Background: DNA polymerase η (POLH) whose deficiency is responsible for XPV is essential for translesion DNA synthesis.
Results: GCN5-deficiency in DT40 causes both enhanced sensitivity to UV-irradiation and down-regulation of POLH expression.
Conclusion: GCN5 protects cells against UV-irradiation via controlling POLH gene expression.
Significance: This is the first study that reveals involvement of GCN5 in UV-tolerance through transcription activation of POLH.
Keywords: Chromatin Histone Modification, DNA Damage, DNA Damage Response, DNA Repair, Gene Knockout, Histone Acetylase, DT40
Abstract
By UV-irradiation, cells are subjected to DNA damage followed by mutation, cell death and/or carcinogenesis. DNA repair systems such as nucleotide excision repair (NER) and translesion DNA synthesis (TLS) protect cells against UV-irradiation. To understand the role of histone acetyltransferase GCN5 in regulation of DNA repair, we studied the sensitivity of GCN5-deficient DT40, GCN5−/−, to various DNA-damaging agents including UV-irradiation, and effects of GCN5-deficiency on the expression of NER- and TLS-related genes. After UV-irradiation, cell death and DNA fragmentation of GCN5−/− were appreciably accelerated as compared with those of DT40. Interestingly, GCN5−/− showed a remarkable sensitivity to only UV-irradiation but not to other DNA-damaging agents tested. Semiquantitative RT-PCR showed that transcription of DNA polymerase η (POLH) gene whose deficiency is responsible for a variant form of xeroderma pigmentosum was drastically down-regulated in GCN5−/− (to ∼25%). In addition, ectopic expression of human POLH in GCN5−/− dramatically reversed the sensitivity to UV-irradiation of GCN5−/− to almost the same level of wild type DT40. Moreover, chromatin immunoprecipitation assay revealed that GCN5 binds to the chicken POLH gene 5′-flanking region that contains a typical CpG island and acetylates Lys-9 of histone H3, but not Lys-14 in vivo. These data suggest that GCN5 takes part in transcription regulation of POLH gene through alterations in the chromatin structure by direct interaction with its 5′-flanking region, and protects vertebrate cells against UV-induced DNA damage via controlling POLH gene expression.
Introduction
Most organisms on the earth are subjected to UV-irradiation from the sun. UV shows strong genotoxic effects inducible to DNA damage, mutation, cell death, and/or carcinogenesis (1). UV produces specific DNA damage such as cyclobutane pyrimidine dimers (CPDs)2 and pyrimidine(6-4)pyrimidine photoproducts (6-4PDs) at dipyrimidine sites in DNA (1). Both CPDs and 6-4PDs perturb the stability of DNA structure, and cause mutations in cells. Nucleotide excision repair (NER) system removes these DNA lesions to protect cells from DNA damage (2, 3). There are two pathways in NER; transcription-coupled repair (TCR) and global genome repair (GGR). DNA damage in the transcribed strand in transcriptional active regions is repaired by TCR, and that in non-transcribed regions of the genome is removed by GGR at relatively slower rate than TCR. However, failure in NER leads to a stall of replication at the damage site, and causes DNA break followed by cell death. To avoid the catastrophe, remaining damaged bases are bypassed by specialized DNA polymerases, such as DNA polymerase η (POLH), κ (POLK), and ζ (POLZ), and DNA replication is completed (4). This process is called translesion DNA synthesis (TLS) (4, 5). The importance of these DNA repair systems (NER and TLS) is emphasized by genetic disorders known as xeroderma pigmentosum (XP), Cockayne syndrome, trichothiodystrophy, and so on (6, 7). XP is an autosomal recessive disease characterized by high sensitivity to sunlight and UV-induced skin cancers. XP consists of eight different complementation groups; groups A through G (XPA∼XPG) and a variant (XPV). The former seven XP groups result from mutations in their corresponding genes; XPA∼XPG, respectively, and their protein products are all involved in NER. XPC and XPE proteins are required only for GGR, whereas other XP proteins are required for both TCR and GGR (6, 7). In contrast, XPV has a defect in TLS which is caused by POLH deficiency (6–8). Thus, both NER and TLS are essential systems for protecting cells from UV-irradiation. However, the mechanism of epigenetic control including participation of histone acetylation in regulation of NER- and TLS-related gene expression remains to be resolved.
GCN5, a prototypical histone acetyltransferase (HAT), was first identified as a global coactivator and transcription-related HAT (9). GCN5-deficiency in mice led to early embryonic lethality with increased apoptosis in mesodermal lineages (10, 11). To further investigate physiological roles of GCN5, we generated chicken homozygous DT40 mutants, GCN5−/−, by gene targeting techniques in the chicken B cell line DT40, which are excellent methods to study physiological roles of genes involved in histone modifications including acetylation and deacetylation (12, 13). GCN5-deficiency caused not only delayed growth rate and suppressed cell cycle progression at G1/S phase transition, but also down- or up-regulation of various G1/S phase transition-related genes (14). Moreover, GCN5-deficiency caused drastic resistance against apoptosis induced by the B cell receptor-mediated stimulation through transcription regulation of various apoptosis-related genes (15), and GCN5 activated phosphatidylinositol 3-kinase (PI3K)/Akt survival pathway in cells exposed to oxidative stress via controlling gene expression of Syk and Btk (16). In addition, GCN5 promoted the superoxide-generating system in leukocytes via controlling the gp91-phox gene expression through acetylation of Lys-9 of histone H3 (H3K9) surrounding its promoter region (17).
To understand the role of GCN5 in regulation of DNA repair, in this study we studied the sensitivity of GCN5−/− to various DNA damaging agents including UV-irradiation, and also effects of GCN5-deficiency on regulations of NER- and TLS-related gene expression. Our results obtained revealed that GCN5 acts as a positive modulator for the gene expression of POLH to protect cells from UV-irradiation.
EXPERIMENTAL PROCEDURES
Materials
Bovine aprotinin, aphidicolin (APC), camptothecin (CPT), cisplatin (CDDP), methyl methanesulfonate (MMS), mitomycin C (MMC), and anti-Flag antibody (Ab) were from Sigma-Aldrich. Bleomycin (BLEO) (Invitrogen), etoposide (ETO) (Calbiochem), PMSF (Wako), monoclonal anti-GCN5 Ab (Chemicon), KOD FX DNA polymerase (Toyobo), all anti-acetylated histone antisera, and protein G-agarose/salmon sperm DNA (Millipore) were obtained.
Cell Cultures, UV-irradiation, and Treatments with DNA-damaging Agents
Generation of GCN5−/− was described in our previous report (14). DT40 cells and all subclones were cultured essentially as described (14–17). UV-irradiation was performed as follows: cells (5 × 105) in 1 ml of PBS were irradiated with UV from a 254 nm germicidal lamp at doses of 150 J/m2, resuspended in culture medium, and cultured at 37 °C up to 6 h. Treatments with DNA-damaging agents were carried out as follows: cells (2 × 106) in 10 ml of culture medium were incubated with 20 μm APC, 400 μg/ml BLEO, 30 μm CDDP, 100 nm CPT, 20 μm ETO, 1 μg/ml MMC, or 130 μg/ml MMS at 37 °C for indicated times. Viable cells were counted by the trypan blue dye exclusion method. DNA fragmentation assay was carried out as described (18).
Semiquantitative RT-PCR
Total RNAs were isolated from DT40 and its subclones. Semiquantitative RT-PCR was performed using appropriate sense and antisense primers listed in supplemental Table S1, which were synthesized according to the sequence data deposited in GenBankTM as described (14–17). Expression of NER- and TLS-related genes found in chicken was analyzed. Chicken GAPDH gene was used as internal controls. PCR products were subjected to 1.5% agarose gel electrophoresis. Data obtained by semiquantitative RT-PCR before reaching the plateau were analyzed by Image Gauge software Profile mode (densitometrical analysis mode) using a luminescent image analyzer LAS-1000plus (Fujifilm).
Ectopic Expression of Human POLH (hPOLH) in GCN5−/−
To obtain the Flag-tagged hPOLH expression vector, a full-length hPOLH cDNA (kindly provided by Dr. S. Takeda at Radiation Genetics, Graduate School of Medicine, Kyoto University, Japan) was inserted into pApuro, carrying the chicken β-actin promoter upstream of the cloning site and a marker gene (the puro-resistant gene) under the control of the SV40 promoter (19, 20). It was reported that ectopic expression of hPOLH could reverse the phenotype of POLH-deficient DT40 cells (21). The GCN5−/− cells were transfected with the vector using electroporation and the transfectant clones were selected in the presence of puromycin (0.4 μg/ml). PCR was performed using primers corresponding to common sequences between chicken POLH and hPOLH (supplemental Table S1). Immunoblotting using anti-Flag Ab was carried out as described (19, 20).
5′-Rapid Amplification of cDNA Ends (5′-RACE)
Transcription initiation site of chicken POLH was determined by 5′-RACE using a 5′-RACE Kit (Takara) according to manufacturer's instructions. Oligonucleotide 5′-CTGCACGAAGGTGGT-3′ was used as a RT-primer. The primers used in primary and secondary PCR reactions were RACE-1 and -2 primers, respectively (supplemental Table S1).
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assay was performed using Chromatin Immunoprecipitation Assay Kit (Millipore). Formaldehyde-fixed cells (1 × 106) were lysed in the presence of 1 mm PMSF and 100 μg/ml aprotinin. Lysates were sonicated with Biorupter UCD-250 (Cosmo Bio) to shear DNA to lengths between 200 and 1000 bp and centrifuged for 10 min at 12,000 × g at 4 °C. Immunoprecipitation with 2 μg anti-GCN5 Ab (or 2 μg irrelevant IgG as negative control) or 2 μl anti-acetylated histone antisera (or 2 μl normal rabbit serum as negative control) was carried out as described (17, 18). Immunoprecipitated and input DNAs were analyzed by PCR using appropriate primers (ChIP primer, see supplemental Table S1) corresponding to the 5′-flanking region of the chicken POLH gene. PCRs using KOD FX DNA polymerase were carried out at 98 °C for 20 s, 59 °C for 20 s and 68 °C for 30 s, for 32∼36 cycles, and stopped before reaching the plateau. PCR products were subjected to 1.5% agarose gel electrophoresis and analyzed using a luminescent image analyzer LAS-1000plus.
RESULTS
GCN5-deficiency Enhances the Sensitivity to UV-irradiation
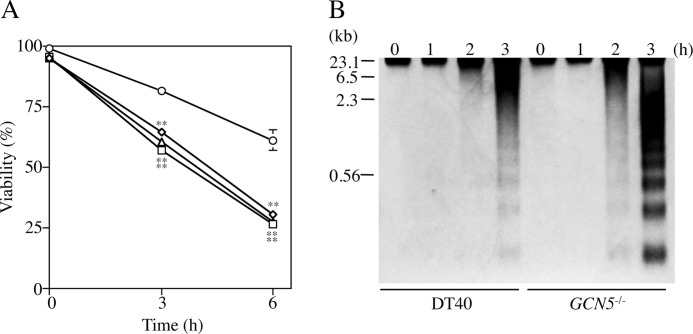
To know influences of GCN5-deficiency on UV-induced damage, we examined effects of UV-irradiation on cell viability and DNA fragmentation in GCN5−/− and wild type DT40. After UV-irradiation, the viability of three independent GCN5−/− clones was appreciably reduced (to ∼25% at 6 h) as compared with that of DT40 (to ∼65% at 6 h) (Fig. 1A). In addition, the DNA fragmentation in GCN5−/− clone 1 was more prominent compared with that of DT40 (Fig. 1B). Similar results were obtained in two other GCN5−/− clones (data not shown). These results suggested that GCN5 is necessary for cell survival against UV-irradiation in DT40.
FIGURE 1.
Influences of GCN5-deficiency on sensitivity to UV-irradiation. A, cell viability. After UV-irradiation (150 J/m2), DT40 (circles) and three independent GCN5−/− clones 1, 2, and 3 (squares, triangles, and ovals) were cultured at 37 °C up to 6 h. Viable cells were counted by the trypan blue dye exclusion method. Data represent the average of three separate experiments. Error bars indicate S.D. Statistical differences were calculated using t test. **, p < 0.01. B, DNA fragmentation. After UV-irradiation, the cells (DT40 and clone 1) were cultured at 37 °C for 0, 1, 2, and 3 h. DNAs were isolated from the cells and analyzed by 1.5% agarose gel electrophoresis. The sizes of λ-DNA digested with HindIII are indicated in kilobase pairs.
GCN5-deficiency Shows Insignificant Influences on the Sensitivity to Various DNA-damaging Agents
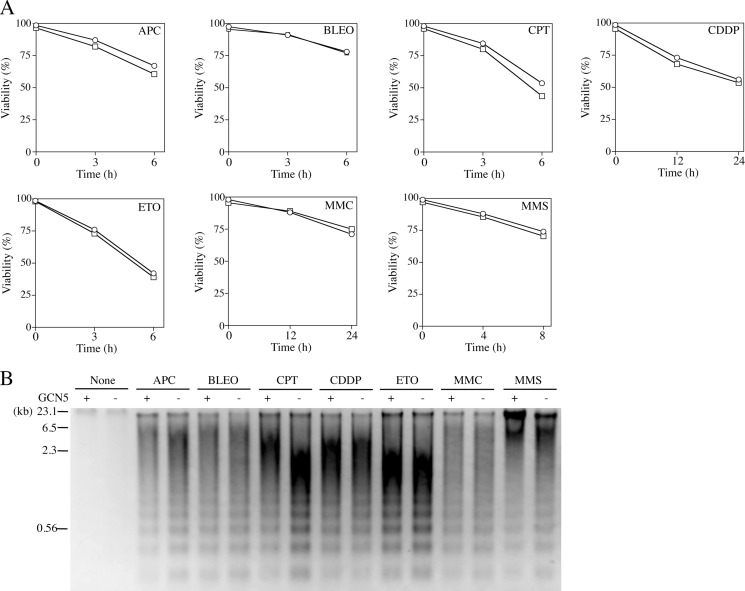
Next, we examined effects of GCN5-deficiency on the sensitivity to various DNA-damaging agents; APC, BLEO, CPT, CDDP, ETO, MMC, and MMS. As shown in Fig. 2, GCN5−/− clone 1 showed insignificant effects of GCN5-deficiency on the sensitivity to all agents tested. Interestingly, GCN5−/− showed a remarkable sensitivity to only UV-irradiation but not to other DNA-damaging agents. Similar results were obtained using two other GCN5−/− clones (data not shown). These data suggested that GCN5-deficiency may affect the expression of DNA repair, especially NER- and/or TLS-related genes.
FIGURE 2.
Influences of GCN5-deficiency on sensitivity to various DNA-damaging agents. A, cell viability. DT40 (circles) and GCN5−/− clone 1 (squares) were cultured with various DNA-damaging agents; 20 μm APC, 400 μg/ml BLEO, 30 μm CDDP, 100 nm CPT, 20 μm ETO, 1 μg/ml MMC, or 130 μg/ml MMS at 37 °C up to indicated times, respectively. Viable cells were counted by the trypan blue dye exclusion method. Data represent the average of three separate experiments. B, DNA fragmentation. DT40 (+) and GCN5−/− clone 1 (−) were cultured with various DNA-damaging agents; 20 μm APC (6 h), 400 μg/ml BLEO (6 h), 30 μm CDDP (24 h), 100 nm CPT (6 h), 20 μm ETO (6 h), 1 μg/ml MMC (24 h), or 130 μg/ml MMS (8 h) at 37 °C. DNAs were isolated from the cells and analyzed by 1.5% agarose gel electrophoresis. The sizes of λ-DNA digested with HindIII are indicated in kilobase pairs.
GCN5-deficiency Down-regulates Gene Expression of POLH
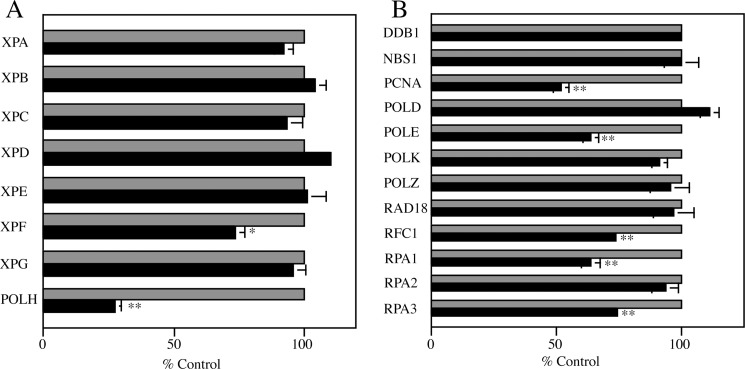
To study the influences of GCN5-deficiency on expression of NER- and TLS-related genes; XPA∼XPG, POLH, DNA-damage-binding protein 1 (DDB1), Nijmegen Breakage Syndrome 1 (NBS1), proliferation cell nuclear antigen (PCNA), DNA polymerase δ (POLD), DNA polymerase ϵ (POLE), POLK, POLZ, RAD18, replication factor 1 (RFC1) and replication protein A 1∼3 (RPA1∼3), we carried out semiquantitative RT-PCR on total RNAs prepared from three independent GCN5−/− clones and DT40 (Fig. 3). As expected, GCN5-deficiency showed significant influences on transcription of some NER- and TLS-related genes; XPF (to ∼75%), PCNA (to ∼55%), POLE (to ∼65%), RFC1 (to ∼75%), RPA1 (to ∼65%) and RPA3 (to ∼75%). In particular, transcription of POLH gene whose deficiency is responsible for XPV was drastically down-regulated in GCN5−/− (to ∼25%). As mentioned above, GCN5−/− showed an enhanced sensitivity to UV-irradiation but not to other DNA-damaging agents used. Very interestingly, the phenotype of GCN5−/− is similar to that of POLH-deficient DT40 cells (21). These findings, combined, suggested that the remarkable decrease in gene expression of POLH in GCN5−/− probably resulted in accelerated sensitivity to UV-irradiation as compared with that of DT40.
FIGURE 3.
Influences of GCN5-deficiency on expression of NER- and TLS-related genes. A, XP-related genes. B, other NER- and TLS-related genes. Total RNAs were extracted from DT40 and three independent GCN5−/− clones, and mRNA levels of NER- and TLS-related genes found in chicken were determined by semiquantitative RT-PCR using appropriate primers (see supplemental Table S1). Chicken GAPDH gene was used as an internal control for calibration. Data represent averages of three independent GCN5−/− clones (solid bars), and indicated as percentages of control values (100%) obtained from DT40 cells (gray bars). Error bars indicate S.D. Statistical differences were calculated using t test. *, p < 0.05; **, p < 0.01.
Ectopic Expression of hPOLH Rescues GCN5−/− from the Enhanced Sensitivity to UV-irradiation
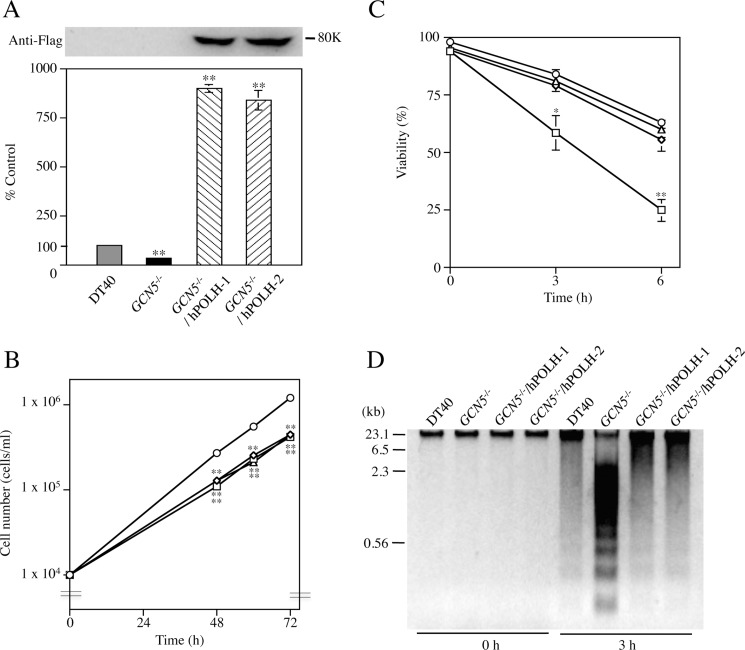
To investigate whether or not the down-regulation of POLH caused the enhanced sensitivity to UV-irradiation in GCN5−/−, ectopic expression of POLH was performed using hPOLH expression vector. Two resultant transfectant clones GCN5−/−/hPOLH-1 and -2 expressed hPOLH gene in large excess (∼800%) compared with endogenous POLH gene in the wild-type DT40 and synthesized definite amounts of Flag-tagged hPOLH (Fig. 4A). Ectopic expression of hPOLH showed insignificant effect on the growth rate of GCN5−/− (Fig. 4B). Next, to study the influences of ectopic expression of hPOLH on UV-induced damage in GCN5−/−, we examined effects of UV-irradiation on cell viability and DNA fragmentation in GCN5−/−/hPOLH-1, -2, GCN5−/−, and wild type DT40. As expected, both cell viability and DNA fragmentation were significantly reversed by ectopic expression of hPOLH to almost the same levels of wild type DT40 (Fig. 4, C and D), suggesting that the remarkable decrease of POLH mainly caused high sensitivity to UV-irradiation in GCN5−/−.
FIGURE 4.
Recovery of the sensitivity to UV-irradiation by ectopic expression of hPOLH in GCN5−/−. A, ectopic expression of hPOLH in GCN5−/−. Upper panel: immunoblotting. Flag-tagged hPOLH was detected using anti-Flag M2 Ab. Lower panel: semiquantitative RT-PCR. Total RNAs were extracted from DT40 (gray bar), GCN5−/− (solid bar), and two independent GCN5−/−/hPOLH clones-1 and -2 (striped bars). PCR was performed using primers corresponding to common sequences between chicken POLH and hPOLH (see supplemental Table S1). Chicken GAPDH gene was used as an internal control for calibration. Data represent averages of three separate experiments, and indicated as percentages of control values (100%) obtained from DT40. Error bars indicate S.D. Statistical differences were calculated using t test. **, p < 0.01. B, growth curves of DT40 and its subclones. DT40 (circles), GCN5−/− (squares), and two independent GCN5−/−/hPOLH clones-1 and -2 (triangles and ovals) were grown, and cell numbers were determined at indicated times. The numbers are plotted on a log phase. The values are averages for three independent experiments. Statistical differences were calculated using t test. **, p < 0.01. C, effects of ectopic expression of hPOLH on cell viability of GCN5−/− after UV-irradiation. After UV-irradiation (150 J/m2), DT40 (circles), GCN5−/− (squares), and two independent GCN5−/−/hPOLH clones-1 and -2 (triangles and ovals) were cultured at 37 °C up to 6 h. Viable cells were counted by the trypan blue dye exclusion method. Data represent averages of three separate experiments. Error bars indicate S.D. Statistical differences were calculated using t test. *, p < 0.05; **, p < 0.01 compared with DT40 and two independent GCN5−/−/hPOLH clones-1 and -2. D, effects of ectopic expression of hPOLH on DNA fragmentation in GCN5−/− after UV-irradiation. After UV-irradiation, the cells were cultured at 37 °C for 0 and 3 h. DNAs were isolated from the cells and analyzed by 1.5% agarose gel electrophoresis. The sizes of λ-DNA digested with HindIII are indicated in kilobase pairs.
GCN5 Binds to the 5′-Flanking Region of the Chicken POLH Gene and Contributes to Acetylation of H3K9 Residues within Chromatin Surrounding the Region
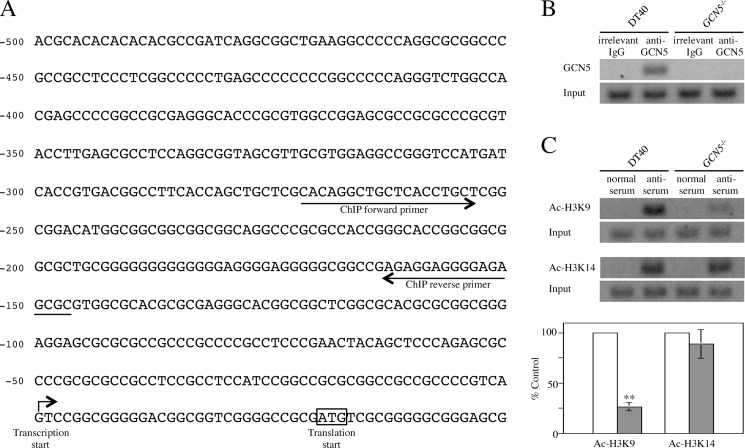
We examined by ChIP assay whether or not GCN5 interacts with regulatory region of the chicken POLH gene. First, to determine the transcription initiation site of the gene, we carried out 5′-RACE method (Fig. 5A). Data obtained revealed that the transcription initiation site G was located at position −30 from the translation start codon ATG, and a typical CpG island was located in the 5′-flanking region of the gene (C+G content = 80.6%, observed CpG/expected CpG = 0.986, length = 500 bp) (22). Next, we performed ChIP assay using anti-GCN5 Ab and anti-acetylated histone (H3K9 and H3K14) antisera. These two Lys residues of histone H3 are typical acetylation sites catalyzed by GCN5 (17, 23–28). Cross-linked chromatins were co-precipitated from lysates of DT40 and GCN5−/− with these three Ab agents. Precipitated chromatins were amplified by PCR using ChIP forward and reverse primers (Fig. 5A and supplemental Table S1) for the 5′-flanking region of the chicken POLH gene. Concerning GCN5, as expected, amplified DNA fragment could be detected in DT40 but not in GCN5−/− using these ChIP primers (Fig. 5B). Regarding acetylated histones, acetylation of H3K9 within chromatin surrounding the 5′-flanking region of the gene was remarkably decreased in GCN5−/− (to ∼25% of control value obtained from DT40), whereas GCN5-deficiency showed insignificant effect on acetylation of H3K14 (Fig. 5C). For both GCN5 and acetylated histones, similar results were obtained using two other GCN5−/− clones (data not shown). These data revealed that GCN5 binds to the 5′-flanking region of the chicken POLH gene and acetylates H3K9 residue in vivo, suggesting that gene expression of POLH is certainly regulated by GCN5 in vertebrate cells.
FIGURE 5.
Interaction of GCN5 with 5′-flanking region of chicken POLH gene and acetylation of Lys-9 and Lys-14 residues of histone H3 within chromatin surrounding the region. A, schematic diagram for the 5′-flanking region of chicken POLH gene. The transcription start site of POLH gene determined by 5′-RACE is indicated by an arrow. The translation start codon ATG is enclosed by an open box. ChIP forward- and reverse-primers used for PCR in ChIP assay are shown. B, interaction of GCN5 with the 5′-flanking region of chicken POLH gene. The cross-linked chromatins from cell lysates of DT40 and GCN5−/− were co-precipitated by anti-GCN5 Ab. Irrelevant IgG was used as a negative control. After decross-linking, co-precipitated chromatin and input samples were amplified by PCR using ChIP primers. PCR products were analyzed by 1.5% agarose gel electrophoresis. Typical patterns are shown. C, acetylation of Lys residues of histone H3 within chromatin surrounding the 5′-flanking region of chicken POLH gene. ChIP assay was carried out as in B using antisera specific for acetylated H3K9 (Ac-H3K9) and H3K14 (Ac-H3K14). The cross-linked chromatins from cell lysates of DT40 (open bars) and GCN5−/− (gray bars) were co-precipitated by these antisera. Rabbit normal serum was used as a negative control. Typical patterns are shown. Quantitative data are indicated as percentages of control values (100%) obtained from DT40, and represent the averages of three separate experiments. Error bars indicate S.D. Statistical differences were calculated using t test. **, p < 0.01.
DISCUSSION
Our findings in this study reveal that GCN5 is involved in UV-tolerance through controlling gene expression of POLH in vertebrate cells. GCN5, one of the most important HATs, plays specialized roles in DNA repair and transcription activation by changing chromatin structure (26–29). Concerning the former, as is well known, GCN5 promotes recruitment of DNA repair-related protein factors to damaged sites and efficient DNA repair. For example, the human TFTC complex containing a homolog of DDB1 preferentially binds to nucleosomes on UV-damaged DNA and acetylates histones (30, 31). GCN5 in human STAGA complex also plays a role in p53-dependent gene activation by mediating coactivator recruitment after UV-irradiation (32). In addition, the GCN5-mediated histone H3 hyperacetylation promotes efficient NER at MFA2 in UV-irradiated yeast (33, 34). Recently, it was reported that GCN5 cooperated with E2F1 to stimulate NER by promoting H3K9 acetylation at UV-induced DNA damage sites (35). Thus, GCN5 acetylates core histones around the damaged DNA in response to UV-irradiation, and the enhanced histone acetylation stimulates recruitment of NER factors, resulting in efficient DNA repair by NER. However, physiological roles of GCN5 for regulation of DNA repair systems via transcription activation of specific genes concerning NER or TLS remain poorly defined in vertebrate cells.
Interestingly, the GCN5-deficient DT40 mutants, GCN5−/−, established by us showed a remarkably enhanced sensitivity to UV-irradiation but not to other DNA-damaging agents (Figs. 1 and 2), suggesting that GCN5-deficiency may have an effect on expression of NER- and/or TLS-related genes. We carried out semiquantitative RT-PCR to study influences of GCN5-deficiency on expression of these genes. As expected, GCN5-deficiency caused significant influences on transcriptions of various genes tested (Fig. 3). In particular, gene expression of POLH whose deficiency is responsible for XPV (8) was significantly down-regulated (to ∼25%) in GCN5−/− (Fig. 3A). It was reported that POLH-deficient DT40 cells are sensitive to UV but not to several genotoxic stresses (21). As mentioned above, GCN5−/− showed a high sensitivity to only UV-irradiation among the DNA-damaging agents tested. Thus, very interestingly, the phenotype of GCN5−/− resembles POLH-deficient DT40 cells concerning the sensitivity to DNA-damaging agents including UV-irradiation.
Next, to determine whether or not the down-regulation of POLH was really involved in the enhanced sensitivity to UV-irradiation in GCN5−/−, hPOLH whose ectopic expression could reverse the phenotype of POLH-deficient DT40 cells (21) was expressed in GCN5−/− (Fig. 4A). Ectopic expression of hPOLH in large excess (∼800%) in GCN5−/− dramatically reversed sensitivity to UV-irradiation of GCN5−/− to almost the same level of wild type DT40 (Fig. 4, C and D), while it exerted insignificant effects on cell growth (Fig. 4B). Since GCN5-deficiency caused delayed growth rate of DT40 cells (14), there was a possibility that the difference in sensitivity to UV between GCN5−/− and DT40 may be reflected in the difference in growth rate. However, despite the delayed growth rate, sensitivity to UV-irradiation of GCN5−/− was recovered to almost the same level of DT40 by ectopic expression of hPOLH. The possibility that the enhanced sensitivity to UV of GCN5−/− was attributable to their delayed growth rate could be nearly eliminated by these data. In addition, after cell cycle synchronization with hydroxyurea, GCN5−/− also showed a remarkably enhanced sensitivity to UV-irradiation than DT40 (data not shown). Therefore, our data, together, suggested that the drastic decrease in the POLH gene expression mainly resulted in remarkably enhanced sensitivity to UV-irradiation of GCN5−/−. In contrast, the deficiency of p300/CBP-associated factor (PCAF), another HAT belonging to GCN5-family, showed insignificant influences on both the sensitivity to UV-irradiation and POLH gene expression (data not shown). As shown previously (14), the gene expression of PCAF was extremely increased in GCN5−/−, suggesting that PCAF could play in part complementary roles in chromatin dynamics linked to the acetylation state of core histones in absence of GCN5. Probably, transcriptional regulation of the POLH gene, similar to several genes such as gp91-phox (14–17), is an inherent function of GCN5, and cannot be appreciably complemented by PCAF or any other HATs.
A typical CpG island, which has been found in promoters and exonic regions of ∼40% of mammalian genes (36), was located in the 5′-flanking region of chicken POLH gene. We examined interaction between GCN5 and the 5′-flanking region of the POLH gene, and histone acetylation within chromatin surrounding the region by ChIP assay. The results obtained revealed that GCN5 binds to the 5′-flanking region of the POLH gene and acetylates H3K9 residue but not H3K14 in vivo (Fig. 5), and agreed with our previous data; GCN5-deficiency in DT40 cells led to decreased bulk acetylation level of H3K9 but not H3K14 (14). As is well known, GCN5-mediated acetylation of H3K9 participates in the transcription activation of various genes (17, 23–28). Therefore, the gene expression of POLH is certainly regulated by GCN5 through acetylation of H3K9 in vertebrate cells.
In conclusion, data obtained in this study, together with previous reports, indicate not only that GCN5 promotes recruitment of DNA repair-related protein factors to the UV-damaged sites and efficient DNA repair (30–35), but also that it takes part in transcriptional regulation of POLH gene through alterations in the chromatin structure by direct interaction with its 5′-flanking region and protects cells against UV-induced DNA damage. As is well known, POLH is essential for TLS over UV-induced DNA damage. It was recently showed that POLH contributes to a much wide range of TLS events than had been predicted by the phenotype of XPV cells (21). Moreover, POLH is also involved in somatic hypermutation (SHM) mechanism (37). SHM introduces nucleotide substitution into immunoglobulin variable genes to increase antibody diversity. In the recent 10 years, HATs and histone deacetylases have become subjects of interest as targets of drug innovation (38, 39). Expression of tens of thousands of genes should be regulated by HATs, though less than 20 HATs have been found in vertebrates (28). Therefore, the functions of HATs show redundancy, for example, PCAF can play in part complementary roles in absence of GCN5 (14). Interestingly, mice lacking PCAF are developmentally normal without a distinct phenotype, while animals lacking GCN5 die during embryogenesis (13). These results suggested that GCN5 has specialized functions necessary for life support systems in cells. In fact, we have revealed specialized functions of GCN5; down-regulation of B cell receptor-mediated signaling (15), up-regulation of PI3K/Akt survival pathway (16), and control of superoxide-generating system in leukocytes (17). Thus, studies on specific and overlapped functions of HATs would be very important for applied science such as drug innovation, although these molecular mechanisms should be elucidated in the future. Here, in addition to specialized functions mentioned above, we revealed a new inherent function of GCN5; GCN5 may be involved in TLS and SHM via controlling POLH gene expression. Our results will significantly help in the understanding of epigenetic regulation specific for XPV, molecular mechanisms for network of DNA repair, SHM of immunoglobulin genes and drug innovation.
Supplementary Material
Acknowledgments
We thank M. Nakayama, H. Madhyastha, and R. Madhyastha for editorial reading of the manuscript. We also thank R. Masuya and N. Nagamatsu-Yamamoto for technical assistance.
This work was supported in part by Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 22510203) (to H. K. and T. N.).

This article contains supplemental Table S1.
- CPD
- cyclobutane pyrimidine dimer
- 6-4PD
- pyrimidine(6-4)pyrimidine photoproduct
- NER
- nucleotide excision repair
- TCR
- transcription-coupled repair
- GGR
- global genome repair
- POLH
- DNA polymerase η
- POLK
- DNA polymerase κ
- POLZ
- DNA polymerase ζ
- TLS
- translesion DNA synthesis
- XP
- xeroderma pigmentosum
- HAT
- histone acetyltransferase
- PI3K
- phosphatidylinositol 3-kinase
- H3K9
- Lys-9 of histone H3
- APC
- aphidicolin
- BLEO
- bleomycin
- CPT
- camptothecin
- CDDP
- cisplatin
- MMS
- methyl methanesulfonate
- MMC
- mitomycin C
- ETO
- etoposide
- Ab
- antibody
- hPOLH
- human POLH
- RACE
- rapid amplification of cDNA ends
- ChIP
- chromatin immunoprecipitation
- DDB1
- DNA-damage-binding protein 1
- NBS1
- Nijmegen Breakage Syndrome 1
- PCNA
- proliferation cell nuclear antigen
- POLD
- DNA polymerase δ
- POLE
- DNA polymerase ϵ
- RFC
- replication factor
- RPA
- replication protein A
- PCAF
- p300/CBP-associated factor
- SHM
- somatic hypermutation.
REFERENCES
- 1. Ikehata H., Ono T. (2011) The mechanisms of UV mutagenesis. J. Radiat. Res. 52, 115–125 [DOI] [PubMed] [Google Scholar]
- 2. Balajee A. S., Bohr V. A. (2000) Genomic heterogeneity of nucleotide excision repair. Gene 250, 15–30 [DOI] [PubMed] [Google Scholar]
- 3. Lans H., Marteijn J. A., Schumacher B., Hoeijmakers J. H., Jansen G., Vermeulen W. (2010) Involvement of grobal genome repair, transcription coupled repair, and chromatin remodeling in UV DNA damage response changes during development. PLoS Genet. 6, e1000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lehmann A. R., Niimi A., Ogi T., Brown S., Sabbioneda S., Wing J. F., Kannouche P. L., Green C. M. (2007) Translesion synthesis: Y-family polymerases and the polymerase switch. DNA Repair 6, 891–899 [DOI] [PubMed] [Google Scholar]
- 5. Lehmann A. R. (2006) Translesion synthesis in mammalian cells. Exp. Cell Res. 312, 2673–2676 [DOI] [PubMed] [Google Scholar]
- 6. DiGiovanna J. J., Kraemer K. H. (2012) Shining a light on xeroderma pigmentosum. J. Invest. Dermatol. 132, 785–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lehmann A. R., McGibbon D., Stefanini M. (2011) Xeroderma pigmentosum. Orphanet. J. Rare Dis. 6, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Masutani C., Kusumoto R., Yamada A., Dohmae N., Yokoi M., Yuasa M., Araki M., Iwai S., Takio K., Hanaoka F. (1999) The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature 399, 700–704 [DOI] [PubMed] [Google Scholar]
- 9. Brownell J. E., Zhou J., Ranalli T., Kobayashi R., Edmondson D. G., Roth S. Y., Allis C. D. (1996) Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 84, 843–851 [DOI] [PubMed] [Google Scholar]
- 10. Xu W., Edmondson D. G., Evrard Y. A., Wakamiya M., Behringer R. R., Roth S. Y. (2000) Loss of Gcn5l2 leads to increased apoptosis and mesodermal defects during mouse development. Nat. Genet. 26, 229–232 [DOI] [PubMed] [Google Scholar]
- 11. Yamauchi T., Yamauchi J., Kuwata T., Tamura T., Yamashita T., Bae N., Westphal H., Ozato K., Nakatani Y. (2000) Distinct but overlapping roles of histone acetylase PCAF and of the closely related PCAF-B/GCN5 in mouse embryogenesis. Proc. Natl. Acad. Sci. U.S.A. 97, 11303–11306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buerstedde J. M., Takeda S. (1991) Increased ration of targeted to random integration after transfection of chicken B cell lines. Cell 67, 179–188 [DOI] [PubMed] [Google Scholar]
- 13. Kikuchi H., Barman H. K., Nakayama M., Takami Y., Nakayama T. (2006) Participation of histones, histone modifying enzymes and histone chaperones in vertebrate cell functions. Subcell. Biochem. 40, 225–243 [DOI] [PubMed] [Google Scholar]
- 14. Kikuchi H., Takami Y., Nakayama T. (2005) GCN5: a supervisor in all-inclusive control of vertebrate cell cycle progression through transcription regulation of various cell cycle-related genes. Gene 347, 83–97 [DOI] [PubMed] [Google Scholar]
- 15. Kikuchi H., Nakayama T. (2008) GCN5 and BCR signalling collaborate to induce pre-mature B cell apoptosis through depletion of ICAD and IAP2 and activation of caspase activities. Gene 419, 48–55 [DOI] [PubMed] [Google Scholar]
- 16. Kikuchi H., Kuribayashi F., Takami Y., Imajoh-Ohmi S., Nakayama T. (2011) GCN5 regulates the activation of PI3K/Akt survival pathway in B cells exposed to oxidative stress via controlling gene expressions of Syk and Btk. Biochem. Biophys. Res. Commun. 405, 657–661 [DOI] [PubMed] [Google Scholar]
- 17. Kikuchi H., Kuribayashi F., Kiwaki N., Takami Y., Nakayama T. (2011) GCN5 regulates the superoxide-generating system in leukocytes via controlling gp91-phox gene expression. J. Immunol. 186, 3015–3022 [DOI] [PubMed] [Google Scholar]
- 18. Kikuchi H., Imajoh-Ohmi S. (1995) Activation and possible involvement of calpain, a calcium-activated cysteine protease, in down-regulation of apoptosis of human monoblast U937 cells. Cell Death Differ. 2, 195–199 [PubMed] [Google Scholar]
- 19. Kikuchi H., Yamashita K., Nakayama M., Toyonaga K., Tsuneyoshi I., Takasaki M., Nakayama T. (2009) Lacking of Aiolos accelerates pre-mature B cell apoptosis mediated by BCR signaling through elevation in cytochrome c release. Biochim. Biophys. Acta 1793, 1304–1314 [DOI] [PubMed] [Google Scholar]
- 20. Kikuchi H., Nakayama M., Takami Y., Kuribayashi F., Nakayama T. (2012) EBF1 acts as a powerful repressor of Blimp-1 gene expression in immature B cells. Biochem. Biophys. Res. Commun. 422, 780–785 [DOI] [PubMed] [Google Scholar]
- 21. Hirota K., Sonoda E., Kawamoto T., Motegi A., Masutani C., Hanaoka F., Szüts D., Iwai S., Sale J. E., Lehmann A., Takeda S. (2010) Simultaneous disruption of two DNA polymerases, Polη and Polζ, in avian DT40 cells unmasks the role of Polη in cellular response to various DNA lesions. PLoS Genet. 6, e1001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takai D., Jones P. A. (2002) Comprehensive analysis of CpG islands in human chromosomes 21 and 22. Proc. Natl. Acad. Sci. U.S.A. 99, 3740–3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grant P. A., Eberharter A., John S., Cook R. G., Turner B. M., Workman J. L. (1999) Expanded lysine acetylation specificity of Gcn5 in native complexes. J. Biol. Chem. 274, 5895–5900 [DOI] [PubMed] [Google Scholar]
- 24. Suka N., Suka Y., Carmen A. A., Wu J., Grunstein M. (2001) Highly specific antibodies determine histone acetylation site usage in yeast heterochromatin and euchromatin. Mol. Cell 8, 473–479 [DOI] [PubMed] [Google Scholar]
- 25. Shimada M., Niida H., Zineldeen D. H., Tagami H., Tanaka M., Saito H., Nakanishi M. (2008) Chk1 is a histone H3 threonine 11 kinase that regulates DNA damage-induced transcriptional repression. Cell 132, 221–232 [DOI] [PubMed] [Google Scholar]
- 26. Kouzarides T. (2007) Chromatin modifications and their function. Cell 128, 693–705 [DOI] [PubMed] [Google Scholar]
- 27. Lee K. K., Workman J. L. (2007) Histone acetyltransferase complexes: one size dosen't fit all. Nat. Rev. Mol. Cell Biol. 8, 284–295 [DOI] [PubMed] [Google Scholar]
- 28. Allis C. D., Berger S. L., Cote J., Dent S., Jenuwien T., Kouzarides T., Pillus L., Reinberg D., Shi Y., Shiekhattar R., Shilatifard A., Workman J., Zhang Y. (2007) New nomenclature for chromatin-modifiying enzymes. Cell 131, 633–636 [DOI] [PubMed] [Google Scholar]
- 29. Waters R., Reed S. H., Yu Y., Teng Y. (2008) Chromatin modifications and nucleotide excision repair. SEB Exp. Biol. Ser. 59, 189–201 [PubMed] [Google Scholar]
- 30. Brand M., Moggs J. G., Oulad-Abdelghani M., Lejeune F., Dilworth F. J., Stevenin J., Almouzni G., Tora L. (2001) UV-damaged DNA-binding protein in the TFTC complex links DNA damage recognition to nucleosome acetylation. EMBO J. 20, 3187–3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martinez E., Palhan V. B., Tjernberg A., Lymar E. S., Gamper A. M., Kundu T. K., Chait B. T., Roeder R. G. (2001) Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo. Mol. Cell Biol. 21, 6782–6795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gamper A. M., Roeder R. G. (2008) Multivalent binding of p53 to the STAGA complex mediates coactivator recruitment after UV damage. Mol. Cell Biol. 28, 2517–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Teng Y., Yu Y., Waters R. (2002) The Saccharomyces cerevisiae histone acetyltransferase Gcn5 has a role in the photoreactivation and nucleotide excision repair of UV-induced cyclobutane pyrimidine dimers in the MFA2 gene. J. Mol. Biol. 316, 489–499 [DOI] [PubMed] [Google Scholar]
- 34. Yu Y., Teng Y., Liu H., Reed S. H., Waters R. (2005) UV irradiation stimulates histone acetylation and chromatin remodeling at a repressed yeast locus. Proc. Natl. Acad. Sci. U.S.A. 102, 8650–8655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guo R., Chen J., Mitchell D. L., Johnson D. G. (2011) GCN5 and E2F1 stimulate nucleotide excision repair by promoting H3K9 acetylation at sites of damage. Nucleic Acids Res. 39, 1390–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fatemi M., Pao M. M., Jeong S., Gal-Yam E. N., Egger G., Weisenberger D. J., Jones P. A. (2005) Footprinting of mammalian promoters: use of a CpG DNA methyltransferase revealing nucleosome positions at a single molecule level. Nucleic Acids Res. 33, e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steele E. J. (2009) Mechanism of somatic hypermutation: critical analysis of strand biased mutation signatures at A:T and G:C base pairs. Mol. Immunol. 46, 305–320 [DOI] [PubMed] [Google Scholar]
- 38. Furdas S. D., Kannan S., Sippl W., Jung M. (2012) Small molecule inhibitors of histone acetyltransferases as epigenetic tools and drug candidates. Arch. Pharm. 345, 7–21 [DOI] [PubMed] [Google Scholar]
- 39. Gray S. G., Dangond F. (2006) Rationale for the use of histone deacetylase inhibitors as a dual therapeutic modality in multiple sclerosis. Epigenetics 1, 67–75 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.