Background: Little is known regarding the role of PARP-1 in UVR-induced photolesion repair.
Results: PARP inhibitors decrease PARP-1-XPA associations and reduce chromatin binding of XPA.
Conclusion: PARP activation promotes XPA association with PARP-1 and chromatin.
Significance: These data provide a mechanistic basis for the contribution of PARP-1 to nucleotide excision repair and expands the role of poly(ADP-ribose) in DNA repair pathways.
Keywords: ADP-ribosylation, Chromatin, DNA Nucleotide Excision Repair, DNA-Protein Interaction, Keratinocytes, XPA, Poly(ADP-ribose) Polymerase, Ultraviolet Radiation
Abstract
Exposure to ultraviolet radiation (UVR) promotes the formation of UVR-induced, DNA helix distorting photolesions such as (6-4) pyrimidine-pyrimidone photoproducts and cyclobutane pyrimidine dimers. Effective repair of such lesions by the nucleotide excision repair (NER) pathway is required to prevent DNA mutations and chromosome aberrations. Poly(ADP-ribose) polymerase-1 (PARP-1) is a zinc finger protein with well documented involvement in base excision repair. PARP-1 is activated in response to DNA damage and catalyzes the formation of poly(ADP-ribose) subunits that assist in the assembly of DNA repair proteins at sites of damage. In this study, we present evidence for PARP-1 contributions to NER, extending the knowledge of PARP-1 function in DNA repair beyond the established role in base excision repair. Silencing the PARP-1 protein or inhibiting PARP activity leads to retention of UVR-induced photolesions. PARP activation following UVR exposure promotes association between PARP-1 and XPA, a central protein in NER. Administration of PARP inhibitors confirms that poly(ADP-ribose) facilitates PARP-1 association with XPA in whole cell extracts, in isolated chromatin complexes, and in vitro. Furthermore, inhibition of PARP activity decreases UVR-stimulated XPA chromatin association, illustrating that these relationships occur in a meaningful context for NER. These results provide a mechanistic link for PARP activity in the repair of UVR-induced photoproducts.
Introduction
Keratinocytic tumors (basal cell and squamous cell carcinomas) are the most common cancers in the United States, and solar ultraviolet radiation (UVR)2 is the major etiologic factor (1). Solar UVR exposure forms DNA photoproducts such as cyclobutane pyrimide dimers (CPDs) and (6-4) pyrimidine-pyrimidone photoproducts (6-4 PPs), which are helix distorting lesions repaired predominantly by nucleotide excision repair (NER) (2, 3). If such lesions are retained, they may lead to mutations, chromosome aberrations, and cellular malfunctions including cell death, senescence, and cancer (4, 5).
Poly(ADP-ribose) polymerase-1 (PARP-1) has numerous functions in cells including orchestration of DNA damage responses (6). Although the involvement of PARP-1 in single strand break repair and base excision repair is established, less is known regarding the contributions of PARP-1 to NER. Chemical inhibition of PARP activity or overexpression of the PARP-1 DNA-binding domain decreased CPD repair rate in a transformed cell line (7) and PARP depletion by RNAi decreased host cell reactivation of a UVR-damaged reporter gene in fibroblasts (8). Although these studies provide evidence that PARP enzymes may modulate NER, little insight exists into a mechanism to account for these observations.
PARP-1 is rapidly activated in response to DNA damage leading to consumption of NAD+ as a substrate to form poly(ADP-ribose) (PAR) subunits and accounts for more than 70% of PAR production by PARP enzymes (9, 10). PAR residues bind to acceptor proteins including PARP-1, histones, and various proteins involved in DNA processing and repair. PAR fosters protein-protein associations (11–13), and a PAR-binding motif has been identified in certain proteins, including xeroderma pigmentosum complementation group A protein (XPA) (14). XPA is part of a group of core proteins that are essential for the initial phase of the NER process (15, 16). The data suggest that loss of this protein, through silencing or chemical suppression, can decrease repair of UVR-induced photoproducts and lead to increased cell sensitivity to DNA damaging agents, therefore illustrating that loss of the XPA protein is rate-limiting to NER (17, 18). Additionally, XPA binding, in conjunction with RPA, is proposed to be important in a secondary recognition step that verifies the presence of DNA lesions. Along these lines, XPA may also provide a checkpoint to control three-dimensional organization of NER complexes (19, 20). These data support the critical role for the XPA protein, as well as its function in NER. Biochemical studies established the location of a PAR-binding motif in XPA and confirmed that the motif conferred PAR binding (14, 21), but the functional significance of this motif has not been defined.
In this study, we demonstrate that inhibition of PARP activity, or PARP-1 knockdown, causes retention of UVR-induced photoproducts in human keratinocytes. UVR exposure stimulated PARP activity and promoted association between XPA and PAR, as well as XPA and PARP-1. Inhibition of PARP activity: 1) decreased the association between XPA and PARP-1 in whole cell extracts and in vitro, 2) decreased the association between XPA and chromatin-bound PARP-1, and 3) blocked UVR-induced XPA association with chromatin, suggesting that these associations and XPA recruitment to chromatin are dependent on poly(ADP-ribosyl)ation. These results not only confirm a role for PARP-1 in NER but suggest a mechanistic link for PARP activity in the repair of UVR-induced photoproducts.
EXPERIMENTAL PROCEDURES
Cell Lines
The human keratinocyte cell line (HaCaT) was generously provided by Dr. Mitch Denning (Loyola University Medical Center, Maywood, IL). HaCaT cells were maintained as described previously (22). PARP-1 HuSH cells were created by transfecting HaCaT cells with PARP-1 shRNA (Origene; HuSH 29-mer). Stable clones were selected using 0.5 μg/ml puromycin and maintained in growth medium supplemented with 0.3 μg/ml puromycin. Decreased PARP-1 protein and mRNA were confirmed by Western blot and Northern blot analysis, respectively. Human embryonic kidney 293 cells were cultured in DMEM supplemented with 10% FBS, 2 mm l-glutamine and antibiotics (penicillin, 100 units/ml; streptomycin, 100 μg/ml). The cells were cultured at 37 °C in 95% air, 5% CO2 humidified incubator.
Antibodies
Antibodies used include: Anti-PAR (Alexis Biochemical/Enzo Life Science), anti-thymine dimer clone KTM53 (CPD) and anti-(6-4) photoproducts clone KTM50 (Kamiya Biomedical Company), anti-XPA (Abcam; ab2352), anti-XPA (Abcam; ab85914 for immunoprecipitation), anti-PARP (Cell Signaling; catalogue number 9542), anti-PARP (Cell Signaling; catalogue number 9532 for immunocytochemistry), anti-GAPDH (Millipore), anti-β-tubulin (Santa Cruz), anti-rabbit and anti-mouse IgG, HRP-conjugated (Promega), goat anti-rabbit IgG FITC-conjugated, and donkey anti-mouse IgG, Cy3-conjugated (Millipore).
UVR Exposure and DPQ Treatments
Cells at 50–60% confluent density were placed in PBS and exposed to 3 kJ/m2 solar-simulated ultraviolet radiation (ssUVR) using an Oriel 300W solar ultraviolet simulator (Newport Corporation, Irvine, CA). This solar simulator produces a high intensity UVR beam with 91% UVA (320–400 nm) and 9% UVB (280–320 nm). The number of minimal erythema doses for 3 kJ/m2 is 0.042 as measured by the Erythema UV and UVA intensity meter (model 3D; Solar Light Company, Glenside, PA). This dose resulted in 88% viability at 24 h. After UVR exposure, PBS was replaced with growth medium for the times indicated in the figures. Levels of UVR-induced photoproducts at zero time were performed, and there was no difference in initial photoproduct formation between HaCaT and PARP-1 HuSH cells. For the indicated studies, the cells were exposed to 10 μm DPQ (Santa Cruz Biotechnology) for 30 min before UVR exposure. DPQ was present in the postexposure incubation medium. Cells that were not treated are labeled as NT.
Western Blotting
For whole cell lysates, cells were collected in PARP lysis buffer (20 mm Tris base (pH 7.5), 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 25 mm sodium pyrophosphate, 1 mm β-glycerol phosphate, 1 mm sodium vanadate, 1 μg/ml leupeptin, and 2 mm PMSF), and extracts were clarified by centrifugation (8,000 rpm at 4 °C for 5 min). Cytoplasmic and nuclear cell fractions were obtained as described for the CellLytic NuCLEAR extraction kit (Sigma). Protein concentrations were measured using the BCA protein assay (Thermo Scientific). 30 μg of protein in loading buffer (3×, 187.5 mm Tris-HCl, pH 6.8, 6% (w/v) SDS, 30% glycerol, 150 mm DTT and 0.03% (w/v) bromphenol blue) was heated at 100 °C for 5 min, resolved on a 10% SDS-polyacrylimide gel, and transferred to nitrocellulose or PVDF membranes. The proteins were detected as previously described (23). Band signal intensity was obtained using a Kodak 440CF Imager digital science image station. To control sample loading and protein transfer, the membranes were stripped and reprobed to detect GAPDH. GAPDH was tested and found not to change following treatment conditions.
NAD Assay
Experiments were conducted according to the manufacturer's (Cell Technology Inc., Mountain View, CA) protocol for the fluorescent NAD/NADH detection kit.
Detection of UVR-induced Photoproducts
6-4 PPs or CPDs were detected as described in Ref. 22 (protocol for 8-hydroxyl-2′-deoxyguanine) with the following modifications. Fixed cells were not treated with RNase or proteinase K. The cells were incubated in 10% normal horse serum in PBS overnight. Anti-6-4 PP or CPD antibody was incubated with cells at a 1:1000 or 1:200 dilution, respectively, for 1 h at 37 °C in a humid chamber. The cells were washed with PBS then incubated with secondary antibody (1:300) for 1 h at 37 °C in a humid chamber. Following incubation with secondary antibody, the cells were washed with PBS and mounted with Vectashield mounting medium containing 2 μg/ml DAPI (Vector Laboratories). The images were obtained using a Zeiss Axioscope 40 using a 40× objective with an Optronics MacroFire camera and PictureFrame 2.1 picture software. The images used for comparison were acquired with the same instrument settings and exposure times. Three to five images per cell type and time point were obtained, and intensity measurements were quantified using Image J (National Institutes of Health).
In addition, the levels of genomic photoproducts were measured using a slot blot (Minifold II; Whatman International) immunoassay using antibodies to 6-4 PPs (1:1000) and CPDs (1:2000). Sample preparation and slot blot procedure were conducted according to the Bio-Dot SF instruction manual (Bio-Rad). Nitrocellulose membranes were placed in blocking solution for 1 h (5% dried milk made in Tris-buffered saline with 0.05% Tween 20), and primary antibodies were incubated for 1 h followed by incubation with secondary antibodies for 1 h. The membranes were washed for 20 min, and signal intensity was obtained using a Kodak 440CF Imager digital science image station. After imaging, the membranes were stained with methylene blue for 5 min to obtain total DNA in each well. Intensity measurements were normalized to total DNA in each well.
Immunoprecipitation
PAR, PARP-1, or XPA were immunoprecipitated from 750 to 1000 μg of protein in PARP lysis buffer as described in Ref. 24 with the following modifications. Primary antibodies (1:100 dilution) were incubated with protein for 1 h at 4 °C followed by the addition of protein A-agarose beads (Invitrogen) and further incubation for 1 h at 4 °C. The beads were isolated by centrifugation (4,500 rpm at 4 °C for 5 min) and washed three times with PARP lysis buffer. To elute protein, loading buffer (see Western blotting) was added to pelleted beads and heated at 100 °C for 5 min and resolved by SDS-polyacrylimide gel as described above in the Western blotting section.
Immunocytochemistry
In situ detection of XPA and PARP-1 was conducted as described in Ref. 25 with the following modifications. XPA (1:50) and PARP-1 (1:150) antibodies were diluted in washing buffer (0.5% bovine albumin, 0.05% Tween 20). Secondary antibodies were used at 1:300 (FITC) and 1:500 (cy3) dilutions. Primary and secondary antibodies were added simultaneously during the appropriate steps. Five images/group were obtained using an LSM 510-META confocal with a 63× objective. For co-localization analysis, cy3 (XPA) and FITC (PARP-1) intensity measurements were obtained with individual masks for the respective channels, and co-localization was determined in Slidebook 5.0 (Intelligent Imaging Innovations Inc., Denver, CO) using percent co-localization or Pearson's correlation coefficient.
Chip-on-Western
Chromatin preparation was adapted from Ref. 26 and collection protocol was followed as stated. Following collection, chromatin suspension was sonicated on ice (1 × 90 s) in radioimmune precipitation assay buffer (0.01 m Tris-HCl, pH 8.0, 0.14 m NaCl, 1% Triton X-100, 0.1% deoxycholate, 1% SDS) using a Branson Sonifer (output, 5; duty, 40%; pulsed). The samples were isolated by centrifugation (13,200 rpm at 4 °C for 15 min) with the supernatant containing cross-linked chromatin. A 50-μl aliquot of the supernatant was used to determine DNA concentration using the DNeasy blood and tissue kit (Qiagen). For each sample, an equal amount of cross-linked chromatin (40–50 μg) was immunoprecipitated with 1:100 dilution of specific antibody (XPA 12F5 or PARP-1 catalogue number 9542). For immunoprecipitation of PARP-1, the samples were incubated in primary antibody for 1 h at 4 °C. The immunocomplexes were absorbed onto precleared protein A-Sepharose beads (GE Healthcare) for 1 h at 4 °C. The samples were washed three times in PARP lysis buffer and lastly in LiCl buffer (0.02 m Tris, pH 8.0, 0.25 m LiCl, 0.5% Triton X-100, 0.5% sodium deoxycholate), resuspended in loading buffer (see Western blotting), and boiled for 30 min at 100 °C before loading onto a 10% polyacrylamide gel. The Western blotting protocol (above) was followed. The protocol for immunoprecipitation of XPA was adjusted as follows; the samples were incubated in primary antibody for 3 h at 4 °C. The immunocomplexes were absorbed onto precleared protein A and protein G beads (1:1 ratio; Invitrogen) for 3 h at 4 °C. The beads were washed as stated in Ref. 26, resuspended in loading buffer (see Western blotting), and boiled for 30 min at 100 °C before loading onto a 10% polyacrylamide gel. The Western blotting protocol (above) was followed. For analysis, band signal intensity was obtained using a Kodak 440CF Imager digital science image station. All of the negative control samples were incubated with normal rabbit IgG-AC (Santa Cruz) and processed similarly to samples incubated with primary antibody. We confirmed the presence of photolesions in the same soluble chromatin fraction used to perform immunoprecipitations by blotting the fraction onto a membrane using a slot blot apparatus (see above protocol under “Detection of UVR-induced Photoproducts,” slot blot).
In Vitro Pulldown Assay
Purified PARP protein (1 μg, high specific activity, Trevigen, Gaithersburg, MD) was diluted in PARP lysis buffer (see Western blotting) in a final volume of 200 μl. PARP was activated by addition of activated DNA (1 μl, 10×; Trevigen) and NAD+ (1.5 μl; Trevigen) followed by incubation at room temperature for 15 min. PARP inhibition was achieved by preincubating purified PARP-1 with the PARP inhibitor, AG-014699 (1 μm; Selleck Chemicals, Tx [Rucaparib]) at room temperature for 30 min prior to activation. His-tagged XPA (2 μg; Proteintech, Chicago, IL) was added to appropriate tubes and incubated for 1 h at 4 °C with rotation. For pulldown of XPA, EcoMagTM His-Co magnetic particles (10 μl; Bioclone Inc., San Diego, CA) were added to the solution, and the proteins were isolated according to the manufacturer's protocol with a slight modification through use of PARP lysis buffer for the bead washing steps. The beads were washed a total of three times. The protein was eluted from the beads using 1× elution buffer (15 μl, 100 mm HEPES, 0.5 m imidazole, pH 8.0). Loading buffer (see Western blotting) was added to eluted samples, and the samples were heated to 100 °C for 5 min followed by Western blotting procedures.
Statistical Analysis
All of the graphs and statistical data were completed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA). Analysis used were unpaired t test, two-way analysis of variance with Bonferroni's correction, and one-way analysis of variance followed by Tukey's post hoc multiple comparison test.
RESULTS
UVR-induced Photoproducts Are Retained Following Reduction of PARP Activity through Protein Silencing or Chemical Inhibition
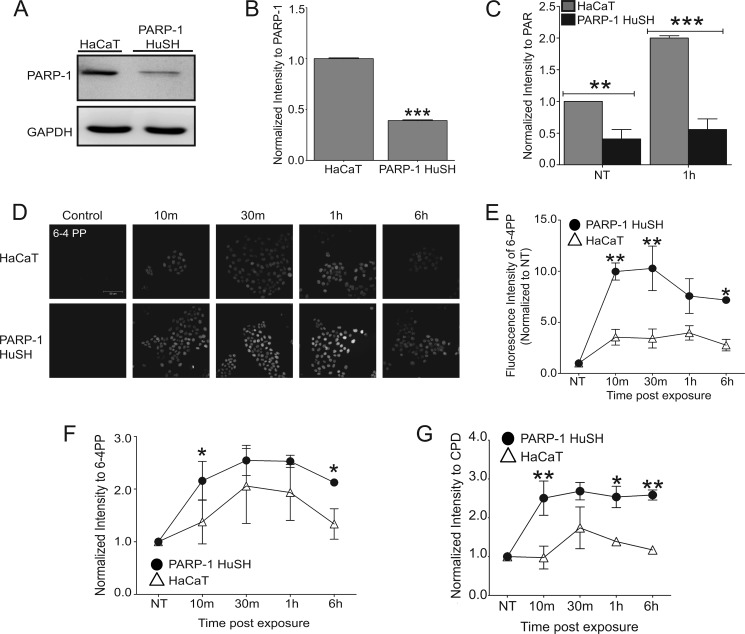
To investigate the impact of PARP-1 depletion on retention of UVR-induced photoproducts in cancer-relevant target cells, immortalized human keratinocytes (HaCaT) were transfected with a short hairpin RNA directed toward the PARP-1 protein (PARP-1 HuSH). A 60% reduction in PARP-1 protein levels was detected by Western blotting (Fig. 1, A and B), which corresponded to a 60% reduction in basal PAR levels (Fig. 1C, NT) and a 70% reduction in PARP activation following a single dose of 3 kJ/m2 solar-simulated UVR (Fig. 1C, 1h). The accumulation of 6-4 PPs, as measured by fluorescent intensity, in PARP-1 HuSH cells remained elevated compared with HaCaT cells over a 6-h time frame (Fig. 1, D and E), suggesting decreased efficiency in repair mechanisms. Similar results were obtained using the slot blot technique for UVR-induced 6-4 PPs (Fig. 1F) and CPDs (Fig. 1G), further illustrating a role for PARP-1 in the repair of UVR-induced lesions.
FIGURE 1.
Effects of PARP activity on retention of UVR-induced photoproducts. A, representative Western blot comparing PARP-1 protein in HaCaT and PARP-1 HuSH cells. GAPDH is used as a loading control. B, quantification of A by densitometry. PARP-1 intensity was normalized to GAPDH. The data are presented as the means ± S.E., n = 3. C, quantification of PAR Western blots by densitometry in HaCaT and PARP-1 HuSH cells following UVR exposure. The data are presented as the means ± S.E., n = 3. NT = not treated. D, HaCaT cells and PARP-1 HuSH cells were exposed to a single dose of ssUVR (3 kJ/m2) and collected at various times postexposure. Immunofluorescence was used to obtain images of 6-4 PPs. Initial UVR-induced photoproducts did not differ between HaCaT and PARP-1 HuSH cells. E, fluorescence intensity obtained from images in D. Open triangles, HaCaT; closed circles, PARP-1 HuSH. Intensities were normalized to NT sample. The data are presented as the means ± S.E., n = 4. F and G, slot blot was performed on DNA extracted from HaCaT and PARP-1 HuSH cells. Quantification of lesion intensity was done by densitometry. The intensities were normalized to NT. F, intensity of 6-4 PPs formation. The data are presented as the means ± S.E., n = 3. G, intensity of CPD formation. The data are presented as the means ± S.E., n = 3. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Scale bar, 50 μm.
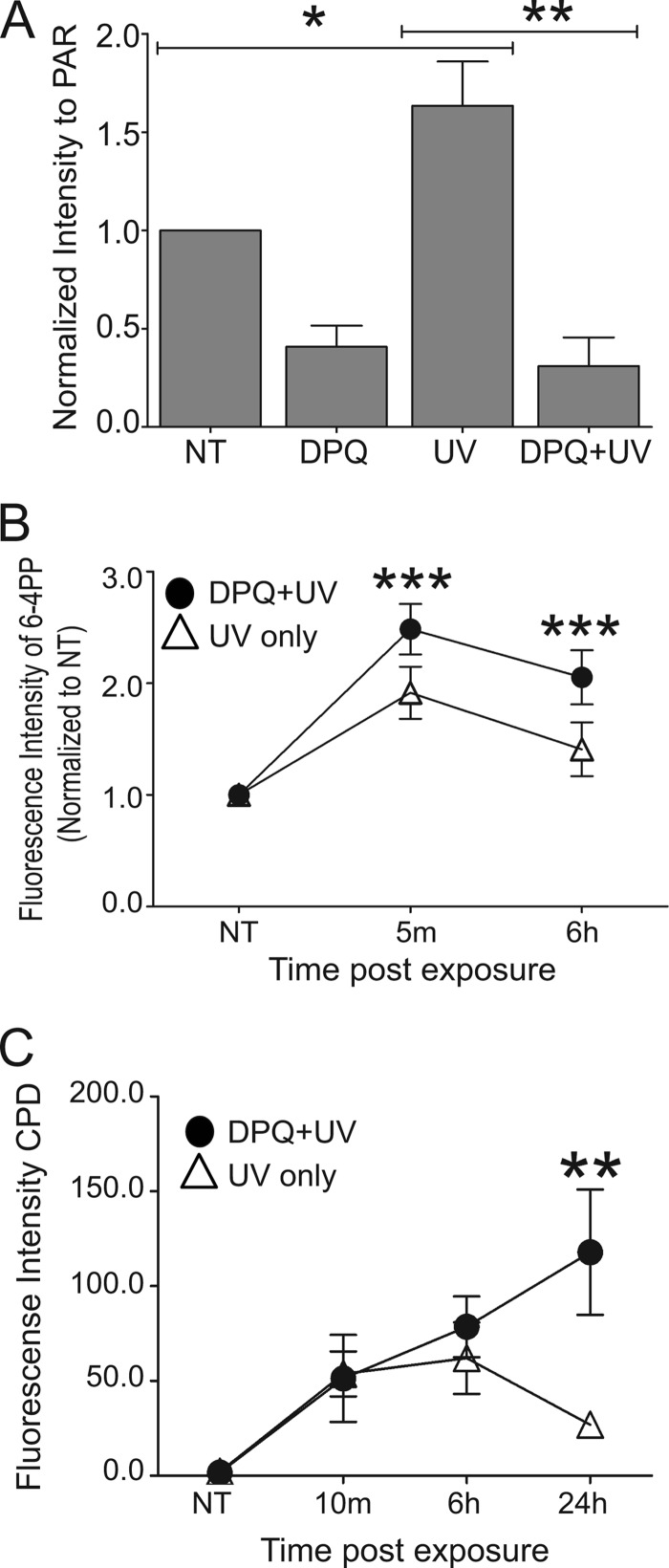
To expand on the above findings and examine the role of PARP activity during the repair process, the PARP inhibitor DPQ (27, 28) was used. This inhibitor significantly decreased UVR-stimulated PARP activity as measured by PAR production (Fig. 2A) and resulted in retention of 6-4 PPs (Fig. 2B) and CPDs (Fig. 2C). Taken together, these findings demonstrate that reduction of PARP activity, either by PARP-1 silencing or chemical inhibition, promotes retention of UVR-induced photolesions.
FIGURE 2.
Inhibition of PARP activity results in retention of UVR-induced lesions. HaCaT cells were pre-exposed to a PARP inhibitor, DPQ, 30 min prior to a single dose of ssUVR (3 kJ/m2) or exposed to UVR alone and collected at the indicated times postexposure. A, quantification of PAR Western blots by densitometry. The data are presented as the means ± S.E., n = 3. B, quantification of 6-4 PPs obtained from immunofluorescent images. UV only samples (open triangles) and DPQ+UV samples (closed circles). Fluorescence intensity was normalized to untreated (NT) sample. The data are presented as the means ± S.E., n = 4. C, quantification of CPDs obtained from immunofluorescent images. Fluorescence intensity of raw data is shown. The data are presented as the means ± S.E., n = 3. *, p < 0.05; **, p < 0.01; ***, p < 0.01.
UVR Promotes Association between PARP-1 and XPA
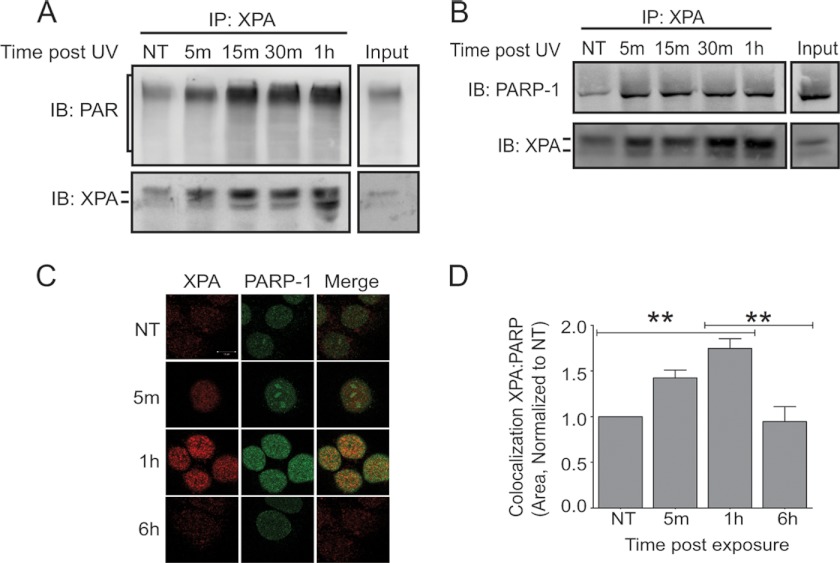
PARP-1 activity was stimulated by UVR, as measured by decreased cellular NAD+ (supplemental Fig. S1A) and a significant increase in PAR production (supplemental Fig. S1, B and C). Given that biochemical assays established that XPA contains a PAR-binding motif (14), we examined whether association between XPA and PAR could be detected within an intact cellular system using co-immunoprecipitation of endogenous proteins. Association between XPA and PAR was detectable in unstimulated cells (Fig. 3A, NT), and this association was rapidly increased following UVR exposure (Fig. 3A and supplemental Fig. S2A). Because PARP-1 is self-modified by PAR, we investigated the possible association between XPA and PARP-1. Co-immunoprecipitation revealed UVR-induced association between XPA and PARP-1 following immunoprecipitation of XPA (Fig. 3B) and the reciprocal immunoprecipitation with PARP-1 (supplemental Fig. S2B). Immunofluorescence detection was performed to evaluate co-localization of XPA and PARP-1 in situ. In agreement with the co-immunoprecipitation findings, a significant increase in XPA and PARP-1 co-localization was detected 1 h after UVR exposure (Fig. 3, C and D). The co-localization was transient with significant reduction apparent 6 h after UVR (Fig. 3D). These results demonstrate that the identified PAR-binding motif in XPA is functional within cells and suggest that it promotes UVR-induced associations of XPA with PAR and PARP-1.
FIGURE 3.
UVR-induced association between XPA and PARP-1. HaCaT cells were exposed to a single dose of ssUVR (3 kJ/m2) and collected at the indicated times postexposure. A, representative image of co-immunoprecipitation. XPA was immunoprecipitated (IP) from cells, and the membranes were subsequently immunoblotted (IB) for PAR. The membranes were stripped and immunoblotted for XPA, as confirmation for immunoprecipitation, n = 3. B, representative image of co-immunoprecipitation. XPA was immunoprecipitated from cells, and the membranes were subsequently immunoblotted for PARP-1. The membranes were stripped and immunoblotted for XPA, as confirmation for immunoprecipitation, n = 3. C, dual staining with antibodies against XPA (red) and PARP-1 (green) was performed to assess the amount of co-localization (merge, yellow). D, quantification of intensities from C. The percentage of co-localization was determined as stated under “Experimental Procedures.” The intensities were normalized to the untreated (NT) sample. The data are presented as the means ± S.E., n = 3. **, p < 0.01. Scale bar, 10 μm.
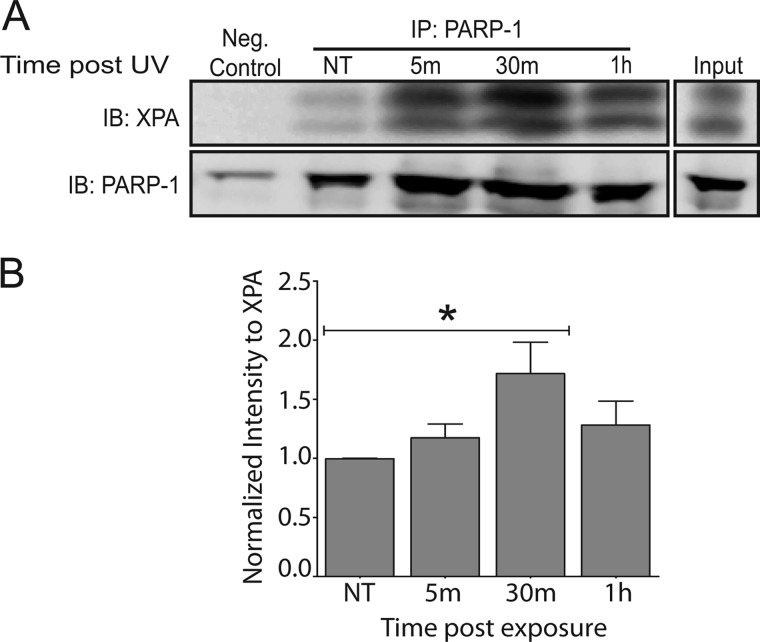
To observe this association in a more direct context for PARP-1 function, we isolated chromatin complexes using in vivo cross-linking followed by chromatin immunoprecipitation, as described in Ref. 26. Chromatin fragments were immunoprecipitated with a PARP-1 specific antibody. Following UVR exposure, increased binding of PARP-1 with chromatin complexes was detected (Fig. 4A, IB: PARP-1), which was expected based on the known function of PARP-1 (13). Furthermore, under conditions where PARP-1 had increased binding with chromatin, it also associated with XPA. This association was significant 30 min after UVR exposure (Fig. 4B). The observed association between PARP-1 and XPA occurs under relevant conditions for NER proteins, thus demonstrating its potential to be meaningful in lesion repair.
FIGURE 4.
UVR-induced association between XPA and chromatin bound PARP-1. HEK 293 cells were exposed to a single dose of ssUVR (3 kJ/m2) and collected at the indicated times postexposure. A modified chromatin immunoprecipitation method (ChIP-on-Western) was then performed. A, representative image of co-immunoprecipitation. PARP-1 was immunoprecipitated (IP) from cells, and the membranes were subsequently immunoblotted (IB) for XPA. The membranes were stripped and immunoblotted for PARP-1, as confirmation for immunoprecipitation. B, quantification of Western blot by densitometry. NT, untreated. The data are presented as the means ± S.E., n = 5. *, p < 0.05.
Decreased PARP Activity through Silencing or Chemical Inhibition Results in Decreased Association between PARP-1 and XPA
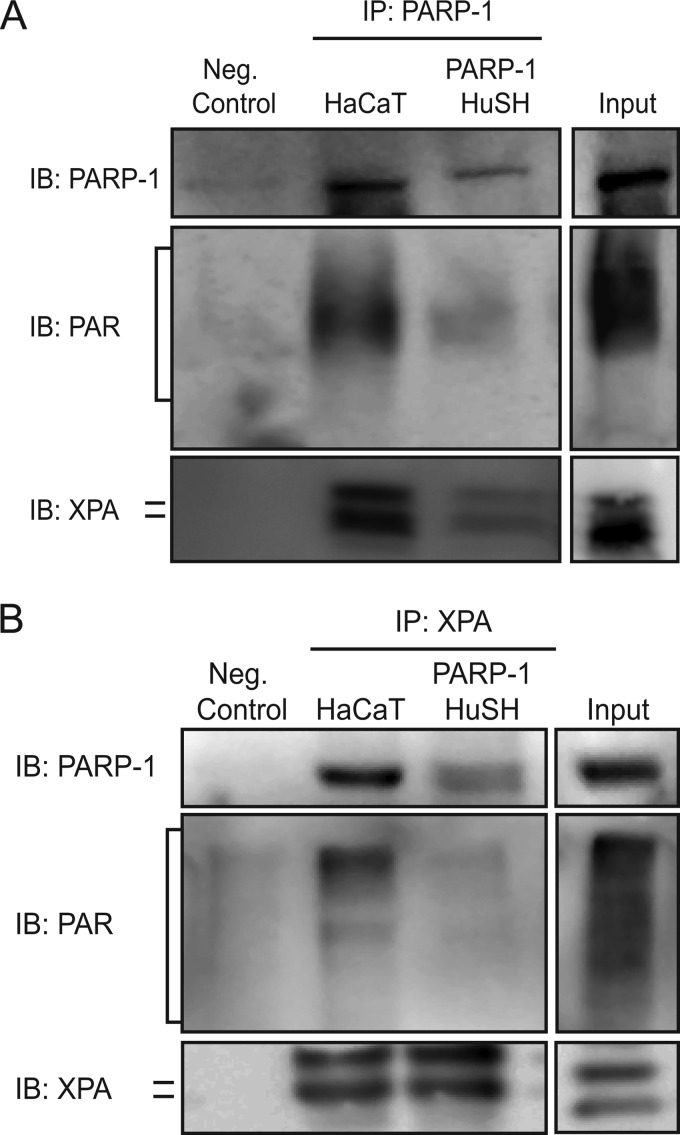
Next, we wanted to observe the association between PARP-1 and XPA within PARP-1 HuSH cells. The results in Fig. 1 show that decreasing PARP-1 protein and therefore PARP activity results in retention of UVR-induced lesions. To better understand how PARP-1 associations with XPA may contribute to these findings, we conducted immunoprecipitations with a PARP-1 specific antibody in the PARP-1 HuSH cells. Immunoblotting for PARP-1 following its immunoprecipitation from PARP-1 HuSH cells showed a significant decrease in PARP-1 protein (Fig. 5A and Table 1), and PAR bound to PARP-1 itself (Fig. 5A and Table 1). These data corroborate results from Fig. 1 (A–C). More importantly, following immunoprecipitation of PARP-1 from PARP-1 HuSH cells, there was a significant decrease in its association with XPA when compared with HaCaT cells (Fig. 5A and Table 1). Reciprocal immunoprecipitations also showed a decreased association between XPA and PARP-1 in PARP-1 HuSH cells (Fig. 5B and Table 1). Taken together, these data suggest a contribution of the PARP-1 protein and/or its activity in its association with XPA.
FIGURE 5.
Silencing PARP-1 protein leads to decreased association between PARP-1 and XPA. Co-immunoprecipitations were performed in HaCaT and PARP-1 HuSH cells. A, representative image of co-immunoprecipitations. PARP-1 was immunoprecipitated (IP) from cells, and the membranes were subsequently immunoblotted (IB) for PARP-1, PAR, and XPA. B, representative image of reciprocal co-immunoprecipitations. XPA was immunoprecipitated from cells, and the membranes were subsequently immunoblotted for PARP-1, PAR, and XPA.
TABLE 1.
Impact of PARP-1 silencing on immunoprecipitations
Shown is a quantification summary of the immunoprecipitation data from HaCaT and PARP-1 HuSH cells. Statistical analysis of data was obtained from Western blots as shown in Fig. 5 (n = 3). NS, not significant.
| Decrease | p value | |
|---|---|---|
| % | ||
| Immunoprecipitation with PARP-1 specific antibody | ||
| PARP-1 | 75 | <0.05 |
| PAR | 54 | <0.05 |
| XPA | 82 | <0.01 |
| Immunoprecipitation with XPA specific antibody | ||
| PARP-1 | 50 | <0.05 |
| PAR | 40 | <0.01 |
| XPA | 10 | NS |
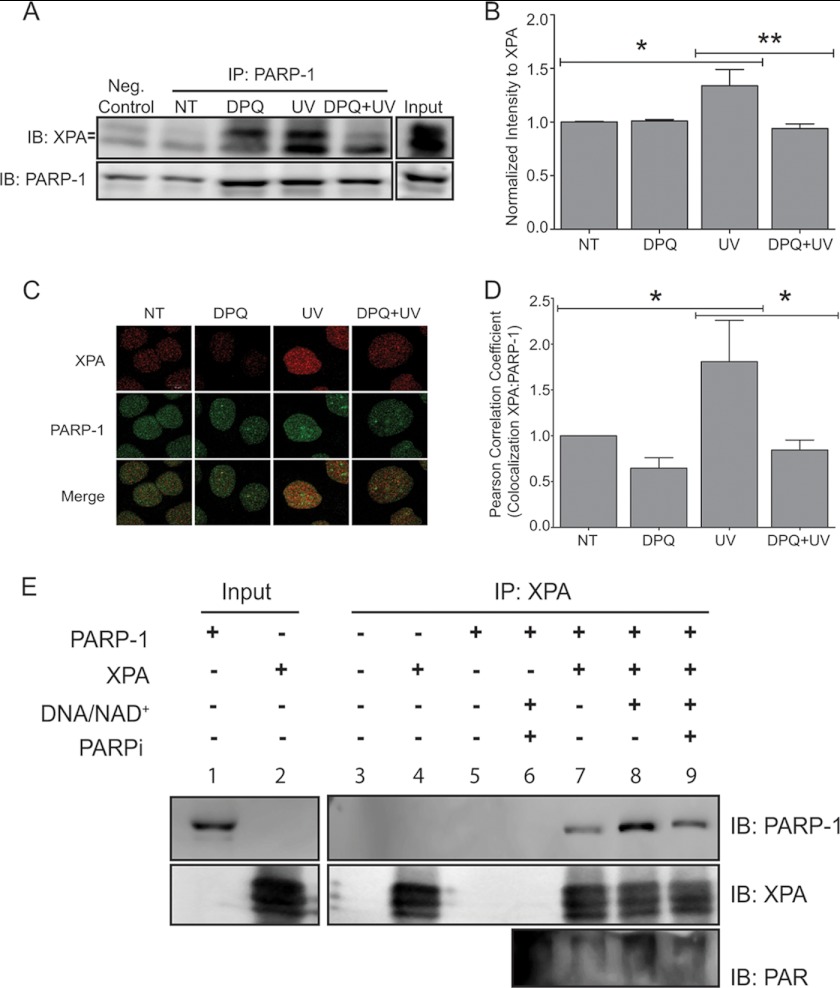
To further investigate the contribution of PARP activity, we performed experiments using the pharmacological PARP inhibitor DPQ. Chromatin binding experiments confirmed a significant increase in the association between chromatin-bound PARP-1 and XPA (Fig. 6, A and B, UV), which was abolished following DPQ exposure (Fig. 6, A and B, DPQ+UV), suggesting a role for PARP activation and PAR formation in the association between XPA and PARP-1. This decrease in association was also observed when XPA was immunoprecipitated from whole cell extracts and subsequently immunoblotted for PARP-1 (supplemental Fig. S3). Immunofluorescent detection provided similar results. Following UVR exposure, there was increased co-localization between PARP-1 and XPA (Fig. 6C, UV); again, following DPQ exposure this association was significantly decreased (Fig. 6D, DPQ+UV). These findings further demonstrate the importance of PARP activity in the association between PARP-1 and XPA.
FIGURE 6.
Inhibition of PARP-1 activity leads to decreased association between PARP-1 and XPA. HaCaT cells were pre-exposed to a PARP inhibitor, DPQ, 30 min prior to UVR exposure. A, cells were collected 30 min post-UVR. Representative Western blot obtained from modified chromatin immunoprecipitation method (ChIP-on-Western). PARP-1 was immunoprecipitated (IP) from chromatin complexes. The membranes were immunoblotted (IB) for XPA and subsequently immunoblotted for PARP-1 as confirmation for immunoprecipitation. B, quantification of Western blot by densitometry. The data are presented as the means ± S.E., n = 3. C, HaCaT cells were pre-exposed to a PARP inhibitor, DPQ, 30 min prior to UVR exposure and cells were fixed 1 h post-UVR. Dual staining with antibodies against XPA (red) and PARP-1 (green) was performed to assess the amount of co-localization (merge, yellow). D, graph representing co-localization between XPA and PARP-1. The percentage of co-localization was determined using Pearson's correlation coefficient. The data are presented as the means ± S.E., n = 3. E, purified PARP-1 and His-XPA were incubated alone (lane 7), with activated PARP-1 (addition of activated DNA and NAD+, lane 8), or in the presence of the PARP inhibitor AG-014699 (lane 9). Following the various treatments, His-XPA was pulled down (IP) from each sample using cobalt-conjugated magnetic beads. Following pulldown, the membranes were subsequently immunoblotted (IB) for PARP-1, XPA, and PAR, NT=untreated. n = 3. *, p < 0.05; **, p < 0.01. Scale bar, 10 μm.
To determine whether PAR facilitates this association in vitro, we conducted studies using purified proteins. To observe the level of basal interaction between the two proteins, purified PARP-1 was incubated with purified His-tagged XPA alone (Fig. 6E, IB: PARP-1, lane 7). The addition of activated DNA and NAD+ stimulated PARP activity (Fig. 6E, IB: PAR, lane 8), and resulted in a 3-fold increase in association between PARP-1 and XPA (Fig. 6E, IB: PARP-1, lane 8). In contrast, preincubation of purified PARP-1 with AG-014699, a potent PARP inhibitor (29–31), prior to PARP-1 activation led to decreased association between PARP-1 and XPA (Fig. 6E, IB: PARP-1, lane 9). Together, the in vitro and in vivo data strongly suggest that PARP activity and the subsequent formation of PAR facilitates the association between PARP-1 and XPA.
PARP Inhibition Leads to Changes in XPA Function
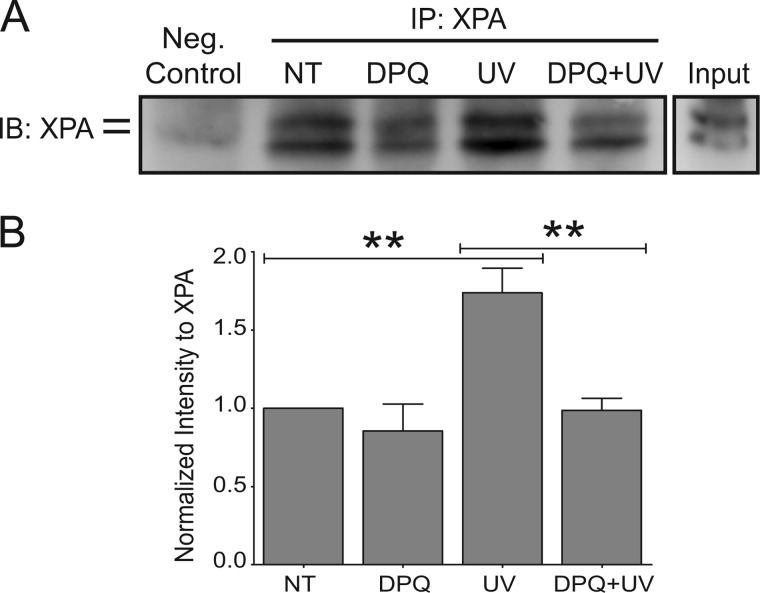
The main functions of XPA are to bind DNA and interact with other NER proteins, thereby promoting DNA repair (32). To ascertain whether there were any changes to the DNA binding ability of XPA as a function of PARP activity, the cells were exposed to UVR with or without DPQ treatment and collected 5 min after UVR. UVR exposure significantly increased XPA binding to chromatin compared with unexposed cells (Fig. 7, A and B, UV), and DPQ significantly decreased UVR-induced XPA association with chromatin (Fig. 7B, DPQ+UV). These data demonstrate that PARP activity regulates UVR-induced XPA association with chromatin, which suggests a mechanistic link between PARP activity and NER.
FIGURE 7.
Inhibition of PARP activity affects XPA function. HEK 293 cells were pre-exposed to a PARP inhibitor, DPQ, 30 min prior to UVR exposure and collected 5 min postexposure. A, representative Western blot obtained from modified chromatin immunoprecipitation (ChIP-on-Western). XPA was immunoprecipitated (IP) from samples, and its ability to bind chromatin was assessed by subsequent immunoblotting (IB). B, quantification by densitometry from ChIP-on-Westerns. XPA intensity was normalized to NT. The data are presented as the means ± S.E., n = 4.; **, p < 0.01.
DISCUSSION
Production of PAR by PARP enzymes modulates the association of DNA repair proteins with sites of DNA damage and is important for the orchestration of DNA repair. Although the involvement of PARP-1 in base excision repair is well characterized, there is limited knowledge regarding the contributions of PARP-1 to NER. Evidence to support a role for PARP-1 in NER includes retention of UVR-induced photoproducts previously described (7, 8) and our data in keratinocytes (Fig. 1) following disruption of PARP activity by expression of the PARP-1 DNA-binding domain, chemical inhibitors, or RNAi. Current literature places a role for PARP-1 in the transcriptional coupled repair arm of NER with studies supporting a cooperative interplay between PARP-1 and Cockane syndrome B protein (7, 8). Our studies provide a novel alternative mechanism, which demonstrates an association between XPA and PARP-1. Because XPA is a core NER protein and both transcription coupled repair and global genomic repair converge at an XPA-dependent step, these findings support the hypothesis that activated PARP contributes to the retention of UVR-induced DNA photoproducts through modulation of XPA.
PARP-1 enzymatic activity is stimulated following DNA damage, and the consequent assembly of PAR subunits recruits DNA repair proteins to the lesion. There are three main PAR-binding motifs responsible for mediating associations between PAR and acceptor proteins. One PAR-binding motif is a highly conserved domain known as the “macro domain” of ∼130–190 amino acid residues. This motif is present in several PARP family members such as PARP-9, PARP-14, and PARP-15 (33, 34). Another PAR-binding motif is known as the PBZ domain. This domain is a putative C2H2 zinc finger and has been identified in the checkpoint protein CHFR (checkpoint protein with forkhead-associated and RING domains) and the DNA damage response protein APLF (aprataxin PNK-like factor) (35, 36). The best characterized PAR-binding motif contains ∼20–25 amino acids with an N-terminal basic amino acid cluster followed by hydrophobic residues interspersed with basic amino acids. This motif has been identified in several of the core histones, p53, cyclin-dependent kinase inhibitor 1 (p21), XRCC1, and other proteins including XPA (14, 37, 38). The presence of a PAR-binding motif in XPA was predicted by sequence alignments that identified the 20–25-amino acid motif in the C terminus of XPA. Polymer blot experiments confirmed PAR binding properties of amino acids between positions 215 and 237 (14). Further in vitro analysis determined that polymers of ∼16 PAR units were necessary for XPA binding and high affinity XPA binding was evident using immobilized long PAR chains (63-mer) (21). Although these studies suggested that PAR might influence XPA activity, the biochemical approaches did not demonstrate in vivo significance.
The current studies reveal that UVR promotes association of XPA with PAR subunits (Fig. 3). Additionally, PAR production by activated PARP enhances UVR-dependent XPA association with chromatin-bound PARP-1 (Fig. 6, A and B), which illustrates that XPA-PAR associations occur in cells at endogenous protein levels. Additionally, in vitro experiments independently confirm the importance of PAR formation in the PARP-1-XPA association (Fig. 6E). UVR-stimulated XPA association with chromatin (Fig. 7) illustrates the relevance of this association in vivo, as well as modulation of UVR-dependent XPA-DNA binding as a function of PARP enzymatic activity. Although the above data highlight a role for PAR-facilitated interactions, there may be additional contributions caused by PARP-1 itself. PARP-1 silencing (Figs. 1, A–C, and 5) decreased the association between PARP-1 and XPA to a greater magnitude than pharmacologic PARP inhibition by DPQ. Additionally, PARP inhibition in vitro decreased the association between PARP-1 and XPA to near basal levels, suggesting a residual association through direct interactions. These data indicate a possible dual mechanism that relies upon PARP activation, and PAR production that may be augmented by a direct protein interaction. More detailed biochemical experiments will help elucidate the exact contributions of PAR and PARP-1 and their roles in NER.
NER is regulated by multiple post-translational modifications including PARylation, polyubiquitination, and phosphorylation (3). The breadth of protein modification and regulation by PAR is currently unknown; however, a mass spectrometry-based proteome-wide search for PAR-binding proteins and PAR-associated complexes using the 20–25-amino acid PAR-binding sequence identified hundreds of putative PAR-binding proteins (39). Fewer proteins have been identified with the alternate PAR-binding motifs, but database searches predicted 27 proteins containing the PAR-binding macro domain and four proteins with the PBZ domain (39). Although only a fraction of putative PAR acceptor proteins have been analyzed, it is clear that in addition to PAR-dependent recruitment of base excision repair proteins such as XRCC1 and DNA ligase III to sites of DNA damage, PARP activation modulates multiple steps in the repair of DNA damage. PARylation of chromatin-associated proteins including histones and certain chromatin remodeling factors is believed to modify local chromatin structure at sites of DNA strand breaks or damage (40–42), and recently, Iduna was identified as a PAR-dependent ubiquitin E3 ligase (43). These findings suggest that PARP activity and PAR production may influence numerous aspects of DNA repair.
Overall, our findings further extend the impact of PARP activation in DNA repair beyond the established role in base excision repair by providing evidence for PAR-facilitated modulation of XPA as had been predicted by the identification of its PAR-binding motif. Because of the centrality of XPA within NER, modulation of XPA by PAR provides a novel mechanism to account for the observed relationship between loss of PARP function and retention of UVR-induced photolesions, thus solidifying a role for PARP in NER. Further studies to assess the functional significance of identified or predicted PAR binding sites in other proteins will be necessary to delineate the scope of PARP activation in DNA repair and other regulatory pathways.
Supplementary Material
Acknowledgments
We thank Dr. Graham Timmins for knowledge and assistance with the UV Solar-Simulator. The images in this paper were generated in the Fluorescence Microscopy Shared Resource of the University of New Mexico Cancer Center (P30CA118100).
This work was supported, in whole or in part, by National Institutes of Health Grants RO1ES015826 (to L. G. H. and K. J. L.) and F31ES019823 (to B. S. K.). This work was also supported by University of New Mexico Initiative to Maximize Student Diversity Grant R25GM060201.

This article contains supplemental Figs. S1–S3.
- UVR
- ultraviolet radiation
- XPA
- xeroderma pigmentosum complementation group A
- PARP-1
- poly(ADP-ribose) polymerase-1
- 6-4 PPs
- (6-4)pyrimidine-pyrimidone photoproducts
- CPD
- cyclobutane pyrimidine dimer
- NER
- nucleotide excision repair
- PAR
- poly(ADP-ribose)
- DPQ
- 3,4-dihydro-5-[4-(1-piperidinyl)butoxyl]-1(2H)-isoquinolinone
- ssUVR
- solar-simulated UVR.
REFERENCES
- 1. Narayanan D. L., Saladi R. N., Fox J. L. (2010) Ultraviolet radiation and skin cancer. Int. J. Dermatol. 49, 978–986 [DOI] [PubMed] [Google Scholar]
- 2. Park C. J., Choi B. S. (2006) The protein shuffle. Sequential interactions among components of the human nucleotide excision repair pathway. FEBS J. 273, 1600–1608 [DOI] [PubMed] [Google Scholar]
- 3. Nouspikel T. (2009) DNA repair in mammalian cells. Nucleotide excision repair. Variations on versatility. Cell Mol. Life Sci. 66, 994–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pfeifer G. P., You Y. H., Besaratinia A. (2005) Mutations induced by ultraviolet light. Mutat. Res. 571, 19–31 [DOI] [PubMed] [Google Scholar]
- 5. Sinha R. P., Häder D. P. (2002) UV-induced DNA damage and repair. A review. Photochem. Photobiol. Sci. 1, 225–236 [DOI] [PubMed] [Google Scholar]
- 6. Woodhouse B. C., Dianov G. L. (2008) Poly ADP-ribose polymerase-1. An international molecule of mystery. DNA Repair 7, 1077–1086 [DOI] [PubMed] [Google Scholar]
- 7. Flohr C., Bürkle A., Radicella J. P., Epe B. (2003) Poly(ADP-ribosyl)ation accelerates DNA repair in a pathway dependent on Cockayne syndrome B protein. Nucleic Acids Research 31, 5332–5337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghodgaonkar M. M., Zacal N., Kassam S., Rainbow A. J., Shah G. M. (2008) Depletion of poly(ADP-ribose) polymerase-1 reduces host cell reactivation of a UV-damaged adenovirus-encoded reporter gene in human dermal fibroblasts. DNA Repair 7, 617–632 [DOI] [PubMed] [Google Scholar]
- 9. Schreiber V., Amé J. C., Dollé P., Schultz I., Rinaldi B., Fraulob V., Ménissier-de Murcia J., de Murcia G. (2002) Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J. Biol. Chem. 277, 23028–23036 [DOI] [PubMed] [Google Scholar]
- 10. Bürkle A. (2001) Physiology and pathophysiology of poly(ADP-ribosyl)ation. Bioessays 23, 795–806 [DOI] [PubMed] [Google Scholar]
- 11. Schreiber V., Dantzer F., Ame J. C., de Murcia G. (2006) Poly(ADP-ribose). Novel functions for an old molecule. Nat. Rev. Mol. Cell Biol. 7, 517–528 [DOI] [PubMed] [Google Scholar]
- 12. Malanga M., Althaus F. R. (2005) The role of poly(ADP-ribose) in the DNA damage signaling network. Biochem. Cell Biol. 83, 354–364 [DOI] [PubMed] [Google Scholar]
- 13. Hassa P. O., Hottiger M. O. (2008) The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front. Biosci. 13, 3046–3082 [DOI] [PubMed] [Google Scholar]
- 14. Pleschke J. M., Kleczkowska H. E., Strohm M., Althaus F. R. (2000) Poly(ADP-ribose) binds to specific domains in DNA damage checkpoint proteins. J. Biol. Chem. 275, 40974–40980 [DOI] [PubMed] [Google Scholar]
- 15. Aboussekhra A., Biggerstaff M., Shivji M. K., Vilpo J. A., Moncollin V., Podust V. N., Protić M., Hübscher U., Egly J. M., Wood R. D. (1995) Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell 80, 859–868 [DOI] [PubMed] [Google Scholar]
- 16. Mu D., Park C. H., Matsunaga T., Hsu D. S., Reardon J. T., Sancar A. (1995) Reconstitution of human DNA repair excision nuclease in a highly defined system. J. Biol. Chem. 270, 2415–2418 [DOI] [PubMed] [Google Scholar]
- 17. Köberle B., Roginskaya V., Wood R. D. (2006) XPA protein as a limiting factor for nucleotide excision repair and UV sensitivity in human cells. DNA Repair 5, 641–648 [DOI] [PubMed] [Google Scholar]
- 18. Kang T. H., Reardon J. T., Sancar A. (2011) Regulation of nucleotide excision repair activity by transcriptional and post-transcriptional control of the XPA protein. Nucleic Acids Res. 39, 3176–3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Missura M., Buterin T., Hindges R., Hübscher U., Kaspárková J., Brabec V., Naegeli H. (2001) Double-check probing of DNA bending and unwinding by XPA-RPA. An architectural function in DNA repair. EMBO J. 20, 3554–3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bartels C. L., Lambert M. W. (2007) Domains in the XPA protein important in its role as a processivity factor. Biochem. Biophys. Res. Commun. 356, 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fahrer J., Kranaster R., Altmeyer M., Marx A., Bürkle A. (2007) Quantitative analysis of the binding affinity of poly(ADP-ribose) to specific binding proteins as a function of chain length. Nucleic Acids Res. 35, e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ding W., Liu W., Cooper K. L., Qin X. J., de Souza Bergo P. L., Hudson L. G., Liu K. J. (2009) Inhibition of poly(ADP-ribose) polymerase-1 by arsenite interferes with repair of oxidative DNA damage. J. Biol. Chem. 284, 6809–6817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qin X. J., Hudson L. G., Liu W., Timmins G. S., Liu K. J. (2008) Low concentration of arsenite exacerbates UVR-induced DNA strand breaks by inhibiting PARP-1 activity. Toxicol. Appl. Pharmacol. 232, 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou X., Sun X., Cooper K. L., Wang F., Liu K. J., Hudson L. G. (2011) Arsenite interacts selectively with zinc finger proteins containing C3H1 or C4 motifs. J. Biol. Chem. 286, 22855–22863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwerdtle T., Ebert F., Thuy C., Richter C., Mullenders L. H., Hartwig A. (2010) Genotoxicity of soluble and particulate cadmium compounds. Impact on oxidative DNA damage and nucleotide excision repair. Chem. Res. Toxicol. 23, 432–442 [DOI] [PubMed] [Google Scholar]
- 26. Fousteri M., Vermeulen W., van Zeeland A. A., Mullenders L. H. (2006) Cockayne syndrome A and B proteins differentially regulate recruitment of chromatin remodeling and repair factors to stalled RNA polymerase II in vivo. Mol. Cell 23, 471–482 [DOI] [PubMed] [Google Scholar]
- 27. Suto M. J., Turner W. R., Arundel-Suto C. M., Werbel L. M., Sebolt-Leopold J. S. (1991) Dihydroisoquinolinones. The design and synthesis of a new series of potent inhibitors of poly(ADP-ribose) polymerase. Anticancer Drug Des. 6, 107–117 [PubMed] [Google Scholar]
- 28. Moroni F., Meli E., Peruginelli F., Chiarugi A., Cozzi A., Picca R., Romagnoli P., Pellicciari R., Pellegrini-Giampietro D. E. (2001) Poly(ADP-ribose) polymerase inhibitors attenuate necrotic but not apoptotic neuronal death in experimental models of cerebral ischemia. Cell Death Differ. 8, 921–932 [DOI] [PubMed] [Google Scholar]
- 29. Kimbung S., Biskup E., Johansson I., Aaltonen K., Ottosson-Wadlund A., Gruvberger-Saal S., Cunliffe H., Fadeel B., Loman N., Berglund P., Hedenfalk I. (2012) Co-targeting of the PI3K pathway improves the response of BRCA1 deficient breast cancer cells to PARP1 inhibition. Cancer Lett. 319, 232–241 [DOI] [PubMed] [Google Scholar]
- 30. Hunter J. E., Willmore E., Irving J. A., Hostomsky Z., Veuger S. J., Durkacz B. W. (2012) NF-κB mediates radio-sensitization by the PARP-1 inhibitor, AG-014699. Oncogene 31, 251–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Plummer R., Jones C., Middleton M., Wilson R., Evans J., Olsen A., Curtin N., Boddy A., McHugh P., Newell D., Harris A., Johnson P., Steinfeldt H., Dewji R., Wang D., Robson L., Calvert H. (2008) Phase I study of the poly(ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors. Clin. Cancer Res. 14, 7917–7923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Camenisch U., Nägeli H. (2008) XPA gene, its product and biological roles. Adv. Exp. Med. Biol. 637, 28–38 [DOI] [PubMed] [Google Scholar]
- 33. Han W., Li X., Fu X. (2011) The macro domain protein family. Structure, functions, and their potential therapeutic implications. Mutat. Res. 727, 86–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karras G. I., Kustatscher G., Buhecha H. R., Allen M. D., Pugieux C., Sait F., Bycroft M., Ladurner A. G. (2005) The macro domain is an ADP-ribose binding module. EMBO J. 24, 1911–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahel I., Ahel D., Matsusaka T., Clark A. J., Pines J., Boulton S. J., West S. C. (2008) Poly(ADP-ribose)-binding zinc finger motifs in DNA repair/checkpoint proteins. Nature 451, 81–85 [DOI] [PubMed] [Google Scholar]
- 36. Li G. Y., McCulloch R. D., Fenton A. L., Cheung M., Meng L., Ikura M., Koch C. A. (2010) Structure and identification of ADP-ribose recognition motifs of APLF and role in the DNA damage response. Proc. Natl. Acad. Sci. U.S.A. 107, 9129–9134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmitz A. A., Pleschke J. M., Kleczkowska H. E., Althaus F. R., Vergères G. (1998) Poly(ADP-ribose) modulates the properties of MARCKS proteins. Biochemistry 37, 9520–9527 [DOI] [PubMed] [Google Scholar]
- 38. Malanga M., Pleschke J. M., Kleczkowska H. E., Althaus F. R. (1998) Poly(ADP-ribose) binds to specific domains of p53 and alters its DNA binding functions. J. Biol. Chem. 273, 11839–11843 [DOI] [PubMed] [Google Scholar]
- 39. Gagné J. P., Isabelle M., Lo K. S., Bourassa S., Hendzel M. J., Dawson V. L., Dawson T. M., Poirier G. G. (2008) Proteome-wide identification of poly(ADP-ribose) binding proteins and poly(ADP-ribose)-associated protein complexes. Nucleic Acids Res. 36, 6959–6976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Polo S. E., Kaidi A., Baskcomb L., Galanty Y., Jackson S. P. (2010) Regulation of DNA-damage responses and cell-cycle progression by the chromatin remodelling factor CHD4. EMBO J. 29, 3130–3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gottschalk A. J., Timinszky G., Kong S. E., Jin J., Cai Y., Swanson S. K., Washburn M. P., Florens L., Ladurner A. G., Conaway J. W., Conaway R. C. (2009) Poly(ADP-ribosyl)ation directs recruitment and activation of an ATP-dependent chromatin remodeler. Proc. Natl. Acad. Sci. U.S.A. 106, 13770–13774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ahel D., Horejsí Z., Wiechens N., Polo S. E., Garcia-Wilson E., Ahel I., Flynn H., Skehel M., West S. C., Jackson S. P., Owen-Hughes T., Boulton S. J. (2009) Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science 325, 1240–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kang H. C., Lee Y. I., Shin J. H., Andrabi S. A., Chi Z., Gagné J. P., Lee Y., Ko H. S., Lee B. D., Poirier G. G., Dawson V. L., Dawson T. M. (2011) Iduna is a poly(ADP-ribose) (PAR)-dependent E3 ubiquitin ligase that regulates DNA damage. Proc. Natl. Acad. Sci. U.S.A. 108, 14103–14108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.