that this results in altered CREB-dependent reporter gene expression, it is conceivable that other regulatory pathways predominate at the level of endogenous gene expression. Therefore, we used immunoblotting to explore whether stress-mediated gene expression is affected by Rit silencing. We chose to study the Bcl-2 family members, Bcl-2 and BclXL, because a number of studies have shown that the CREB/CRE transcriptional pathway regulates Bcl-2/BclXL expression (10, 34) and elevated levels of Bcl-2/BclXL are positively correlated with cell survival (35, 36). We were unable to identify commercial antibodies that detected endogenous Bcl-2 proteins from PC6 cells. However, these same reagents recognized endogenous human protein, necessitating a change to HEK293 cells for the remaining studies. Exposure of HEK293 cells to hydrogen peroxide (H2O2; 100 μm) resulted in a 3.8-fold increase in Bcl-2 protein levels, which remained elevated for 24 h (Fig. 6A). We also examined the BclXL protein, with similar results. Consistent with its known role in promoting cellular survival, expression of activated Rit was alone sufficient to promote a ∼3-fold increase in Bcl-2 (3-fold) and BclXL (3.3-fold) levels in PC6 cells, and further enhanced hydrogen peroxide-mediated Bcl-2/BclXL expression to >5-fold (Fig. 6A). In keeping with our earlier analysis, inhibition of p38-MSK1/2 signaling, but not blockade of MEK/ERK, PI3-kinase, or Akt activity, significantly blunted Rit-dependent increases in Bcl-2 and BclXL (Fig. 6B). As expected, the induction of CREBSer133 phosphorylation in HEK293 cells by either overexpression of active Rit or stimulation with H2O2 was inhibited by inhibition of p38 or MSK1/2, but not by blockade of MEK, PI3K, or Akt activity (Fig. 6C). Taken together, these studies suggest that the p38-MSK1/2-CREB pathway plays a critical role in Rit-mediated cell survival, in part by regulating the expression of the anti-apoptotic Bcl-2 and BclXL proteins (Fig. 6D).
FIGURE 6.
Rit-dependent regulation of Bcl-2/BclXL expression requires p38-MSK1/2 signaling. A, Rit stimulates expression of pro-survival Bcl-2 family members. HEK293T cells expressing either Flag-EV or Flag-RitQ79L (2 μg) were subjected to H2O2 (100 μm, 6, 16, or 24 h) treatment beginning 24 h after transfection, and expression of Bcl-2 and BclXL determined by immunoblotting. Average fold induction was calculated from three individual experiments as described under “Materials and Methods.” B, Rit-mediated Bcl-2 expression is disrupted by p38 and MSK1/2 inhibition. HEK293T cells were transfected with Flag-RitQ79L (2 μg) after pretreatment with kinase inhibitors as described in panel A. Following transfection (48 h) immunoblotting was used to examine Bcl-2 and BclXL expression. Representative data from three separate experiments are shown. Average fold induction was calculated as described under “Material and Methods.” C, Rit- and H2O2-dependent CREB activation relies upon p38-MSK signaling in HEK293 cells. HEK293T cells were pretreated with the kinase inhibitors, SB203580 (10 μm), PD98059 (10 μm), LY294002 (10 μm), Ro-31–8220 (2.5 μm), or Akt inhibitor V (2 μm) for 30 min, and then transfected with either Flag-EV (lanes 1 and 8–13) or Flag-RitQ79L (lanes 2–7) (2 μg). Cells were allowed to recover (36 h) and then starved in serum-free DMEM (5 h) prior to stimulation with or without H2O2 (100 μm) for 30 min. Phosphorylated CREB was detected by immunoblotting of total cell lysates. Results are representative of three independent experiments. D, schematic diagram of putative Rit-p38-dependent survival signaling cascades.
DISCUSSION
In this study, we have demonstrated that activation of a Rit-p38-MSK signaling pathway participates in the regulation of CREB-dependent transcription in response to environmental stress, playing a role in cellular adaptation to stress, by stimulating a CREB-mediated pro-survival transcriptional program. These data build upon earlier studies demonstrating a crucial role for Rit in the survival of cells in response to cellular stress (22, 24). Thus, in addition to regulating a p38-dependent Akt survival signaling cascade (22, 24), Rit acts via MSK to stimulate CREB transcriptional activity, resulting in the increased expression of anti-apoptotic members of the Bcl-2 family, including Bcl-2 and BclXL.
CREB and CREB-dependent gene expression has been implicated in promoting survival in a variety of tissues in response to protective stimuli, and CREB synthesis and phosphorylation promotes the expression of a large and diverse number of genes (6, 8, 9, 11). Within the central nervous system (CNS), CREB is essential for normal neuronal development and for neuroprotection against a range of pathophysiological stimuli (7, 8, 37). Previous studies have demonstrated that a variety of kinase signaling pathways can contribute to stress-mediated phosphorylation of CREB in PC12 cells and neurons (6, 11), and disruption of CREB-phosphorylation at Ser-133 has been shown to increase the vulnerability of neurons to cytotoxic stress (38). The exact molecular mechanisms involved in stress-dependent CREB activation depend upon both cell-type and stimulus-specific factors (6). MAPK signaling pathways are known to play critical roles in both mitogen- and stress-mediated CREB activation (39) by regulating the actions of a second tier of ERK and p38-regulated kinases known to mediate direct CREBSer133 phosphorylation (6). The p90rsk kinases are regulated in an ERK-dependent fashion and several RSK isoforms have been shown to mediate CREB phosphorylation. Indeed, RSK-2 has been reported to mediate CREB phosphorylation in response to nerve growth factor (NGF) stimulation (15). While Rit has been found to contribute to NGF-mediated ERK activation in pheochromocytoma cell (21), inhibitor studies indicate that Rit-dependent CREB activation does not rely upon ERK signaling in response to stress (Fig. 3, A and B). However, it will be important in future to examine whether Rit might contribute to neurotrophin-mediated CREB activation and the role of Rit-ERK signaling to survival. Indeed, Rit has been found to contributes to PACAP38-dependent CREB transcriptional activation (28).
In pheochromocytoma cells adapting to a variety of stresses, we found that Rit stimulated CREB predominantly via a p38-dependent signaling mechanism. Rit silencing generated a block in stress-mediated p38 activation, blunting the subsequent activation of both the p38 subordinate kinases MK2 and MSK1 (Fig. 1), but did not alter the amplitude or duration of stress-dependent ERK activation. Examining the potential contribution of these two kinase pathways to CREB regulation, we demonstrate a role central for MSK1/2, but not MK2 activity in Rit-dependent and stress-mediated CREB activation (Fig. 3). Since recent studies have shown that Rit regulates a p38-MK2-HSP27-Akt survival cascade in cells adapting to stress (22), and Akt has been identified as an upstream regulator of CREB (40), we anticipated a role for p38-MK2 signaling in CREB activation. However, inhibition of this cascade using overexpression of dominant negative mutant proteins and shRNAi methods failed to significantly disrupt Rit-mediated CREB activation (Fig. 3, C and D). The relative contributions of MK2-Akt and MSK1-CREB-mediated pathways to Rit-p38-mediated pro-survival signaling await further characterization.
Our data suggest that the p38-MSK-CREB signaling axis contributes to Rit-mediated cellular stress resistance, at least in part, through regulation of the pro-survival Bcl-2 family members, Bcl-2 and BclXL (Figs. 4 and 5), although these studies do not preclude Rit-mediated control of translational or post-translational regulatory mechanisms. Indeed, we have previously shown that Bad, a pro-apoptotic member of the Bcl-2 family, is phosphorylated at Ser-136 by Rit-p38-Akt signaling, which leads to sequestration of phosphorylated Bad in the cytosol (24). This prevents Bad from associating with mitochondria where it disrupts the interaction between Bax and Bcl-2/BclXL proteins, leading to outer membrane permeability and apoptosis (41). Thus, Rit signaling appears to promote cell survival in response to oxidative stress by both increasing cellular levels of anti-apoptotic Bcl-2 family members and inactivating pro-apoptotic members of the family (Fig. 6D).
A major finding of the present study is that Rit plays a significant upstream role in coupling a variety of cellular stresses to the MSK-CREB signaling pathway (Fig. 1, B and C), with p38-MSK1/2 signaling playing a critical role in Rit-dependent CREBSer133 phosphorylation and cell survival (Figs. 2 and 3). This is consistent with recent studies using knock-out animals that have shown that MSK1 and MSK2 are required for stress-induced CREB activation, but that CREB phosphorylation has reduced but not eliminated following mitogen-stimulation (17). While MSK1 and MSK2 have been shown to be activated via both ERK and p38 MAPK pathways (4, 42), and Rit has been found to contribute to regulation of both MAPK cascades downstream of neurotrophin stimulation (21–23), our analysis indicates that p38 is the critical kinase operating downstream of Rit (Fig. 3, B and C). This is in agreement with a variety of studies in which the requirement for ERK or p38 signaling in MSK1/2 activation depends largely upon the nature of the cellular stimuli. For example, MSK activation by stimuli such as EGF, which predominantly activates ERK but not p38, is blocked by disruption of ERK activation (13, 17, 43), while MSK activation by stress stimuli such as anisomyosin is disrupted by p38 inhibitor treatment (13, 17). In PC6 cells, we previously identified a role for Rit in PACAP38-mediated CREB activation (28), and again p38 was shown to be critical. While, several Ras family GTPases have been identified as regulators of MAPK-dependent CREB activation, including Ras and Rap1 (10, 44), these GTPases regulate CREB in an ERK-dependent fashion. Thus, Rit might play a particularly important role in coupling stress to p38-MSK-dependent CREB activation. However, it is currently unclear whether ERK and p38 are functionally related or represent two distinct signaling cascades that regulate CREBSer133 phosphorylation, and how individual Ras family GTPases may be involved in directing stimulus-specific transcriptional responses. Whereas cAMP-dependent protein kinase (PKA) was the first CREBSer133 kinase to be described, reports have implicated additional kinase cascades, including PKC, and PI-3 kinase, in the regulation of CREBSer133 phosphorylation (12), and it is likely that a complex interplay of these kinase cascades are involved in generating the stimulus and cell-specific differences in CREB phosphorylation. Rit is known to be activated by a novel cAMP-Epac signaling cascade (28), and previous work indicates that Rit serves as a critical regulator of cAMP-mediated CREB activation. These data indicate that Rit-p38 signaling is important for the regulation of CREB function by both cAMP and diverse stress signals. Studies are underway to explore how the Rit-p38-MSK cascade is integrated into the complex interplay of kinases that appear to under CREB regulation.
In conclusion, we find that stimulation of a Rit-p38-MSK signaling pathway is important for the regulation of CREB function in response to a variety of stresses. Activation of the Rit-p38-MSK-CREB pathway leads to an increase in the anti-apoptotic proteins Bcl-2 and BclXL, promoting pheochromocytoma cell survival, and suggests that the Rit-p38 signaling pathway is important for controlling cellular adaptation to stress. Within the CNS, CREB has been shown to be essential for normal neuronal development, and is also a key regulator of neuroprotective signaling, promoting survival against a range of cellular stresses (10, 13, 45–47). CREB-dependent gene expression has also been implicated in activity dependent modulation of synaptic plasticity, which is critical for adaptive processes like learning and memory (7, 48). Because Rit has been found to contribute to neuronal morphogenesis by determining axonal versus dendritic growth responses (20), it is of great interest to examine whether Rit-p38-MSK signaling may contribute to the regulation of axonal versus dendritic growth in response to environmental cues. Further study of Rit knock-out mice, particularly stress-mediated gene expression should help clarify the role of Rit signaling in these processes.
Acknowledgment
We thank Dr. Matthew Gentry for critical reading of the manuscript.
This work was supported by the United States Public Health Service Grant NS045103 (to D. A. A.) from the National Institute of Neurological Disorders and Stroke and in part by National Institutes of Health Grant P20GM103486 from the National Institute of General Medical Sciences.
- CRE
- cyclic AMP response element
- Bcl-2
- B-cell lymphoma 2
- CKT
- C-terminal kinase domain truncation
- CREB
- CRE-binding protein
- MEFs
- mouse embryonic fibroblasts
- MSK1/2
- mitogen- and stress-activated protein kinases 1 and 2
- RSK
- ribosomal S6 kinase.
REFERENCES
- 1. Pearson G., Robinson F., Beers Gibson T., Xu B. E., Karandikar M., Berman K., Cobb M. H. (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22, 153–183 [DOI] [PubMed] [Google Scholar]
- 2. Kyriakis J. M., Avruch J. (2001) Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81, 807–869 [DOI] [PubMed] [Google Scholar]
- 3. Wagner E. F., Nebreda A. R. (2009) Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 9, 537–549 [DOI] [PubMed] [Google Scholar]
- 4. Roux P. P., Blenis J. (2004) ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68, 320–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cuadrado A., Nebreda A. R. (2010) Mechanisms and functions of p38 MAPK signalling. Biochem. J. 429, 403–417 [DOI] [PubMed] [Google Scholar]
- 6. Altarejos J. Y., Montminy M. (2011) CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 12, 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lonze B. E., Ginty D. D. (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35, 605–623 [DOI] [PubMed] [Google Scholar]
- 8. Benito E., Barco A. (2010) CREB's control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci. 33, 230–240 [DOI] [PubMed] [Google Scholar]
- 9. Benito E., Valor L. M., Jimenez-Minchan M., Huber W., Barco A. (2011) cAMP response element-binding protein is a primary hub of activity-driven neuronal gene expression. J. Neurosci. 31, 18237–18250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Riccio A., Ahn S., Davenport C. M., Blendy J. A., Ginty D. D. (1999) Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 286, 2358–2361 [DOI] [PubMed] [Google Scholar]
- 11. Sands W. A., Palmer T. M. (2008) Regulating gene transcription in response to cyclic AMP elevation. Cell. Signal. 20, 460–466 [DOI] [PubMed] [Google Scholar]
- 12. Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. (1988) Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature 334, 494–498 [DOI] [PubMed] [Google Scholar]
- 13. Deak M., Clifton A. D., Lucocq L. M., Alessi D. R. (1998) Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 17, 4426–4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Impey S., Obrietan K., Wong S. T., Poser S., Yano S., Wayman G., Deloulme J. C., Chan G., Storm D. R. (1998) Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron 21, 869–883 [DOI] [PubMed] [Google Scholar]
- 15. Xing J., Ginty D. D., Greenberg M. E. (1996) Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science 273, 959–963 [DOI] [PubMed] [Google Scholar]
- 16. Ronkina N., Menon M. B., Schwermann J., Arthur J. S., Legault H., Telliez J. B., Kayyali U. S., Nebreda A. R., Kotlyarov A., Gaestel M. (2011) Stress induced gene expression: a direct role for MAPKAP kinases in transcriptional activation of immediate early genes. Nucleic Acids Res. 39, 2503–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wiggin G. R., Soloaga A., Foster J. M., Murray-Tait V., Cohen P., Arthur J. S. (2002) MSK1 and MSK2 are required for the mitogen- and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Mol. Cell. Biol. 22, 2871–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Colicelli J. (2004) Human RAS superfamily proteins and related GTPases. Sci. STKE 2004, RE13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andres D. A., Shi G. X., Bruun D., Barnhart C., Lein P. J. (2008) Rit signaling contributes to interferon-γ-induced dendritic retraction via p38 mitogen-activated protein kinase activation. J. Neurochem. 107, 1436–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lein P. J., Guo X., Shi G. X., Moholt-Siebert M., Bruun D., Andres D. A. (2007) The novel GTPase Rit differentially regulates axonal and dendritic growth. J. Neurosci. 27, 4725–4736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi G. X., Andres D. A. (2005) Rit contributes to nerve growth factor-induced neuronal differentiation via activation of B-Raf-extracellular signal-regulated kinase and p38 mitogen-activated protein kinase cascades. Mol. Cell. Biol. 25, 830–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi G. X., Jin L., Andres D. A. (2011) A rit GTPase-p38 mitogen-activated protein kinase survival pathway confers resistance to cellular stress. Mol. Cell. Biol. 31, 1938–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spencer M. L., Shao H., Andres D. A. (2002) Induction of neurite extension and survival in pheochromocytoma cells by the Rit GTPase. J. Biol. Chem. 277, 20160–20168 [DOI] [PubMed] [Google Scholar]
- 24. Cai W., Rudolph J. L., Harrison S. M., Jin L., Frantz A. L., Harrison D. A., Andres D. A. (2011) An evolutionarily conserved Rit GTPase-p38 MAPK signaling pathway mediates oxidative stress resistance. Mol. Biol. Cell 22, 3231–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mao Z., Bonni A., Xia F., Nadal-Vicens M., Greenberg M. E. (1999) Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science 286, 785–790 [DOI] [PubMed] [Google Scholar]
- 26. Thornton T. M., Pedraza-Alva G., Deng B., Wood C. D., Aronshtam A., Clements J. L., Sabio G., Davis R. J., Matthews D. E., Doble B., Rincon M. (2008) Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science 320, 667–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi G. X., Jin L., Andres D. A. (2010) Src-dependent TrkA transactivation is required for pituitary adenylate cyclase-activating polypeptide 38-mediated Rit activation and neuronal differentiation. Mol. Biol. Cell 21, 1597–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shi G. X., Rehmann H., Andres D. A. (2006) A novel cyclic AMP-dependent Epac-Rit signaling pathway contributes to PACAP38-mediated neuronal differentiation. Mol. Cell. Biol. 26, 9136–9147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gonzalez G. A., Montminy M. R. (1989) Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59, 675–680 [DOI] [PubMed] [Google Scholar]
- 30. Ahn S., Olive M., Aggarwal S., Krylov D., Ginty D. D., Vinson C. (1998) A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol. Cell. Biol. 18, 967–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caivano M., Cohen P. (2000) Role of mitogen-activated protein kinase cascades in mediating lipopolysaccharide-stimulated induction of cyclooxygenase-2 and IL-1β in RAW264 macrophages. J. Immunol. 164, 3018–3025 [DOI] [PubMed] [Google Scholar]
- 32. Rane M. J., Pan Y., Singh S., Powell D. W., Wu R., Cummins T., Chen Q., McLeish K. R., Klein J. B. (2003) Heat shock protein 27 controls apoptosis by regulating Akt activation. J. Biol. Chem. 278, 27828–27835 [DOI] [PubMed] [Google Scholar]
- 33. Arthur J. S., Fong A. L., Dwyer J. M., Davare M., Reese E., Obrietan K., Impey S. (2004) Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins. J. Neurosci. 24, 4324–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilson B. E., Mochon E., Boxer L. M. (1996) Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol. Cell. Biol. 16, 5546–5556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bonfanti L., Strettoi E., Chierzi S., Cenni M. C., Liu X. H., Martinou J. C., Maffei L., Rabacchi S. A. (1996) Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing bcl-2. J. Neurosci. 16, 4186–4194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang E., Korsmeyer S. J. (1996) Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood 88, 386–401 [PubMed] [Google Scholar]
- 37. Merz K., Herold S., Lie D. C. (2011) CREB in adult neurogenesis–master and partner in the development of adult-born neurons? Eur. J. Neurosci. 33, 1078–1086 [DOI] [PubMed] [Google Scholar]
- 38. Hardingham G. E., Fukunaga Y., Bading H. (2002) Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nature Neurosci. 5, 405–414 [DOI] [PubMed] [Google Scholar]
- 39. Xing J., Kornhauser J. M., Xia Z., Thiele E. A., Greenberg M. E. (1998) Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol. Cell. Biol. 18, 1946–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Du K., Montminy M. (1998) CREB is a regulatory target for the protein kinase Akt/PKB. J. Biol. Chem. 273, 32377–32379 [DOI] [PubMed] [Google Scholar]
- 41. Yang E., Zha J., Jockel J., Boise L. H., Thompson C. B., Korsmeyer S. J. (1995) Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell 80, 285–291 [DOI] [PubMed] [Google Scholar]
- 42. Gaestel M. (2006) MAPKAP kinases - MKs - two's company, three's a crowd. Nat. Rev. Mol. Cell Biol. 7, 120–130 [DOI] [PubMed] [Google Scholar]
- 43. McCoy C. E., Campbell D. G., Deak M., Bloomberg G. B., Arthur J. S. (2005) MSK1 activity is controlled by multiple phosphorylation sites. Biochem. J. 387, 507–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grewal S. S., Fass D. M., Yao H., Ellig C. L., Goodman R. H., Stork P. J. (2000) Calcium and cAMP signals differentially regulate cAMP-responsive element-binding protein function via a Rap1-extracellular signal-regulated kinase pathway. J. Biol. Chem. 275, 34433–34441 [DOI] [PubMed] [Google Scholar]
- 45. Bonni A., Brunet A., West A. E., Datta S. R., Takasu M. A., Greenberg M. E. (1999) Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286, 1358–1362 [DOI] [PubMed] [Google Scholar]
- 46. Lee B., Butcher G. Q., Hoyt K. R., Impey S., Obrietan K. (2005) Activity-dependent neuroprotection and cAMP response element-binding protein (CREB): kinase coupling, stimulus intensity, and temporal regulation of CREB phosphorylation at serine 133. J. Neurosci. 25, 1137–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Walton M., Woodgate A. M., Muravlev A., Xu R., During M. J., Dragunow M. (1999) CREB phosphorylation promotes nerve cell survival. J. Neurochem. 73, 1836–1842 [PubMed] [Google Scholar]
- 48. Barco A., Alarcon J. M., Kandel E. R. (2002) Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell 108, 689–703 [DOI] [PubMed] [Google Scholar]