Background: Asp isomers in lens crystallins are one of the triggers of cataracts.
Results: Multiple highly isomeric Asp sites in insoluble crystallins from cataract lenses were identified by LC-MS using the corresponding synthetic peptides as standards.
Conclusion: Asp isomers induce insolubilization of crystallin, leading to cataracts.
Significance: This study opens up a new field of protein biochemistry in age-related diseases.
Keywords: Aggregation, Aging, Aspartate, Cataract, Crystallin, LC-MS, Isomerization
Abstract
Cataracts are caused by clouding of the eye lens and may lead to partial or total loss of vision. The mechanism of cataract development, however, is not well understood. It is thought that abnormal aggregates of lens proteins form with age, causing loss of lens clarity and development of the cataract. Lens proteins are composed of soluble α-, β-, and γ-crystallins, and as long lived proteins, they undergo post-translational modifications including isomerization, deamidation, and oxidation, which induce insolubilization, aggregation, and loss of function that may lead to cataracts. Therefore, analysis of post-translational modifications of individual amino acid residues in proteins is important. However, detection of the optical isomers of amino acids formed in these proteins is difficult because optical resolution is only achieved using complex methodology. In this study, we describe a new method for the analysis of isomerization of individual Asp residues in proteins using LC-MS and the corresponding synthetic peptides containing the Asp isomers. This makes it possible to analyze isomers of Asp residues in proteins precisely and quickly. We demonstrate that Asp-58, -76, -84, and -151 of αA-crystallin and Asp-62 and -96 of αB-crystallin are highly converted to lβ-, dβ-, and dα-isomers. The amount of isomerization of Asp is greater in the insoluble fraction at all Asp sites in lens proteins, therefore indicating that isomerization of these Asp residues affects the higher order structure of the proteins and contributes to the increase in aggregation, insolubilization, and disruption of function of proteins in the lens, leading to the cataract.
Introduction
A cataract is caused by clouding of the eye lens and may lead to blindness. By age 80, more than 90% of people either have a cataract or have had cataract surgery. Although cataracts are among the most common age-related diseases, the mechanism of cataract development is not well understood. However, it is thought that proteins of the eye lens aggregate abnormally, resulting in clumping that scatters the light and interferes with focusing on the retina. Human lens proteins are mainly composed of α-, β-, and γ-crystallins, and the overall structure, stability, and short range interactions of these proteins are thought to contribute to the transparent properties of the lens. α-Crystallin is a large molecule with a molecular mass of ∼800 kDa and comprises two kinds of polypeptides: αA and αB. Because each αA- or αB-crystallin monomer has a mass of ∼20 kDa, the α-crystallin molecule is an aggregate containing ∼40–50 subunits. The β/γ-crystallin superfamily comprises oligomeric β-crystallin and monomeric γ-crystallin (1). αA- or αB-crystallin are members of the small heat-shock protein family and function as molecular chaperones to protect β- and γ-crystallins from aggregation (2). Along with β- and γ-crystallin, lens crystallin accounts for about 90% of total water-soluble (WS)2 protein in a highly concentrated form and constitutes the refractive medium. Lenses are constantly subjected to UV light and oxidative stress, and therefore damaged proteins accumulate in water-insoluble (WI) fractions in the lens because there is no turnover of the lens proteins. It is well known that WI proteins increase in aged and cataractous lenses (3). Furthermore, the lens crystallins undergo various post-translational modifications such as isomerization and inversion of aspartic acid (Asp) residues, that is lβ-, dβ-, and dα-formation (4–6); deamidation of asparagine (Asn) or glutamine (Gln) residues (7–11); disulfide bonding of cysteine (12); oxidation of methionine or tryptophan (13, 14); backbone cleavage (15); phosphorylation (16); and glycation (17) during the aging process. These modifications may induce a decrease in crystallin solubility, alter lens transparency, diminish vision, and lead to development of a cataract. Indeed, there is a strong relationship among post-translational modifications, aggregation, and loss of solubility of crystallin. Of the post-translational modifications, we have proposed that the appearance of dβ-Asp residues may be responsible for the change in the higher order structure and the loss of function of crystallins. This is due to the fact that if d-Asp is formed in the protein the configuration of the Asp residue would be opposite to the usual configuration, and hence this would induce a change in the higher order structure of the protein. In addition, the β-linkage produced with Asp formation may affect the quaternary structure of crystallin because the main chain of the protein would be elongated. Therefore, the presence of the Asp isomers may be one of the triggers of the insolubilization, abnormal aggregation, and induction of partial unfolding of protein and lead to a disease state. In fact, in our previous study, we clearly showed that α-crystallin containing large amounts of dβ-Asp undergoes abnormal aggregation to form massive and heterogeneous aggregates, leading to loss of its chaperone activity (18).
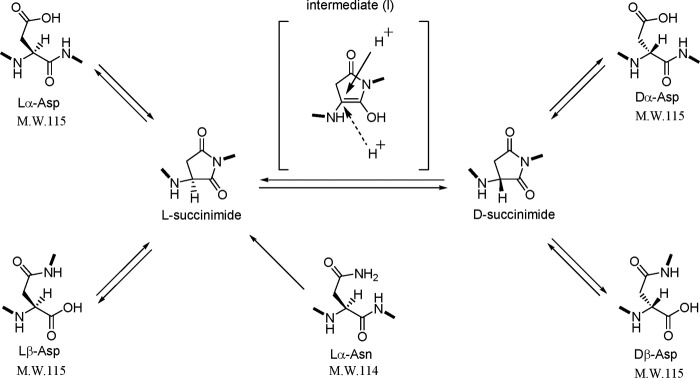
Deamidation of Asn or Gln introduces a negative charge at physiological pH by replacing an amide with a carboxyl group to form Asp or Glu, respectively, and causes some isomerization (4, 19). Isomerization and deamidation are major non-enzymatic post-translational modifications of proteins under physiological conditions. We have clarified the mechanism by which d-Asp residues are spontaneously formed in proteins (4–6). As shown in Fig. 1, the simultaneous formation of β- and d-Asp residues in the protein could be explained as follows. (i) When the carbonyl group of the side chain of the lα-Asp/lα-Asn residue is attacked by the nitrogen of the amino acid residue following the Asp residue, l-succinimide is formed by intramolecular cyclization. (ii) l-Succinimide may be converted to d-succinimide through an intermediate that has the prochiral α-carbon in the plane of the ring. (iii) Protonation of the intermediate may proceed from the upper or lower side of the plane in an ordinary peptide or protein. (iv) d- and l-succinimides are hydrolyzed at either side of their two carbonyl groups, yielding both β- and α-Asp residues, respectively. Thus, four isomers, lα-Asp, lβ-Asp, dα-Asp, and dβ-Asp, are simultaneously formed in the protein. The rate of succinimide formation is expected to depend on the neighboring residue of the Asp. When the neighboring amino acid of the Asp residue has a small side chain such as glycine, alanine, or serine, the formation of succinimide occurs easily because there is no steric hindrance. The deamidation of Asn residues in proteins is detected easily because the formation of Asp from Asn induces a +1 mass shift. Therefore, this mass shift caused by deamidation can be easily detected using mass spectrometry analysis. However, it was not possible to detect the d- or β-Asp isomers in proteins by mass spectrometry because the mass of the peptide containing Asp isomers is exactly the same as the normal peptide. Recently, however, we found that MS/MS analysis using postsource decay with a curved field reflectron could distinguish between the β-Asp- and α-Asp-containing peptides (20). The relative content of β-Asp in a peptide was successfully estimated from the unique ratio, yn:yn + 1, derived from tryptic peptides of a protein. However, there remained the problem of distinguishing between d-Asp and l-Asp in the peptide precisely and quickly. In conventional analysis of Asp isomers, the following steps are required to determine the site of dβ-Asp in a protein. (i) The protein is digested with a protease such as trypsin. (ii) The resulting peptides are separated by reverse-phase high performance liquid chromatography (RP-HPLC). (iii) The peptides are identified by mass analysis and/or protein sequencing. (iv) The α- or β-isomer of the identified peptides is determined by Edman degradation reaction. (v) The d/l ratios of the Asp residues are determined after hydrolysis of peptides with 6 n HCl and derivatization. (vi) The diastereoisomers are analyzed by RP-HPLC, and the d/l ratio of amino acids is determined by analysis of the respective peak areas. Hence, the analysis of the isomerization of Asp residues in a protein is a technically demanding process.
FIGURE 1.
Possible reaction pathways for spontaneous isomerization of Asp and deamidation of Asn residues in protein.
In the present study, we propose a new method of analysis for determining the peptides containing Asp isomers at individual sites and for detecting inverted Asp residues in any protein by using LC-mass spectrometry (MS) systems. The advantages of the method are as follows. 1) There is no need for purification of lens proteins from WI and WS fractions. 2) There is no need for the complicated analytical steps iii–vi as described above. Here we present a method for quickly and easily distinguishing d- and l-Asp-containing peptides in non-purified proteins using LC-MS. This is a groundbreaking method for the detection of Asp isomers in proteins.
EXPERIMENTAL PROCEDURES
Preparation of WI and WS Proteins from Human Lenses
Lens samples (one lens sample each) from elderly individuals (60–80 years old) were homogenized in 20 mm Tris/HCl, pH 7.8, 150 mm NaCl, 1 mm phenylmethylsulfonyl fluoride (PMSF), 1 mm EDTA by ultrasonication and fractionated into WI and WS fractions by centrifugation at 16,000 × g for 20 min at 4 °C. The WI proteins were dissolved in 8 m urea, 50 mm Tris/HCl, pH 7.8, 1 mm CaCl2, and then the urea concentration was diluted to less than 1 m in 50 mm Tris/HCl, pH 7.8, 1 mm CaCl2 buffer before enzymatic digestion.
Enzymatic Digestion of WI and WS Proteins
The WI proteins were digested with trypsin (sequencing grade modified trypsin, Promega) for 17 h at 37 °C in 50 mm Tris/HCl, pH 7.8, 1 m urea, 1 mm CaCl2 buffer at an enzyme-to-substrate ratio of 1:50. The WS proteins were digested with trypsin (sequencing grade modified trypsin, Promega) for 17 h at 37 °C in 50 mm Tris/HCl, pH 7.8, 1 mm CaCl2 buffer at an enzyme-to-substrate ratio of 1:50.
Isolation of Tryptic Peptides of the WI and WS Proteins from Human Lenses by LC-MS
LC used a nanoflow HPLC system (Paradigm MS4, Michrom Bioresources). MS was performed on an ion trap system (LCQ Fleet, Thermo). The peptides (about 500 ng) resulting from digestion with trypsin were separated by nanoflow RP-HPLC using a C18 column (L-column, 0.1 × 150 mm; Chemicals Evaluation and Research Institute, Japan) with a linear gradient of 5–60% acetonitrile in the presence of 0.1% formic acid at a flow rate of 0.5 μl/min and analyzed by Proteome Discoverer 1.0 software. MS analysis was carried out alternating between full MS and MS/MS scans. The MS/MS scan used the collision-induced dissociation mode with dynamic exclusion function.
Synthesis of Peptides Containing Four Different Asp Isomers
Synthetic peptides containing four different Asp isomers were made using Fmoc solid-phase chemistry using an automated solid-phase peptide synthesizer (21) (PSSM-8, Shimadzu, Japan). Fmoc-l-aspartic acid β-t-butyl ester (Fmoc-l-Asp(OtBu)-OH), Fmoc-d-Asp(OtBu)-OH, Fmoc-l-Asp-OtBu, and Fmoc-d-Asp-OtBu were used as building blocks to synthesize lα-, dα-, lβ-, and dβ-isomers, respectively. The coupling reaction was carried out using each Fmoc amino acid (10 eq), 1H-benzotriazol-1-yloxy-tri(pyrrolidino)phosphonium hexafluorophosphate (10 eq), 1-hydroxybenzotriazole (10 eq), and N-methylmorpholine (7.5 eq) in dimethylformamide (22). The N-terminal Fmoc group was deblocked with 20% piperidine in dimethylformamide. Simultaneous cleavage of the peptide from the resin and removal of the protective groups were achieved by treatment with a mixture containing 90% trifluoroacetic acid (TFA), 5% 1,2-ethanedithiol, and 5% thioanisole for 2 h. The cleavage of the peptide containing Arg and its protective groups from the resin was performed with 82.5% TFA, 5% water, 5% thioanisole, 3% ethylmethylsulfide, 2.5% 1,2-ethanedithiol, and 2% thiophenol for 6 h. The cleavage of the peptide containing Trp and its protective groups from the resin was carried out with the above reagents plus 1 mg/ml 2-methylindole. The crude peptides were purified by RP-HPLC using a C18 column (Capcell Pak C18 ACR, 30 × 250 mm; Shiseido, Japan) with a linear gradient of 0–50% acetonitrile in the presence of 0.1% TFA at a flow rate of 3.0 ml/min with monitoring at 230 or 280 nm. The purity of each peptide was confirmed to be >95% by analytical RP-HPLC and mass spectrometry. The yields of the purified peptides were about 50%.
RESULTS
A New General Survey of Isomeric Asp Residues in Crystallins by LC-MS
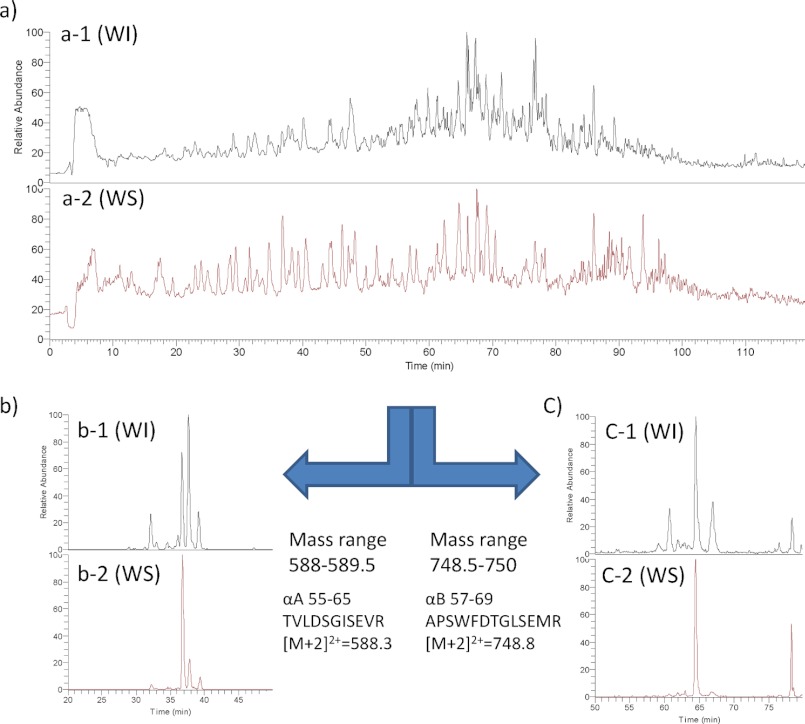
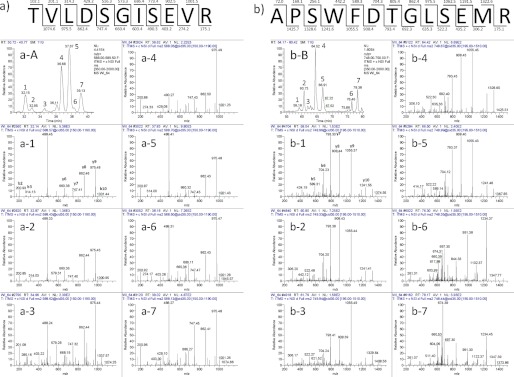
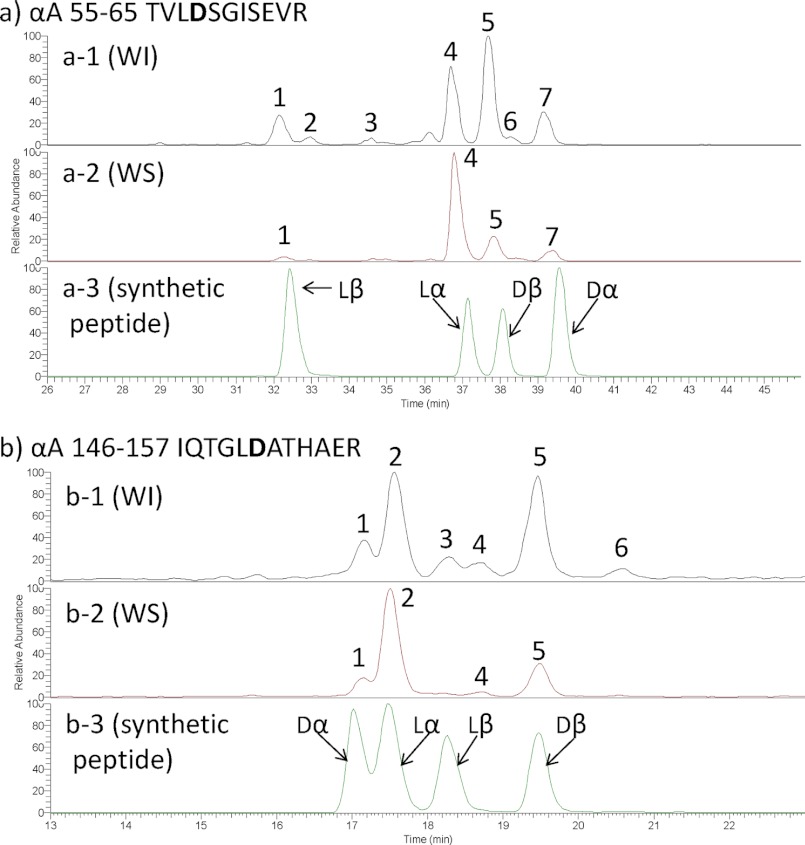
Fig. 2 shows a set of typical LC-MS chromatograms from tryptic digests of the WI and WS proteins derived from the lens of a 64-year-old individual. Fig. 2, a-1 and a-2, show typical full LC-MS chromatograms of the tryptic peptides from the WI and WS proteins, respectively, derived from the lens of a 64-year-old donor. The mass numbers of these peaks were measured and then analyzed by MS/MS, and then all peaks were identified using the Proteome Discover 1.0 software attached to this LC-MS system. Generally, each peptide would be expected to elute as one peak with one mass number. However, some peptides that contain Asp residues were often separated into multiple peaks, and they eluted at different retention times during the LC-MS run even though it was entirely the same peptide. For example, the peptide predicted to be positions 55–65 of αA-crystallin (αA 55–65; TVLDSGISEVR; [M + 2H]2+ = 588.3) as identified by the database was mainly separated into four different peaks and eluted at different elution times between 32 and 40 min of the LC-MS run as shown in Fig. 2, b-1 and b-2. Fig. 2, b-1 and b-2, show the peaks from WI and WS proteins that have an MS range of 588–589.5 m/z extracted from the full mass data shown in Fig. 2, a-1 and a-2. As shown in Fig. 2b, the number of peptides with [M + 2H]2+ = 588.3 (Fig. 2, b-1) was much greater from WI protein than that from WS protein (Fig. 2, b-2). Similarly, we extracted multiple peptides with an MS range of 748.5–750 from the full MS data in Fig. 2a, and these data are shown in Fig. 2c. These peaks correspond to residues 57–69 of αB-crystallin (αB 57–69; APSWFDTGLSEMR; [M + 2H]2+ = 748.8) peptide. The peptide with [M + 2H]2+ = 748.8 from WI protein (Fig. 2, c-1) was mainly separated into four peaks, whereas the peptide [M + 2H]2+ = 748.8 from WS protein (Fig. 2, c-2) was mainly separated into two peaks. We further analyzed these multiple peptides from the WI fraction of the 64-year-old donor using MS/MS analysis. Fig. 3a-A shows the LC-MS chromatogram of αA 55–65 (TVLDSGISEVR; [M + 2H]2+ = 588.3) from the WI fraction of lens proteins from the 64-year-old donor. Fig. 3, a-1 to a-7, correspond to the MS/MS analysis of peak numbers 1–7 in Fig. 3a-A, respectively. Fig. 3, a-1 to a-7, clearly show that these MS/MS analyses are entirely the same. Therefore, peak numbers 1–7 in Fig. 3a-A were all assigned as the peptide TVLDSGISEVR, contained in four main peaks (peak numbers 1, 4, 5, and 7) and minor peptides (peak numbers 2, 3, and 6). Fig. 3b-B shows the LC-MS chromatogram of αB 57–69 (APSWFDTGLSEMR; [M + 2H]2+ = 748.8) from the WI fraction of lens proteins from the 64-year-old donor. Fig. 3, b-1 to b-7, correspond to the MS/MS analysis of peak numbers 1–7 in Fig. 3b-B, respectively. As shown in Fig. 3, b-1 to b-7, b-1 to b-5 are entirely the same, but b-6 and b-7 are different from b-1 to b-5. The results clearly show that peak numbers 1–5 in Fig. 3b-B are all αB 57–69 but that peaks 6 and 7 are not the αB 57–69 peptide but rather are unknown peptides.
FIGURE 2.
LC-MS chromatograms of the tryptic peptides of WI and WS fractions of lens proteins from 64-year-old donor. a, total intensity of WI and WS fractions. b, MS range 588–589.5 m/z of WI and WS fractions. c, MS range 748.5–750 m/z of WI and WS fractions.
FIGURE 3.
LC-MS chromatograms and MS/MS spectra of the tryptic peptides of αA 55–65 (TVLDSGISEVR) (a-A) and αB 57–69 (APSWFDTGLSEMR) (b-B) of the WI fraction of lens proteins from 64-year-old donor. a-1 to a-7 correspond to the MS/MS spectra of peaks 1–7 of a-A, respectively. b-1 to b-7 correspond to the MS/MS spectra of peaks 1–7 of b-B, respectively.
Similar multiple peak separation of the various peptides containing Asp residues was found for both WI and WS proteins as shown in Table 1. This separation was thought to be due to the presence of different Asp, Glu, or Pro isomers and deamidation of Asn/Gln in the peptides. MS analysis should be able to distinguish deamidation of Asn/Gln on the basis of a mass difference of 1, but the peaks obtained from the LC-MS were detected as divalent or trivalent ions, and therefore a mass difference of 1 would be detected as only 0.5 or 0.3. This small difference could not accurately be detected in our LC-MS system. Therefore, we analyzed the peptides containing Asn/Gln by MS/MS to distinguish between deamidated peptides and isomerized peptides.
 |
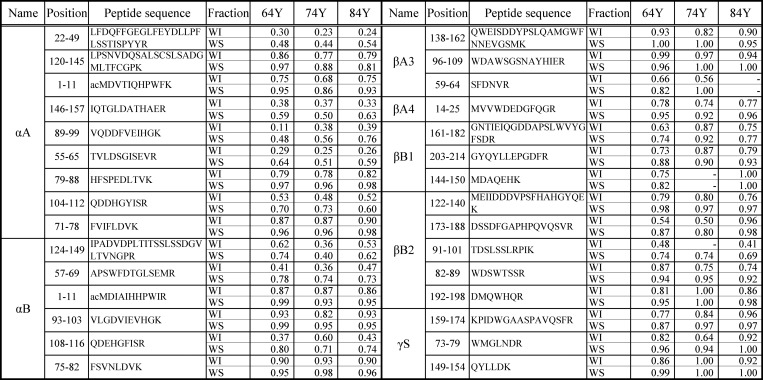
The above equation indicates that the closer the rI value is to 1.0 the less isomerization occurs in the peptide. The rI values are indicators of the presence of the isomeric peptides, and the smaller the rI, the more isomers are present. Table 1 shows the rI values of the tryptic peptides from αA-, αB-, βA3-, βA4-, βB1-, βB2-, and γS-crystallins from the WS and WI fractions of 64-, 74-, and 84-year-old donors. The rI values of the peptides from the WI faction are smaller than those of the peptides from the WS fraction in all samples. The isomeric Asp residues are much more frequent in the WI fraction than in the WS fraction in all crystallins. The results clearly indicate that isomerization occurs in the proteins of the WI fraction in the lenses of donors of any age. In the WI fraction, the peptides 146–157, 55–65, 89–99,104–112, and 22–49 of αA-crystallin and 57–69 and 108–116 of αB-crystallin contain the isomers with highest percentages. The amount of Asp isomers in β- and γ-crystallin is smaller than in αA- or αB-crystallins.
TABLE 1.
rI values of the tryptic peptides from αA-, αB-, βA3-, βA4-, βB1-, βB2-, and γS-crystallins
The rI value is an indicator of the quantity of modified peptides in the WI and WS fractions from the 64- (64Y), 74- (74Y), and 84-year-old (84Y) donors. The numerical value 1.00 means one peak in LC-MS. -, not detected or not identified; ac, acetyl.
Identification of Asp Isomers in Crystallins from WI and WS Fractions of Lens
The peptide 55TVLDSGISEVR65 was separated into four main peaks and three minor peaks from both WI (Figs. 2b and 3a) and WS proteins (Fig. 2b) even though it is the same peptide in each peak. This separation was predicted to be due to the presence of Asp and Glu isomers in this peptide. In particular, lα-Asp residues are well known to invert to lβ-Asp, dα-Asp, and dβ-Asp residues through a succinimide in proteins under physiological conditions. Our previous studies have shown that separation of the same peptide by RP-HPLC was caused by a difference in the Asp isomers in the peptide. However, which peak corresponds to which isomeric peptide is not known. To identify the peptides of each peak on the RP-HPLC, synthetic peptides are necessary. The identification of the sample peptides becomes possible by using the synthetic peptides as standards. Therefore, in the present study, we synthesized crystallin-derived normal peptides and their isomeric peptides containing three different Asp isomers (lβ-Asp-, dα-Asp, and dβ-Asp) and applied them to the LC-MS system. Fig. 4a shows the set of LC-MS chromatograms of αA 55–65 (TVLDSGISEVR; [M + 2H]2+ = 588.3) from the WI fraction (Fig. 4, a-1) and WS fraction (Fig. 4, a-2) of lens proteins from the 64-year-old donor and those of the synthetic peptides (Fig. 4, a-3). As shown in Fig. 4a-3, the TVLDSGISEVR peptides containing lβ-Asp, lα-Asp, dβ-Asp, and dα-Asp eluted at 32.5, 37.2, 38, and 39.6 min, respectively. By comparison with the elution time of these synthetic peptides, peaks 1, 4, 5, and 7 in Fig. 4, a-1 and a-2, were identified to be the TVLD(lβ-Asp)SGISEVR, TVLD(lα-Asp)SGISEVR, TVLD(dβ-Asp)SGISEVR, and TVLD(dα-Asp)SGISEVR peptides, respectively. Fig. 4, a-1 and a-2, show that the intensities of the isomeric peptides in the WI fraction are larger than those in the WS fraction. In the WS fraction, TVLD(lα-Asp)SGISEVR is the highest peak, whereas in the WI fraction, TVLD(dβ-Asp)SGISEVR is the highest peak. Fig. 4b shows the set of LC-MS chromatograms of αA 146–157 (IQTGLDATHAER; [M + 2H]2+ = 656.0) from the WI fraction (Fig. 4, b-1) and the WS fraction (Fig. 4, b-2) of lens proteins from the 64-year-old donor and the synthetic peptides (Fig. 4, b-3). The synthetic IQTGLDATHAER peptides containing dα-Asp, lα-Asp, lβ-Asp, and dβ-Asp residues eluted at 17.0, 17.5, 18.3, and 19.5 min, respectively (Fig. 4, b-3). Therefore, peaks 1, 2, 3, and 5 in Fig. 4, b-1 and b-2 were identified to be IQTGLD(dα-Asp)ATHAER, IQTGLD(lα-Asp)ATHAER, IQTGLD(lβ-Asp)ATHAER, and IQTGLD(dβ-Asp)ATHAER by fitting with the retention time of the synthetic peptides (Fig. 4, b-3). Supplemental Fig. S1 shows the MS/MS spectrum of the synthetic peptides (TVLDSGISEVR and IQTGLDATHAER peptides) with the Asp isomers. Fig. 4, b-1 and b-2, indicate that the isomeric peptides occur more in the WI fraction than in the WS fraction. In the WI fraction, the amounts of IQTGLD(dβ-Asp)ATHAER were increased relative to that in the WS fraction. In the crystallin protein from the WI fraction, Asp isomers are clearly increased relative to those found in the WS protein.
FIGURE 4.
LC-MS chromatograms of the tryptic peptides of αA 55–65 (TVLDSGISEVR) (a) and αA 146–157 (IQTGLDATHAER) (b) from the WI (a-1 and b-1) and WS fractions (a-2 and b-2) of 64-old-donor. a-3 and b-3 are LC-MS chromatograms of the synthetic peptides of αA 55–65 and αA 146–157, respectively.
Quantification of the Asp Isomers in Protein by LC-MS
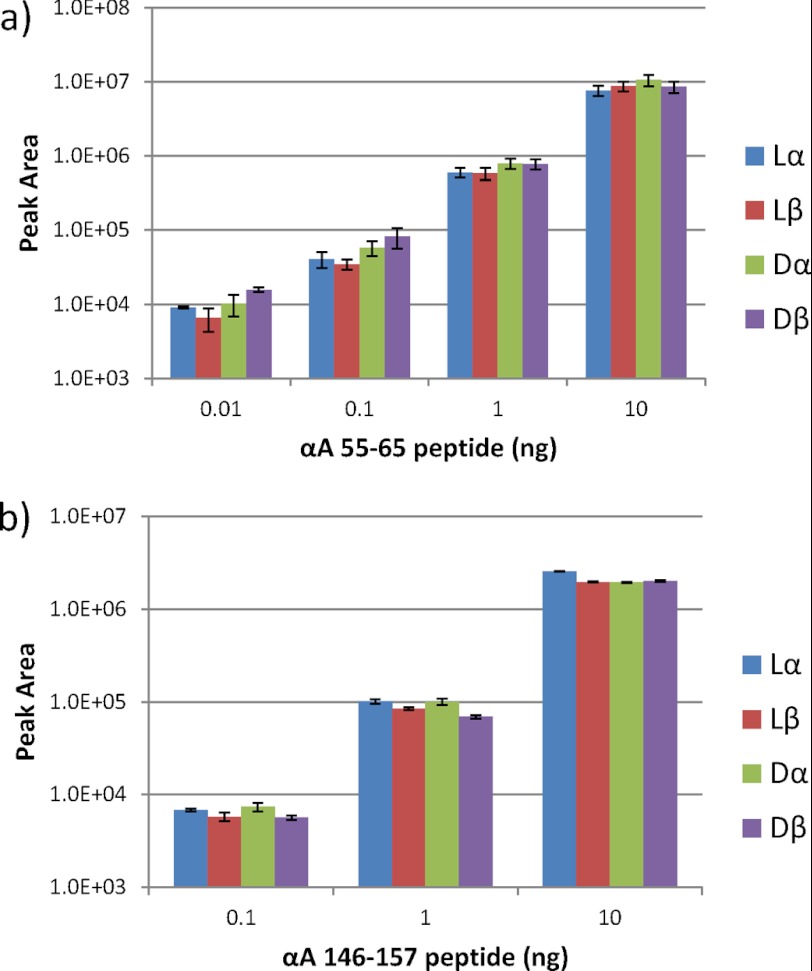
To confirm whether there are differences between the intensities of the fragment ions of the lα-Asp-containing peptide and those of the other three isomeric peptides, the four synthetic peptides were applied to LC-MS in various quantities. Fig. 5, a and b, show the relationship between the intensities of the divalent ions and the amounts of the αA 55–65 peptide plus isomeric peptides and αA 146–157 peptide plus isomeric peptides, respectively. As shown in Fig. 5, a and b, there were no significant differences between the intensities of the divalent ions of the peptides and the isomeric peptides in either set of samples. The intensities of the divalent ions of αA 55–65 peptide including the isomeric peptides can be expressed as the linearity of the amounts of the sample peptides ranging from 1.0e+4 to 1.0e+7 for the intensity and from 0.01 to 10 ng for the amounts, respectively (Fig. 5a). Similarly, for αA 146–157 peptide and its isomeric peptides, the intensities and the amounts of the peptides can be expressed as the linearity ranging from 1.0e+4 to 1.0e+7 for the intensity and from 0.1 to 10 ng for the amounts, respectively (Fig. 5b).
FIGURE 5.
The relationship between the intensities of the fragment ions and the amounts of αA 55–65 peptide plus isomeric peptides (a) and αA 146–157 peptide plus isomeric peptides (b), respectively. The results presented are the mean plus S.D. of three measurements.
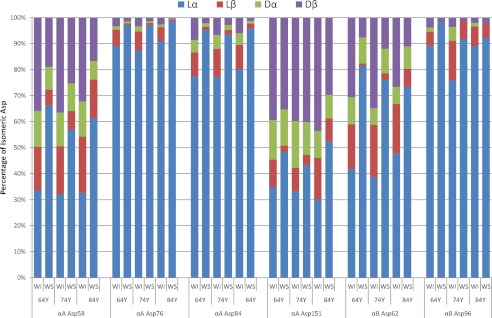
Furthermore, we synthesized the following peptides and their three isomers: αA 79–88 (HFSPED(lα-Asp)LTVK, HFSPED(lβ-Asp)LTVK, HFSPED(dβ-Asp)LTVK, and HFSPED(dα-Asp)LTVK), αA 71–78 (FVIFLD(lα-Asp)VK, FVIFLD(lβ-Asp)VK, FVIFLD(dβ-Asp)VK, and FVIFLD(dα-Asp)VK), αB 57–69 (APSWFD(lα-Asp)TGLSEMR, APSWFD(lβ-Asp)TGLSEMR, APSWFD(dβ-Asp)TGLSEMR, and APSWFD(dα-Asp)TGLSEMR), and αB 93–103 (VLGD(lα-Asp)VIEVHGK, VLGD(lβ-Asp)VIEVHGK, VLGD(dβ-Asp)VIEVHGK, and VLGD(dα-Asp)VIEVHGK). These peptides also show the linearity between the intensities of the divalent ions and the amounts of the peptides at an intensity ranging from 1.0e+4 to 1.0e+7. These results clearly indicate that the quantitative analysis of the isomeric peptides containing four different Asp residues should be possible on the basis of the intensity of the fragment ion of the peptide by LC-MS. Therefore, on the basis of this valuable result, we estimated the relative amounts of the Asp isomers at the Asp-58, Asp-76, Asp-84, and Asp-151 residues of αA-crystallin and at Asp-62 and Asp-96 of αB-crystallin in WI and WS proteins from the elderly donors (Fig. 6). In all samples, the isomerization of Asp residues is increased more in the WI fraction than in the WS faction. In particular, the ratios of the total of the three Asp isomers to the normal lα-Asp form at Asp-58 and Asp-151 of αA-crystallin and Asp-62 of αB-crystallin in the WI fraction are ∼1:1 or greater than 1.0. The isomerization of Asp-76 and Asp-84 of αA-crystallin and Asp-96 of αB-crystallin in the WS fraction was not observed, but the isomers increased in the WI fractions. These results clearly indicate that the isomerization of Asp residues affects the higher order structure of crystallin through the inversion of the side chain to the d-form and through the extension of the backbone via formation of the β-linkage, which causes the crystallins in the soluble fraction to lose their solubility and move to the WI fraction of the lens.
FIGURE 6.
The relative amounts of Asp isomers at αA Asp-58, Asp-76, Asp-84, and Asp-151 and αB Asp-62 and Asp-96 from the WI and WS fractions of lenses from 64-, 74-, and 84-year-old donors.
DISCUSSION
Homochirality is essential for the development and maintenance of life. Until recently, the homochirality of proteins composed of l-amino acids was believed to be maintained, and the inversion of l-amino acids to d-amino acids was not thought to occur throughout the entire lifespan of an organism. However, d-amino acids were recently detected in various living higher organisms in the form of free amino acids, peptides, and proteins. Free d-Asp may be related to differentiation and development in living organisms (23), and d-serine plays a role as a coagonist of NMDA receptors in the brain (24). Some d-amino acid-containing peptides were found in opioid peptides (25–28). In proteins, in addition to the lens proteins described in this study, d-Asp has been widely detected in various tissues such as teeth (29, 30), bone (31, 32), aorta (33), ligament (34), brain (35, 36), retina (37), conjunctiva (38), cornea (39), and skin (40, 41) of elderly individuals. It is therefore no longer uncommon to find d-amino acids in living organisms. The presence of d-asp in aged tissues of living organisms is thought to result from the racemization of the Asp residues in these particular proteins. Of all the naturally occurring amino acids, the Asp residue is the most susceptible to racemization. The racemization of Asp residues in proteins does not occur uniformly but does so at specific Asp residues on the basis of the sequence context or structural considerations that make the specific residues more susceptible to reaction than others. Therefore, it is necessary to determine the nature of the Asp residues at specific sites within particular proteins.
The separation of the optical isomers of amino acids has been considered to be difficult because the physical and chemical properties of the optical isomers are the same. Conventional enantioseparation of amino acids has been established by gas chromatography (GC) or RP-HPLC. The GC analysis involves direct enantioseparation through the use of a chiral capillary column, whereas RP-HPLC analysis involves indirect enantioseparation of the diastereoisomeric derivatives of the amino acid samples produced by chiral derivatizing reagents. Both methods require the appropriate amino acid derivatization or preparation in advance of the analysis; the former requires changing the samples into the gaseous state before injection for GC, and the latter requires production of diastereoisomeric derivatives in the case of the non-chiral column. The process for free d-amino acid analysis is very complex. In addition, to analyze the specific sites of d-amino acids in a protein, other steps more complicated than free d-amino acid analysis are required. These steps are as follows. (i) The protein is digested with an appropriate enzyme. (ii) The resulting peptides are separated by RP-HPLC. (iii) The peptides are identified by mass analysis and/or protein sequencing. (iv) The α- or β-isomer of the identified peptides is determined by Edman degradation reaction. (v) The d/l ratio of the identified peptides is determined after hydrolysis with 6 n HCl and derivatization. (vi) The diastereoisomers are analyzed by RP-HPLC, and the d/l ratio of amino acids is determined by analysis of the respective peak areas. The resulting analysis of the isomerization of Asp residues in a protein can be accurate, but it is a technically demanding process. Consequently, there has been little study of the presence and function of d-amino acids in living organisms.
In the present study, we propose a new accurate and quick LC-MS-based analysis for determining the specific sites having Asp isomers and quantifying the amounts of Asp isomers at the individual sites of all lens crystallins in the WI and WS fractions without the need for complicated purification from the lens tissues. The isomeric peptides containing Asp isomers can be resolved because peptides with the same mass are separated into several peaks by LC-MS. Fig. 2, b and c, are typical LC-MS runs showing αA 55–65 and αB 57–69 separated into several peaks. These are expected to be the isomeric peptides resulting from isomerization of Asp-58 of αA-crystallin and Asp-62 of αB-crystallin with their amounts increased in the WI fraction over the WS fraction. However, there is still the possibility that different peptides with the same mass as αA 55–65 and αB 57–69 may be present in the profiles in Fig. 2, b and c. Therefore, to confirm the presence of the isomers, these peptides were analyzed by MS/MS analysis. The results clearly indicated that peaks 1–7 of Fig. 3a-A are all αA 55–65 peptide, whereas peaks 1–5 of Fig. 3b-B are αB 57–69 peptide, and peaks 6 and 7 are different peptides.
The detection of the isomeric Asp residues in the protein was achieved by the combination of finding peptides with the same mass that are separated into multiple peaks in LC-MS and their MS/MS analysis. Furthermore, we found isomeric peptides of all crystallins, that is αA-, αB-, βA3-, βA4-, βB1-, βB2-, and γS-crystallins, in both the WI and WS fractions of the lenses. The rI values are indicators of the presence of the isomeric peptides, and the smaller the rI value, the more isomers are present. The isomeric Asp residues are present in greater abundance in the WI fraction than in the WS fraction in all crystallins. The isomerization sites are Asp-24 and/or -35, -58, -91 and/or -92, -105 and/or -106, and -151 of αA-crystallin, whereas Asp-62 and -109 of αB-crystallin have a high isomeric percentage. The amounts of Asp isomers in β- and γ-crystallin are smaller than those in αA- or αB-crystallins. To determine which types of Asp isomers among dβ, dα, lβ, and lα are present, standard peptides were synthesized and applied to LC-MS. Fig. 4, a and b, show typical LC-MS chromatograms of αA 55–65 and αA 146–157 peptides, respectively. Peaks 1, 4, 5, and 7 in Fig. 4, a-1 and a-2, were identified to be the TVLD(lβ-Asp)SGISEVR, TVLD(lα-Asp)SGISEVR, TVLD(dβ-Asp)SGISEVR, and TVLD(dα-Asp)SGISEVR by comparison with the elution time of the synthetic peptides. Peak 3 of Fig. 4a-1 was identified to be TVLD(lα-Asp)SGISE(lγ-Glu)VR by matching with the elution time of the synthetic peptides (supplemental Fig. S2). The other minor peaks may be the peptides containing the combination of Asp-58 isomers (dβ, lβ, and dα) and a lγ-Glu residue at position 63. Peaks 1, 2, 3, and 5 in Fig. 4, b-1 and b-2, were identified to be IQTGLD(dα-Asp)ATHAER, IQTGLD(lα-Asp)ATHAER, IQTGLD(lβ-Asp)ATHAER, and IQTGLD(dβ-Asp)ATHAER. The intensities of the normal lα-Asp-containing peptides and the other three isomeric peptides containing dβ-Asp, lβ-Asp, and dα-Asp were the same in both peptides (Fig. 5). Therefore, it is possible for the ratio of the isomeric peptides to be calculated from the areas of LC-MS peaks. The most abundant isomers of Asp-58 and Asp-151 of αA-crystallin in the WS fraction were dβ-isomers, which is consistent with our previous results using conventional methods (4). The ratios of the dβ-Asp isomers to the normal lα-Asp form at both Asp-58 and Asp-151 of αA-crystallin in the WI fraction are ∼1:1 or greater.
Fig. 6 shows the relative amounts of the various Asp isomers of αA- and αB-crystallins from WI and WS fractions. The Asp-58 and Asp-151 of αA-crystallin and Asp-62 of αB-crystallin are highly inverted to the isomers in the WS fraction, which is entirely consistent with our previous results (4, 5), and their isomeric ratios increased dramatically in the WI fraction. In recent work, Hooi et al. (42) reported that Asp-151 of αA-crystallin converted to dβ, lβ, and dα in a ratio of 3:1:0.5 until age 15 and that the percentage of dβ-Asp-151 is 40% by age 20 with a fixed ratio to age 80. These results are consistent with the present study. As shown in Fig. 1, the lα-Asp residue spontaneously converts to lβ-, dβ-, and dα-isomers in proteins through a succinimide intermediate. The rate of the succinimide formation depends on the neighboring residue of the Asp residue as well as the surrounding structure. Because the succinimide is unstable, it is hydrolyzed to form lβ-Asp or inverted to d-succinimide. If the deprotonation rate of the l-succinimide is faster than the hydrolysis rate of l-succinimide, the amounts of d-succinimide should increase. In the case of crystallin, the deprotonation of l-succinimide may be predominant. It is well known that succinimide is hydrolyzed predominantly to the dβ form rather than the dα form (19). This is the reason why the dβ-Asp form is the most abundant in the lens crystallins.
The isomerization of Asp-76 and Asp-84 of αA-crystallin and Asp-96 of αB-crystallin was almost absent from the WS fraction, but the isomers significantly increased in the WI fractions. The relative amounts of the isomers at Asp-76 and Asp-84 in the WI fraction were lower than the amounts of Asp-58 and Asp-151; however, the amounts of the isomers are very different between the WS and WI fractions. Therefore, it is proposed that the isomerization of Asp-76 and Asp-84 residues also contributes to the insolubilization and aggregation of αA-crystallin. In our previous study, we synthesized peptides corresponding to residues 70–88 (KFVIFLD76VKHFSPED84LTVK) of human αA-crystallin and its corresponding diastereoisomers in which lα-Asp was replaced with lβ-Asp, dα-Asp, and dβ-Asp at position 76 and compared their biochemical properties with that of the lα-Asp-containing peptide (43). The isomeric peptides containing lβ-Asp, dα-Asp, and dβ-Asp residues were more hydrophilic than the lα-Asp-containing peptide, and the isomeric peptides lost β-sheet structure and changed to random structures (43). The normal peptide promoted the aggregation of insulin, whereas the other three isomers suppressed the aggregation of insulin (43). Recently, Santhoshkumar et al. (44) showed that the αA-crystallin-derived peptide 66SDRDKFVIFLDVKHF80, which accumulates in the aging lens, can inhibit the chaperone activity of α-crystallin and can cause aggregation and precipitation of lens crystallins. The region including Asp-76 is located in the β-strand structure, and therefore the isomerization of the Asp may affect the secondary structure of the protein and induce insolubilization.
The isomerization of Asp in proteins is thought to occur when 1) the neighboring residue has a small side chain such as Gly, Ser, or Ala and 2) the Asp residues are present in flexible regions of the protein structure. The crystal structure of human αA-crystallin is not known, but that of bovine αA-crystallin has been reported (45). Because the conformations of bovine and human αA-crystallins are considered to be essentially similar due to the amino acid sequence similarity, it is possible to discuss the environment of the isomeric Asp residues in human αA-crystallin. Asp-84 and -151 are located in the unstructured region as shown in supplemental Fig. S3, and Asp-58 is easily accessible as a proteolytic target (46).
The lens crystallins are originally in the water-soluble fraction, but aggregation and insolubilization of the proteins proceed with age or cataract. The cause of the crystallin aggregation is not well understood; however, the increase in the Asp isomers may be one of the triggers for crystallin aggregation and insolubilization because the Asp isomers directly affect the protein structure via inversion of the side chain by the d-form and also via formation of the β-linkage. Consistent with this is that αA-crystallin containing large amounts of dβ-Asp obtained from donors of 80 years has been shown to undergo abnormal aggregation to form massive and heterogeneous aggregates (18). In addition, the chaperone activity of aged αA-crystallin was reduced by 60% relative to that of young αA-crystallin (18). It is therefore necessary to determine the levels of isomeric aspartyl residues at specific sites in all crystallins from the WI and WS fractions of the lens.
Here we describe a convenient and robust biochemical method for identifying the isomeric Asp sites in crystallins using LC-MS systems. However, to determine which types of Asp isomers are present, synthetic peptides are required. With the exception of this point, there are many advantages to this new method: 1) no requirement for large amounts of sample proteins, 2) no requirement for the purification of lens proteins from WI and WS fractions, and 3) no requirement for complicated analytical steps, which usually include the hydrolysis of the peptides followed by derivatization to the diastereoisomers of amino acids. This new method is able to search comprehensively for the Asp isomers in damaged or aged proteins from all living tissues and cells. Furthermore, the isomeric Asp sites can be determined, and the amounts of the Asp isomers can be quantified quickly and accurately at the femtomole level. This new method therefore improves the study of the isomerization of any amino acid that occurs spontaneously in living tissues or cells.
Supplementary Material
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan.

This article contains supplemental Figs. S1–S3.
- WS
- water-soluble
- RP
- reverse-phase
- WI
- water-insoluble
- rI
- ratios of isomerization in the peptides
- Fmoc
- N-(9-fluorenyl)methoxycarbonyl
- Asp(OtBu)-OH
- aspartic acid β-t-butyl ester.
REFERENCES
- 1. Bloemendal H., de Jong W., Jaenicke R., Lubsen N. H., Slingsby C., Tardieu A. (2004) Ageing and vision: structure, stability and function of lens crystallins. Prog. Biophys. Mol. Biol. 86, 407–485 [DOI] [PubMed] [Google Scholar]
- 2. Horwitz J. (1992) α-Crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. U.S.A. 89, 10449–10453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lund A. L., Smith J. B., Smith D. L. (1996) Modifications of the water-insoluble human lens α-crystallins. Exp. Eye Res. 63, 661–672 [DOI] [PubMed] [Google Scholar]
- 4. Fujii N., Satoh K., Harada K., Ishibashi Y. (1994) Simultaneous stereoinversion and isomerization at specific aspartic acid residues in αA-crystallin from human lens. J. Biochem. 116, 663–669 [DOI] [PubMed] [Google Scholar]
- 5. Fujii N., Ishibashi Y., Satoh K., Fujino M., Harada K. (1994) Simultaneous racemization and isomerization at specific aspartic acid residues in αB-crystallin from the aged human lens. Biochim. Biophys. Acta 1204, 157–163 [DOI] [PubMed] [Google Scholar]
- 6. Fujii N., Kawaguchi T., Sasaki H., Fujii N. (2011) Simultaneous stereoinversion and isomerization at the Asp-4 residue in βB2-crystallin from the aged human eye lenses. Biochemistry 50, 8628–8635 [DOI] [PubMed] [Google Scholar]
- 7. Takemoto L. (1996) Increase in the intramolecular disulfide bonding of α-A crystallin during aging of the human lens. Exp. Eye Res. 63, 585–590 [DOI] [PubMed] [Google Scholar]
- 8. Lampi K. J., Amyx K. K., Ahmann P., Steel E. A. (2006) Deamidation in human lens βB2-crystallin destabilizes the dimer. Biochemistry 45, 3146–3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lampi K. J., Oxford J. T., Bachinger H. P., Shearer T. R., David L. L., Kapfer D. M. (2001) Deamidation of human βB1 alters the elongated structure of the dimer. Exp. Eye Res. 72, 279–288 [DOI] [PubMed] [Google Scholar]
- 10. Hains P. G., Truscott R. J. (2010) Age-dependent deamidation of lifelong proteins in the human lens. Invest. Ophthalmol. Vis. Sci. 51, 3107–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li X., Lin C., O'Connor P. B. (2010) Glutamine deamidation: differentiation of glutamic acid and γ-glutamic acid in peptides by electron capture dissociation. Anal. Chem. 82, 3606–3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spector A., Roy D. (1978) Disulfide-linked high molecular weight protein associated with human cataract. Proc. Natl. Acad. Sci. U.S.A. 75, 3244–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Truscott R. J. (2005) Age-related nuclear cataract-oxidation is the key. Exp. Eye Res. 80, 709–725 [DOI] [PubMed] [Google Scholar]
- 14. Zhang Z., Smith D. L., Smith J. B. (2003) Human β-crystallins modified by backbone cleavage, deamidation and oxidation are prone to associate. Exp. Eye Res. 77, 259–272 [DOI] [PubMed] [Google Scholar]
- 15. Takemoto L. (1995) Age-dependent cleavage at the C-terminal region of lens βB2 crystallin. Exp. Eye Res. 61, 743–748 [DOI] [PubMed] [Google Scholar]
- 16. Kantorow M., Piatigorsky J. (1998) Phosphorylations of αA- and αB-crystallin. Int. J. Biol. Macromol. 22, 307–314 [DOI] [PubMed] [Google Scholar]
- 17. Hanson S. R., Hasan A., Smith D. L., Smith J. B. (2000) The major in vivo modifications of the human water-insoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation and backbone cleavage. Exp. Eye Res. 71, 195–207 [DOI] [PubMed] [Google Scholar]
- 18. Fujii N., Shimmyo Y., Sakai M., Sadakane Y., Nakamura T., Morimoto Y., Kinouchi T., Goto Y., Lampi K. (2007) Age-related changes of α-crystallin aggregate in human lens. Amino Acids 32, 87–94 [DOI] [PubMed] [Google Scholar]
- 19. Geiger T., Clarke S. (1987) Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J. Biol. Chem. 262, 785–794 [PubMed] [Google Scholar]
- 20. Yamazaki Y., Fujii N., Sadakane Y., Fujii N. (2010) Differentiation and semiquantitative analysis of an isoaspartic acid in human α-crystallin by postsource decay in a curved field reflectron. Anal. Chem. 82, 6384–6394 [DOI] [PubMed] [Google Scholar]
- 21. Carpino L. A., Han G. Y. (1972) 9-Fluorenylmethoxycarbonyl amino-protecting group. J. Org. Chem. 37, 3404–3409 [Google Scholar]
- 22. Coste J., Le-Nguyen D., Castro B. (1990) PyBOP®: A new peptide coupling reagent devoid of toxic by-product. Tetrahedron Lett. 31, 205–208 [Google Scholar]
- 23. Furuchi T., Homma H. (2005) Free d-aspartate in mammals. Biol. Pharm. Bull. 28, 1566–1570 [DOI] [PubMed] [Google Scholar]
- 24. Hashimoto A., Nishikawa T., Oka T., Takahashi K. (1993) Endogenous d-serine in rat brain: N-methyl-d-aspartate receptor-related distribution and aging. J. Neurochem. 60, 783–786 [DOI] [PubMed] [Google Scholar]
- 25. Broccardo M., Erspamer V., Falconieri Erspamer G., Improta G., Linari G., Melchiorri P., Montecucchi P. C. (1981) Pharmacological data on dermorphins, a new class of potent opioid peptides from amphibian skin. Br. J. Pharmacol. 73, 625–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kamatani Y., Minakata H., Iwashita T., Nomoto K., In Y., Doi M., Ishida T. (1990) Molecular conformation of achatin-I, an endogenous neuropeptide containing d-amino acid residue. X-ray crystal structure of its neutral form. FEBS Lett. 276, 95–97 [DOI] [PubMed] [Google Scholar]
- 27. Boehning D., Snyder S. H. (2003) Novel neural modulators. Annu. Rev. Neurosci. 26, 105–131 [DOI] [PubMed] [Google Scholar]
- 28. Amiche M., Sagan S., Mor A., Delfour A., Nicolas P. (1989) Dermenkephalin (Tyr-d-Met-Phe-His-Leu-Met-Asp-NH2): a potent and fully specific agonist for the δ opioid receptor. Mol. Pharmacol. 35, 774–779 [PubMed] [Google Scholar]
- 29. Helfman P. M., Bada J. L. (1976) Aspartic acid racemisation in dentine as a measure of ageing. Nature 262, 279–281 [DOI] [PubMed] [Google Scholar]
- 30. Masuda W., Nouso C., Kitamura C., Terashita M., Noguchi T. (2002) d-Aspartic acid in bovine dentine non-collagenous phosphoprotein. Arch. Oral Biol. 47, 757–762 [DOI] [PubMed] [Google Scholar]
- 31. Ohtani S., Yamamoto T., Matsushima Y., Kobayashi Y. (1998) Changes in the amount of d-aspartic acid in the human femur with age. Growth Dev. Aging 62, 141–148 [PubMed] [Google Scholar]
- 32. Cloos P. A., Fledelius C. (2000) Collagen fragments in urine derived from bone resorption are highly racemized and isomerized: a biological clock of protein aging with clinical potential. Biochem. J. 345, 473–480 [PMC free article] [PubMed] [Google Scholar]
- 33. Perry R. E., Swamy M. S., Abraham E. C. (1987) Progressive changes in lens crystallin glycation and high-molecular-weight aggregate formation leading to cataract development in streptozotocin-diabetic rats. Exp. Eye Res. 44, 269–282 [DOI] [PubMed] [Google Scholar]
- 34. Ritz-Timme S., Laumeier I., Collins M. (2003) Age estimation based on aspartic acid racemization in elastin from the yellow ligaments. Int. J. Legal Med. 117, 96–101 [DOI] [PubMed] [Google Scholar]
- 35. Foulon C. F., Reist C. J., Bigner D. D., Zalutsky M. R. (2000) Radioiodination via d-amino acid peptide enhances cellular retention and tumor xenograft targeting of an internalizing anti-epidermal growth factor receptor variant III monoclonal antibody. Cancer Res. 60, 4453–4460 [PubMed] [Google Scholar]
- 36. Roher A. E., Lowenson J. D., Clarke S., Wolkow C., Wang R., Cotter R. J., Reardon I. M., Zürcher-Neely H. A., Heinrikson R. L., Ball M. J. (1993) Structural alterations in the peptide backbone of β-amyloid core protein may account for its deposition and stability in Alzheimer's disease. J. Biol. Chem. 268, 3072–3083 [PubMed] [Google Scholar]
- 37. Kaji Y., Oshika T., Takazawa Y., Fukayama M., Takata T., Fujii N. (2007) Localization of d-β-aspartic acid-containing proteins in human eyes. Invest. Ophthalmol. Vis. Sci. 48, 3923–3927 [DOI] [PubMed] [Google Scholar]
- 38. Kaji Y., Oshika T., Okamoto F., Fujii N. (2009) Immunohistochemical localisation of d-β-aspartic acid in pingueculae. Br. J. Ophthalmol. 93, 974–976 [DOI] [PubMed] [Google Scholar]
- 39. Kaji Y., Oshika T., Takazawa Y., Fukayama M., Fujii N. (2009) Immunohistochemical localisation of d-β-aspartic acid-containing proteins in climatic droplet keratopathy. Br. J. Ophthalmol. 93, 977–979 [DOI] [PubMed] [Google Scholar]
- 40. Fujii N., Tajima S., Tanaka N., Fujimoto N., Takata T., Shimo-Oka T. (2002) The presence of d-β-aspartic acid-containing peptides in elastic fibers of sun-damaged skin: a potent marker for ultraviolet-induced skin aging. Biochem. Biophys. Res. Commun. 294, 1047–1051 [DOI] [PubMed] [Google Scholar]
- 41. Mori Y., Aki K., Kuge K., Tajima S., Yamanaka N., Kaji Y., Yamamoto N., Nagai R., Yoshii H., Fujii N., Watanabe M., Kinouchi T. (2011) UV B-irradiation enhances the racemization and isomerization of aspartyl residues and production of Nϵ-carboxymethyl lysine (CML) in keratin of skin. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879, 3303–3309 [DOI] [PubMed] [Google Scholar]
- 42. Hooi M. Y., Raftery M. J., Truscott R. J. (2012) Racemization of two proteins over our lifespan: deamidation of asparagine 76 in γS crystallin is greater in cataract than in normal lenses across the age range. Invest. Ophthalmol. Vis. Sci. 53, 3554–3561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fujii N., Fujii N., Kida M., Kinouchi T. (2010) Influence of lβ-, dα- and dβ-Asp isomers of the Asp-76 residue on the properties of αA-crystallin 70–88 peptide. Amino Acids 39, 1393–1399 [DOI] [PubMed] [Google Scholar]
- 44. Santhoshkumar P., Raju M., Sharma K. K. (2011) αA-crystallin peptide SDRDKFVIFLDVKHF accumulating in aging lens impairs the function of α-crystallin and induces lens protein aggregation. PLoS One 6, e19291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Laganowsky A., Benesch J. L., Landau M., Ding L., Sawaya M. R., Cascio D., Huang Q., Robinson C. V., Horwitz J., Eisenberg D. (2010) Crystal structures of truncated αA and αB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Sci. 19, 1031–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shimizu K., Kita A., Fujii N., Miki K. (2012) Structural features of isomerizable aspartyl residues in human α-crystallins. Mol. Vis. 18, 1823–1827 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.