Background: (Glyco)lipid translocation across the plasma membrane plays a pivotal role in the biogenesis of the mycobacterial cell envelope.
Results: The SMR-likes transporter Rv3789 appears to be involved in reorienting decaprenol phosphate arabinose to the periplasm.
Conclusion: Rv3789 participates in the buildup of the arabinan domains of arabinogalactan and lipoarabinomannan.
Significance: This is the first lipid-linked sugar translocase ever identified in mycobacteria.
Keywords: Cell Wall, Glycoconjugate, Lipid Transport, Mycobacterium Tuberculosis, Polysaccharide, Arabinogalactan, Decaprenol Phosphate Arabinose, Lipoarabinomannan, Translocase
Abstract
The biosynthesis of the major cell envelope glycoconjugates of Mycobacterium tuberculosis is topologically split across the plasma membrane, yet nothing is known of the transporters required for the translocation of lipid-linked sugar donors and oligosaccharide intermediates from the cytoplasmic to the periplasmic side of the membrane in mycobacteria. One of the mechanisms used by prokaryotes to translocate lipid-linked phosphate sugars across the plasma membrane relies on translocases that share resemblance with small multidrug resistance transporters. The presence of an small multidrug resistance-like gene, Rv3789, located immediately upstream from dprE1/dprE2 responsible for the formation of decaprenyl-monophosphoryl-β-d-arabinose (DPA) in the genome of M. tuberculosis led us to investigate its potential involvement in the formation of the major arabinosylated glycopolymers, lipoarabinomannan (LAM) and arabinogalactan (AG). Disruption of the ortholog of Rv3789 in Mycobacterium smegmatis resulted in a reduction of the arabinose content of both AG and LAM that accompanied the accumulation of DPA in the mutant cells. Interestingly, AG and LAM synthesis was restored in the mutant not only upon expression of Rv3789 but also upon that of the undecaprenyl phosphate aminoarabinose flippase arnE/F genes from Escherichia coli. A bacterial two-hybrid system further indicated that Rv3789 interacts in vivo with the galactosyltransferase that initiates the elongation of the galactan domain of AG. Biochemical and genetic evidence is thus consistent with Rv3789 belonging to an AG biosynthetic complex, where its role is to reorient DPA to the periplasm, allowing this arabinose donor to then be used in the buildup of the arabinan domains of AG and LAM.
Introduction
Similar to (lipo)polysaccharide, glycoprotein and peptidoglycan synthesis in any prokaryotic organism, the biogenesis of the cell envelope glycoconjugates of Mycobacterium tuberculosis involves catalytic steps on both sides of the plasma membrane. In the biosynthesis of phosphatidyl-myo-inositol mannosides (PIMs)6 and metabolically related lipomannan (LM) and lipoarabinomannan (LAM), for instance, it is now clear that whereas the first two to three mannosylation steps of PIM occur on the cytoplasmic side of the membrane, further steps in the biosynthesis of the more highly mannosylated PIMs LM and LAM rely upon polyprenyl-phospho-mannose (PPM) and -arabinose-dependent integral membrane glycosyltransferases and take place on the periplasmic face of the membrane (1). Likewise, enzymatic evidence points to biosynthetic compartmentalization in the case of the major cell wall polysaccharide of mycobacteria, arabinogalactan (AG). AG synthesis is initiated in the cytoplasm on a decaprenyl phosphate (Dec-P) molecule with formation of the “linker unit” (Dec-P-P-GlcNAc-Rha) followed by the addition of Galf residues from UDP-Galf by two cytosolic galactosyltransferases (GalTs), GlfT1 and GlfT2. Polyprenyl-monophosphoryl-β-d-arabinose (DPA) being the only known Araf donor in mycobacteria (2), it is expected that the arabinosylation of AG, like that of LAM, is next catalyzed by membrane-associated polyprenyl-dependent arabinosyltransferases (AraTs) on the periplasmic side of the plasma membrane (1, 3). Because the unassisted transbilayer movement of polar (glyco)lipids across the plasma membrane is energetically unfavorable (4, 5) and certainly insufficient to satisfy the requirement for glycoconjugate biosynthesis in mycobacteria, such a compartmentalization implies that translocases (or “flippases”) translocate polyprenyl-phospho-sugars, PIM intermediates, and AG precursors from the cytoplasmic to the periplasmic side of the plasma membrane. In part because of the poor sequence similarity shared by prokaryotic transporters and a general lack of identifiable motifs in their primary sequence, none of the transporters involved in these processes have yet been identified in mycobacteria.
Despite the structural diversity of glycoconjugates produced by bacteria, similarities in the mechanisms of polymerization and transport of LPS, capsular polysaccharides, peptidoglycan, and N-linked glycosylated proteins are known, and to date, only four major mechanisms of translocation of the lipid-linked intermediates involved in their biosynthesis have been described (6–13). One of them relies on EmrE-like small multidrug resistance (SMR) transporters (14), typically 105–121 amino acids in size and containing four transmembrane domains, to translocate lipid-linked sugars from the cytosolic to the periplasmic side of the plasma membrane. One example includes the hetero-oligomeric undecaprenyl phosphate 4-amino-4-deoxy-l-arabinose translocase encoded by arnE/arnF in Escherichia coli (15). Although biochemically not as well characterized, another example is the GtrA protein produced by the bacteriophage SfX of Shigella flexneri, which is required for O-antigen glucosylation and was proposed to mediate the translocation of undecaprenyl phosphate glucose across the plasma membrane (16, 17). The presence of an SMR transporter-like gene, Rv3789, located within a cell wall biosynthetic cluster of M. tuberculosis adjacent to the DPA-forming genes dprE1/dprE2 led us to investigate its potential involvement in the biogenesis of the major arabinosylated cell envelope glycopolymers of mycobacteria.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
M. tuberculosis H37Rv ATCC 25618 were grown in minimal Sauton's medium, Middlebrook 7H11-OADC agar, and 7H9-OADC medium supplemented with 0.05% Tween 80. Mycobacterium smegmatis mc2155 was grown on 7H11-OADC or LB agar and in LB broth supplemented with 0.05% Tween 80. Kanamycin and hygromycin were added to final concentrations of 20 and 50 μg ml−1, respectively.
Disruption of Rv3789 in M. tuberculosis and Ortholog in M. smegmatis
The Ts/sacB method was used to achieve allelic replacement at the Rv3789 locus of M. tuberculosis H37Rv and orthologous MSMEG_6372 locus of M. smegmatis (18). To this end, the Rv3789 gene and flanking regions was PCR-amplified from M. tuberculosis H37Rv genomic DNA using primers Rv3789.fwd (5′-cagttctagatgaggaactcgacacccacggc-3′) and Rv3789.rev (5′-agcttctagacgaacatgtccagcggatggtag-3′), and a disrupted allele, Rv3789::kan, was obtained by inserting the Tn903 kanamycin resistance cassette at the BsrGI restriction site of Rv3789. Rv3789::kan was then cloned into the XbaI-cut pPR27-xylE (18), yielding pPR27Rv3789KX. MSMEG_6372 was PCR-amplified from M. smegmatis mc2155 genomic DNA with primers smg3789.1 (5′-ggtctagagctcgggggtgcgaaacggcg-3′) and smg3789.2 (5′-cctctagagacgccgtcatccagcccacc-3′), and a disrupted allele, MSMEG_6372::kan, was obtained by inserting the Tn903 kanamycin resistance cassette at the unique AgeI restriction site of MSMEG_6372. MSMEG_6372::kan was then cloned into the XbaI-cut pPR27-xylE, yielding pPR27SMEG_6372KX. The replicative plasmid used to complement the knock-out mutants, pVV16-Rv3789, was constructed by PCR-amplifying the entire coding sequence of Rv3789 from M. tuberculosis H37Rv genomic DNA using primers Rv3789.1 (5′-gggcccgcatatgcggttcgttgtcaccggc-3′) and Rv3789.2 (5′-cccaagcttgcggatccggaagatcacggc-3′) and cloning this fragment into the expression vector pVV16 (19). This construct leads to the constitutive production of a C-terminal His6-tagged recombinant Rv3789 protein under control of the hsp60 promoter.
Expression of Gram-negative Flippases and Other Mycobacterial Inner Membrane Transporters in M. smegmatis
Genes encoding Gram-negative polyprenol-linked oligosaccharide translocases (pglK from Campylobacter jejuni, and wzxO16, wzxO7, wzxE, and arnEF from E. coli) (10, 15, 20, 21) and M. tuberculosis inner membrane proteins (mmr, mmpL3, and Rv3779) (18, 22, 23) were constitutively expressed in M. smegmatis from pVV16. Primer sequences are available upon request.
AG and Lipoglycan Preparation and Analysis
mAGP, soluble AG, and lipoglycans were prepared from M. smegmatis WT and recombinant strains cultured on 7H11-OADC plates and from Sauton's medium-grown M. tuberculosis strains harvested at the same growth stage. Their monosaccharide composition was determined by capillary electrophoresis monitored by laser-induced fluorescence as described previously (18). Lipoglycans were analyzed by SDS-PAGE and visualized by periodic acid/Schiff reagent staining. Glycosyl linkage composition was determined as described earlier (24). The total lipids were extracted from bacterial cells and analyzed by TLC in a variety of solvent systems (19). Mild acid and mild alkali hydrolysis of lipids followed earlier procedures (18).
Enzyme Assays
Enzymatically active mycobacterial membranes and cell envelopes (P60) (cell wall plus membranes) were prepared essentially as described (25). Briefly, the cells were suspended in buffer A (50 mm MOPS buffer, pH 7.9, containing 5 mm 2-mercaptoethanol and 10 mm MgCl2), subjected to probe sonication, and centrifuged at 23,000 × g for 20 min at 4 °C. The pellet was resuspended in buffer A, and Percoll (Amersham Biosciences) was added to achieve a 60% suspension, which was centrifuged at 23,000 × g for 60 min at 4 °C. The white upper band was isolated, and the Percoll was removed by repeated suspension in buffer A and centrifugation. The membranes were obtained by centrifugation of the 23,000 × g supernatant at 100,000 × g for 2 h at 4 °C. The cell envelope and membrane fractions were resuspended in buffer A to final protein concentrations of 10–12 mg ml−1 and 30 mg ml−1, respectively. The reaction mixture and processing of the products for incorporation of GDP-[14C]mannopyranosyl (specific activity 275 mCi mmol−1; GE Healthcare) into mannolipids essentially followed earlier protocols (19). The typical reaction mixture for monitoring the incorporation of [14C]Gal from UDP-[U-14C]Galp to AG intermediates contained 1.0 μCi of UDP-[U-14C]Galp (specific activity 285 mCi mmol−1; GE Healthcare), 0.1 mm ATP, 0.25 mm NADH, 62.5 μm UDP-GlcNAc, TDP-Rha (prepared as described in Ref. 25), cytosol (0.1 mg of proteins), membrane fraction (1.0 mg of protein), cell envelope fraction (3.0 mg of proteins), and buffer A in a final volume of 480 μl. Alternatively, in AraT assays, 0.18 μCi of 14C-labeled 5-phosphoribosyl-1-pyrophosphate [P[14C]RPP], prepared enzymatically from [14C]glucose as described (26), replaced UDP-[U-14C]Galp as the radioactive precursor in the reaction mixture described above, and 62.5 μm (nonradiolabeled) UDP-Galp was added. After 2 h of incubation at 37 °C, the reactions were stopped by the addition of CHCl3/CH3OH (8:5, v/v) and the lipid-linked, TT3-soluble (CHCl3/CH3OH/H2O, 10:10:3), and E-soak-soluble (water/ethanol/diethyl ether/pyridine/concentrated NH4OH, 15:15:5:1:0.017) polymers were extracted as described earlier (27). Lipid-linked oligosaccharides were dissolved in CHCl3/CH3OH/concentrated NH4OH/H2O (65:25:0.5:3.6) prior to TLC analysis on silica gel 60-precoated plates. TT3 and E-soak products were separated by SDS-PAGE on Tricine gels (Invitrogen) and transferred onto nitrocellulose membranes. Radiolabeled products on TLC plates and nitrocellulose membranes were visualized by autoradiography using Biomax MR-1 films (Kodak), and the radioactivity incorporated by these products was quantified by scintillation counting. All of the assays were repeated at least twice using different membrane and cell envelope preparations, and the results of representative experiments are shown.
Two-hybrid System
Bacterial two-hybrid experiments were performed using the BACTH (Bacterial Adenylate Cyclase Two-Hybrid) System kit (Euromedex, France). The M. tuberculosis Rv3789, dprE1, dprE2, glfT1, and glfT2 genes were thus expressed from plasmids pUT18, pUT18C, pKT25, and pKNT25 as recombinant proteins bearing N- or C-terminal T25 and T18 fusions, and E. coli BTH101 was transformed with the resulting plasmids in different combinations. β-Galactosidase activity was assayed as recommended by the manufacturer.
Statistical Analysis
The results are expressed as the means ± S.D. and were analyzed using one-way analysis of variance followed by Tukey test to determine significant differences between samples.
RESULTS
Relationship of Rv3789 to SMR Transporters
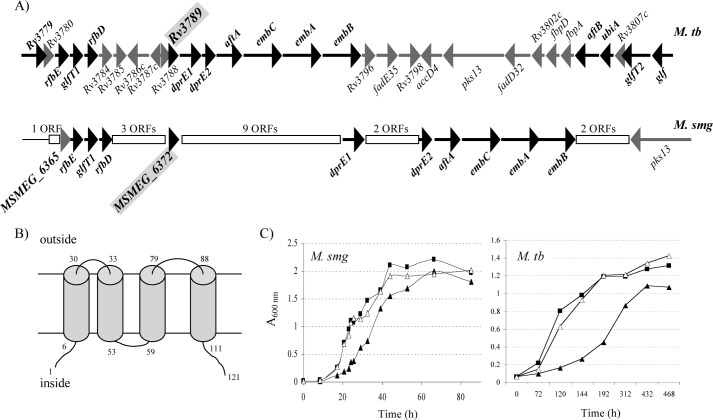
The Rv3789 gene of M. tuberculosis H37Rv is located in a cell wall biosynthetic gene cluster (Fig. 1A). Orthologs of this gene are found in all available quickly and slowly growing mycobacterial genomes, as well as in Rhodococcus, Corynebacterium, and Nocardia species, and map in the immediate or close vicinity of the decaprenyl phosphoribose 2′ epimerase genes dprE1 and dprE2 required for the formation of the arabinose donor, DPA, and in that of one or more AraT genes involved in the arabinosylation of AG and LAM (1) (Fig. 1A). In M. tuberculosis, however, Rv3789 is transcribed independently from the downstream four-gene operon made of dprE1-dprE2-aftA and embC (Rv3790–Rv3793) (28). Rv3789 encodes a 121-amino acid protein with many of the characteristics of bacterial SMR transporters (14). In particular, it is predicted to be an integral membrane protein with four transmembrane helices and N- and C-terminal ends located in the cytoplasm (Fig. 1B). SMR are proton-dependent transporters that have the ability to transport a broad range of lipophilic compounds, including a variety of antibiotics, antiseptics, and disinfectants (14), but also physiological substrates such as lipid-linked sugar donors (15–17). Among SMR and SMR-like transporters, Rv3789 shows the best sequence similarities with the putative undecaprenyl phosphate glucose flippase GtrA produced by the bacteriophage SfX of S. flexneri (16, 17) and a GtrA-like protein (pfam04138) from Saccharopolyspora erythraea (24.8 and 42% identity on an 121-amino acid overlap, respectively). It is more distantly related to the hetero-oligomeric SMR-like ArnE/ArnF transporter of E. coli responsible for the translocation of undecaprenyl phosphate 4-amino-4-deoxy-l-arabinose across the plasma membrane (<15.7% identity and 22.3% similarity) (15). Altogether, these observations thus suggested that Rv3789 might function as a translocase for DPA.
FIGURE 1.
Genomic localization of Rv3789 and SMEG_6372 (A), predicted topology of Rv3789 from M. tuberculosis H37Rv (B), and growth characteristics of the Rv3789 and MSMEG_6372 knock-out mutants (C). A, genes involved or thought to be involved in AG and LAM biosynthesis are represented by black arrows, and their names are in bold type. aftA, aftB, embC, embA, and embB are AraTs; glfT1 and glfT2 are GalTs. dprE1/dprE2, decaprenyl-phosphoribose 2′ epimerase; Rv3779, M. tuberculosis-specific (N-acetyl)galactosaminyltransferase; ubiA, decaprenyl-phosphoryl-5-phosphoribose synthase; glf, UDP-Galp mutase; rfbE/rfbD, ABC transporter thought to be involved in the translocation of lipid-linked AG precursors. Rearrangements (i.e., ORF insertions) in the corresponding genomic region of M. smegmatis are indicated by white rectangles; the inserted ORFs are apparently not related to AG and LAM biosynthesis. B, the topology of the Rv3789 protein of M. tuberculosis was predicted using TMHMM server v. 2.0. The numbers indicate amino acid residues. C, growth characteristics of WT mc2155 and M. tuberculosis H37Rv (closed rectangles), the MSMEG_6372 and Rv3789 knock-out mutants (closed triangles), and the complemented mutant strains mc2ΔMSMEG_6372/pVV16-Rv3789 and H37RvΔRv3789/pVV16-Rv3789 (open triangles) in LB-Tween 80 medium (M. smegmatis (M. smg)) or 7H9-OADC-Tween 80 (M. tuberculosis (M. tb)) at 37 °C.
Disruption of MSMEG_6372 in M. smegmatis and Rv3789 in M. tuberculosis H37Rv
To investigate the putative involvement of Rv3789 in the synthesis of mycobacterial glycoconjugates, Rv3789 from M. tuberculosis H37Rv and its ortholog in M. smegmatis, MSMEG_6372, were disrupted by homologous recombination. MSMEG_6372 (135 amino acids) shares 68% amino acid sequence identity (83% similarity) with Rv3789 from M. tuberculosis H37Rv. Allelic replacement was confirmed in both species by Southern hybridization and PCR (supplemental Fig. S1, A and B). Both H37RvΔRv3789 and mc2ΔMSMEG_6372 grew slightly slower than their respective WT parents in liquid broth. Normal growth was restored upon complementation with a WT copy of the M. tuberculosis Rv3789 gene expressed from pVV16-Rv3789 (Fig. 1C). Suggestive of alterations affecting its cell envelope permeability, mc2ΔMSMEG_6372 was 8-fold more susceptible than its WT parent to rifampicin (Table 1). That this difference in rifampicin MIC was the result of the involvement of MSMEG_6372 in the efflux of this drug is unlikely given the lack of effect of carboxyl cyanide m-chlorophenylhydrazone (a proton motive force uncoupler) and reserpine (an inhibitor of ATP-dependent efflux pumps) on the MIC of rifampicin against M. smegmatis. The susceptibility of H37RvΔRv3789 to isoniazid, streptomycin, ciprofloxacin, rifampicin, ethambutol, and acriflavin were, in contrast, identical to that of WT M. tuberculosis H37Rv (Table 1).
TABLE 1.
MIC values of various antibiotics against WT M. smegmatis mc2155 and M. tuberculosis H37Rv and their respective MSMEG_6372 and Rv3789 knock-out mutants
MIC values for M. smegmatis were determined in LB-Tween 80 broth at 37 °C in microtiter plates by visually scanning for growth. Carbonyl cyanide p-chlorophenylhydrazone (CCCP) and reserpine (reserp) were used at concentrations of 1.56 and 40 μg ml−1, respectively. M. tuberculosis MIC values were determined on 7H11-OADC agar. MIC values are in μg ml−1. ND, not determined; EMB, ethambutol; CIP, ciprofloxacin; RIF, rifampicin; INH, isoniazid; AMP, ampicillin; STR, streptomycin; ACR, acriflavine.
| Strain | EMB | CIP | RIF | RIF + CCCP | RIF + reserp | INH | AMP | STR | ACR |
|---|---|---|---|---|---|---|---|---|---|
| mc2155 WT | 7.5 | 0.2 | 12 | 12 | 12 | 2.5 | 500 | 2 | 15.6 |
| ΔMSMEG6372 | 7.5 | 0.2 | 1.5 | 1.5 | 1.5 | 1.25 | 250 | 2 | 15.6 |
| Complemented | 7.5 | 0.2 | 12 | ND | ND | 2.5 | 500 | 2 | 15.6 |
| H37Rv WT | 5 | 0.2 | 0.2 | ND | ND | 0.1 | ND | 1 | 1.6 |
| Η37RvΔRv3789 | 5 | 0.2 | 0.2 | ND | ND | 0.1 | ND | 0.5 | 1.6 |
Phenotypic Characterization of the M. smegmatis ΔSMEG_6372 and M. tuberculosis H37RvΔRv3789 Mutant Strains
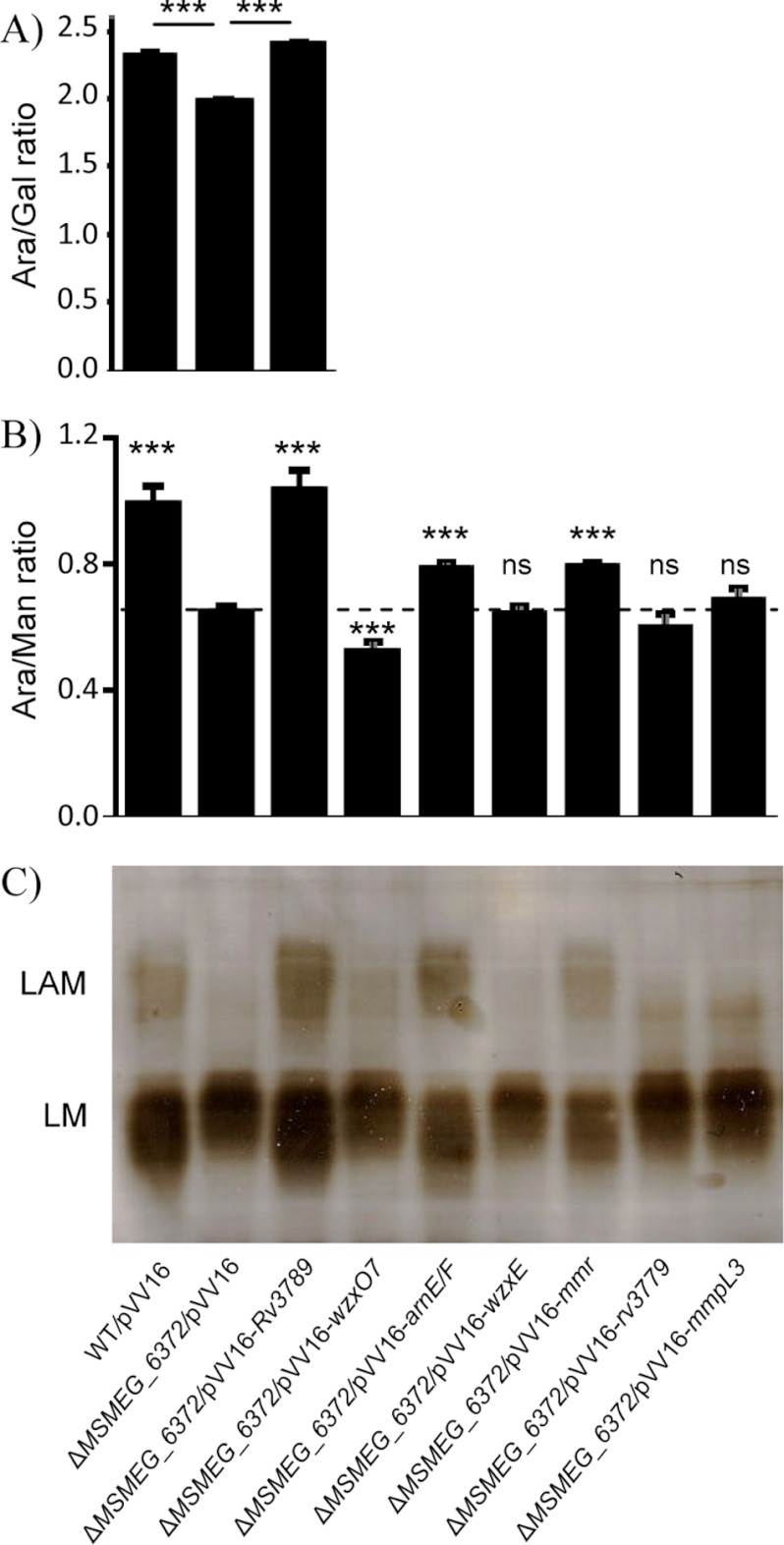
Soluble AG and lipoglycans were prepared from the M. smegmatis WT, mutant, and complemented mutant strains, and the Ara/Gal and Ara/Man ratios of these fractions were determined by capillary electrophoresis analysis upon total acid hydrolysis and fluorescent labeling. Compared with the WT and complemented strains and consistent with the general decrease (∼36%) of the Ara content of mutant cells (supplemental Table S1), the AG prepared from mc2ΔMSMEG_6372 consistently exhibited a ∼15% decrease in its Ara/Gal ratio (Fig. 2A). Similarly, but in a more pronounced fashion, the relative amount of Ara in the lipoglycan fraction of the mutant was reduced by ∼35% (Fig. 2B). Glycosyl linkage analysis indicated an overall truncation of the arabinan domain of LAM, with shorter side chains, as revealed by the marked reduction of 2-Araf units (supplemental Table S2). In contrast, mannan branching remained unaffected. Lipoglycans were further analyzed by SDS-PAGE and showed a dramatically altered profile in the mutant in that, compared with the WT and complemented strains, mc2ΔMSMEG_6372 displayed a much reduced LAM content (Fig. 2C). In striking contrast to the situation in M. smegmatis, M. tuberculosis H37RvΔRv3789 failed to show any significant changes at the level of lipoglycans or AG under the growth conditions used in this study (supplemental Fig. S1C and Tables S3 and S4). Further analyses thus focused on the M. smegmatis mutant.
FIGURE 2.
Analysis of the AG and lipoglycans produced by wild-type mc2155, mc2ΔMSMEG_6372 and mc2ΔMSMEG_6372 expressing various mycobacterial and Gram-negative inner membrane proteins. A and B, Ara/Gal ratio of AG (A) and Ara/Man ratio of the lipoglycan fraction (B) determined by capillary electrophoresis analysis upon total acid hydrolysis and fluorescent labeling. C, SDS-PAGE analysis of the lipoglycan fractions prepared from the various recombinant strains. The results reported in A and B are the means ± S.D. of triplicate assays from one representative culture batch of each strain and were analyzed using one-way analysis of variance followed by Tukey test to determine significant differences between samples. ***, p < 0.001; ns, not significant. In B, the p value is given relative to mc2ΔMSMEG_6372/pVV16. The analyses presented here are representative of the arabinosylation defect observed in at least two to four independent culture batches prepared from each strain.
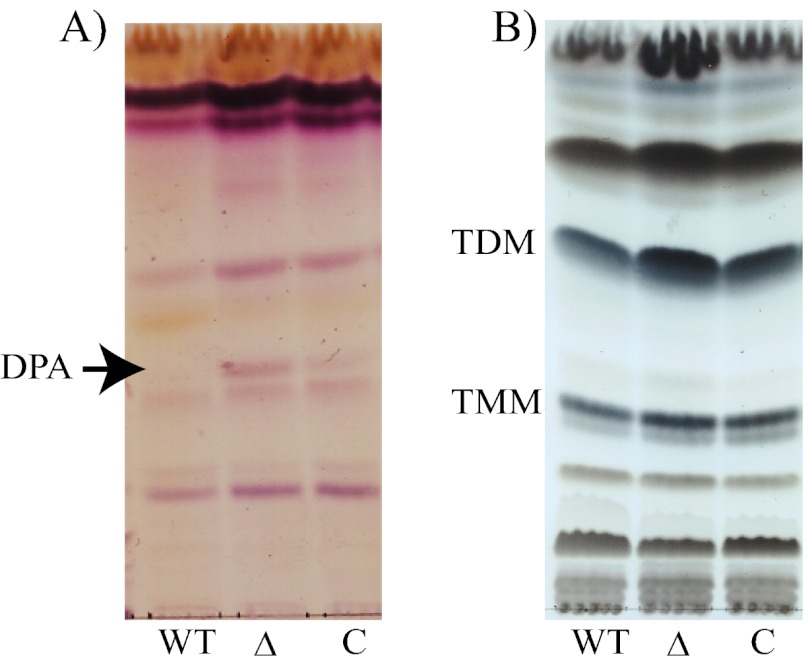
Comparative analysis of total extractable lipids revealed an accumulation of three glycolipids in the mutant that were restored to WT levels in the complemented strain (Fig. 3). One of them was mild acid labile and mild alkali stable, consistent with a precursor of the polyisoprenyl-P class (Fig. 3A). It was identified by co-migration with a radiolabeled standard by TLC and by gas chromatography as DPA (supplemental Fig. S2). The two other glycolipids were attributed to trehalose monomycolates (TMMs) and trehalose dimycolates (TDMs) (Fig. 3B) based on their staining properties with cupric sulfate and α-naphthol and co-migration with authentic standards (22). The synthesis of PIMs (supplemental Fig. S3) and other (glyco)lipids appeared otherwise unaffected in the mutant. It is likely that the accumulation of TMM and TDM in mc2ΔMSMEG_6372 is a consequence of the arabinosylation defect of AG, resulting in a lack of mycolic acid attachment sites and, thus, the channeling of mycolates into outer membrane lipids. Consistent with the apparent WT structure of AG in M. tuberculosis H37RvΔRv3789, the TMM and TDM contents of this mutant were similar to those of the WT and complemented mutant strains (supplemental Fig. S1D). The fact that the M. smegmatis mutant complemented with a WT copy of the M. tuberculosis Rv3789 gene displayed WT lipid, AG, and LM/LAM contents clearly indicates that MSMEG_6372 and Rv3789 have analogous functions.
FIGURE 3.
Comparative analysis of the lipid content of wild-type mc2155 (lanes WT), the MSMEG_6372 knock-out mutant (lanes Δ), and the complemented mutant strain, mc2ΔMSMEG_6372/pVV16-Rv3789 (lanes C). Equal amounts of mild alkali-treated lipids (TLC, panel A) or untreated total lipids (TLC, panel B) were loaded per lane. TLC plates were run in the solvent systems CHCl3/CH3OH/NH4OH/H2O (65:25:0.5:3.6) (A) and CHCl3/CH3OH/H2O (20:4:0.5) (B).
Arabinogalactan and Mannolipid Synthesis in Vitro
To gain insight into the possible causes of the alterations in the arabinosylation of AG and LAM in mc2ΔMSMEG_6372, we next compared cell-free extracts from the WT, mutant, and complemented mutant strains for their ability to synthesize the lipid-linked sugar donors PPM and DPA, and biosynthetic precursors of LAM and AG in vitro. The in vitro incorporation of radioactive mannose from GDP-[14C]mannopyranosyl by mycobacterial membranes and cell wall produces PPM and PIMs (i.e., precursors of LM and LAM) (19). Likewise, the early steps of the galactan and arabinan polymerization of AG can be monitored in vitro using mycobacterial cell wall, membranes, and cytosol as enzyme source and UDP-[14C]Galp or [14C]DPA (generated in situ from p[14C]Rpp) as radiolabeled precursors (27).
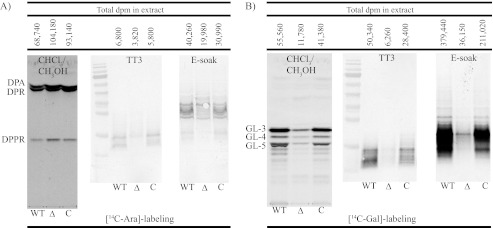
Although mannosyltransferase assays using GDP-[14C]mannopyranosyl did not point to any significant defect in PPM and PIM synthesis in the mutant (supplemental Fig. S4), important differences between strains were observed in the AraT and GalT assays. Reflecting the phenotypic alterations noted in whole mutant cells, ∼50% more radiolabeled DPA, decaprenyl-phosphoribose, and decaprenyl-phosphoryl-5-phosphoribose were recovered from the reaction mixtures that used p[14C]Rpp and mutant extracts than from those run in the presence of WT and complemented mutant extracts (Fig. 4A). In contrast, the incorporation of [14C]DPA (generated in situ from p[14C]Rpp) into AG precursors isolated in the TT3 and E-SOAK fractions (i.e. lipid-linked oligosaccharide polymers consisting of polyprenyl-P-P-GlcNAc-Rha-Galn) was reduced by 43 and 50%, respectively, in the mutant extracts (Fig. 4A). A dramatic decrease (79–90%) in the galactosylation of AG was also noted with the mutant extracts in the reactions that used UDP-[14C]Gal as the radiolabeled precursor (Fig. 4B). Galactosylation and arabinosylation activities were partially restored in the complemented mutant. It is likely that the defect in galactosyl polymerization in the mutant extracts, which reduces the availability of galactan acceptors, accounts, at least in part, for the dramatically decreased incorporation of [14C]Araf observed in the AraT assay.
FIGURE 4.
In vitro biosynthesis of AG precursors by cell-free extracts from wild-type mc2155 (lanes WT), the MSMEG_6372 knock-out mutant (lanes Δ), and the complemented mutant strain mc2ΔMSMEG_6372/pVV16-Rv3789 (lanes C). AraT assays using p[14C]Rpp (A) and GalT assays using UDP-[14C]Galp (B) as radiolabeled precursors were run, and the products of the reactions were extracted as described under “Experimental Procedures.” The total number of dpm recovered in the CHCl3/CH3OH-, TT3-, and E-soak-soluble products are indicated above the autoradiograms. The same volumes of samples from each assay were loaded per lane. CHCl3/CH3OH-soluble products were analyzed by TLC in the solvent system CHCl3/CH3OH/1 m CH3COONH4/NH4OH/H2O (180:140:9:9:23) followed by autoradiography. TT3- and E-soak-soluble products were analyzed by SDS-PAGE followed by autoradiography. In the AraT assay, the CHCl3/CH3OH-soluble products correspond to the simpler lipid-linked sugars: DPA, decaprenyl-phosphoribose, and decaprenyl-phosphoryl-5-phosphoribose. In the GalT assay, they correspond to the lipid-linked oligosaccharides, polyprenyl-P-P-GlcNAc-Rha-Galn (n = 1: GL-3; n = 2, GL-4; n = 3, GL-5). The products of both assays extracted in the TT3 and E-soak fractions correspond to polyprenyl phosphate-linked AG precursors, the TT3 products being incompletely glycosylated versions of the E-soak-soluble ones (27).
We conclude from the phenotypic analyses of whole bacterial cells and the cell-free assays described above that although the mutant has retained the ability to synthesize PPM, DPA, and PIMs and to elongate LM, its ability to arabinosylate AG and LM are greatly reduced. Although a notable effect of the mutation on the galactosylation of AG was clearly visible in vitro, the formation of the galactan domain of AG appeared qualitatively and quantitatively unaffected in whole mutant cells (supplemental Tables S1 and S5).
Complementation of the MSMEG_6372 Knock-out Mutant with Gram-negative Polyprenol-linked Sugar Donor and Oligosaccharide Flippases
With the primary sequence of Rv3789/MSMEG_6372 and phenotypic characterization of mc2ΔMSMEG_6372 pointing to an involvement of this protein in the translocation of DPA, we next sought to complement the M. smegmatis mutant with different types of Gram-negative polyprenol-linked sugar/oligosaccharide flippases. Despite the low sequence similarity displayed by these transporters and structural diversity of the substrates they translocate, published reports indeed indicate that they may display relaxed substrate specificity with respect to oligosaccharide structures. Wzx proteins, for instance, can complement each other in the translocation of different O-antigen subunits or even translocate single sugars linked to undecaprenyl pyrophosphate (6, 21). Likewise, the ABC-transporter-like protein PglK from C. jejuni whose physiological substrate is an undecaprenyl-pyrophosphate-linked heptasaccharide intermediate involved in N-linked protein glycosylation was shown to complement a wzx deficiency in E. coli O-antigen biosynthesis (10).
Replicative plasmids allowing for the constitutive expression in mycobacteria of the ABC transporter-like pglK gene from C. jejuni, the wzx-type wzxO16, wzxO7, and wzxE genes from E. coli, and the undecaprenyl phosphate 4-amino-4-deoxy-l-arabinose translocase arnE/F genes were thus constructed and electroporated into mc2ΔMSMEG_6372. A plasmid allowing for the expression of the only other putative SMR-like transporter identified in the genome of M. tuberculosis to date, Mmr (23), and two negative control plasmids expressing unrelated M. tuberculosis integral membrane proteins—the glycosyltransferase Rv3779 (18) and the inner membrane TMM transporter MmpL3 (22)—were also prepared. Whereas the expression of pglK and wzxO16 was apparently toxic to mc2ΔMSMEG_6372, transformants were obtained in all other cases. Analysis of their lipoglycan fractions showed, as expected, no evidence of complementation in the case of Rv3779 and MmpL3. Whether because of poor expression or the inability of the expressed transporters to carry DPA across the M. smegmatis plasma membrane, no complementation was observed in the case of wzxO7 and wzxE (Fig. 2, B and C). In contrast, clear indication of complementation was obtained with ArnE/F from E. coli and the M. tuberculosis multidrug transporter Mmr. This was reflected both in the Ara/Man ratio (Fig. 2B) and in the migration profile (Fig. 2C) of the lipoglycans prepared from the arnE/F and mmr complemented strains.
Interactions of Rv3789 with Components of the AG Biosynthetic Machinery
To investigate potential interactions in vivo between Rv3789 and the DPA-generating DprE1/DprE2 epimerase or other components of the AG machinery, a bacterial two-hybrid assay was employed. This system exploits the fact that the catalytic domain of the adenylate cyclase (CyaA) from Bordetella pertussis consists of two complementary fragments, T25 and T18, that are not active when physically separated but whose functional complementation when fused to interacting polypeptides results in cyclic AMP synthesis in E. coli (29). The reporter for this activity is β-galactosidase. Importantly, this system was shown to be suitable to study protein interactions even if one of the interacting partners is membrane-associated (30, 31). Whereas no evidence of physical interaction between Rv3789 and DprE1 or DprE2 was found in this system, Rv3789 clearly interacted with the GalT GlfT1, which initiates the polymerization of the galactan domain of AG. Co-expression of the fusion proteins Rv3789-T18 and T25-GlfT1 in E. coli BTH101 resulted in blue colonies on LB/X-gal/isopropyl β-d-thiogalactopyranoside plates at 30 °C, indicative of cAMP-dependent induction of the lactose operon. Consistently, significant β-Gal activity over the negative control (containing empty fusion plasmids) was measured in this recombinant strain (Table 2). Similar assays carried out with the second GalT of the AG biosynthetic pathway, GlfT2, yielded negative results, suggesting that Rv3789 specifically interacts with GlfT1 (Table 2).
TABLE 2.
In vivo protein interaction between M. tuberculosis Rv3789 and GlfT1
Fusions proteins made of Rv3789, DprE1, DprE2, GlfT1, and GlfT2 harboring C-terminal or N-terminal T18 and T25 domains were transformed in E. coli BTH101 cells in different combinations, and the transformants were grown at 30 °C for 48 h on LB/X-gal/isopropyl β-d-thiogalactopyranoside agar. The positive control consisted of the fusion proteins combination pKT25-Zip + pUT18C-Zip (provided with the BACTH system kit) and the negative control of the empty plasmids pKT25 + pUT18. Individual colonies were grown in LB broth, and the cultures were processed for β-gal activity. All transformations were repeated at least three times, and the values presented are the mean activities (Miller units) ± standard error from at least three independent measurements.
| β-gal activity | |
|---|---|
| pUT18 + pKT25 (negative control) | 81.8 ± 1.3 |
| pUT18C-Zip + pKT25-Zip (positive control) | 4287.1 ± 26.3 |
| Glft1-pKT25 + Rv3789-pUT18 | 463.9 ± 15.4 |
| Glft2-pKT25 + Rv3789-pUT18 | 79.7 ± 2.0 |
| Rv3790-pUT18 + Rv3791-pKT25 | 80.5 ± 0.6 |
| Rv3790-pUT18 + Rv3791-pKTN25 | 80.8 ± 1.8 |
| Rv3790-pUT18C + Rv3791-pKT25 | 83.3 ± 1.9 |
| Rv3790-pUT18C + Rv3791-pKTN25 | 79.5 ± 1.0 |
DISCUSSION
The pivotal role played by (glyco)lipid translocation across the plasma membrane in the biogenesis of all major mycobacterial cell envelope glycoconjugates contrasts with our limited understanding of the processes involved. The M. tuberculosis Rv3789 gene and orthologs cluster with the DPA-forming genes dprE1/dprE2 in the genome of Mycobacterium species and other AG-producing Corynebacterianeae and encode integral membrane proteins displaying many of the characteristics of SMR transporters. Although not a lipid-linked sugar flippase, the E. coli prototype SMR transporter, EmrE, extrudes a variety of lipophilic cations from the cells, coupled to the inward movement of protons down their electrochemical gradient (14, 32). Importantly, small transporters sharing the same topological features as EmrE and Rv3789, such as the ArnE/ArnF transporter of E. coli (15) and the GtrA protein from bacteriophage SfX of S. flexneri (16, 17), were implicated or, in the case of GtrA, proposed to be implicated in the transbilayer movement of undecaprenyl monophosphate sugars. Suggestive of an involvement of Rv3789 and mycobacterial orthologs in similar functions, a detailed analysis of the cell envelope composition of a MSMEG_6372 knock-out mutant of M. smegmatis revealed a decrease in the arabinosylation of AG (∼15%) and lipoglycans (∼35%) accompanied by the accumulation of the Araf donor, DPA, in the mutant cells. The mycolic acid-containing lipids TMM and TDM also accumulated in the mutant, most likely as a result of the defect in the arabinosylation of AG. Because the mutant synthesized in vivo and in vitro normal if not higher levels of DPA, its reduced AG and lipoglycan arabinose contents are therefore consistent with a defect in the translocation of DPA from its site of biosynthesis on the surface of the inner leaflet of the plasma membrane to the outer leaflet of the membrane. In this regard, it is noteworthy that the accumulation of lipid-linked sugar/oligosaccharide substrates is a common phenotypic trait shared by several bacterial translocase mutants, including wzx and wzxE mutants of E. coli (8, 33).
The residual transfer of Araf residues onto AG and LAM in the M. smegmatis mutant and the fact that MSMEG_6372 and Rv3789 could be knocked out without lethal consequences on M. smegmatis and M. tuberculosis despite the vital requirement of mycobacteria for AG synthesis clearly indicate that other translocases with overlapping substrate specificities exist in these species and probably even more so in M. tuberculosis than in M. smegmatis given the absence of a noticeable arabinosylation phenotype in the M. tuberculosis Rv3789 knock-out mutant. Alternatively, the significantly slower growth rate of M. tuberculosis compared with M. smegmatis may make it easier for compensatory transport mechanisms to maintain a WT arabinosylation of AG and LAM in the first species. The ability of the Mmr transporter of M. tuberculosis to partially restore the arabinosylation of lipoglycans in mc2ΔMSMEG_6372 suggests that this SMR-like transporter, which has been involved in the resistance of M. smegmatis to a number of small cationic dyes and inhibitors (23, 34), plays a role in this function, even though its sequence similarity to Rv3789 is weak (18% similarity), its encoding gene (Rv3065) does not cluster with cell wall biosynthetic genes, and its natural substrate in M. tuberculosis remains to be identified. If this is the case, the lower level of compensatory DPA transport activity in M. smegmatis may also be explained by the absence of a clear ortholog of mmr in this species. Experiments are underway in our laboratory to determine whether knocking out both mmr and Rv3789 in M. tuberculosis would result in arabinosylation defects in this species.
A further strong argument in favor of a translocase activity associated to Rv3789 and orthologs is the partial functional complementation of the mutant with the E. coli SMR-like ArnE/F undecaprenyl phosphate aminoarabinose flippase. This finding was somewhat surprising in view of the different structures of the lipid-linked sugar donors used by Gram-negative bacteria in the biosynthesis of LPS and by mycobacteria in that of AG and LAM. Mycobacteria are indeed unusual in that they produce C50 polyprenol phosphate molecules made of 10 isoprene units of which only one is a trans-(E)-isoprene unit instead of the common bacterial C55 polyprenol phosphate molecule composed of 11 isoprene units (ω,E,E,polyZ-undecaprenyl-P) (35). The results of our complementation studies with ArnE/F thus reinforce and further extend the idea that bacterial translocases have relaxed substrate specificities with regard to not only mono- or oligosaccharide structures (6, 10, 21, 36) but also the structure of the lipid moiety.
With Rv3789 serving to translocate DPA across the plasma membrane, the dramatically reduced polymerization rate of the galactan domain of AG observed in the M. smegmatis mutant cell-free extracts was unexpected. Indeed, the elongation of the galactan domain of AG, which is catalyzed in whole cells by two UDP-Galf-utilizing GalTs (GlfT1 and GlfT2), is expected to take place entirely on the cytosolic side of the plasma membrane and thus not to involve a translocase (1), yet despite not containing any transmembrane domains, both GlfT1 and GlfT2 have been reported to be membrane-associated (25, 27, 37). In light of our two-hybrid experiments showing evidence for a physical interaction between Rv3789 and GlfT1, we propose that Rv3789 helps target and stabilize GlfT1 and perhaps other components of the AG biosynthetic machinery at the periphery of the plasma membrane. Multiprotein complexes made of transporters and biosynthetic enzymes are indeed not unusual in bacterial (lipo)polysaccharide biosynthesis and both the ABC- and the wzy-dependent pathways are example of such systems where proper interactions between translocase, polymerase, and chain regulator are required for the efficient elongation and export of LPS (31, 38). It is thus to be expected that mycobacteria use similar multiprotein complexes in the biogenesis of AG and LM/LAM. As evidenced by the structure and amounts of AG produced by mc2ΔMSMEG_6372, the galactan domain is apparently polymerized normally in intact mutant cells. However, our cell-free assays suggest that the rate at which this domain is formed might be reduced, possibly adding to the growth defects of the M. smegmatis mutant.
Future work will aim at determining whether Rv3789 functions as a homodimer or a heterodimer (i.e., requiring the expression of another SMR-like protein for activity) and interacts with other components of the AG and LAM biosynthetic machineries and at characterizing at the molecular level using cell-free systems the mechanism through which this protein translocates DPA across the plasma membrane.
Supplementary Material
Acknowledgments
We gratefully acknowledge Drs. M. Aebi and C. Neupert (Swiss Federal Institute of Technology, Zurich, Switzerland) for the pglK, wzxE, wzxO7, and wzxO16 expression constructs, C. Raetz (Duke University) for the arnE/F plasmid constructs, and Drs. M. McNeil (Colorado State University) and J. Blaško (Institute of Chemistry, Faculty of Natural Sciences, Comenius University in Bratislava) for assistance with GC and GC-MS analyses. Dr. D. Ladant (Pasteur Institute, Paris, France) is gratefully acknowledged for assistance with the BACTH system.
This work was supported, in whole or in part, by National Institutes of Health Grant AI064798. This work was also supported by Slovak Research and Development Agency Contract RPEU-0012-06.

This article contains supplemental Tables S1–S5 and Figs. S1–S4.
- PIM
- phosphatidyl-myo-inositol mannoside
- AG
- arabinogalactan
- Araf
- arabinofuranosyl
- DPA
- decaprenol phosphate arabinose
- LM
- lipomannan
- LAM
- lipoarabinomannan
- PPM
- polyprenol phosphate mannose
- SMR
- small multidrug resistance
- GalT
- galactosyltransferase
- AraT
- arabinosyltransferase
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- TMM
- trehalose monomycolate
- TDM
- trehalose dimycolate
- X-gal
- 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside
- Galf
- galactofuranose
- MIC
- minimum inhibitory concentration.
REFERENCES
- 1. Kaur D., Guerin M. E., Škovierová H., Brennan P. J., Jackson M. (2009) Biogenesis of the cell wall and other glycoconjugates of Mycobacterium tuberculosis. Adv. Appl. Microbiol. 69, 23–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolucka B. A., McNeil M. R., de Hoffmann E., Chojnacki T., Brennan P. J. (1994) Recognition of the lipid intermediate for arbinogalactan/arabinomannan biosynthesis and its relation to the mode of action of ethambutol on mycobacteria. J. Biol. Chem. 269, 23328–23335 [PubMed] [Google Scholar]
- 3. Berg S., Kaur D., Jackson M., Brennan P. J. (2007) The glycosyltransferases of Mycobacterium tuberculosis. Roles in the synthesis of arabinogalactan, lipoarabinomannan, and other glycoconjugates. Glycobiology 17, 35R–56R [DOI] [PubMed] [Google Scholar]
- 4. Daleke D. L. (2007) Phospholipid flippases. J. Biol. Chem. 282, 821–825 [DOI] [PubMed] [Google Scholar]
- 5. Sanyal S., Frank C. G., Menon A. K. (2008) Distinct flippases translocate glycerophospholipids and oligosaccharide diphosphate dolichols across the endoplasmic reticulum. Biochemistry 47, 7937–7946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feldman M. F., Marolda C. L., Monteiro M. A., Perry M. B., Parodi A. J., Valvano M. A. (1999) The activity of a putative polyisoprenol-linked sugar translocase (Wzx) involved in Escherichia coli O antigen assembly is independent of the chemical structure of the O repeat. J. Biol. Chem. 274, 35129–35138 [DOI] [PubMed] [Google Scholar]
- 7. Raetz C. R., Whitfield C. (2002) Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rick P. D., Barr K., Sankaran K., Kajimura J., Rush J. S., Waechter C. J. (2003) Evidence that the wzxE gene of Escherichia coli K-12 encodes a protein involved in the transbilayer movement of a trisaccharide-lipid intermediate in the assembly of enterobacterial common antigen. J. Biol. Chem. 278, 16534–16542 [DOI] [PubMed] [Google Scholar]
- 9. Whitfield C. (2006) Biosynthesis and assembly of capsular polysaccharides in. Escherichia coli. Annu. Rev. Biochem. 75, 39–68 [DOI] [PubMed] [Google Scholar]
- 10. Alaimo C., Catrein I., Morf L., Marolda C. L., Callewaert N., Valvano M. A., Feldman M. F., Aebi M. (2006) Two distinct but interchangeable mechanisms for flipping of lipid-linked oligosaccharides. EMBO J. 25, 967–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raetz C. R., Reynolds C. M., Trent M. S., Bishop R. E. (2007) Lipid A modification systems in Gram-negative bacteria. Annu. Rev. Biochem. 76, 295–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mohammadi T., van Dam V., Sijbrandi R., Vernet T., Zapun A., Bouhss A., Diepeveen-de Bruin M., Nguyen-Distèche M., de Kruijff B., Breukink E. (2011) Identification of FtsW as a transporter of lipid-linked cell wall precursors across the membrane. EMBO J. 30, 1425–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Henriques A. O., Glaser P., Piggot P. J., Moran C. P., Jr. (1998) Control of cell shape and elongation by the rodA gene in. Bacillus subtilis. Mol. Microbiol. 28, 235–247 [DOI] [PubMed] [Google Scholar]
- 14. Bay D. C., Rommens K. L., Turner R. J. (2008) Small multidrug resistance proteins. A multidrug transporter family that continues to grow. Biochim. Biophys. Acta 1778, 1814–1838 [DOI] [PubMed] [Google Scholar]
- 15. Yan A., Guan Z., Raetz C. R. (2007) An undecaprenyl phosphate-aminoarabinose flippase required for polymyxin resistance in Escherichia coli. J. Biol. Chem. 282, 36077–36089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guan S., Bastin D. A., Verma N. K. (1999) Functional analysis of the O antigen glucosylation gene cluster of Shigella flexneri bacteriophage SfX. Microbiology 145, 1263–1273 [DOI] [PubMed] [Google Scholar]
- 17. Korres H., Mavris M., Morona R., Manning P. A., Verma N. K. (2005) Topological analysis of GtrA and GtrB proteins encoded by the serotype-converting cassette of. Shigella flexneri. Biochem. Biophys. Res. Commun. 328, 1252–1260 [DOI] [PubMed] [Google Scholar]
- 18. Škovierová H., Larrouy-Maumus G., Pham H., Belanová M., Barilone N., Dasgupta A., Mikušová K., Gicquel B., Gilleron M., Brennan P. J., Puzo G., Nigou J., Jackson M. (2010) Biosynthetic origin of the galactosamine substituent of arabinogalactan in Mycobacterium tuberculosis. J. Biol. Chem. 285, 41348–41355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Korduláková J., Gilleron M., Mikušová K., Puzo G., Brennan P. J., Gicquel B., Jackson M. (2002) Definition of the first mannosylation step in phosphatidylinositol synthesis. PimA is essential for growth of mycobacteria. J. Biol. Chem. 277, 31335–31344 [DOI] [PubMed] [Google Scholar]
- 20. Marolda C. L., Feldman M. F., Valvano M. A. (1999) Genetic organization of the O7-specific lipopolysaccharide biosynthetic cluster of Escherichia coli VW187 (O7:K1). Microbiology 145, 2485–2495 [DOI] [PubMed] [Google Scholar]
- 21. Marolda C. L., Vicarioli J., Valvano M. A. (2004) Wzx proteins involved in biosynthesis of O antigen function in association with the first sugar of the O-specific lipopolysaccharide subunit. Microbiology 150, 4095–4105 [DOI] [PubMed] [Google Scholar]
- 22. Grzegorzewicz A. E., Pham H., Gundi V. A., Scherman M. S., North E. J., Hess T., Jones V., Gruppo V., Born S. E., Korduláková J., Chavadi S. S., Morisseau C., Lenaerts A. J., Lee R. E., McNeil M. R., Jackson M. (2012) Inhibition of mycolic acid transport across the Mycobacterium tuberculosis plasma membrane. Nat. Chem. Biol. 8, 334–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Rossi E., Branzoni M., Cantoni R., Milano A., Riccardi G., Ciferri O. (1998) mmr, a Mycobacterium tuberculosis gene conferring resistance to small cationic dyes and inhibitors. J. Bacteriol. 180, 6068–6071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaur D., McNeil M. R., Khoo K. H., Chatterjee D., Crick D. C., Jackson M., Brennan P. J. (2007) New insights into the biosynthesis of mycobacterial lipomannan arising from deletion of a conserved gene. J. Biol. Chem. 282, 27133–27140 [DOI] [PubMed] [Google Scholar]
- 25. Mikušová K., Belánová M., Korduláková J., Honda K., McNeil M. R., Mahapatra S., Crick D. C., Brennan P. J. (2006) Identification of a novel galactosyl transferase involved in biosynthesis of the mycobacterial cell wall. J. Bacteriol. 188, 6592–6598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scherman M. S., Kalbe-Bournonville L., Bush D., Xin Y., Deng L., McNeil M. (1996) Polyprenylphosphate-pentoses in mycobacteria are synthesized from 5-phosphoribose pyrophosphate. J. Biol. Chem. 271, 29652–29658 [DOI] [PubMed] [Google Scholar]
- 27. Mikušová K., Yagi T., Stern R., McNeil M. R., Besra G. S., Crick D. C., Brennan P. J. (2000) Biosynthesis of the galactan component of the mycobacterial cell wall. J. Biol. Chem. 275, 33890–33897 [DOI] [PubMed] [Google Scholar]
- 28. Goude R., Amin A. G., Chatterjee D., Parish T. (2008) The critical role of embC in Mycobacterium tuberculosis. J. Bacteriol. 190, 4335–4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karimova G., Pidoux J., Ullmann A., Ladant D. (1998) A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. U.S.A. 95, 5752–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baulard A. R., Gurcha S. S., Engohang-Ndong J., Gouffi K., Locht C., Besra G. S. (2003) In vivo interaction between the polyprenol phosphate mannose synthase Ppm1 and the integral membrane protein Ppm2 from Mycobacterium smegmatis revealed by a bacterial two-hybrid system. J. Biol. Chem. 278, 2242–2248 [DOI] [PubMed] [Google Scholar]
- 31. Clarke B. R., Greenfield L. K., Bouwman C., Whitfield C. (2009) Coordination of polymerization, chain termination, and export in assembly of the Escherichia coli lipopolysaccharide O9a antigen in an ABC-transporter-dependent pathway. J. Biol. Chem. 284, 30662–30672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schuldiner S. (2009) EmrE, a model for studying evolution and mechanism of ion-coupled transporters. Biochim. Biophys. Acta 1794, 748–762 [DOI] [PubMed] [Google Scholar]
- 33. Liu D., Cole R. A., Reeves P. R. (1996) An O-antigen processing function for Wzx (RfbX). A promising candidate for O-unit flippase. J. Bacteriol. 178, 2102–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ninio S., Rotem D., Schuldiner S. (2001) Functional analysis of novel multidrug transporters from human pathogens. J. Biol. Chem. 276, 48250–48256 [DOI] [PubMed] [Google Scholar]
- 35. Wolucka B. A. (2008) Biosynthesis of d-arabinose in mycobacteria. A novel bacterial pathway with implications for antimycobacterial therapy. FEBS J. 275, 2691–2711 [DOI] [PubMed] [Google Scholar]
- 36. Hug I., Couturier M. R., Rooker M. M., Taylor D. E., Stein M., Feldman M. F. (2010) Helicobacter pylori lipopolysaccharide is synthesized via a novel pathway with an evolutionary connection to protein N-glycosylation. PLoS Pathog. 6, e1000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kremer L., Dover L. G., Morehouse C., Hitchin P., Everett M., Morris H. R., Dell A., Brennan P. J., McNeil M. R., Flaherty C., Duncan K., Besra G. S. (2001) Galactan biosynthesis in Mycobacterium tuberculosis. Identification of a bifunctional UDP-galactofuranosyltransferase. J. Biol. Chem. 276, 26430–26440 [DOI] [PubMed] [Google Scholar]
- 38. Marolda C. L., Tatar L. D., Alaimo C., Aebi M., Valvano M. A. (2006) Interplay of the Wzx translocase and the corresponding polymerase and chain length regulator proteins in the translocation and periplasmic assembly of lipopolysaccharide O antigen. J. Bacteriol. 188, 5124–5135 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.